Abstract
To analyze the pathogenetic mechanism of hematopoietic dysregulation associated with hepatitis A virus (HAV) infections, we studied the influence of HAV on monocyte (MO)-to-macrophage (MAC) maturation in vitro. Exposure of peripheral blood-derived mononuclear cells (MNC) to HAV led to diminished adherence of MO to plastic. Furthermore, HAV inhibited the ability of peripheral blood MO to differentiate toward MAC. Freshly isolated and 14-day-old MO cultures demonstrated reduced differentiation and decreased phagocytic capacity after challenge with HAV. Viral replication in MO/MAC cultures was confirmed by titration of infectious virus. We also determined the influence of HAV on the MO/MAC population in human long-term bone marrow cultures (LTBMCs). Inoculation of bone marrow MNC with HAV suppressed the establishment of an adherent stromal layer containing a reduced number of MAC. Furthermore, increased MO numbers in the nonadherent fraction of HAV-challenged LTBMCs are indicative of the disturbance of MO adherence. These findings suggest that HAV infection leads to a disorder of the mononuclear phagocytic system which may contribute to functional abnormalities of the bone marrow stroma.
Infections with hepatitis A virus (HAV) are associated with perturbations of hematopoietic regulation ranging from transient granulocytopenia to rare cases of aplastic anemia (AA) (5, 7, 9). The clinical syndrome of AA, which may also be caused by exposure to drugs, radiation, chemicals, or other hepatotropic viruses, is characterized by bone marrow hypocellularity and peripheral blood pancytopenia. The pathogenesis of AA associated with viral hepatitis is not well understood, although a variety of hematopoietic and immunoregulatory defects have been described, including direct infection of bone marrow progenitor cells and impairment of stromal function (6, 11, 14).
The bone marrow stroma represents a heterogeneous cell pool, consisting of fibroblasts, endothelial cells, adipocytes, and macrophages (MAC). Growth and differentiation of the hematopoietic cell population is dependent on its close association with stromal cells, which produce regulatory cytokines necessary to control hematopoiesis. In previous studies with human long-term bone marrow cultures (LTBMCs), HAV-induced inhibition of hematopoiesis was demonstrated by granulocyte-macrophage colony formation assays, and sequential determination of virus titers in the supernatant provided evidence for HAV replication in LTBMCs (3, 4, 17). However, our knowledge about which cell types of the heterogeneous stromal cell population are permissive for HAV is limited. Whereas the HAV infection of fibroblasts is well characterized (16), very little is known about the interaction of HAV with MAC, endothelial cells, or adipocytes. In this study, we focused on the interaction of HAV with peripheral blood monocytes (MO) and MAC, using primary cultures of MO and LTBMCs.
MATERIALS AND METHODS
Virus.
The virus used in this study was HAV strain GBM adapted to human fibroblasts.
Cell isolation.
Mononuclear cells (MNC) were isolated from peripheral blood or buffy coats of normal donors by Ficoll-Hypaque density gradient centrifugation (2). MO were isolated by a 1-h plastic adherence procedure followed by extensive washing with Iscove's modified Dulbecco medium (IMDM; Sigma, Deisenhofen, Germany).
Infection of monocytic cells.
Human peripheral blood MO, isolated by plastic adherence, were inoculated with HAV at a multiplicity of infection (MOI) of 1 and incubated for 2 h at 37°C in an atmosphere with 5% CO2. For some experiments, MO had been cultured for 2 weeks before infection. Cells were washed once with IMDM and incubated for up to 3 weeks in IMDM containing 30% fetal calf serum (FCS; Gibco BRL, Eggenstein, Germany), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Controls consisted of supernatants from cultures of uninfected human fibroblasts or UV-inactivated HAV. In some experiments, cells were stained for the MO-specific marker α-naphthyl esterase by using commercial kits (Sigma).
Determination of adherence of human peripheral blood MO.
