Abstract
The brain is remarkably responsive to its interactions with the environment, and its morphology is altered by experience in measurable ways. Histological examination of the brains of animals exposed to either a complex (‘enriched’) environment or learning paradigm, compared with appropriate controls, has illuminated the nature of experience-induced morphological plasticity in the brain. For example, this research reveals that changes in synapse number and morphology are associated with learning and are stable, in that they persist well beyond the period of exposure to the learning experience. In addition, other components of the nervous system also respond to experience: oligodendrocytes and axonal myelination might also be permanently altered, whereas changes in astrocytes and cerebrovasculature are more transient and appear to be activity- rather than learning-driven. Thus, experience induces multiple forms of plasticity in the brain that are apparently regulated, at least in part, by independent mechanisms.
Keywords: enrichment, complex environment, exercise
INTRODUCTION
The temptation to understand behavior in terms of either nature or nurture has become considerably less compelling in recent decades, in part because of the recognition that experience induces measurable, morphological changes in cells of the brain, both neurons and glia, and in their connections with one another. Alterations in gene expression, neurochemistry and the physiological properties of these cells often accompany environment-induced reorganization of brain connectivity, as do measurable adjustments in behavior that are adaptive in the context of the new environment. This dynamic interplay between the environment (nurture) and brain biology (nature) is being called upon to further the understanding of complex psychopathological disorders that are characterized by abnormal brain morphology and/or functional activation, even those that are known to have a strong genetic component. For example, the concordance rate for schizophrenia is 17% in dizygotic twins and 50% in monozygotic twins (reviewed in Tsuang, 2000), which indicates the contribution of both genetic and environmental factors. Similarly, polymorphism in the serotonin transporter gene modulates the onset of major depression in response to stressful life events (Caspi et al., 2003). It is suggested that interaction between an individual’s genetic makeup and his or her environment can account not only for individual differences in susceptibility to psychopathology, but for such things as personality characteristics and neural and behavioral responses to the normal aging process as well.
Elucidation of experience-induced alterations in brain morphology is facilitated by conducting studies in a laboratory setting where environmental conditions can be better controlled. The complex environment paradigm, pioneered by Donald Hebb and his students (e.g. Hebb, 1949; Forgays and Forgays, 1952; Hymovitch, 1952), involves housing a group of animals, typically either rats or mice, in a large cage that contains numerous toys, such as balls, tunnels and ladders, which are changed daily to provide a continuously stimulating environment. This complex environment is sometimes referred to as an ‘enriched condition’ (EC), but it is important to note that these animals are only enriched relative to rats housed in isolation or ‘impoverished condition’ (IC) and rats housed in a group but without toys (‘social condition’ or SC) rather than animals living in the wild. As Hebb’s work first demonstrated, animals exposed to EC demonstrate superior performance in several tests measuring higher-order cognitive ability, such as the Hebb-Williams maze (Mohammed et al., 1986; Galani et al., 1997), the Morris water maze (Whishaw et al., 1984; Mohammed et al., 1990) and the radial-arm maze (Galani et al., 1998).
The first use of the EC paradigm as a tool to study experience-induced morphological plasticity in the brain was by Bennett et al. (1964), who reported that rats raised in EC possessed heavier and thicker cerebral cortices than did IC rats. Because the differences were largest in the visual cortex, much of the subsequent work was aimed at determining the underlying morphology that contributes to the gross difference in size in this region (e.g. Diamond et al., 1964; Diamond et al., 1966; Volkmar and Greenough, 1972). Although not a ubiquitous phenomenon, morphological plasticity following exposure to EC has been demonstrated in several other brain regions that are involved in the processing and/or response to environmental stimuli, including the auditory cortex (Greenough et al., 1973), primary somatosensory cortex (Coq and Xerri, 1998), hippocampus and entorhinal cortex (Fiala et al., 1978; Moser et al., 1997; Rampon et al., 2000), amygdala (Nikolaev et al., 2002), basal ganglia (Comery et al., 1995; Comery et al., 1996) and cerebellar cortex (Greenough et al., 1986). Initially, alterations in neuronal structure were the focus of investigation, however, more recently it has become clear that other components of the nervous system, such as macroglial cells and cerebrovasculature elements, also exhibit robust plasticity in response to experience.
Morphological plasticity in the brain occurring in response to an increase in the complexity of the environment appears to reflect brain substrates of adaptation to the demands and opportunities provided by experience, including both relatively typical forms of learning and memory and adjustments associated with fundamental processes such as sensory, motor and cognitive processing. Some important points concerning how this conclusion has been reached deserve mention, but for a more comprehensive discussion see (Grossman et al., 2002). Following the initial reports of increased cortical thickness in EC rats, it was suggested that the observed plasticity might not be caused by learning-dependent processes, but by changes in overall body growth, increase in social contact, differences in stress that might occur as a result of differential housing and to other hormonal or metabolic responses to behavioral manipulations. The evidence, however, does not support any of these mechanisms. In the original publication by Bennett and colleagues in 1964, it was reported that the increased cortical thickness of EC rats could not be attributed to differences in body size between the groups, because the EC rats weighed ~7% less than the IC rats, presumably because they were more active. The finding that young EC rats have slower somatic growth at the time that brain regions are expanding has been replicated (Black et al., 1989b). Additionally, although somatic growth of adult rats is slow and not very responsive to housing condition, EC still induces plasticity in the adult brain that is similar to that observed in young animals (Black et al., 1989b). The concern about increased social contact has been addressed by the inclusion of SC control animals (housed in a group but not in a complex environment); plasticity observed in EC animals cannot be explained by social contact if comparable changes are not demonstrated by SC animals, as is often the case (e.g. Rosenzweig et al., 1978; Turner and Greenough, 1985; Sirevaag et al., 1988; Sirevaag and Greenough, 1991; Jones and Greenough, 1996; Briones et al., 2004) (see below for discussion of these studies). Neither is experience-dependent plasticity attributable to changes in levels of stress or stress hormones, although stress is capable of altering the morphology of neurons in some brain regions (Sapolsky, 2003). In one study, the adrenal weights of young male EC, SC and IC rats were compared with the density of astrocytic processes in the dentate gyrus of the hippocampus to examine the relationship between group experience and potential individual differences in stress reactivity (Sirevaag et al., 1991). Because experience-induced astrocytic changes typically accompany alterations in dendritic structure, the dentate gyrus was selected for examination because EC had previously been shown to induce little or no effect on the dendritic architecture in this region of the brains of weanling male rats (Juraska et al., 1985). Although there was a positive correlation between the density of astrocytic processes in this region and adrenal weight on an individual animal basis, there were no group differences in either adrenal weight or astrocytic process density (Sirevaag et al., 1991). Thus, changes that correlate with adrenal hypertrophy can be distinguished from changes that correlate with experience. This is not to mitigate the substantial effects of significant exposure to stressors on the brain (reviewed in Sapolsky, 2003), which are evident here at the individual level, but rather to indicate that the effects of the complex environment are, by and large, not mediated in this manner.
One way the potential confounds of stress hormones and other, nonspecific hormonal and metabolic changes accompanied by EC exposure can be addressed is to employ paradigms in which the effects of learning are expected to be restricted to specific brain regions, and to compare plasticity in these regions to other ‘control’ regions. This approach has shown plastic anatomical effects of training to be concentrated on the side of the brain to which training is delivered compared to the opposite ‘untrained hemisphere’ (Chang and Greenough, 1982; Greenough et al., 1985) and to distinguish between activity-induced and learning-induced plasticity (Black et al., 1990; Anderson et al., 1996; Kleim et al., 1996; Kleim et al., 1998) (discussed below). Furthermore, specificity of plasticity is seen even in subpopulations of neurons and synapses within the same brain region (Withers and Greenough, 1989; Kleim et al., 1998); for example, EC causes dendritic hypertrophy of cerebellar Purkinje cells but not granule cells (Floeter and Greenough, 1979). Learning and memory remain the most, if not the only, plausible explanation of brain plasticity observed following complex experience because (1) both social and non-specific hormonal and metabolic factors can be ruled out, (2) direct interaction with the environment, not just visual exposure, is required to induce morphological plasticity in the brain (Ferchmin and Bennett, 1975), and (3) the brain undergoes similar remodeling in response to both training paradigms and EC exposure, specifically in regions that are associated with the processing of either environmental or task-related information.
