Abstract
Asthma is characterized by appearance of eosinophils in the airway. Eosinophils purified from the airway 48 h after segmental antigen challenge are described as exhibiting greater adhesion to albumin-coated surfaces via an unidentified β2 integrin and increased expression of αMβ2 (CD11b/18) compared with purified blood eosinophils. We have investigated the determinants of this hyperadhesive phenotype. Airway eosinophils exhibited increased reactivity with the CBRM1/5 anti-αM activation-sensitive antibody as well as enhanced adhesion to VCAM-1 (CD106) and diverse ligands, including albumin, ICAM-1 (CD54), fibrinogen, and vitronectin. Purified blood eosinophils did not adhere to the latter diverse ligands. Enhanced adhesion of airway eosinophils was blocked by anti-αMβ2. Podosomes, structures implicated in cell movement and proteolysis of matrix proteins, were larger and more common on airway eosinophils adherent to VCAM-1 when compared with blood eosinophils. Incubation of blood eosinophils with IL-5 replicated the phenotype of airway eosinophils. That is, IL-5 enhanced recognition of αM by CBRM1/5; stimulated αMβ2-mediated adhesion to VCAM-1, albumin, ICAM-1, fibrinogen, and vitronectin; and increased podosome formation on VCAM-1. Thus, the hyperadhesion of airway eosinophils after antigen challenge is mediated by upregulated and activated αMβ2.
Keywords: adhesion molecules, cell trafficking, eosinophils, human
Asthma is an inflammatory syndrome of the airway accompanied by recurrent episodes of airway obstruction, mucus hypersecretion, and bronchial smooth muscle contraction (1). The prevalence of asthma worldwide is increasing, but the causes of the increase remain unclear (2–4). In individuals with allergic asthma, recruitment of eosinophils (EOS) into the airway lumen is believed to exacerbate asthmatic symptoms and lead to the chronic character of asthma (5, 6).
How EOS traffic to the airway is incompletely understood and of considerable importance to asthma. Heterodimeric integrin adhesion receptors, which are among the most functionally versatile of known cellular receptors, have generated particular interest as determinants of how EOS are recruited to the asthmatic airway (7, 8). Purified blood EOS express seven integrin heterodimers: α4β1 (CD49d/29), α6β1 (CD49f/29), αMβ2 (CD11b/18), αLβ2 (CD11a/18), αXβ2 (CD11c/18), αDβ2 (αD/18), and α4β7 (CD49d/β7) (9–11). Each kind of heterodimer may adopt several conformational states that regulate integrin-mediated adhesion and movement (12, 13). Integrins are thus believed to mediate rolling and arrest of EOS on endothelium, migration through endothelium and the underlying basement membrane in response to chemotactic stimuli, and traversing of bronchial epithelium into the airway lumen (14, 15).
EOS obtained from bronchoalveolar lavage (BAL) fluid after segmental antigen challenge represent cells that have transmigrated from blood to airway in response to inflammatory mediators. These EOS exhibit elevated adhesion to albumin mediated via an unidentified β2 integrin family member and enhanced migration compared with blood EOS (16). We have done further analyses of airway EOS to identify the β2 integrin responsible for the hyperadhesive phenotype and learn whether increased migration is associated with increased podosomes, transitory cell adhesive contacts that are found in migratory cells and recently have been demonstrated on cytokine-stimulated blood EOS (17). Our investigations led to the important findings that human eosinophils recruited to the airway in response to segmental antigen challenge express an allosterically active form of αMβ2 concomitant with enhanced αMβ2-mediated adhesion to diverse ligands and increased formation of podosomes. Treatment of blood EOS with IL-5 replicates the phenotype of airway EOS recovered after segmental antigen challenge.
MATERIALS AND METHODS
Cells
EOS isolation.
Samples were from blood of unchallenged subjects or from blood or BAL fluid 48 h after segmental bronchoprovocation with antigen in challenge of normal subjects, subjects with allergic rhinitis, or subjects with allergic asthma. The sex, age, antigen type, antigen dose, and percentage of EOS in BAL fluid for each subject are listed in Table E1 in the online supplement. Antigen doses constituting 10–15% of the dose that provoked a 20% fall (Ag PD20) in forced expiratory volume (FEV1) as calculated from a dose–response curve were used for antigen challenges. Cat dander and house dust mite extract were from Hollister Stier (Spokane, WA), and ragweed extract was from Greer (Lenoir, NC). All extracts passed the Food and Drug Administration live animal safety testing in which animals must live and gain weight for at least 2 wk after antigen injection. Bronchoscopy and segmental bronchoprovocation were performed as previously described (18). Human EOS were isolated from peripheral blood by anti-CD16 magnetic bead selection (19, 20). For purification of BAL EOS, BAL fluid collected 48 h after segmental bronchoprovocation was layered over a double Percoll gradient of 1.085/1.100 g/ml density, and the cells at the interface were collected (21). The purity of eosinophils was at least 98% as determined by Diff-Quik staining. Viability was at least 99% as assessed by staining with propidium iodide and annexin V–FITC (BD Biosciences, San Jose, CA). Endotoxin in BAL fluid tested from pre- and post-challenge samples of 13 subjects was either undetectable or < 0.25 endotoxin units (EU)/ml according to the Limulus Amebocyte Lysate assay from BioWhittaker (Walkersville, MD). There were no systematic differences in endotoxin in BAL fluid obtained before and after antigen challenge. The University of Wisconsin Human Subjects Committee approved the study, and informed consent was obtained before participation of volunteers.
Cell lines.
Eosinophilic leukemic cell lines, EoL-3 and AML14.3D10, were cultured as described previously (17). Jurkat T lymphocytic cells were provided by Laura Kiessling (University of Wisconsin–Madison, Madison, WI) and grown in RPMI 1640 with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin and subcultured by dilution to 1 × 105/ml twice a week. Human fibroblasts (17), and human umbilical vein endothelial cells (22) were cultured as described.
Adhesive Ligands
The seven-module form of soluble vascular cell adhesion molecule-1 (VCAM-1) was produced in High 5 insect cells transfected with recombinant baculoviruses. The secreted protein contained a C-terminal histidine tag and was purified from cell medium by Ni-NTA beads (Qiagen, Valencia, CA) as described (17). Recombinant soluble human intercellular adhesion molecule-1 (ICAM-1) and human plasma fibrinogen were purchased from R&D Systems (Minneapolis, MN) and Sigma-Aldrich (St. Louis, MO), respectively. Human plasma fibronectin (FN) was purified in the absence of urea or other denaturant as described (23). Rat collagen type I was from Upstate Biotechnology (Lake Placid, NY), human vitronectin was purified in its native form as described (24), and entactin-free mouse laminin was purchased from Collaborative Biomedical Products (Bedford, MA). Bovine serum albumin (BSA) rendered free of fatty acids was purchased from Sigma-Aldrich. The purity and molecular weight of each ligand was verified by SDS-PAGE as shown in Figure E1.