At several times postinfection (p.i.), mock- and HAV-infected MO/MAC were fixed in phosphate-buffered saline (PBS) containing 4% paraformaldehyde at 4°C overnight. Cells were stained with propidium iodide (50 μg/ml in PBS with 20 U of RNase IIIa/ml). After 30 min at room temperature, cells were washed once in PBS prior to being scraped off with 1 ml of Isoton solution (Coulter, Krefeld, Germany), and fluorescence was measured with a fluorescence-activated cell sorter (FACS; FACScan, Coulter).
NBT dye reduction.
Nitroblue tetrazolium (NBT) studies were performed as described by Sing et al. (15). Briefly, adherent cells were washed three times in PBS, and then PBS was added to a final volume of 500 μl. An equal volume of NBT (Sigma) solution (0.2% in PBS) was added, as well as 200 ng of phorbol myristate acetate. Cells were incubated in the dark for 25 min at 37°C in an atmosphere containing 5% CO2 and washed three times with PBS. They were then fixed with ice-cold methanol for 10 min and counterstained with safranin for 30 s. The percentage of cells containing intracellular blue-black formazan deposits was determined after at least 200 cells were counted.
Detection of HAV replication in MO/MAC.
HAV replication was measured by sequential determination of virus titers in MO/MAC cultures. For this purpose, purified MO were infected with HAV or mock infected, as described above, and disrupted at different times p.i. by repeated freezing and thawing. The 50% tissue culture infective dose (TCID50) was determined by inoculating human fibroblasts (HFS-10) with the lysate. The infection of the HFS-10 cells was determined, 2 weeks after inoculation, by indirect immunofluorescence microscopy with the HAV-specific monoclonal antibody 7E7 (Mediagnost, Reutlingen, Germany) and a fluorescein-labeled anti-mouse antibody (Kirkegaard and Perry, Guildford, United Kingdom). The TCID50 was calculated according to the method of Kärber (11a).
Detection of HAV antigen in MO/MAC.
HAV antigen was detected by indirect immunofluorescence microscopy. For this purpose, mock- or HAV-infected MO/MAC were fixed at 3 weeks p.i. for 20 min with 4% paraformaldehyde in PBS at room temperature, followed by a 6-min incubation with ice-cold methanol at −20°C. After being washed with PBS, cells were incubated with 10% FCS in PBS for 30 min at 37°C to block Fc receptors on cell membranes. MO/MAC were then incubated for 60 min at 37°C with the HAV-specific monoclonal antibody 7E7 (Mediagnost). Cells were washed with PBS and incubated for 60 min with a fluorescein-labeled anti-mouse antibody (Kirkegaard and Perry). After six washes with PBS, cells were analyzed under a fluorescence microscope (Carl Zeiss, Jena, Germany).
LTBMCs.
Mononuclear bone marrow cells (BMNC) from patients undergoing hip surgery were obtained by density gradient centrifugation over Ficoll (density 1.077 g/ml). BMNC were inoculated with HAV (MOI = 1) for 2 h at 37°C. For LTBMC, mock- or HAV-infected cells (2 × 106/ml) were suspended in IMDM supplemented with 12.5% FCS, 12.5% horse serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10−6 M hydrocortisone sodium hydrogen succinate (Sigma), according to a modification of the method described by Gartner and Kaplan (10). Cultures were set up in 12.5-cm2 flasks in a total volume of 2 ml and incubated in a fully humidified atmosphere of 5% CO2 in air. Cultures were fed weekly by removal of half of the culture supernatant and replacement with an equal volume of fresh medium.
Phagocytosis assay.
The percentage of functional MAC in the stromal layer was determined by a latex ingestion procedure. Stromal cells were incubated with green-fluorescent latex particles in PBS (Sigma) for 2 h at 37°C. Cells were washed twice with PBS, and ingestion of particles was determined using fluorescence microscopy (Olympus, Hamburg, Germany).
Staining of monocytic surface markers.