Neuronal plasticity
Synapse number
Synaptogenesis in response to exposure to a complex environment has been demonstrated many times. Animals raised in EC have greater dendritic arborization, increased dendritic spine density and more synapses per neuron in a number of brain areas as compared with IC animals (reviewed by Greenough and Chang, 1988). For example, both dendritic branching and the number of synapses per neuron in the visual cortex are greater in rats raised in EC compared with either IC or SC rats, which are equivalent on this measure (Volkmar and Greenough, 1972; Turner and Greenough, 1985; Sirevaag and Greenough, 1987). Interestingly, the magnitude of these two effects is similar (20–25% increase in EC), which might indicate that synaptogenesis in the visual cortex in response to visual experience is the result of elongation and/or elaboration of dendritic branches on which new synapses are formed, rather than an increase in the packing density of synapses along an existing length of dendrite. Dendritic elaboration as a result of EC also occurs in other neocortical areas (e.g. Greenough et al., 1973; Kolb et al., 2003) and in the dentate gyrus and area CA3 of the hippocampus, although, interestingly, the direction of the changes in dendritic arbor here varies by sex (Juraska et al., 1985; Juraska et al., 1989). This increase in dendritic length can be detected after as few as 4 days in EC in the visual cortex (Wallace et al., 1992), and contributes to the greater thickness of the visual cortex in EC animals that was reported initially (Bennett et al., 1964). However, increases in dendritic spine density in response to EC exposure are also reported (e.g. Globus et al., 1973; Comery et al., 1995; Rampon et al., 2000; Kolb et al., 2003). Also, repeated in vivo imaging of dendritic spines on pyramidal neurons in the adult mouse barrel cortex (using transgenic mice that express green fluorescent protein in a subset of cortical neurons) revealed that spine turnover is influenced heavily by sensory experience (Trachtenberg et al., 2002). Sensory deprivation (whisker trimming) increased the proportion of spines that were transient (present for ≤1 day) and decreased the proportion of spines observed to be stable over several days (Trachtenberg et al., 2002).
Experience-induced synaptogenesis persists well beyond the exposure to EC and can also be induced later in life. A recent study illustrates both findings: rats that were exposed to EC for either 30 or 60 days as adults had significantly (and equivalently) more synapses per neuron in layer IV of the visual cortex than did IC animals of the same age, as revealed by electron microscopy. Importantly, animals that had experienced EC for 30 days and then were placed in individual housing (IC conditions) for a subsequent 30 days also exhibited the full EC effect, reflecting the persistence of EC-induced synaptic plasticity (Fig. 1) (Briones et al., 2004). Similarly, animals raised in EC for 30 days beginning at weaning followed by 30 days of IC housing conditions had comparably greater dendritic arborization of visual cortical neurons (both stellate neurons of layer IV and pyramidal neurons of layer III) compared with IC animals, as did animals that were examined immediately after 30 days of EC housing (Camel et al., 1986). Finally, increases in synapse number and dendritic branching of neurons have been demonstrated in response to EC in aging rats (Green et al., 1983; Greenough et al., 1986).
Fig. 1. Persistence of the EC-induced increase in the number of synapses per neuron in the adult rat visual cortex.
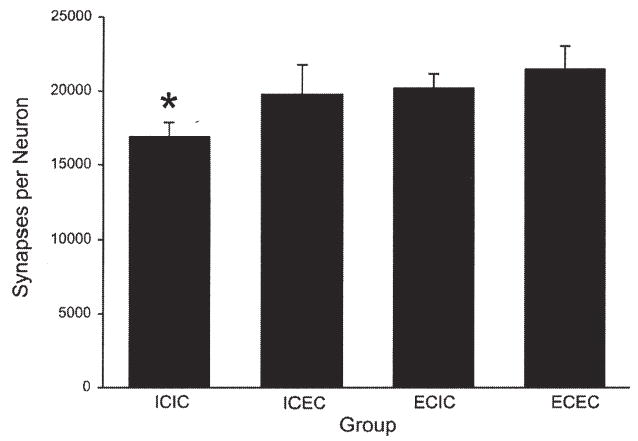
The EC-induced increase in the number of synapses per neuron in the adult rat visual cortex persists for at least 30 days after animals are removed from EC. ICIC animals, which were individually caged (IC) for 60 days, were significantly different (*, P<0.05) from each of the three other groups: ICEC animals (housed in IC for 30 days followed by EC housing for 30 days); ECIC animals (housed in EC for 30 days followed by IC housing for 30 days); and ECEC animals (housed in EC for 60 days). Modified, with permission, from Briones et al. (2004).
Training animals results in similar increases in synapses, which indicates that the effects of EC on this measure are a result of the learning process and not merely increases in general activity levels. In a study designed to tease apart morphological changes associated with learning versus those associated with general activity, a group of adult female rats that had been trained on a motor skill-learning task (using a challenging ‘acrobatic’ course) were compared with animals allowed to exercise freely (on a treadmill) but with minimal opportunity for learning. The number of synapses per neuron in both motor and cerebellar cortices was greater in rats trained on the acrobat course when compared to both rats that exercised but did not engage in learning and to rats who were sedentary during the experiment (Black et al., 1990; Kleim et al., 1996). These learning-induced changes in synapse number persist for at least 4 weeks after training has completed (Fig. 2B) (Kleim et al., 1997). Thus, it appears that learning, not merely neural activity, is required to induce synaptogenesis.
Fig. 2. The glial response to motor-skill learning.
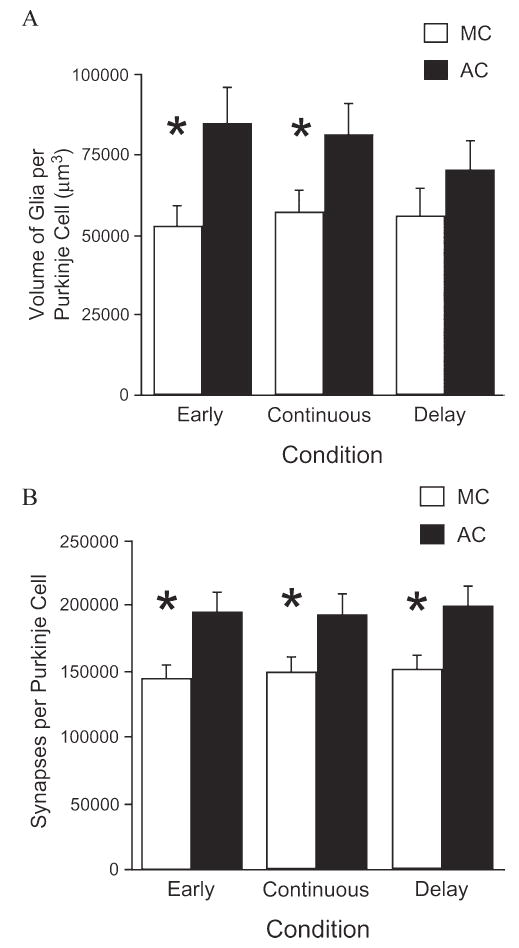
The glial response to motor-skill learning (A) is transient and requires persistent motor skill training for maintenance, whereas the increase in synapses per neuron (B) is stable in the absence of continued training. AC (acrobat) rats were trained on a motor skill learning task, whereas MC (motor control) animals ran on a treadmill but were not given an opportunity for learning. Animals in the Early group either participated in training (AC) or exercised (MC) for 10 days, animals in the Continuous group participated for 38 days, and animals in the Delay group participated for 10 days and then training (or exercise) was discontinued for the following 28 days before histological examination. * indicates P<0.05 for the comparison between the MC and AC animals of a particular group (Early, Continuous and Delay). Modified, with permission, from Kleim et al. (1997) and from Kleim et al. (in preparation).