Other Materials
Sources of the following mAbs have been described previously: anti-α4 mAb HP2/1 (mouse IgG1), anti-α4 mAb 9F10 (mouse IgG1), anti-αD mAb 240I (mouse IgG1), anti-β2 mAb TS1/18 (mouse IgG1), anti-β7 mAb Fib504 (rat IgG2a), isotype control mouse IgG1 (anti-keyhole limpet hemocyanin, clone A112–2), isotype control rat IgG2a (anti-keyhole limpet hemocyanin, clone A110–2), FITC-conjugated goat anti-mouse, anti-gelsolin mAb GS-2C4 (mouse IgG1), and rhodamine-phalloidin (17). The following mAbs were purchased from BD Biosciences: activation-specific mAbs against β1, HUTS-21 (mouse IgG1) and 9EG7 (mouse IgG1); anti-β1 mAb MAR4 (mouse IgG1), and mouse IgG2a (anti-keyhole limpet hemocyanin, clone G155–178). The latter mAb was used as an isotype control in adhesion studies. Anti-αL clone 38 (mouse IgG2a) and anti-αX clone 3.9 (mouse IgG1) were from Calbiochem (San Diego, CA). Anti-αM mAb 2LPM19c (mouse IgG1) was from Biomeda (Foster City, CA). Anti-β1 4B4 (mouse IgG1) was from Coulter (Miami, FL). The N29 activation-specific mAb against β1 (mouse IgG1) was from Chemicon (Temecula, CA). The mAb recognizing the activated form of αMβ2, CBRM1/5 (mouse IgG1), was purchased from BioLegend (San Diego, CA). Anti-αD mAb 217I (mouse IgG1) was a gift from ICOS (Bothell, WA). Eotaxin and RANTES were from Cell Sciences (Canton, MA).
Cell Adhesion
Polystyrene 96-well non–tissue culture treated plates (Becton Dickinson, Franklin Lakes, NJ) were coated in triplicate with 100 μl per well of 10 μg/ml protein solution, unless otherwise noted, in TBS, pH 8.0, for 3 h at 37°C and then blocked with heat-inactivated FBS for 20 min at 37°C. The protein coating amount was determined to support maximum adhesion of fibroblasts or airway EOS. Endotoxin in wells coated with proteins was either undetectable or < 0.06 EU/well according to the Limulus Amebocyte Lysate assay (BioWhittaker). FBS was heat-inactivated by heating to 56°C for 30 min, mixed every 10 min, and then rapidly cooled in an ice water bath. For inhibition experiments, mAbs were added to cells at 10 μg/ml final concentration and incubated 5 min at room temperature before cell addition to wells. Cells (1 × 104 per well) were added to wells in volumes of 100 μl/well and allowed to adhere for 1 h at 37°C, then washed three times with TBS, pH 8.0. For quantitation of adherent EOS, 100 μl HBSS was added to wells with 100 μl of solution containing 0.1% Triton-X100, 1 mM o-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich), 1 mM H2O2 and 55 mM Tris, pH 8.0 (25). After 30 min of color development at room temperature, 50 μl of 4 M H2SO4 was added to quench the reaction. Absorbances were read at 490 nm in an EL microplate reader (Bio-Tek Instruments, Winooski, VT). Percentages of adherent EOS were calculated by dividing the absorbance per experimental well by the absorbance from uncoated wells containing 100% of input EOS and multiplying by 100. Absorbance and signal values were within the linear range of the detector as determined by a standard curve. For inhibition studies, values are given as percentages of the absorbances obtained in the presence of respective isotype control mAbs.
Flow Cytometric Analysis of Integrin Expression
EOS (1–5 × 105) were incubated in 0.25 ml HBSS along with primary mAb or isotype control for 30 min at 4°C, washed with 0.25 ml HBSS, resuspended in 0.25 ml HBSS, and then incubated with secondary mAb for 30 min at 4°C in the dark. Primary mAbs were added in final amounts of 0.5 μg. FITC-conjugated goat anti-mouse IgG was added at final concentrations of 20 μg/ml. After secondary mAb incubation, cell lines were pelleted and fixed by resuspension in 1% paraformaldehyde, 67.5 mM sodium cacodylate, 113 mM NaCl, pH 7.2, stored at 4°C in the dark, and within 1 wk washed with RPMI buffer and analyzed. EOS were always analyzed after fixation. Fluorescence measurements were collected within 1 h on a FACSCalibur (BD Biosciences; available through the Flow Cytometry Facility, Comprehensive Cancer Center, University of Wisconsin, Madison). Data were collected from 10,000 cells per condition and analyzed using the CellQuest software (BD Biosciences) or FlowJo software (Tree Star, Inc., Ashland, OR). Fixed cells were gated based on forward and side scatter. The specific geometric mean channel fluorescence (gMCF) is reported. The gMCF was obtained by subtracting the gMCF of the isotype control from the gMCF of the experimental sample according to the following equation, where gMF = geometric mean fluorescence: gMCF = [1,024 × (log gMF of experimental sample)] – [1,024 × (log gMF of isotype control sample)]. The percentages of EOS from different donors that reacted with mAb CBRM1/5 compared with isotype control mAb was calculated from histogram plots using the Overton cumulative histogram subtraction algorithm in FlowJo, which subtracts histograms on a channel-by-channel basis to provide a percent of positive cells (26).
Fluorescent Staining
Immunofluorescence was performed as previously described (17).
Statistical Analysis
Results were analyzed by one-way or repeated measures ANOVA with Dunnett's or Tukey-Kramer multiple comparison post test or two-tailed t test on GraphPad Prism software (San Diego, CA). Results are the mean and SD or SEM of assays involving several different donors and multiple replicates as noted in the tables and figure legends.