The percentage of MO in the nonadherent fraction of LTBMCs was determined using a monoclonal antibody against the monocytic surface marker CD14 (Immunotech, Marseille, France). Surface immunofluorescence was analyzed by flow cytometry.
RESULTS
Effects of HAV on morphological differentiation of MO/MAC.
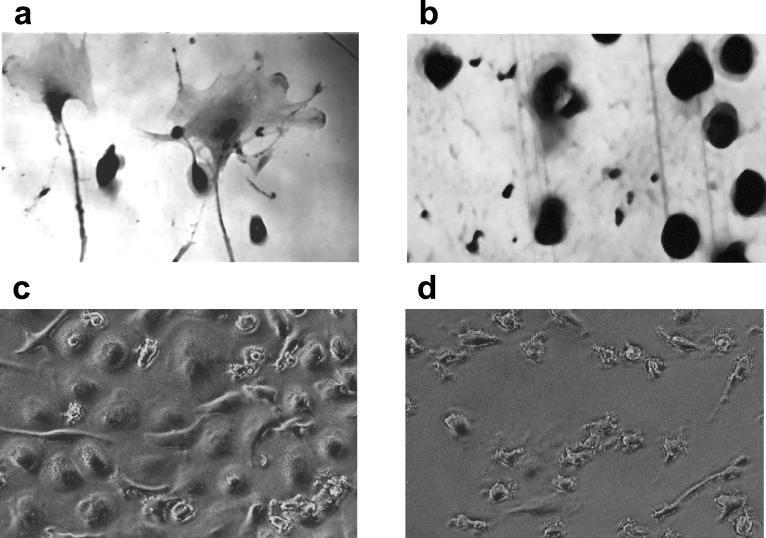
HAV- or mock-infected human peripheral blood MO, isolated by plastic adherence, were cultured for 3 weeks in IMDM-30% FCS. Mock-infected MO differentiated in the presence of serum, resulting in morphological changes characteristic of the MAC phenotype (Fig. 1a). However, MO that were infected with HAV prior to culture were unable to differentiate and remained predominantly as MO (Fig. 1b). Similar findings were obtained when MO were cultured for 14 days prior to infection with HAV. Terminal differentiation steps toward a MAC phenotype, evident in the mock-infected cultures (Fig. 1c), were blocked in HAV-inoculated cells (Fig. 1d).
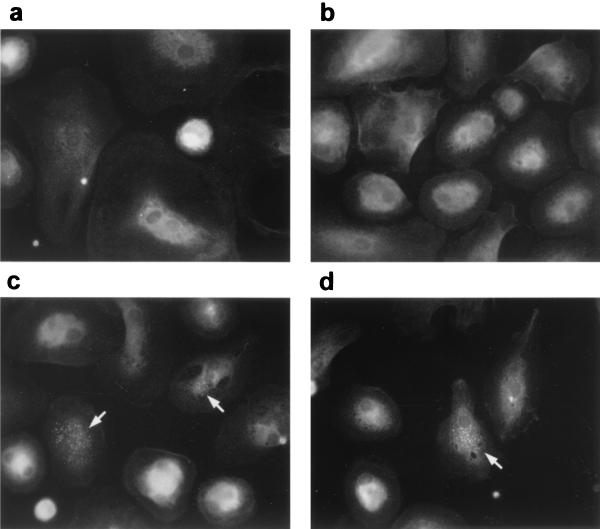
FIG. 1.
Morphological differentiation of HAV-infected MO. Cells were isolated from buffy coats by plastic adherence. MO were then incubated with supernatant of mock-infected fibroblasts (a) or infected with HAV (MOI = 1) (b), cultured for 21 days, and stained for the monocytic marker α-naphthyl esterase. Alternatively, isolated MO were cultured for 14 days prior to mock infection (c) or infection with HAV (d) and were analyzed without specific staining at 7 day p.i. by phase-contrast microscopy. Magnifications, ×400 (a and b) and ×200 (c and d).