Synapse morphology
In addition to inducing the formation of new synapses, experience can also either modify the morphology of existing synapses or induce the formation and/or loss of synapses exhibiting particular characteristics (reviewed in Greenough and Chang, 1988). Animals exposed to EC have larger synaptic components, both pre- and post-synaptic. For example, the average size of the postsynaptic density (PSD), which is proportional to dendritic-spine volume (Sorra and Harris, 2000), is increased by ~5–8% in the visual cortex following exposure of rats to EC for 1 month beginning around weaning (West and Greenough, 1972; Diamond et al., 1975; Sirevaag and Greenough, 1985; Turner and Greenough, 1985). In synaptic boutons, the cross-sectional area of vesicle aggregate profiles in layer IV of the visual cortex is ~one-third greater in EC compared with IC rats (SC rats were intermediate) (Sirevaag and Greenough, 1987). The size of the synapse is thought to be related to its strength, and in support of this notion, it has been found that in monocularly-deprived cats, synapses innervated by fibers carrying information from the non-deprived eye are larger, both pre- and post-synaptically, than synapses that are innervated by fibers carrying information from the deprived eye (Tieman, 1984; Tieman, 1985).
In addition to size, the shape of dendritic spines, which are the primary location of excitatory synaptic input onto principal neurons in the neocortex and hippocampus, is important for their function, because shape influences a spine’s conductive properties (Sorra and Harris, 2000). Reflective of the spine’s relative maturational state, spine shape changes in similar ways over development (from the initial sessile shape, to exhibiting a clearly discernible head or neck and, finally, to the large mushroom shape with a mature spine apparatus) (Harris et al., 1992) and in response to EC (Sirevaag and Greenough, 1985) and to LTP (Chang and Greenough, 1984) (reviewed by Greenough and Chang, 1988). For example, Comery et al. (1996) reported 60% greater density of multiple-headed dendritic spines on spiny neurons in the striatum of EC compared with IC rats. On the pre-synaptic side, boutons in animals exposed to EC are more concave than those in IC rats, with SC rats intermediate (Wesa et al., 1982). Additionally, Tieman (1985) showed that in monocularly-deprived cats, synapses associated with the deprived eye are more presynaptically convex than those associated with the open eye. Finally, Dyson and Jones (1980) reported that synaptic contacts in the rat visual cortex become increasingly less convex with age. Therefore it appears that the shapes of both pre- and post-synaptic components indicate the maturational state of a synapse.
Recently, it has become clear that other aspects of synaptic morphology are also sensitive to experience. For instance, perforated synapses (those whose PSD has enlarged, resulting in discontinuities) are associated with synaptic plasticity, in part, because they increase in the visual cortex both across development and in response to EC (Greenough et al., 1978; Jones and Calverley, 1991), in the motor cortex following training on a motor skill learning task (Jones, 1999), and in the hippocampus in response to either kindling or LTP induction (Geinisman et al., 1990; Geinisman et al., 1991). Additionally, postsynaptic expression of AMPA receptors, the number of which is considered to be the major determinant of synaptic efficacy, was found to be a ubiquitous characteristic of perforated synapses in the hippocampus using immunogold electron microscopy (Ganeshina et al., 2004). By contrast, only a fraction (64%) of the non-perforated synapses examined expressed these receptors (Ganeshina et al., 2004). Experience also induces the formation of multiple synaptic boutons (MSBs; two post-synaptic contacts innervated by the same pre-synaptic varicosity). Specifically, animals trained on a motor skill task had more MSBs in the cerebellum than animals who either exercised without the opportunity for learning or were sedentary during the course of the experiment (Federmeier et al., 2002). Similarly, the number of MSBs per neuron that contacted both a dendritic spine and a dendritic shaft were increased greatly in layer IV of the visual cortex of rats exposed to EC for 60 days compared with either SC or IC controls (Jones et al., 1997). From these examples it is clear that the formation of novel dendritic contacts onto existing axonal boutons and varicosities is a common form of experience-driven synaptic plasticity, one that would seem to have the effect of altering the efficacy of a pre-existing pathway rather than creating novel connections. Although the steps leading to the formation of perforated synapses and MSBs, and their ultimate function, is less clear, some interesting hypotheses have been proposed. Carlin and Siekevitz (1983) advanced the model of the dividing synapse to account for the induction of perforated synapses. Initially, the synaptic junction was thought to enlarge, then develop a perforation, then split into two separate synaptic junctions within a single synaptic terminal and, finally, the spine itself would divide into two spines, each containing one synaptic junction. However, when Kristin Harris’ group examined synapses carefully in CA1 of hippocampus using unbiased stereological techniques as applied to electron microscopy, they failed to observe a single branched (‘multiple-headed’ spine) with the different ‘heads’ in synaptic contact with the same presynaptic bouton (Sorra et al., 1998), an intermediate stage that is predicted by the splitting hypothesis. Subsequently, they examined the issue of spine splitting directly by serially reconstructing synapses on hippocampal dendrites and the surrounding neuropil across development and in response to hippocampal LTP (Fiala et al., 2002). Their results indicate that the postsynaptic components of MSBs actually frequently arise from separate dendritic processes. When two postsynaptic components from the same dendrite contact the same presynaptic bouton, they do not appear to have derived originally from the same dendritic spine, because other stable structures, such as mature axons, pass between them. This topic is still hotly debated and is an exciting avenue for future research in the neuroanatomical correlates of plasticity.
Neurogenesis
Most neurons in the brain proliferate during gestation and, until recently, the notion that neurogenesis does not occur in the adult mammalian brain (outside of the olfactory bulb) was part of neuroscience dogma. Although there were earlier indications to the contrary (Altman, 1962; Altman, 1963; Kaplan, 1981), these were largely ignored until several key studies were published within the last decade. These studies confirm the phenomenon of adult neurogenesis in the dentate gyrus of rodents and primates; the question of whether neurogenesis occurs in the adult primate neocortex remains controversial (Eriksson et al., 1998; Gould et al., 1999b; Kornack and Rakic, 1999; Gould et al., 2001).
Although the number of neurons added to the adult brain is small in comparison to both total neuron number and glial cell genesis, several environmental factors influence this process. In general, stress (during both development and adulthood), and stress hormones, alcohol exposure and the aging process decrease the number of new neurons added to the adult brain, whereas antidepressants, estrogen, exercise and EC increase it (Gould et al., 1997; Cameron et al., 1998; Kempermann et al., 1998; Tanapat et al., 1999; van Praag et al., 1999; Malberg et al., 2000; Brown et al., 2003; Mirescu et al., 2004). The mechanisms by which voluntary exercise and environmental complexity result in greater numbers of new cells added to the dentate gyrus of the adult rodent appear to be different: exercise increases the rate of neurogenesis, whereas EC exposure promotes the survival of new neurons (Kempermann et al., 1998; van Praag et al., 1999).
Recently, our laboratory has investigated the interacting influences of voluntary exercise and aging on adult neurogenesis for the first time in a primate model. In collaboration with Judy Cameron’s group at the University of Pittsburgh, young adult (10–12 years) and mature adult (15–17 years) female M. fascicularis monkeys were assigned to one of three treatment groups: Runners, who ran on a treadmill for one hour a day, five days a week, for 24 weeks; sedentary Controls, who sat on an immobile treadmill for an equivalent amount of time for 24 weeks; and Run-Stops, who exercised for 24 weeks exactly as the runners did, but then subsequently sat on an immobile treadmill for the allotted time for an additional 12 weeks. Preliminary results confirm that significant neurogenesis occurs in the dentate gyrus of adult monkeys. Additionally, our findings to date indicate that neurogenesis is increased in this area in young adult monkeys in response to exercise, but that the ability of exercise to increase neurogenesis in this region might be reduced with age (Kohler, Cameron, Williams and Greenough, unpublished).