RESULTS
αMβ2 Mediates Adhesion of Airway EOS to Diverse Integrin Ligands
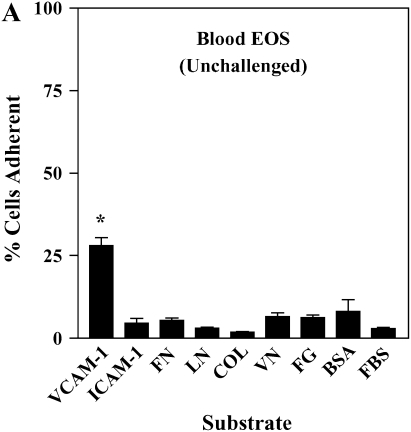
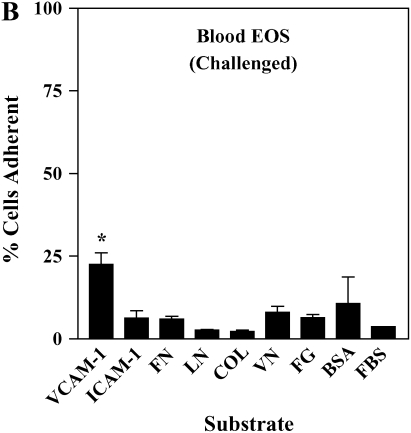
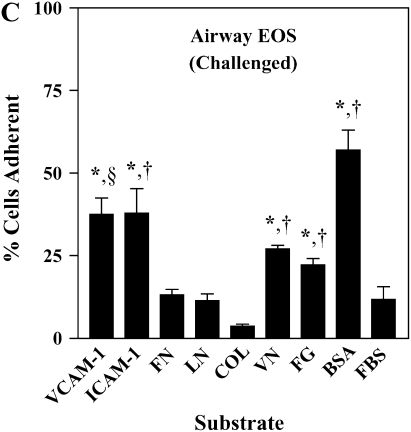
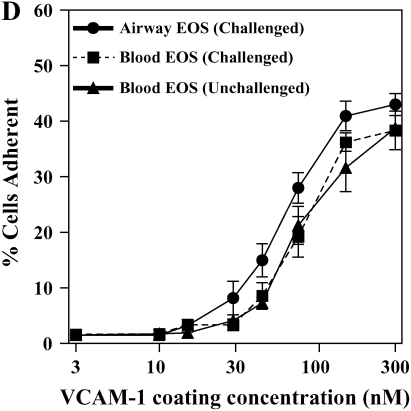
It has been shown previously that airway EOS purified from antigen-challenged subjects exhibit elevated adhesion to surface-coated albumin via an unidentified β2 integrin (16). We hypothesized that airway EOS exhibit increased β2-dependent adhesion to diverse ligands expressed on or within airway endothelium and basement membrane. Blood EOS purified either before or after antigen challenge did not adhere specifically to albumin, ICAM-1, fibrinogen, fibronectin, laminin, collagen type I, or vitronectin (Figures 1A and 1B). In contrast, airway EOS purified from subjects after segmental antigen challenge with three different antigens (cat dander, ragweed, or house dust mite; Table E1) adhered to albumin, ICAM-1, fibrinogen, or vitronectin (Figure 1C). Both blood and airway EOS adhered to the seven-module form of soluble VCAM-1; adhesion of airway EOS was 1.6-fold greater (P < 0.05) and required a lesser coating of VCAM-1 (Figure 1D). The percentage of airway EOS that adhered to albumin, in contrast, was 6-fold greater than the percent adhesion of blood EOS to albumin. Airway EOS, like blood EOS, did not adhere specifically to fibronectin, laminin, or collagen type I (Figure 1), to which fibroblasts or endothelial cells adhered readily (not shown). Thus, airway EOS adhered specifically to a surprising spectrum of adhesive ligands and exhibited an adhesive profile not shared by blood EOS.
Figure 1.
Adhesion of purified blood and airway EOS to diverse integrin ligands. Adhesion of purified blood EOS of unchallenged subjects (A), blood EOS of challenged subjects (B), or airway EOS obtained after antigen challenge (C) to diverse integrin ligands coated at 10 μg/ml or with a solution of 1% BSA and blocked with FBS. Results are the mean adhesion from six separate donors performed in triplicate wells on six different occasions (18 wells) with the SEM indicated. *P < 0.001, comparing adhesion to ligands versus the FBS blocker; one-way ANOVA with Dunnett's post test. †P < 0.001, §P < 0.05, comparing adhesion of airway EOS to blood EOS of unchallenged or challenged subjects; one-way ANOVA with Dunnett's post test. (D) Specific adhesion of blood EOS of unchallenged and challenged subjects and airway EOS of challenged subjects to increasing coatings of VCAM-1. Specific adhesion to VCAM-1 was calculated by subtracting nonspecific adhesion to FBS blocker from adhesion to VCAM-1. Results are the mean from five separate donors performed in triplicate wells on five different occasions (15 wells) with the SEM indicated. COL, collagen type I; FG, fibrinogen; FN, fibronectin; LN, laminin; VN, vitronectin.
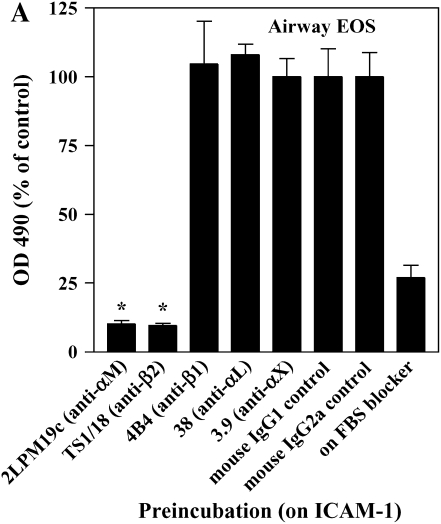
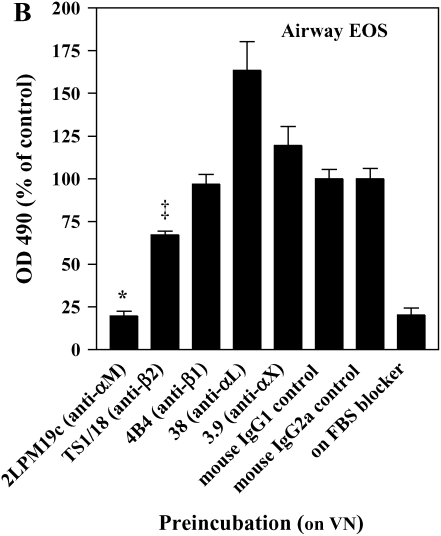
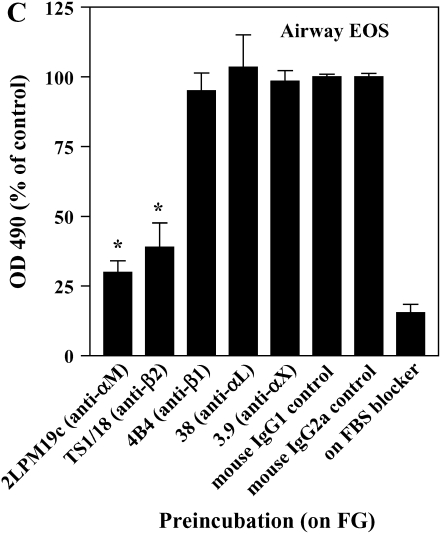
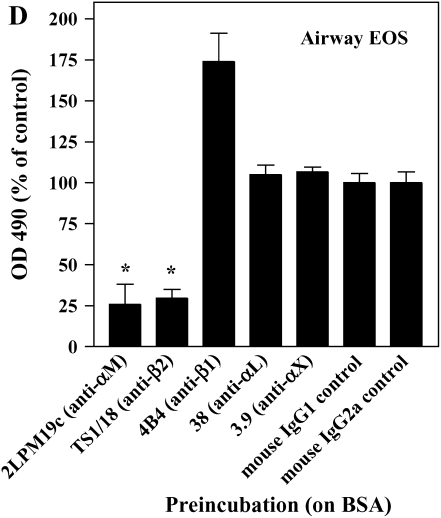
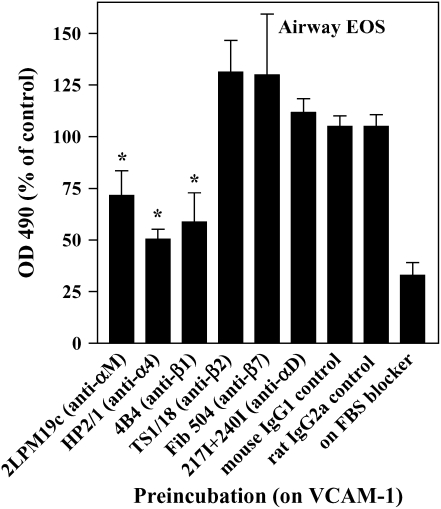