Effects of HAV on MO/MAC adherence.
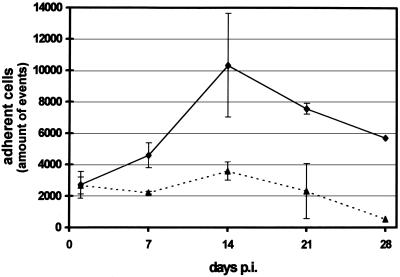
MO, isolated by plastic adherence, were infected with HAV or mock infected. At several times p.i., MO/MAC cultures were fixed with paraformaldehyde, stained with propidium iodide, scraped off, and analyzed by flow cytometry. One day after infection, the numbers of adherent cells measured by FACS were equal for mock- and HAV-infected cultures. However, beginning at day 7 p.i., the numbers of adherent cells dropped in HAV-infected cultures; at day 21 p.i., they were threefold lower than those of the mock-infected controls (Fig. 2).
FIG. 2.
Adherence of HAV-infected MO/MAC. MO isolated by plastic adherence were mock infected (—⧫—) or HAV infected (MOI = 1) (· · ·▴· · ·). At the times indicated, cells were fixed with 4% paraformaldehyde, treated with propidium iodide (50 μg/ml) and RNase (20 U/ml), scraped off, and analyzed by flow cytometry. Each data point is an average of values obtained in two separate experiments. Error bars indicate standard deviations of the means.
To exclude the possibility that the morphological findings and the decreased number of adherent MO/MAC in HAV cultures were due to accelerated cell death, we determined cell viability of both virus-infected and control cultures by trypan blue exclusion. Over a period of 14 days, we found no significant difference in cell viability between the adherent MO/MAC and cells from the supernatant. The viability of the adherent cells in mock- or HAV-infected cultures was more than 99% at 6 days p.i. The viability of cells in the supernatant of HAV- or mock-infected cultures was approximately 80% at 6 days and 96% at 14 days p.i. However, 14 days after infection, the total number of nonadherent cells in the HAV-infected culture was 50% higher than that in the mock-infected control culture (data not shown). Taken together, these data show that the reduced number of adherent MO/MAC is not due to accelerated cell death but is caused by a significant impairment of MO adherence in HAV-infected cultures.
Effects of HAV on phagocytic function of MO/MAC.
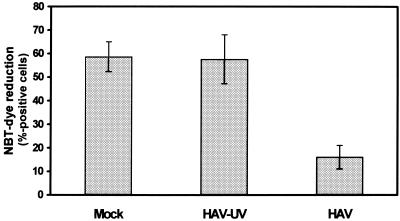
MO differentiation toward MAC is accompanied by increased phagocytic activity. Phagocytes take up the water-soluble dye NBT, which is converted to insoluble intracellular blue formazan particles in the presence of oxygen radicals, which are necessary for the digestion of the particle. Using this assay, high NBT activities were detected in mock- and UV-inactivated-HAV-infected MO after 14 days in culture (average proportion of NBT-positive cells, 58%), whereas the percentage of NBT-positive cells in HAV-infected MO cultures (16%) was markedly reduced (Fig. 3). Thus, HAV interferes with the functional maturation of MO.
FIG. 3.
Phagocytic functionality of HAV-infected MO/MAC cultures. MO were isolated by plastic adherence and either were mock infected or were infected with HAV (MOI = 1) or UV-inactivated HAV. MO were cultured for 14 days, and the percentage of active phagocytes was determined by NBT dye reduction as described in Materials and Methods. Each data point is an average of values obtained in two separate experiments. Error bars indicate standard deviations of the means.
HAV replication in MO/MAC cultures.