Aided by the discovery that brain-derived neurotrophic factor (BDNF), which is known to be crucial for use-dependent synaptic plasticity, is common to many of the factors known to impact adult neurogenesis, the potential functions of adult-generated hippocampal neurons are beginning to be explored. Stress is a risk-factor for depression, and both exercise and antidepressants can relieve behavioral correlates of depression (Cotman and Berchtold, 2002). BDNF can alleviate behavioral symptoms in animal models of depression (e.g. Siuciak et al., 1997; Shirayama et al., 2002) and is increased by factors that increase neurogenesis in the adult dentate gyrus (i.e. exercise, estrogen and antidepressants) and decreased by those factors known to reduce neurogenesis (i.e. stress, aging) (reviewed by Cotman and Berchtold, 2002). Furthermore, antidepressant administration either blocks or attenuates the stress-induced decrease of neurogenesis in the dentate gyrus and BDNF levels (Nibuya et al., 1995; Xu et al., 2004). Interestingly, the time-course necessary for antidepressant administration to influence hippocampal neurogenesis is similar to that required for therapeutic benefit (i.e. chronic rather than acute) (Nibuya et al., 1995; Russo-Neustadt et al., 2000). Finally, inhibiting hippocampal neurogenesis blocks the behavioral effects of antidepressant drug administration (Santarelli et al., 2003). Thus, hippocampal neurogenesis might play a role in the behavioral effects of mood-stabilizing factors, such as antidepressants and exercise, in the adult brain.
Increases in BDNF levels have also been found in the dentate gyrus and cerebral cortex of rats housed in EC (e.g. Ickes et al., 2000). Because EC rats exhibit superior learning and memory ability compared to IC rats, and because exercise and EC-exposure during adulthood increase neurogenesis in the dentate gyrus but not in the olfactory bulb (Brown et al., 2003), a learning-specific role for neurons added to the adult brain has also been proposed. It has been shown that training on associative learning tasks that require the hippocampal formation, but not training on hippocampal-independent tasks, increases the number of new neurons in the dentate gyrus (Gould et al., 1999a). Because exposure to EC is known to improve animals’ performance on tests of learning and memory, it seems likely that EC-generated hippocampal neurons participate in the improved memory performance. Earlier this year, Rampon’s group confirmed the benefit conferred on both memory performance and hippocampal neurogenesis by EC exposure, and furthermore reported that blocking adult neurogenesis with the antimitotic agent methylazoxy-methanol acetate abolished the EC-induced improvement in hippocampal-dependent memory (Bruel-Jungerman et al., 2005). Interestingly, it might be the neurons born prior to the learning experience, and not those generated by the learning experience itself, that are crucial for memory performance. Mild irradiation, which inhibits adult neurogenesis, disrupts performance on the spatial (hippocampal-dependent) version of the Morris water maze (but is without effect on performance of the hippocampal-independent, visible platform version of the maze) when administered 4–28 days before maze training, but not when administered just before or immediately following maze training (Snyder et al., 2005). This finding is perhaps unsurprising in light of the fact that the brain must rely on past experiences to predict future ones. Thus, cells may be added to the adult hippocampus in anticipation of their need to mediate the acquisition, storage, and/or consolidation of future memories.
Astrocytes
Although the focus on environmentally-driven plasticity in the brain has traditionally been on altered morphology of neurons and in particular the synapse, astrocytic glia also demonstrate robust changes in response to experience. Exposure to EC, which was originally designed to investigate the relationship between neuronal and behavioral changes, was also shown in some early studies to induce changes in astroyctic morphology (Diamond et al., 1964; Szeligo and Leblond, 1977). However, limitations in quantification techniques available at the time resulted in inconsistent findings that made subsequent interpretation difficult. With the advent of improved quantification methods, in general referred to as unbiased stereological methodology, EC-induced increases astrocytic-cell size (hypertrophy) and number (hyperplasia) have been confirmed (Sirevaag and Greenough, 1987; Sirevaag and Greenough, 1991).
The hypertrophy of astrocytic processes in response to EC appears to depend on both duration of EC exposure and the cortical layer (reviewed in Jones, 2002). In general, morphological plasticity of astrocytes in response to EC occurs on a time scale that is comparable to observed neuronal changes in this paradigm. For example, 4 days of EC housing during adolescence increases the surface density of glial fibrillary acidic protein-immunoreactive astrocytic processes in layer II/III of the rat visual cortex (Jones et al., 1996), an exposure duration that induced detectable dendritic growth in this same layer (Wallace et al., 1992). Additionally, after 30 days of differential housing, the astrocyctic volume per neuron in the visual cortex increased in EC rats by an amount comparable to the previously established increase in synapse number (Sirevaag and Greenough, 1985; Jones and Greenough, 1996). This might indicate that the astrocytic hypertrophy in the visual cortex of the EC rat is driven by synapse formation, as is the case in the cerebellar cortex following motor-skill learning (Anderson et al., 1994). Because exercise without skill learning does not induce either synaptogenesis or astrocytic hypertrophy, and astrocytic and synaptic changes in the cerebellar cortex correlate on an animal-by-animal basis, increased astrocytic volume can be inferred to arise in association with learning-specific synaptogenesis, and not merely constitute a response to a general increase in neural activity (Anderson et al., 1994).
It is not just the morphology of astrocytes that is altered by experience; the relationship between astrocytes and neurons is also refined. In the neocortex, astrocytes cover pre- and post-synaptic elements of axodendritic synapses but, typically, only partial covering is observed. The degree of synaptic ensheathement by fine astrocytic processes, as observed by electron microscopy, increases in the visual cortex of EC rats (Fig. 3) (Jones and Greenough, 1996), indicating that the function of demonstrated alterations in astrocytic processes in response to EC is related to enhancing synaptic function. Clearly there is an experience-dependent enhancement of astrocytic–synaptic communication, an important finding in light of the fact that perisynaptic astrocytes modulate synaptic transmission in response to synaptically released neurotransmitters, and themselves release neurotransmitter (Oliet et al., 2001; Zhang and Haydon, 2005). Additionally, astrocytes are involved in GABA and glutamate re-uptake and metabolism (Schousboe et al., 1992; Bezzi et al., 1999) and can conduct excitation via propagated Ca2+ waves which can directly influence neuronal activity (reviewed by Zhang and Haydon, 2005). In CA1 of the hippocampus, where nearly 60% of the synapses are contacted directly by astrocytic processes (Ventura and Harris, 1999), postsynaptic glutamate receptors are activated by glutamate spillover from neighboring terminals (in addition to glutamate released from the presynaptic terminal) (see Kullmann et al., 1999). Astrocytic coverage of synapses may thus also serve to enhance input specificity. Although to our knowledge it has not been examined whether activity influences the degree of astrocytic ensheathement of synapses in the hippocampus, it has been demonstrated conclusively in the supraoptic nucleus of the hypothalamus that the degree of synaptic coverage by astrocytes is associated with the degree of glutamate clearance, which, in turn, influences glutamate concentration and diffusion in the synapse (Oliet et al., 2001). Thus the presence of astrocytes at the synapse has a profound influence on synaptic efficacy.
Fig. 3. Astrocytic processes in layer IV of the rat visual cortex.
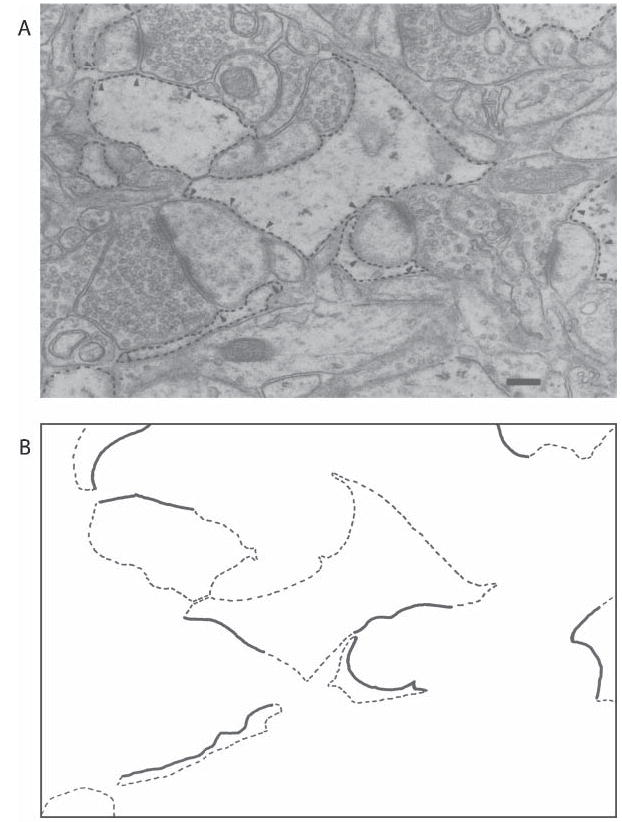
Astrocytic processes (dashed outline) in layer IV of the rat visual cortex revealed by electron microscopy (A), and tracing of these processes (B). Processes in direct apposition to synaptic elements are indicated (arrows, A; solid lines, B). Scale bar, 0.2 μm. Reprinted, with permission, from Jones and Greenough (1996).