The adhesion of airway EOS to ICAM-1, fibrinogen, albumin, or vitronectin, all of which are potential targets of αMβ2 (27–29), prompted us to determine whether αMβ2 was involved in the adhesion to all the proteins. Antibody-blocking experiments identified αMβ2 as the integrin responsible for the specific adhesion of airway EOS to the four recognized αMβ2 ligands (Figure 2). That is, inhibitory mAbs recognizing αM and β2 subunits blocked adhesion of airway EOS to all four ligands, whereas inhibitory mAbs recognizing β1, αL, or αX did not inhibit adhesion (Figure 2). In experiments not shown, EoL-3 and AML14.3D10 eosinophilic leukemic cells, which do not express αMβ2 but do express αLβ2, did not adhere to albumin, ICAM-1, fibrinogen, or vitronectin, consistent with the conclusion that adhesion of airway EOS to these ligands is mediated by αMβ2. Adhesion of blood (not shown) and airway (Figure 3) EOS to VCAM-1 was mostly blocked by anti-α4 or anti-β1. In addition, there was partial inhibition of adhesion of airway EOS to VCAM-1 with an antibody to αM (Figure 3). These results identify αMβ2 as the integrin receptor that mediates the hyperadhesion of airway EOS to diverse ligands, including known αMβ2-reactive ligands—ICAM-1, fibrinogen, albumin, and vitronectin—and one that is not an already recognized αMβ2-ligand— VCAM-1.
Figure 2.
Antibody blocking of adhesion of purified airway EOS to diverse integrin ligands. Antibody inhibition of purified airway EOS on (A) ICAM-1, (B) vitronectin, (C) fibrinogen, or (D) BSA. Results for assays on each substrate are the mean and SEM of inhibition assays performed in triplicate for four different donors (12 wells). *P < 0.001 or ‡P < 0.05 represents an inhibition of adhesion compared with the isotype control mAb; one-way ANOVA with Dunnett's post test.
Figure 3.
Antibody blocking of adhesion of purified airway EOS to VCAM-1. Antibody inhibition of adhesion of purified airway EOS on VCAM-1. Results are the mean and SEM of inhibition assays performed in triplicate from six separate donors (18 wells). *P < 0.01, represents an inhibition of adhesion compared with the isotype control mAb; one-way ANOVA with Dunnett's post test.
αMβ2 Is Allosterically Activated on EOS Purified from Airway of Antigen-Challenged Human Subjects
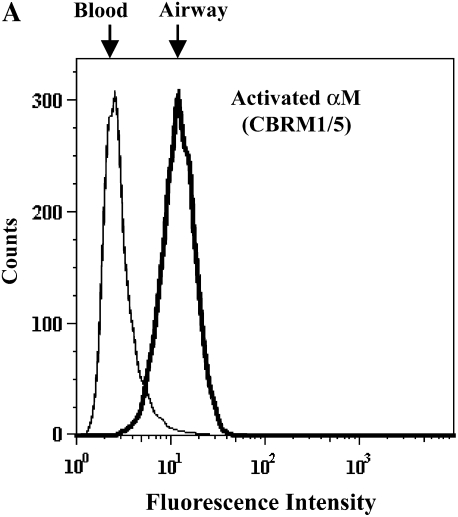
The conformation-sensitive CBRM1/5 mAb, which recognizes an epitope in the ligand-binding “insert” (I) domain of the αM subunit (30), reacted 3- to 4-fold higher with airway EOS purified from antigen-challenged subjects compared with blood EOS purified before or after challenge (Figure 4 and Table 1). These results indicate that antigen challenge allosterically activates αMβ2 on airway EOS, consistent with the notion that the enhanced adhesion of airway EOS to diverse integrin ligands is mediated by αMβ2.
Figure 4.
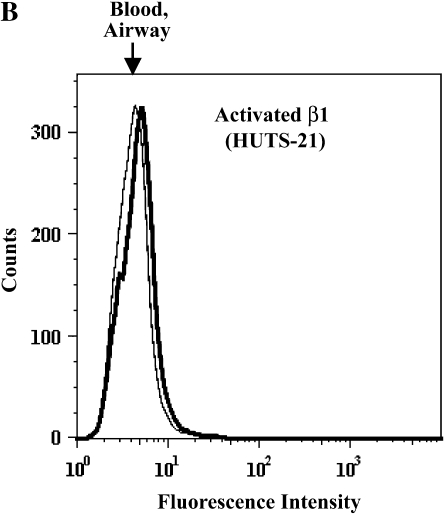
αMβ2 and α4β1 activation states on purified blood and airway EOS. Representative flow cytometric histograms of (A) activated αM assessed by CBRM1/5 or (B) activated β1 as assessed by HUTS-21 of purified blood EOS of an unchallenged individual (thin line) or of airway EOS obtained after antigen challenge (bold line).
TABLE 1.
ACTIVATION STATES OF INTEGRINS OF PURIFIED EOS
| Mean Specific gMCF* ± SD
|
||||
|---|---|---|---|---|
| Activated αM
|
Activated β1
|
|||
| CBRM1/5 | N29 | HUTS-21 | 9EG7 | |
| Unchallenged blood EOS | < 110 (7) | 140 ± 130 (9) | < 110 (6) | < 110 (6) |
| Challenged blood EOS | 150 ± 120 (5) | 170 ± 110 (6) | < 110 (6) | < 110 (5) |
| Challenged airway EOS | 460 ± 250† (5) | < 110 (8) | < 110 (4) | < 110 (4) |
Definition of abbreviations: EOS, eosinophils; gMCF, geometric mean channel fluorescence.
Results from different donors for purified EOS are shown.
Detected with FITC-conjugated secondary mAb; data are shown as the mean specific gMCF in assays performed in HBSS buffer with standard deviations indicated. Epitopes exhibiting a specific gMCF less than 110 are counted as not recognized.