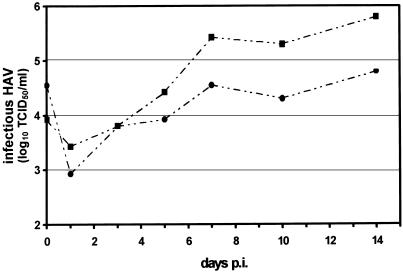
HAV replication in MO/MAC cultures was measured by sequential determination of virus titers in HFS-10 cells (Fig. 4). The TCID50s of MO/MAC cell lysates harvested at various time points were determined by indirect immunofluorescence microscopy in HFS-10 cells. Viral titers in MO/MAC cultures established from two different donors continuously rose from day 1 up to day 7 p.i. and reached a maximum 14 days after infection. The data show that HAV can replicate in MO/MAC. Furthermore, indirect immunofluorescence microscopy revealed the presence of HAV antigen in the cytoplasm of MO/MAC 3 weeks after infection (Fig. 5).
FIG. 4.
Replication of HAV in MO/MAC cultures. MO/MAC were isolated from two different buffy coat samples by plastic adherence [BC(A), —· ·•· ·—; BC(B), —· ·▪· ·—] and infected with HAV (MOI = 1). At the times indicated, the level of infectious HAV was determined by titration of the cell lysates in HFS-10 cells followed by HAV-specific immunofluorescence microscopy. The TCID50 was calculated according to the method of Kärber (11a).
FIG. 5.
Detection of HAV antigen by immunofluorescence microscopy. Mock-infected (a and b) or HAV-infected (MOI = 1) (c and d) MO/MAC cultures were stained at 3 weeks p.i. with the HAV-specific antibody 7E7 and a fluorescein-labeled anti-mouse antibody. Cells were analyzed by fluorescence microscopy. Arrows indicate HAV antigen in the cytoplasm of infected cells. Magnification, ×400.
Effects of HAV on morphological differentiation of human LTBMCs.
Human bone marrow MNC, isolated from spongiosa, were infected prior to onset of human LTBMC. Mock-infected cells and cultures inoculated with UV-inactivated HAV developed a confluent bone marrow stroma within 14 days of culture. Adherent hematopoietic cells covered wide areas of the stromal layer, concentrating in several so-called cobblestone areas, indicating sites of active hematopoiesis. However, in HAV-inoculated cultures, the development of the stromal layer was markedly reduced compared to that of control LTBMCs, and cobblestone areas did not appear throughout the culture period (Fig. 6).
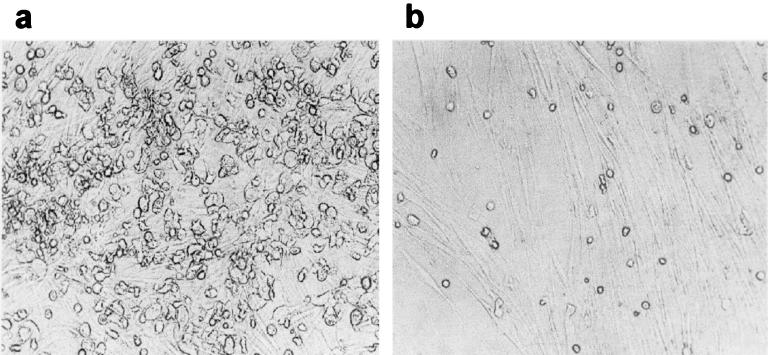
FIG. 6.
Morphological characterization of HAV- and mock-infected LTBMCs. Bone marrow cells were isolated from human spongiosa. Cells were either mock inoculated (a) or inoculated with HAV (MOI = 1) (b) and cultured for 4 weeks. Cells were analyzed by light microscopy. Magnification, ×100.
Effects of HAV on stromal MAC in LTBMCs.
The presence of phagocytic stromal cells in mock- or HAV-infected LTBMCs was determined 5 weeks after onset of culture, using a latex phagocytosis assay. Fluorescence microscopy showed that there were more phagocytic cells in the stromal layers of mock-infected cultures than in those of HAV-infected LTBMCs (Fig. 7).