Uniquely anatomically and functionally positioned to manage these diverse roles at the synapse, astrocytes exhibit plasticity that, in addition to neuronal plasticity, might be crucial to the processes of learning and memory. However, their role in enhancing learning-dependent synaptic plasticity may be transient; astrocytic changes appear to fade rapidly in the absence of continued behavioral and environmental stimulation. For example, when animals were first trained on a motor skill learning task for 10 days and then left idle for the following 28 days, training-induced effects on astrocytes were reduced and no longer statistically significant, although synaptogenesis that had occurred during learning remained evident in these animals (Fig. 2A,B) as it paralleled that observed in animals that were trained continuously for the entire 38 days of the experiment (Kleim et al., 1997; Kleim, in revision). It is tempting to speculate that astrocytic changes might be necessary to induce, but not to maintain, adaptive changes in the brain’s ‘wiring diagram’ in response to experience.
Oligodendrocytes and myelination
There is evidence that both oligodendrocytes and the myelination process itself are sensitive to developmental experience. Early work in the optic nerve showed that visual deprivation resulted in reduced myelination (Gyllensten and Malmfors, 1963) and, conversely, that premature eyelid opening accelerated the onset of myelination (Tauber et al., 1980). However, it should be noted that not all studies find a relationship between early visual experience and myelination in the optic nerve (Moore et al., 1976; Fukui et al., 1991). Szeligo and Leblond (1977), who were the first to examine the influence of rearing environment on brain fiber tracts, found increases in oligodendrocytes in the visual cortex of EC rats. Subsequently, Sirevaag and Greenough (1987) also found the volume fraction of oligodendrocyte nuclei in the visual cortex to be greater among EC-raised rats, compared with their IC littermates. The influence of developmental experience on oligodendrocytes is not limited to the visual cortex; raising rats in EC increases the number of myelinated axons (measured using electron microscopy) in the splenial corpus callosum, which contains axons of visual cortical neurons (the responses of these cells to EC are reviewed above) (Juraska and Kopcik, 1988). The positive effect of a complex rearing environment on the size of the corpus callosum has also been demonstrated in rhesus monkeys (Sanchez et al., 1998).
Despite studies indicating that myelination continues well into adulthood in rodents and humans (Yakovlev, 1967; Benes et al., 1994; Nunez et al., 2000), this process is still often treated as if it were a strictly developmental phenomenon. The question of whether myelination remains sensitive to experience during adulthood is interesting and largely unexplored. Recently, our laboratory has begun to investigate this. Preliminary data indicate that the corpus callosum of rats exposed to EC during adulthood contains increased numbers of myelinated axons in the splenial portion and that these changes persist for at least one month after the animal has been removed from the complex environment (Briones, 1999). Thus, the ability of oligodendrocytes and the myelination process to respond to the demands of an animal’s environment apparently extends beyond the developmental timeframe, and the experience-induced changes in the adult brain appear to be stable. At this time it is unknown whether the increased myelination in the EC rat is due to an increase in the number of axons that a typical oligodendrocyte helps to myelinate or by an increase in the proliferation and/or survival of new oligodendrocytes. Although many questions remain to be answered, the discovery that adult myelination is regulated by experience provides a functional correlate to continued myelination in the brain across the lifespan.
Several potential, activity-dependent axonal signals have been proposed to initiate myelination, including ATP and the neurotransmitters glutamate and aspartate (receptors for which are expressed by oligodendrocytes) (Butt and Tutton, 1992; Brady et al., 1999; Stevens and Fields, 2000; Stevens et al., 2002). The communication between axons and myelinating glial cells is also bidirectional and myelination leads to local changes in axonal cytoarchictecture (de Waegh et al., 1992). Although the mechanism by which an increase in environmental complexity triggers myelination is uncertain, it is clear that some form of communication between neurons and oligodendrocytes is affected by experience.
One might speculatively view the addition of new axons to the myelinated fiber pool as a form of plasticity with a potential comparable to either the addition or strengthening of synapses, with one additional feature: speed rather than efficacy of communication is enhanced. Typical myelinated fibers, which are generally larger in axonal diameter than non-myelinated axons, conduct at velocities 50–100 times faster than their non-myelinated counterparts (see Brinley, 1980). Assuming a velocity of 60 msec for a large myelinated fiber, conduction of an action potential from one hemisphere to the other might require 1–2 msec, whereas a typical unmyelinated fiber conducting at 1 msec might take 50–100 msec to travel the same distance. During the difference in time of arrival between the myelinated and unmyelinated axonal input, a typical large cortical pyramidal neuron could have fired a burst of several action potentials in response to the earlier input. Hence, it is hard to imagine that these two fiber types are working in synchrony on the same behavioral and thought processes. Moreover, if an unmyelinated fiber were to be recruited to the myelinated pool, as seems to occur during exposure to a complex environment, its input would subsequently arrive some 50–100 msec earlier than before –seemingly a qualitative difference in an asynchronously operating system in which the response to inputs does not typically wait until all afferents have had their say. In short, where relatively long-distance communication is involved (and for these purposes, communication between adjacent gyri in the human brain would be ‘long distance’, with disparities of perhaps 30–40 msec between myelinated and unmyelinated fiber inputs), myelinated fibers should largely determine the early response, if there is one. Hence, addition to the myelinated pool connecting brain regions involved in a particular task might qualitatively alter performance. One might wonder about the purpose of the vastly more numerically prevalent unmyelinated fibers. One possibility is that they allow utilization of a richer supply of information in cases where a rapid response is not essential.
Cerebrovasculature
In contrast to earlier studies (e.g. Diamond et al., 1964; Rowan and Maxwell, 1981), data from our laboratory indicate that the brain’s vasculature is responsive to experience. Animals raised in EC have larger, more elaborately branched capillaries in the visual cortex compared with IC- and SC-raised animals (Black et al., 1987; Sirevaag et al., 1988; Black et al., 1991). The relative contribution of motor skill learning and increased motor activity to plasticity of cerebrovasculature cannot be distinguished using the EC paradigm. Physical exercise has since been shown to induce angiogenesis in motor brain regions; however, because motor skill learning is required to induce synaptogenesis, it can be inferred that angiogenesis is driven more by the repeated performance of unskilled movements rather than by the learning process per se (Black et al., 1990). Also, the plasticity of cerebrovasculature in response to behavioral demands appears to be far greater than that of synapses because the volume fraction of capillaries (which combines diameter and density effects) nearly doubles following EC exposure (Black et al., 1987; Sirevaag et al., 1988). Additionally, the capacity of the vasculature in the primary motor cortex to supply blood in response to increased demand is greater in exercised animals, using functional magnetic resonance imaging to specifically address blood flow (versus the more common BOLD signal arising from deoxyhemoglobin) and reserve capacity under load in anesthetized rats (Swain et al., 2003). Recently, our laboratory has investigated the interacting influences of exercise and aging on cerebrovasculature. Capillary volume in the precentral gyrus was found to be increased in mature (15–17 years) but not young (10–12 years) female M. fascicularis monkeys who had exercised for 1 hour a day, 5 days a week for 24 weeks before tissue collection, compared with sedentary controls (Fig. 4) (Rhyu, 2003). Interestingly, capillary volume fraction in monkeys that had a 12 week period of inactivity following the 24 weeks of exercise returned to inactive control levels, indicating that exercise-induced changes in cerebrovasculature are short-lived (Rhyu, 2003). Aside from this study, the persistence of experience-induced angiogenesis has not been well investigated; however the magnitude of the effect and its activity-dependent nature indicate that the impact of experience on cerebrovasculature is likely to be considerably more short-lived than the quite stable changes in synaptic reorganization and myelination discussed above. Because, like exercise, experimentally induced hypoxia also induces rapid angiogenesis (Harik et al., 1995), information concerning blood oxygen levels or a related metabolic demand may be the physiological signal that triggers vascular proliferation. Although the precise signal is unknown, it is known that similar to synaptogenesis, angiogenesis in response to experience is greatest during development, is maintained during adulthood, and remains present, although diminished, during aging (Black et al., 1989a).