Reactivity is significantly different from Purified Unchallenged and Challenged Blood EOS, P < 0.001, repeated measures ANOVA.
We assayed effects of segmental antigen challenge on the allosteric structure and expression level of a second integrin heterodimer, α4β1, an important adhesion receptor involved in recognition by EOS of VCAM-1 (9, 16, 31). We probed β1 conformation with three conformation-sensitive mAbs: N29, HUTS-21, and 9EG7 (32–35). The locations of these epitopes in various allosteric conformations assumed by β1, based on the αVβ3 and αIIbβ3 structural models, suggest that the mAbs recognize increasingly activated forms of β1 in the order N29 < HUTS-21 < 9EG7 (12, 36). HUTS-21 (Figure 3B and Table 1) and 9EG7 (Table 1) failed to recognize β1, and N29 exhibited low or undetectable recognition of purified blood or airway EOS (Table 1). All three mAbs reacted robustly with β1 on Jurkat cells (not shown). Thus, in contrast to αMβ2, the structure of α4β1 on purified EOS is not detectably altered in response to antigen challenge. Indeed, airway EOS expressed less total α4 subunit compared with purified blood EOS (Table E2) as reported previously (37), and β1 and β7 subunits were similarly decreased on purified airway EOS (Table E2).
Airway EOS Exhibit Robust Formation of Podosomes
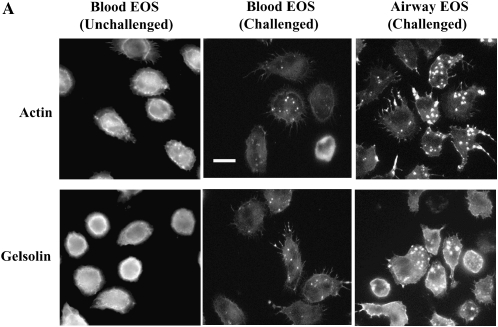
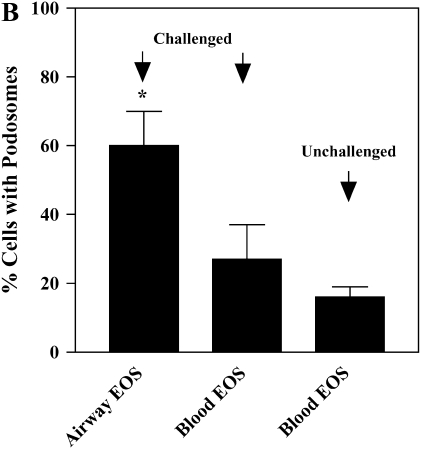
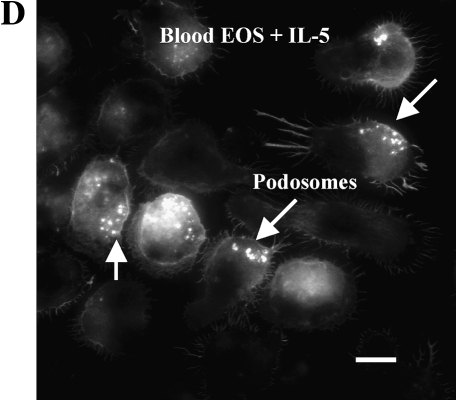
Treatment of blood EOS with IL-5, especially when TNF-α is present, enhances the number and size of podosomes that form when adherent to VCAM-1 (17). Podosomes are transient assemblies of cytoskeletal and membrane proteins that are believed to be important in cell movement and localization of proteolytic machinery (38, 39). We analyzed whether airway EOS purified from antigen-challenged subjects, which are more migratory than unchallenged blood EOS (16), demonstrate enhanced podosome formation compared with blood EOS. Actin and gelsolin, markers of podosomes, were localized to punctate structures that were coarser in challenged airway EOS compared with blood EOS from unchallenged subjects (Figure 5A). Blood EOS purified after challenge exhibited podosomes that were variable in size and number compared with challenged airway EOS and unchallenged blood EOS. Gelsolin co-localized with actin in airway EOS adherent to VCAM-1 (not shown). By virtue of the punctate staining and co-localization by gelsolin and actin (38), the structures have the characteristics of podosomes. The percentage of airway EOS that contained podosomes was ∼ 2- to 3-fold greater than the percentage of blood EOS that contained the podosome structure (Figure 5B). Podosome formation was also detected in airway EOS adherent to albumin, ICAM-1, fibrinogen, and vitronectin (not shown). There was no detectable change in β1 distribution of airway or blood EOS adherent to VCAM-1 in immunofluorescent analyses, consistent with the notion that α4β1 activity and distribution are not altered on purified EOS in response to segmental antigen challenge. These results indicate that EOS recruited to the airway in response to antigen challenge exhibit changes in the cytoskeleton compared with EOS in blood. That is, airway EOS display increases in formation of podosomes, which may partly explain the enhanced mobility of airway EOS in comparison to blood EOS (16).
Figure 5.
Podosome formation of purified blood and airway EOS. (A) Micrographs of separate fields of purified blood EOS of an unchallenged individual (left), of blood EOS of a challenged individual (middle), or of airway EOS of a challenged individual (right) adherent to VCAM-1 assayed by immunofluorescent staining with rhodamine-phalloidin for actin or anti-gelsolin. Fields representative of experiments with six donors are shown. Scale bar = 10 μm. (B) The percentages of EOS that contain podosomes were calculated. EOS were counted as podosome-positive if they had clear and bright actin condensations. Podosomes were quantified by involved and naïve laboratory workers after visualization of at least 200 EOS in each experiment, and similar results were obtained in all cases. Results represent the mean and SEM from assays performed in triplicate from six separate donors (18 coverslips), *P < 0.05 and P < 0.01, comparing the percentage of airway EOS containing podosomes to blood EOS of challenged or unchallenged individuals, respectively, one-way ANOVA with Tukey-Kramer multiple comparison post test.