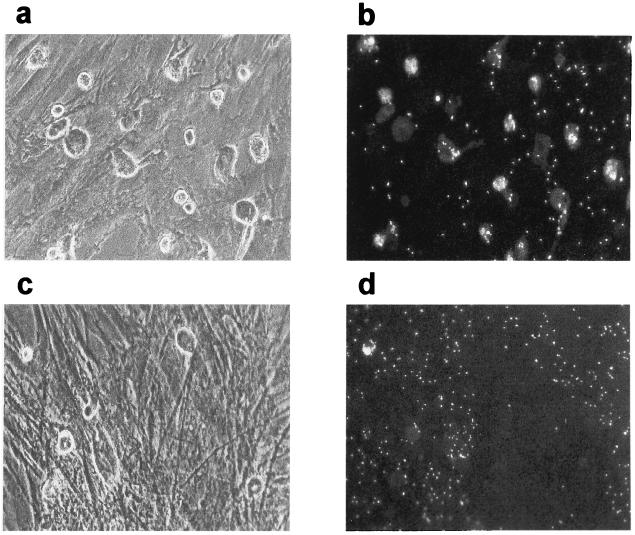
FIG. 7.
Detection of phagocytic cells in the stroma of HAV-infected LTBMCs. BMNC, isolated from patients undergoing hip surgery, were mock infected or infected with HAV (MOI = 1). For long-term culture, cultures were initiated in 12.5-cm2 flasks and incubated at 37°C in an atmosphere containing 5% CO2. The stromal layers of mock-infected (a and b) or HAV-infected (c and d) cultures were analyzed at 5 weeks p.i. for the presence of phagocytic cells, using the latex phagocytosis assay as described in Materials and Methods. Cells were analyzed by light (a and c) and fluorescence (b and d) microscopy. Magnification, ×200.
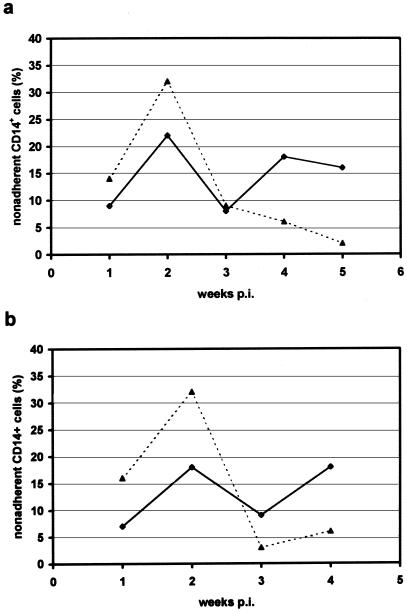
To analyze whether the observed reduction in number of active phagocytes in HAV-infected LTBMCs is induced by disturbance of MO adherence, we determined the numbers of MO in the supernatants of mock- or HAV-infected cultures. Cells that were removed with the supernatant during the weekly feeding were stained for CD14 monocytic surface protein and analyzed by flow cytometry (Fig. 8). Within the first 2 weeks, the average number of MO in the supernatant was up to 20% higher in HAV-infected cultures than in control cultures. Thereafter, MO counts dropped in HAV-infected cultures and were subsequently lower than in mock-infected LTBMCs. Thus, these data show that HAV interferes with MO adherence in LTBMC cultures, resulting in a depletion of stromal MAC.
FIG. 8.
Quantification of MO in the supernatants of HAV-infected LTBMCs. BMNC were mock infected or infected with HAV (MOI = 1) and cultured in IMDM medium. Nonadherent cells, removed from HAV-infected (· · ·▴· · ·) and mock-infected (—⧫—) LTBMCs in the course of weekly feeding, were stained for CD14 monocytic antigen and analyzed by flow cytometry. (a) Data obtained from one LTBM culture; (b) data are means of values from four separately initiated LTBMCs.