Fig. 4. Exercise increases changes in the vascular volume fraction in the cortex of mature, but not young, monkeys.
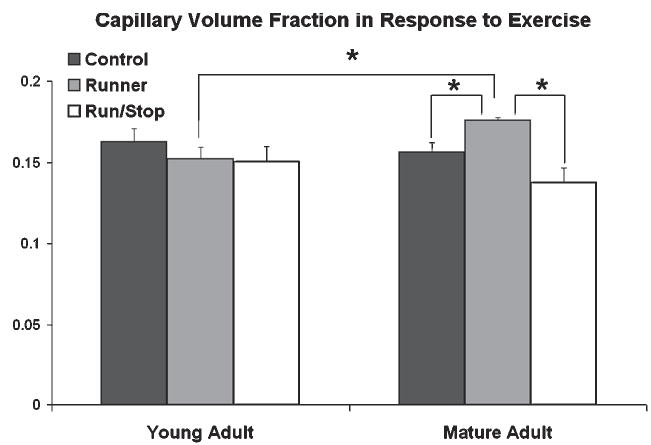
Exercise increases the vascular volume fraction in the cortex of mature (15–17 years) but not young (10–12 years) monkeys. In mature animals, Runners (exercised for 24 weeks) had greater capillary volume fraction compared with both Run/Stop animals (that had a 12 week period of inactivity following 24 weeks of exercise) and sedentary Control animals. Reprinted, with permission, from Rhyu et al. (2003).
CONCLUSIONS
Although much exciting work remains to be done in the field of experience-induced morphological plasticity in the brain, especially in terms of non-neuronal components of the nervous system such as astrocytes, oligodendrocytes and cerebrovasculature, some important observations may be drawn from the information available to date. The most general conclusion that can be made confidently is that the brain is an extremely plastic organ, the structure of which is exquisitely sensitive to experience. A major function of the brain is thus to continuously re-organize itself, and it does so in a way that is specifically tailored to result in behavior that is adaptive in the context of the individual’s own unique environment. The nature of experience-driven plasticity is such that, although it has been demonstrated in many brain regions, in any given instance it is specific to those regions involved in processing the behaviorally-relevant features of the environment. For example, dramatic morphological changes occur in the visual cortex in response to a visually complex environment (EC). By contrast, learning a complex motor skill induces plasticity in motor areas of the brain such as the motor and cerebellar cortices. Such region-specific reorganization is not limited to experimental rodent models because it has also been observed in monkeys (e.g. Recanzone et al., 1992) and humans, for example string musicians (see Pantev et al., 2003) and those who have learned to read Braille (Pascual-Leone and Torres, 1993).
Experience-dependent plasticity is not limited to synapses or, even, to neurons. In fact, most if not every component of the nervous system exhibits robust, reproducible responses to experience. Thus, in addition to synaptogenesis, dendritic reorganization, and neurogenesis, other non-neuronal components are sensitive to experience, resulting for example in angiogenesis, increased myelination, astrocytic hypertrophy and increased astrocytic ensheathement of synapses. There are differences in both the type of experience that drive these changes and in their relative stability in the absence of the driving experience. For example, learning results in synaptogenesis, astrocytic hypertrophy and survival of newly generated dentate gyrus neurons, whereas physical exercise without learning induces angiogenesis and dentate gyrus neurogenesis. Changes in synapses and myelination appear to be more stable and, perhaps, permanent, possibly because they reflect re-organization of the brain’s functional ‘wiring diagram’, in comparison to the more generally-acting transient effects on astrocytes and the brain’s cerebrovasculature. The stability in morphological changes in synapses and myelination might be due to the fact that the brain must rely on past experiences to predict future ones. Therefore, organizational changes in these components of the nervous system at one point in time are very likely to be useful in the future. Another example in this regard is the addition of new cells in the dentate gyrus. As discussed above, whereas physical exercise increases the rate of neurogenesis, EC and, more specifically, learning, appears to enhance the survival of neurons generated earlier. Thus, cells may be added to the adult hippocampus in anticipation of their need to mediate the acquisition, storage and/or consolidation of future memories. From this brief review of the literature it can be inferred that experience induces multiple forms of plasticity in the brain that must be regulated, at least partially, by independent mechanisms. Finally, it should be emphasized that environmentally-induced plasticity in the brain does not simply consist of changes in different classes of cells independently; rather, interactions between neurons and glia are also altered to more optimally meet behavioral demands. The greater ensheathement of synapses by astrocytes in response to a complex environment is one example of this, and we believe the future of the investigation of environmentally-driven plasticity lies in understanding the integrative response of different brain elements to experience and in discovering the nature of their adaptive significance.
Acknowledgments
Portions of this work were supported by NIH grants HD37175, HD07333 (NICHD), AG10154 (NIA), MH35321 (NIMH), by the Illinois-Eastern Iowa District of the Kiwanis International Spastic Paralysis Research Foundation, and by the Retirement Research Foundation. The authors wish to thank the Beckman Visualization, Media, and Imaging Laboratory, Dr. James Churchill and Dr. Im Joo Rhyu for assistance with figures, and Dr. Willie Dong and Shawn Kohler for their thoughtful comments on the manuscript.
References
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anatomical Record. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Alcantara AA, Greenough WT. Motor-skill learning: changes in synaptic organization of the rat cerebellar cortex. Neurobiology of Learning and Memory. 1996;66:221–229. doi: 10.1006/nlme.1996.0062. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia. 1994;11:73–80. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Vesce S, Panzarasa P, Volterra A. Astrocytes as active participants of glutamatergic function and regulators of its homeostasis. Advances in Experimental Medicine and Biology. 1999;468:69–80. doi: 10.1007/978-1-4615-4685-6_6. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proceedings of the National Academy of Sciences of the USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Polinsky M, Greenough WT. Progressive failure of cerebral angiogenesis supporting neural plasticity in aging rats. Neurobiology of Aging. 1989a;10:353–358. doi: 10.1016/0197-4580(89)90048-1. [DOI] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neuroscience Letters. 1987;83:351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Wallace CS, Savin MH, Greenough WT. Effects of complex experience on somatic growth and organ development in rats. Developmental Psychobiology. 1989b;22:727–752. doi: 10.1002/dev.420220707. [DOI] [PubMed] [Google Scholar]
- Black JE, Zelazny AM, Greenough WT. Capillary and mitochondrial support of neural plasticity in adult rat visual cortex. Experimental Neurology. 1991;111:204–209. doi: 10.1016/0014-4886(91)90008-z. [DOI] [PubMed] [Google Scholar]
- Brady ST, Witt AS, Kirkpatrick LL, de Waegh SM, Readhead C, Tu PH, et al. Formation of compact myelin is required for maturation of the axonal cytoskeleton. Journal of Neuroscience. 1999;19:7278–7288. doi: 10.1523/JNEUROSCI.19-17-07278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F.J. (1980) Excitation and conduction in nerve fibers. In: Mountcastle, V.B. (Ed.) Medical Physiology. The C.V. Mosby Co. 1, pp. 46–81.