IL-5 Causes Blood EOS to Resemble Airway EOS
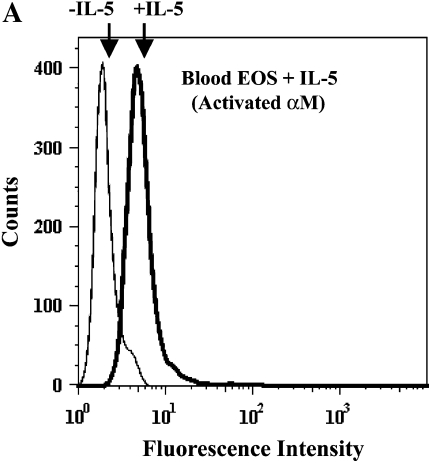
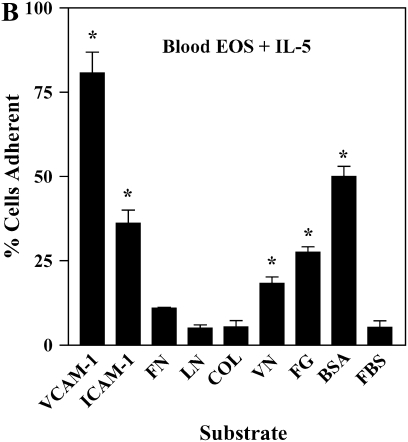
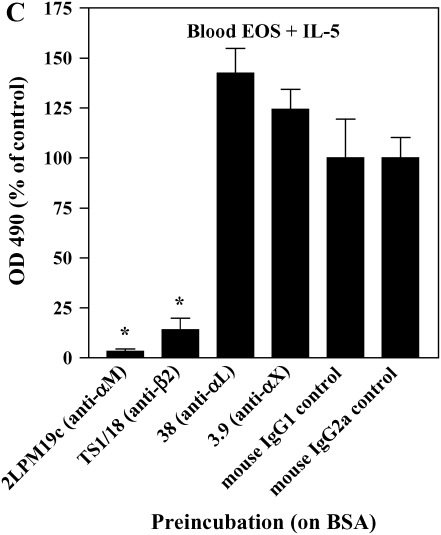
IL-5 is a critical cytokine expressed in individuals with asthma and is important in EOS recruitment to airway. Specifically, IL-5 expression is increased in the airway mucosa after segmental antigen challenge and is associated with airway eosinophilia (18, 40). IL-5 induces enhanced adhesion of EOS to endothelial cells (41) and albumin (9, 16, 42) and formation of podosomes (17). We, therefore, determined whether IL-5 treatment of purified blood EOS mimicked the enhanced activity of αMβ2 and prominent podosomes of airway EOS. Blood EOS obtained from either normal or atopic subjects and treated with IL-5 exhibited increased reactivity with the CBRM1/5 anti-αM conformation-specific mAb (Figure 6A), concomitant with enhanced adhesion to albumin, ICAM-1, fibrinogen, vitronectin, or VCAM-1 (Figure 6B). Blood EOS treated with IL-5 did not adhere to fibronectin, laminin, or collagen type I, consistent with the adhesive phenotype of airway EOS purified from antigen-challenged subjects. Adhesion to albumin (Figure 6C), ICAM-1, fibrinogen, or vitronectin (not shown) after IL-5 stimulation was mediated by αMβ2, since adhesion was blocked by inhibitory mAbs against αM and β2 subunits. There was no detectable difference in adhesion of EOS treated with IL-5 alone compared with IL-5 and eotaxin or RANTES (not shown). As reported previously (17), activation of EOS by IL-5 was accompanied by enhanced formation of podosomes (Figure 6D). Thus, treatment of blood EOS with IL-5 causes the phenotype characteristic of airway EOS after segmental antigen challenge. That is, IL-5 induces the epitope of αM on EOS recognized by CBRM1/5; enhances EOS adhesion to VCAM-1, albumin, ICAM-1, fibrinogen, or vitronectin via an αMβ2-dependent mechanism; and stimulates formation of podosomes of EOS. These results are consistent with the proposal that IL-5 is likely responsible for the allosteric activation of αMβ2, αMβ2-mediated enhanced adhesion, and podosome formation of EOS recruited to the asthmatic airway.
Figure 6.
Effect of IL-5 on αMβ2 conformation, adhesion, and podosome formation of purified blood EOS. (A) Purified blood EOS from normal or atopic subjects were incubated without (thin line) or with (bold line) 50 ng/ml IL-5 for 20 min at 37°C and then placed on ice before addition of CBRM1/5. Similar increases in CBRM1/5 reactivity upon IL-5 treatment of blood EOS were found in four separate donors, P = 0.0417, two-tailed t test. Unchallenged blood EOS from normal or atopic subjects were incubated with 50 pg/ml IL-5 for 5 min before addition of cells to wells coated with (B) 10 μg/ml diverse ligands or (C) 1% BSA in the presence of inhibitory mAbs. Results are the mean and SD of assays performed in triplicate for five separate donors (15 wells). *P < 0.001, comparing adhesion on ligands to the FBS blocker or adhesion in the presence of inhibitory mAbs to adhesion with respective isotype control mAbs, one-way ANOVA with Dunnett's post test. (D) Blood EOS treated with 100 ng/ml IL-5 and adherent to VCAM-1 were assayed for podosome formation by immunofluorescent staining of actin with rhodamine-phalloidin. Scale bar = 10 μm.
DISCUSSION
In this article we report correlative immunochemical and functional studies of integrins of EOS isolated from blood and airway of human subjects in a clinical model of allergic asthma. We identified αMβ2 as the principal heterodimeric integrin adhesion receptor on purified EOS that is structurally and functionally altered in the antigen challenge model. Specifically, airway EOS purified after antigen challenge express an allosterically activated form of αMβ2 as ascertained with the CBRM1/5 activation-specific anti-αM mAb. The allosteric activation of αMβ2 is accompanied by enhanced adhesion of airway EOS to diverse ligands mediated by αMβ2 and by increased formation of podosomes, transitory adhesive contacts implicated in cell movement and proteolysis of matrix proteins. Treatment of blood EOS with IL-5 results in similar allosteric activation of αMβ2, increased αMβ2-dependent adhesion, and induction of podosome formation. IL-5, found in the airway after segmental antigen challenge (40) is, therefore, a likely effector of the enhanced adhesive, podosome, and migratory phenotype of airway EOS. We did not detect enhanced activity of α4β1 of EOS purified from airway as compared with blood, indicating that antigen challenge results in a hyperadhesive phenotype that is specifically as well as primarily αMβ2-dependent.
We initially hypothesized that antigen challenge causes activation of α4β1 and enhanced adhesion to VCAM-1. However, we did not detect an increase in specific adhesion to VCAM-1 that could be attributed to α4β1. These results are strengthened by results from conformation-sensitive mAbs to β1, N29, HUTS-21, and 9EG7, which reacted at either low or undetectable levels with β1 on purified blood or airway EOS. Indeed, total α4 and β1 subunit expression levels were decreased on airway EOS compared with blood EOS, and there was no detectable difference in distribution of β1 on either cell type adherent to VCAM-1. Interestingly, α4β1 on blood or airway EOS did not support adhesion to a second α4β1 ligand, plasma fibronectin. Even after incubation of EOS with potential activators of α4β1, including Mn2+ and PMA, EOS remained unable to recognize fibronectin (not shown). Adhesion of EOS to fibronectin can, however, be induced by incubation with the 8A2 stimulatory mAb (16), which binds to a regulatory region in the βA domain shared by the adhesion blocking mAbs, 4B4 and 13 (43). Incubation with 8A2 exposes the epitope in β1 of the K562 human erythroleukemic cell line recognized by the 9EG7 activation-sensitive mAb (44). Because 9EG7 did not recognize β1 on purified blood or airway EOS, β1 on these cells likely is in a conformational state that is not competent to support adhesion to fibronectin. Indeed, Jurkat cells, which we have found to react with 9EG7, adhered to fibronectin and adhered better to VCAM-1 than EOS (not shown). Blood EOS did not adhere to laminin or fibrinogen, indicating that α6β1, αMβ2, and αXβ2 on blood EOS from unchallenged or challenged subjects are in allosteric states that do not allow for such interactions. Our evidence that attributes the hyperadhesive phenotype of airway EOS solely to αMβ2 leaves unanswered the question of if and when the potential adhesive activities of other integrins on EOS come into play.