DISCUSSION
Despite the well-known hepatotropism of HAV, myelotropic properties of this virus have often been described; a transient suppression of hematopoiesis can be observed regularly in the preicteric phase of HAV infections (5). Additionally, rare fatal courses of AA with serological evidence of HAV being the responsible agent have been reported (7). Previous data addressing the influence of HAV on in vitro hematopoiesis demonstrated virus-induced suppression of the myelomonocytic lineage as well as infection of a subpopulation of stromal cells that were morphologically characterized as fibroblasts and/or MAC (4, 17).
In the present study, we determined the influence of HAV on MO differentiation toward MAC. We found profound abnormalities of peripheral blood MO following HAV infection, including reduced adherence and phagocytic capacity in addition to impaired morphological differentiation toward MAC. Observed alterations of MO function and differentiation might be a consequence of direct viral infection of these cells. Our data demonstrated that human peripheral blood MO are indeed permissive for HAV and that viral replication occurs in MO/MAC in vitro. What might be the significance of these findings in vivo? Circulating blood MO complete their differentiation pathway to mature, functionally competent cells by leaving the capillary bed and transforming into tissue or exudate MAC. In vitro, peripheral blood MO undergo a similar differentiation toward MAC-like cells when cultured in the presence of as-yet-unidentified serum factors (1, 13). The morphological changes that occur during the MO-to-MAC transition closely parallel cytochemical and functional changes. Differentiating MO exhibit increased size, phagocytic capacity, and superoxide anion secretion and very efficiently secrete colony-stimulating factors. Our microscopy studies and NBT assays demonstrated that the morphological maturation and functional maturation of MO are markedly reduced upon challenge with HAV. If the in vitro maturation of MO represents a model for MAC differentiation in vivo, the results presented here may indicate that disorders of the mononuclear phagocyte system occur upon HAV infection.
Functional defects of MO in vivo may lead to changes in immunoregulation and perturbations of hematopoiesis. Mueller et al. (12), who found that the MO of patients with acute viral hepatitis A, B, or C produced markedly less interleukin-1 than those of healthy individuals, suggest a link between virus-induced MO defects and perturbations of immunoregulation and hematopoiesis associated with acute hepatitis. Furthermore, defective MO maturation may lead to a depletion of functionally competent tissue MAC. The tissue MAC are replaced by cells from the blood MO pool with a defined rate of turnover and exist as Kupffer cells in the liver and the splenic sinusoids, as reticulum cells of the bone marrow and the lamina propria of the gastrointestinal tract, and as osteoclasts in bone (8, 18, 19). Adhesion to endothelial cells represents a fundamental step in the differentiation of MO, enabling them to leave the capillary bed and transform into MAC in different tissues. Here we showed that challenge of peripheral blood MNC (PBMCs) with HAV resulted in reduced numbers of MO adhering to plastic, indicating that MO adherence is suppressed by HAV in vitro.
Because bone marrow MAC are fundamental in support of hematopoiesis, their depletion or modulation of function by HAV is very likely to affect hematopoiesis. To gain an understanding of how maturational defects of MO affect hematopoiesis in vitro, we studied the influence of HAV on the development of MO/MAC in human LTBMCs. Our data demonstrated that HAV has an influence on the development of MO/MAC in the bone marrow stroma in vitro. Despite the observed suppression of the development of a confluent stroma in LTBMCs, we also found a reduced number of phagocytic cells in the stromal layer, due to the absence of MO/MAC. Furthermore, increased numbers of MO were determined in the supernatant of HAV-inoculated LTBMCs during the first 2 weeks of culture. These findings are indicative of an impaired MO adherence and are thus in accordance with the data from our adherence studies using PBMCs, as well as with observations of Busch et al., who demonstrated a predominantly monocytic population in the supernatants of HAV-inoculated LTBMCs (4). The subsequent drop in the number of MO observed after 2 weeks of culture can be explained by the absence of an active hematopoiesis due to missing cobblestone areas in HAV-infected LTBMCs. In contrast, mock-infected cultures showed several hematopoietically active areas, suggesting de novo synthesis of monocytic cells.