- Briones T, Shah P, Juraska J, Greenough WT. Effects of prolonged exposure to and subsequent removal from a complex environment on corpus callosum myelination in the adult rat. Society for Neuroscience Abstracts. 1999;25:638. [Google Scholar]
- Briones TL, Klintsova AY, Greenough WT. Stability of synaptic plasticity in the adult rat visual cortex induced by complex environment exposure. Brain Research. 2004;1018:130–135. doi: 10.1016/j.brainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. European Journal of Neuroscience. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. European Journal of Neuroscience. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Butt AM, Tutton M. Response of oligodendrocytes to glutamate and gamma-aminobutyric acid in the intact mouse optic nerve. Neuroscience Letters. 1992;146:108–110. doi: 10.1016/0304-3940(92)90184-9. [DOI] [PubMed] [Google Scholar]
- Camel JE, Withers GS, Greenough WT. Persistence of visual cortex dendritic alterations induced by postweaning exposure to a “superenriched” environment in rats. Behavioral Neuroscience. 1986;100:810–813. doi: 10.1037//0735-7044.100.6.810. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Siekevitz P. Plasticity in the central nervous system: do synapses divide? Proceedings of the National Academy of Sciences of the USA. 1983;80:3517–3521. doi: 10.1073/pnas.80.11.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chang FL, Greenough WT. Lateralized effects of monocular training on dendritic branching in adult split-brain rats. Brain Research. 1982;232:283–292. doi: 10.1016/0006-8993(82)90274-8. [DOI] [PubMed] [Google Scholar]
- Chang FL, Greenough WT. Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Research. 1984;309:35–46. doi: 10.1016/0006-8993(84)91008-4. [DOI] [PubMed] [Google Scholar]
- Comery TA, Shah R, Greenough WT. Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiology of Learning and Memory. 1995;63:217–219. doi: 10.1006/nlme.1995.1025. [DOI] [PubMed] [Google Scholar]
- Comery TA, Stamoudis CX, Irwin SA, Greenough WT. Increased density of multiple-head dendritic spines on medium-sized spiny neurons of the striatum in rats reared in a complex environment. Neurobiology of Learning and Memory. 1996;66:93–96. doi: 10.1006/nlme.1996.0049. [DOI] [PubMed] [Google Scholar]
- Coq JO, Xerri C. Environmental enrichment alters organizational features of the forepaw representation in the primary somatosensory cortex of adult rats. Experimental Brain Research. 1998;121:191–204. doi: 10.1007/s002210050452. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR. The effects of an enriched environment on the histology of the rat cerebral cortex. Journal of Comparative Neurology. 1964;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Law F, Rhodes H, Lindner B, Rosenzweig MR, Krech D, et al. Increases in cortical depth and glia numbers in rats subjected to enriched environment. Journal of Comparative Neurology. 1966;128:117–126. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Lindner B, Johnson R, Bennett EL, Rosenzweig MR. Differences in occipital cortical synapses from environmentally enriched, impoverished, and standard colony rats. Journal of Neuroscience Research. 1975;1:109–119. doi: 10.1002/jnr.490010203. [DOI] [PubMed] [Google Scholar]
- Dyson SE, Jones DG. Quantitation of terminal parameters and their inter-relationships in maturing central synapses: a perspective for experimental studies. Brain Research. 1980;183:43–59. doi: 10.1016/0006-8993(80)90118-3. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Kleim JA, Greenough WT. Learning-induced multiple synapse formation in rat cerebellar cortex. Neuroscience Letters. 2002;332:180–184. doi: 10.1016/s0304-3940(02)00759-0. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Bennett EL. Direct contact with enriched environment is required to alter cerebral weights in rats. Journal of Comparative and Physiological Psychology. 1975;88:360–367. doi: 10.1037/h0076175. [DOI] [PubMed] [Google Scholar]
- Fiala BA, Joyce JN, Greenough WT. Environmental complexity modulates growth of granule cell dendrites in developing but not adult hippocampus of rats. Experimental Neurology. 1978;59:372–383. doi: 10.1016/0014-4886(78)90229-7. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Allwardt B, Harris KM. Dendritic spines do not split during hippocampal LTP or maturation. Nature Neuroscience. 2002;5:297–298. doi: 10.1038/nn830. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Greenough WT. Cerebellar plasticity: modification of Purkinje cell structure by differential rearing in monkeys. Science. 1979;206:227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- Forgays DG, Forgays JW. The nature of the effect of free-environmental experience in the rat. Journal of Comparative and Physiological Psychology. 1952;45:322–328. doi: 10.1037/h0053731. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Hayasaka S, Bedi KS, Ozaki HS, Takeuchi Y. Quantitative study of the development of the optic nerve in rats reared in the dark during early postnatal life. Journal of Anatomy. 1991;174:37–47. [PMC free article] [PubMed] [Google Scholar]
- Galani R, Coutureau E, Kelche C. Effects of enriched postoperative housing conditions on spatial memory deficits in rats with selective lesions of either the hippocampus, subiculum or entorhinal cortex. Restorative Neurology and Neuroscience. 1998;13:173–184. [PubMed] [Google Scholar]
- Galani R, Jarrard LE, Will BE, Kelche C. Effects of postoperative housing conditions on functional recovery in rats with lesions of the hippocampus, subiculum, or entorhinal cortex. Neurobiology of Learning and Memory. 1997;67:43–56. doi: 10.1006/nlme.1996.3745. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. Journal of Comparative Neurology. 2004;468:86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Research. 1991;566:77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Morrell F, de Toledo-Morrell L. Increase in the relative proportion of perforated axospinous synapses following hippocampal kindling is specific for the synaptic field of stimulated axons. Brain Research. 1990;507:325–331. doi: 10.1016/0006-8993(90)90291-i. [DOI] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. Journal of Comparative and Physiological Psychology. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999a;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. Journal of Neuroscience. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999b;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EJ, Greenough WT, Schlumpf BE. Effects of complex or isolated environments on cortical dendrites of middle-aged rats. Brain Research. 1983;264:233–240. doi: 10.1016/0006-8993(83)90821-1. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Chang FLF. Plasticity of synapse structure and pattern in the cerebral cortex. A. Peters, E. G. J. Cerebral Cortex: Development and Maturation of Cerebral Cortex. 1988;7:391–440. [Google Scholar]
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behavioral and Neural Biology. 1985;44:301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- Greenough WT, McDonald JW, Parnisari RM, Camel JE. Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Research. 1986;380:136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR, Juraska JM. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Experimental Neurology. 1973;41:371–378. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978;202:1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, Bates KE, Kleim JA, Greenough WT. A brain adaptation view of plasticity: is synaptic plasticity an overly limited concept? Progress in Brain Research. 2002;138:91–108. doi: 10.1016/S0079-6123(02)38073-7. [DOI] [PubMed] [Google Scholar]
- Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function – a quantitative investigation in mice. Journal of Embryology and Experimental Morphology. 1963;11:255–266. [PubMed] [Google Scholar]
- Harik SI, Hritz MA, LaManna JC. Hypoxia-induced brain angiogenesis in the adult rat. Journal of Physiology. 1995;485:525–530. doi: 10.1113/jphysiol.1995.sp020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. Journal of Neuroscience. 1992;12:2685–705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D.O. (1949) The Organization of Behavior Wiley.
- Hymovitch B. The effects of experimental variations on problem solving in the rat. Journal of Comparative and Physiological Psychology. 1952;45:313–321. doi: 10.1037/h0061535. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Experimental Neurology. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Jones DG, Calverley RK. Frequency of occurrence of perforated synapses in developing rat neocortex. Neuroscience Letters. 1991;129:189–192. doi: 10.1016/0304-3940(91)90458-6. [DOI] [PubMed] [Google Scholar]
- Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. Journal of Comparative Neurology. 1999;414:57–66. [PubMed] [Google Scholar]
- Jones TA, Greenough WT. Ultrastructural evidence for increased contact between astrocytes and synapses in rats reared in a complex environment. Neurobiology of Learning and Memory. 1996;65:48–56. doi: 10.1006/nlme.1996.0005. [DOI] [PubMed] [Google Scholar]
- Jones TA, Hawrylak N, Greenough WT. Rapid laminar-dependent changes in GFAP immunoreactive astrocytes in the visual cortex of rats reared in a complex environment. Psychoneuroendocrinology. 1996;21:189–201. doi: 10.1016/0306-4530(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiology of Learning and Memory. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- Jones T. A. and Greenough W.T. (2002) Behavioural experience-dependent plasticity of glial-neuronal interactions. Volterra, A., Magistretti, P. and Hayden, P.G. (Eds) The Tripartite Synapse: Glia in Synaptic Transmission. Oxford University Press, pp. 248–265.