Considerable evidence indicates that αMβ2 activation may be critical in mediating trafficking of EOS as well as neutrophils to inflammatory foci. αMβ2 mediates transmigration of EOS through endothelial cell monolayers in vitro (45). Migration mediated by αMβ2 is facilitated by the chemokines RANTES, MCP-3, and C5a, which also induce expression of the activation-sensitive epitope of αMβ2 recognized by the CBRM1/5 conformation-sensitive mAb (46–49). Numbers of EOS in blood and airway of humans with asthma are reduced after administration of a humanized mAb against IL-5 (50, 51). Expression of αMβ2 is upregulated on purified human and mouse airway EOS after antigen challenge compared with blood EOS purified before challenge (37, 52–54). αMβ2 expression is increased on migratory EOS (45). Studies of mice null for αM indicate that αMβ2 may be functionally important in a variety of cellular functions. Thus, neutrophils isolated from such mice exhibit reduced respiratory burst (55), defects in degranulation (55), impaired phagocytosis (56), and diminished apoptosis (56). Mice that lack β2 expression are characterized by impaired emigration of neutrophils into inflamed or infected skin (57) and mimic the phenotype of leukocyte adhesion deficiency syndrome in humans (58). Mice that are null for expression of the β2-ligand, ICAM-1, display decreased neutrophil influx in peritonitis (59).
Podosomes are foci of proteolytic activity and play a role in cell locomotion (38). EOS expressing active αMβ2 adhere to diverse ligands, migrate, form podosomes, and remove VCAM-1 (17). One scenario linking functions of αMβ2 and podosomes is that enhanced adhesion mediated by activated αMβ2 nucleates formation of podosomes, leading to enhanced mobility and proteolysis of matrix proteins via clustering of membrane-associated proteases, including ADAM8 (17). αMβ2 interacting with diverse ligands, including some that may become better αMβ2 adhesive and migratory ligands as a result of proteolysis via podosomes, may modulate migration of EOS from blood to the airway wall and finally into the airway lumen. The hyperadhesive and hypermigratory phenotype of airway EOS characterizes most EOS purified from BAL fluid obtained after segmental antigen challenge, as assessed by the uniform increase in CBRM1/5 positivity (Figure 4). One can only speculate whether this phenotype represents the residua of changes required for EOS to move from blood into the airway wall and on into airway lumen or is important for the function of EOS in airway, for example, to allow movement between airway wall and lumen. In either case, an even more marked hyperadhesive phenotype may characterize EOS in the airway wall.
Supplementary Material
Acknowledgments
The authors thank Heather Gerbyshak, Kristyn Jansen, Anne Brooks, and Julie Sedgwick for eosinophil isolation; Ming Lye for help with immunofluorescence experiments; Lara Johansson for help with cell culture; Kathleen Schell, Joan Batchelder, Kristin Elmer, Janet Lewis, Joel Puchalski, and Pamela Whitney for help with flow cytometry data collection and analysis; JoAnn Meerschaert for TS1/18 purification; Pat Hoffman (ICOS), Lynn Allen-Hoffmann, Eva Gordon, Roy Lobb, Richard Lynch, Cassandra Paul, and Kenneth Yamada for providing reagents and cell lines; Ioana Oltean for BAL fluid samples; Becky Kelly and Cheri Swenson for advice; and Mary-Ellen Bates and Paul Bertics for manuscript comments and suggestions.
This work was supported by Specialized Center of Research (SCOR) grant HL56396, Hematology Training grant RTH T32 HL007899, and General Clinical Research Center (GCRC) grant M01 RR03186 from the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2006-0027OC on April 6, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Fireman P. Understanding asthma pathophysiology. Allergy Asthma Proc 2003;24:79. [PubMed] [Google Scholar]
- 2.Busse W, Banks-Schlegel S, Noel P, Ortega H, Taggart V, Elias J. Future research directions in asthma: an NHLBI Working Group report. Am J Respir Crit Care Med 2004;170:683. [DOI] [PubMed] [Google Scholar]
- 3.Woolcock AJ, Peat JK. Evidence for the increase in asthma worldwide. Ciba Found Symp 1997;206:122. [DOI] [PubMed] [Google Scholar]
- 4.Meza C, Gershwin ME. Why is asthma becoming more of a problem? Curr Opin Pulm Med 1997;3:6. [DOI] [PubMed] [Google Scholar]
- 5.Picado C. Early and late-phase asthmatic reactions: a hypothesis. Allergy 1992;47:331. [DOI] [PubMed] [Google Scholar]
- 6.Pawankar R, Yamagishi S, Takizawa R, Yagi T. Mast cell-IgE-and mast cell-structural cell interactions in allergic airway disease. Curr Drug Targets Inflamm Allergy 2003;2:303. [DOI] [PubMed] [Google Scholar]
- 7.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673. [DOI] [PubMed] [Google Scholar]
- 8.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol 2003;201:632. [DOI] [PubMed] [Google Scholar]
- 9.Grayson MH, Van der Vieren M, Sterbinsky SA, Michael Gallatin W, Hoffman PA, Staunton DE, Bochner BS. alphadbeta2 integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1). J Exp Med 1998;188: 2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tachimoto H, Bochner BS. The surface phenotype of human eosinophils. Chem Immunol 2000;76:45. [DOI] [PubMed] [Google Scholar]
- 11.Georas SN, McIntyre BW, Ebisawa M, Bednarczyk JL, Sterbinsky SA, Schleimer RP, Bochner BS. Expression of a functional laminin receptor (alpha 6 beta 1, very late activation antigen-6) on human eosinophils. Blood 1993;82:2872. [PubMed] [Google Scholar]
- 12.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 2004;432:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries MJ. Monoclonal antibodies as probes of integrin priming and activation. Biochem Soc Trans 2004;32:407. [DOI] [PubMed] [Google Scholar]
- 14.Wardlaw AJ. Eosinophil trafficking in asthma. Clin Med 2001;1:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol Rev 2001;179:163. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Sterbinsky SA, Bickel CA, Zhou DF, Kovach NL, Bochner BS. Regulation of alpha 4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1. J Allergy Clin Immunol 1997;99:648. [DOI] [PubMed] [Google Scholar]
- 17.Johansson MW, Lye MH, Barthel SR, Duffy AK, Annis DS, Mosher DF. Eosinophils adhere to vascular cell adhesion molecule-1 via podosomes. Am J Respir Cell Mol Biol 2004;31:413. [DOI] [PubMed] [Google Scholar]
- 18.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol 2002;169:6452. [DOI] [PubMed] [Google Scholar]