- Juraska JM, Fitch JM, Henderson C, Rivers N. Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Research. 1985;333:73–80. doi: 10.1016/0006-8993(85)90125-8. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Fitch JM, Washburne DL. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. II. Effects of gender and the environment. Brain Research. 1989;479:115–119. doi: 10.1016/0006-8993(89)91341-3. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Kopcik JR. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Research. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- Kaplan MS. Neurogenesis in the 3–month-old rat visual cortex. Journal of Comparative Neurology. 1981;195:323–338. doi: 10.1002/cne.901950211. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. Journal of Neuroscience. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. Journal of Neuroscience. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Swain RA, Armstrong KA, Napper RM, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiology of Learning and Memory. 1998;69:274–289. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Vij K, Ballard DH, Greenough WT. Learning-dependent synaptic modifications in the cerebellar cortex of the adult rat persist for at least four weeks. Journal of Neuroscience. 1997;17:717–721. doi: 10.1523/JNEUROSCI.17-02-00717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim J.A., Vij K., Kelly J.L., Ballard D.H and Greenough W.T. (in revision) The persistency of training-induced astrocytic hypertrophy within the cerebellar cortex.
- Kolb B, Gorny G, Soderpalm AH, Robinson TE. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse. 2003;48:149–153. doi: 10.1002/syn.10196. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proceedings of the National Academy of Sciences of the USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Min MY, Asztely F, Rusakov DA. Extracellular glutamate diffusion determines the occupancy of glutamate receptors at CA1 synapses in the hippocampus. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1999;354:395–402. doi: 10.1098/rstb.1999.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. Journal of Neuroscience. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nature Neuroscience. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Mohammed AK, Jonsson G, Archer T. Selective lesioning of forebrain noradrenaline neurons at birth abolishes the improved maze learning performance induced by rearing in complex environment. Brain Research. 1986;398:6–10. doi: 10.1016/0006-8993(86)91243-6. [DOI] [PubMed] [Google Scholar]
- Mohammed AK, Winblad B, Ebendal T, Larkfors L. Environmental influence on behaviour and nerve growth factor in the brain. Brain Research. 1990;528:62–72. doi: 10.1016/0006-8993(90)90195-h. [DOI] [PubMed] [Google Scholar]
- Moore CL, Kalil R, Richards W. Development of myelination in optic tract of the cat. Journal of Comparative Neurology. 1976;165:125–136. doi: 10.1002/cne.901650202. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Egeland T, Andersen P. Spatial training in a complex environment and isolation alter the spine distribution differently in rat CA1 pyramidal cells. Journal of Comparative Neurology. 1997;380:373–381. doi: 10.1002/(sici)1096-9861(19970414)380:3<373::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. Journal of Neuroscience. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev E, Kaczmarek L, Zhu SW, Winblad B, Mohammed AH. Environmental manipulation differentially alters c-Fos expression in amygdaloid nuclei following aversive conditioning. Brain Research. 2002;957:91–98. doi: 10.1016/s0006-8993(02)03606-5. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Nelson J, Pych JC, Kim JH, Juraska JM. Myelination in the splenium of the corpus callosum in adult male and female rats. Brain Research. Developmental Brain Research. 2000;120:87–90. doi: 10.1016/s0165-3806(99)00193-5. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Pantev C, Ross B, Fujioka T, Trainor LJ, Schulte M, Schulz M. Music and learning-induced cortical plasticity. Annals of the New York Academy of Sciences. 2003;999:438–450. doi: 10.1196/annals.1284.054. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nature Neuroscience. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. Journal of Neurophysiology. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Rhyu IJ, Boklewski J, Ferguson B, Lee KJ, Lange H, Bytheway J, et al. Exercise training is associated with increased cortical vascularization in adult female cynomolgus monkeys. Society for Neuroscience Abstracts. 2003;29:920.1. [Google Scholar]
- Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Research. 1978;153:563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- Rowan RA, Maxwell DS. Patterns of vascular sprouting in the postnatal development of the cerebral cortex of the rat. American Journal of Anatomy. 1981;160:247–255. doi: 10.1002/aja.1001600303. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochemical Research. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Yu AC, Hertz L. Regulatory role of astrocytes for neuronal biosynthesis and homeostasis of glutamate and GABA. Progress in Brain Research. 1992;94:199–211. doi: 10.1016/s0079-6123(08)61751-3. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. Journal of Neuroscience. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag AM, Black JE, Greenough WT. Astrocyte hypertrophy in the dentate gyrus of young male rats reflects variation of individual stress rather than group environmental complexity manipulations. Experimental Neurology. 1991;111:74–79. doi: 10.1016/0014-4886(91)90052-e. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Black JE, Shafron D, Greenough WT. Direct evidence that complex experience increases capillary branching and surface area in visual cortex of young rats. Brain Research. 1988;471:299–304. doi: 10.1016/0165-3806(88)90107-1. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. II. Synaptic morphometry. Brain Research. 1985;351:215–226. doi: 10.1016/0165-3806(85)90193-2. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Research. 1987;424:320–332. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Research. 1991;540:273–278. doi: 10.1016/0006-8993(91)90517-y. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Anti-depressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacology, Biochemistry and Behavior. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Fiala JC, Harris KM. Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. Journal of Comparative Neurology. 1998;398:225–240. [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. Journal of Comparative Neurology. 1977;172:247–263. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. Journal of Neuroscience. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber H, Waehneldt TV, Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neuroscience Letters. 1980;16:235–238. doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- Tieman SB. Effects of monocular deprivation on geniculocortical synapses in the cat. Journal of Comparative Neurology. 1984;222:166–176. doi: 10.1002/cne.902220203. [DOI] [PubMed] [Google Scholar]
- Tieman SB. The anatomy of geniculocortical connections in monocularly deprived cats. Cellular and Molecular Neurobiology. 1985;5:35–45. doi: 10.1007/BF00711084. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biological Psychiatry. 2000;47:210–20. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Research. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. Journal of Neuroscience. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Wallace CS, Kilman VL, Withers GS, Greenough WT. Increases in dendritic length in occipital cortex after 4 days of differential housing in weanling rats. Behavioral and Neural Biology. 1992;58:64–68. doi: 10.1016/0163-1047(92)90937-y. [DOI] [PubMed] [Google Scholar]
- Wesa JM, Chang FL, Greenough WT, West RW. Synaptic contact curvature: effects of differential rearing on rat occipital cortex. Brain Research. 1982;256:253–257. doi: 10.1016/0165-3806(82)90049-9. [DOI] [PubMed] [Google Scholar]
- West RW, Greenough WT. Effect of environmental complexity on cortical synapses of rats: preliminary results. Behavioral Biology. 1972;7:279–284. doi: 10.1016/s0091-6773(72)80207-4. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Zaborowski JA, Kolb B. Postsurgical enrichment aids adult hemidecorticate rats on a spatial navigation task. Behavioral and Neural Biology. 1984;42:183–190. doi: 10.1016/s0163-1047(84)91046-x. [DOI] [PubMed] [Google Scholar]
- Withers GS, Greenough WT. Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27:61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]
- Xu H, Luo C, Richardson JS, Li XM. Recovery of hippocampal cell proliferation and BDNF levels, both of which are reduced by repeated restraint stress, is accelerated by chronic venlafaxine. Pharmacogenomics Journal. 2004;4:322–331. doi: 10.1038/sj.tpj.6500265. [DOI] [PubMed] [Google Scholar]
- Yakovlev P. a. L., A. (1967) The myelinogenetic cycles of regional maturation of the brain. In: Minkowski, A. (Ed.) Retional Development of the Brain Early in Life. Blackwell Scientific Publications, pp. 3–70.
- Zhang Q, Haydon PG. Roles for gliotransmission in the nervous system. Journal of Neural Transmission. 2005;112:121–125. doi: 10.1007/s00702-004-0119-x. [DOI] [PubMed] [Google Scholar]