- 19.Hansel TT, De Vries IJ, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods 1991;145:105. [DOI] [PubMed] [Google Scholar]
- 20.Meerschaert J, Vrtis RF, Shikama Y, Sedgwick JB, Busse WW, Mosher DF. Engagement of alpha4beta7 integrins by monoclonal antibodies or ligands enhances survival of human eosinophils in vitro. J Immunol 1999;163:6217. [PubMed] [Google Scholar]
- 21.Busse WW, Nagata M, Sedgwick JB. Characteristics of airway eosinophils. Eur Respir J Suppl 1996;22:132s. [PubMed] [Google Scholar]
- 22.Panetti TS, Nowlen J, Mosher DF. Sphingosine-1-phosphate and lysophosphatidic acid stimulate endothelial cell migration. Arterioscler Thromb Vasc Biol 2000;20:1013. [DOI] [PubMed] [Google Scholar]
- 23.McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol 1983;97:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bittorf SV, Williams EC, Mosher DF. Alteration of vitronectin: characterization of changes induced by treatment with urea. J Biol Chem 1993;268:24838. [PubMed] [Google Scholar]
- 25.Strath M, Warren DJ, Sanderson CJ. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods 1985;83:209. [DOI] [PubMed] [Google Scholar]
- 26.Overton WR. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry 1988;9:619. [DOI] [PubMed] [Google Scholar]
- 27.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol 1999;66:876. [DOI] [PubMed] [Google Scholar]
- 28.Davis GE. The Mac-1 and p150,95 beta 2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Exp Cell Res 1992; 200:242. [DOI] [PubMed] [Google Scholar]
- 29.Kanse SM, Matz RL, Preissner KT, Peter K. Promotion of leukocyte adhesion by a novel interaction between vitronectin and the beta2 integrin Mac-1 (alphaMbeta2, CD11b/CD18). Arterioscler Thromb Vasc Biol 2004;24:2251. [DOI] [PubMed] [Google Scholar]
- 30.Oxvig C, Lu C, Springer TA. Conformational changes in tertiary structure near the ligand binding site of an integrin I domain. Proc Natl Acad Sci USA 1999;96:2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh GM, Symon FA, Lazarovils AL, Wardlaw AJ. Integrin alpha 4 beta 7 mediates human eosinophil interaction with MAdCAM-1, VCAM-1 and fibronectin. Immunology 1996;89:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mould AP, Travis MA, Barton SJ, Hamilton JA, Askari JA, Craig SE, Macdonald PR, Kammerer RA, Buckley PA, Humphries MJ. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J Biol Chem 2005;280:4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem 1996;271:11067. [DOI] [PubMed] [Google Scholar]
- 34.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci USA 1993;90:9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem 1995;270:25570. [DOI] [PubMed] [Google Scholar]
- 36.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science 2001;294:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georas SN, Liu MC, Newman W, Beall LD, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial cell activation accompany the recruitment of human granulocytes to the lung after segmental antigen challenge. Am J Respir Cell Mol Biol 1992;7:261. [DOI] [PubMed] [Google Scholar]
- 38.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 2003;13:376. [DOI] [PubMed] [Google Scholar]
- 39.Linder S, Kopp P. Podosomes at a glance. J Cell Sci 2005;118:2079. [DOI] [PubMed] [Google Scholar]
- 40.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol 2002;169:6459. [DOI] [PubMed] [Google Scholar]
- 41.Walsh GM, Hartnell A, Wardlaw AJ, Kurihara K, Sanderson CJ, Kay AB. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leucocyte integrin (CD11/18)-dependent manner. Immunology 1990;71:258. [PMC free article] [PubMed] [Google Scholar]
- 42.Sano M, Leff AR, Myou S, Boetticher E, Meliton AY, Learoyd J, Lambertino AT, Munoz NM, Zhu X. Regulation of interleukin-5- induced beta2-integrin adhesion of human eosinophils by phosphoinositide 3-kinase. Am J Respir Cell Mol Biol 2005;33:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takada Y, Puzon W. Identification of a regulatory region of integrin beta 1 subunit using activating and inhibiting antibodies. J Biol Chem 1993;268:17597. [PubMed] [Google Scholar]
- 44.Faull RJ, Wang J, Leavesley DI, Puzon W, Russ GR, Vestweber D, Takada Y. A novel activating anti-beta1 integrin monoclonal antibody binds to the cysteine-rich repeats in the beta1 chain. J Biol Chem 1996; 271:25099. [DOI] [PubMed] [Google Scholar]
- 45.Jia GQ, Gonzalo JA, Hidalgo A, Wagner D, Cybulsky M, Gutierrez-Ramos JC. Selective eosinophil transendothelial migration triggered by eotaxin via modulation of Mac-1/ICAM-1 and VLA-4/VCAM-1 interactions. Int Immunol 1999;11:1. [DOI] [PubMed] [Google Scholar]
- 46.Weber C, Kitayama J, Springer TA. Differential regulation of beta 1 and beta 2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA 1996;93:10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med 1992;176:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, Minty A, Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med 1994;179:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today 1994;15:127. [DOI] [PubMed] [Google Scholar]
- 50.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000; 356:2144. [DOI] [PubMed] [Google Scholar]
- 51.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 2003;167:199. [DOI] [PubMed] [Google Scholar]
- 52.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol 1992;149:3710. [PubMed] [Google Scholar]
- 53.Kroegel C, Liu MC, Hubbard WC, Lichtenstein LM, Bochner BS. Blood and bronchoalveolar eosinophils in allergic subjects after segmental antigen challenge: surface phenotype, density heterogeneity, and prostanoid production. J Allergy Clin Immunol 1994;93:725. [DOI] [PubMed] [Google Scholar]
- 54.Hisada T, Hellewell PG, Teixeira MM, Malm MG, Salmon M, Huang TJ, Chung KF. alpha4 integrin-dependent eotaxin induction of bronchial hyperresponsiveness and eosinophil migration in interleukin-5 transgenic mice. Am J Respir Cell Mol Biol 1999;20:992. [DOI] [PubMed] [Google Scholar]
- 55.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest 1997;99: 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, Mayadas TN. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 1996;5:653. [DOI] [PubMed] [Google Scholar]
- 57.Sheppard D. In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol 2000;19:203. [DOI] [PubMed] [Google Scholar]
- 58.Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol 2002;9:30. [DOI] [PubMed] [Google Scholar]
- 59.Rosenkranz AR, Mayadas TN. Leukocyte-endothelial cell interactions - lessons from knockout mice. Exp Nephrol 1999;7:125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.