In summary, we found a maturational defect of human peripheral blood MO upon challenge with HAV in vitro, which may explain mild perturbations of immunoregulation and hematopoiesis observed during acute HAV infections in vivo. However, the clinical picture of AA associated with HAV infections may be associated with a persistent infection of stromal cells of the bone marrow. An infection of the stroma combined with a direct infection of hematopoietic cells and MO/MAC may result in a scenario in which hematopoiesis ceases.
Acknowledgments
S. Wünschmann and B. Becker contributed equally to this work.
This work was supported by the Tönjes-Vagt-Stiftung (Bremen, Germany).
We thank Mediagnost (Reutlingen, Germany) for providing monoclonal anti-HAV antibody 7E7 and Gerhard Schönermarck, Thorsten Volkmann, and Hubert Wöllenstein (Blood Transfusion Service, DRK; Springe, Germany) for providing buffy coats. We also thank Gerhard P. Lenz (Diako, Orthopedic Hospital, Bremen, Germany) for providing human spongiosa.
REFERENCES
- 1.Andreesen, R., W. Brugger, R. Kunze, W. Stille, and H. von Briesen. 1990. Defective monocyte to macrophage maturation in human immunodeficiency virus infection. Res. Virol. 141:217-224. [DOI] [PubMed] [Google Scholar]
- 2.Bøyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. J. Clin. Lab. Investig. 97:77-89. [PubMed] [Google Scholar]
- 3.Busch, F. W., S. de Vos, B. Flehmig, F. Herrmann, C. Sandler, and A. Vallbracht. 1987. Inhibition of in vitro hematopoiesis by hepatitis A virus. Exp. Hematol. 15:978-982. [PubMed] [Google Scholar]
- 4.Busch, F. W., A. Kunst, B. Flehmig, H. G. Mergenthaler, G. Pawelec, and A. Vallbracht. 1992. Myelopoiesis in vitro is suppressed by hepatitis A virus. Ann. Hematol. 64:132-136. [DOI] [PubMed] [Google Scholar]
- 5.Conrad, M. E., F. D. Schwartz, and A. A. Young. 1964. Infectious hepatitis—a generalized disease. Am. J. Med. 37:789-801. [DOI] [PubMed] [Google Scholar]
- 6.Da, W. M. 1988. In vitro studies on the pathogenesis of aplastic anemia in Chinese patients. Exp. Hematol. 16:336-339. [PubMed] [Google Scholar]
- 7.Domenech, P., A. Palomeque, A. Martinez-Gutierrez, N. Vinolas, E. Vela, and R. Jimenez. 1986. Severe aplastic anemia following hepatitis A. Acta Haematol. 76:227-229. [DOI] [PubMed] [Google Scholar]
- 8.Douglas, S. D., and R. A. Musson. 1986. Phagocytic defects—monocytes/macrophages. Clin. Immunol. Immunopathol. 40:62-68. [DOI] [PubMed] [Google Scholar]
- 9.Firkin, F. C., K. Nicholls, and G. Whelan. 1978. Transient myeloid and erythroid aplasia associated with infectious hepatitis. Br. Med. J. 2:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartner, S., and H. S. Kaplan. 1980. Long-term culture of human bone marrow cells. Proc. Natl. Acad. Sci. USA 77:4756-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmberg, L. A., K. Seidel, W. Leisenring, and B. Torok-Storb. 1994. Aplastic anemia: analysis of stromal cell function in long-term marrow cultures. Blood 84:3685-3690. [PubMed] [Google Scholar]
- 11a.Kärber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 12.Mueller, C., P. Knoflach, and C. C. Zielinski. 1993. Reduced production of immunoreactive interleukin-1 by peripheral blood monocytes of patients with acute and chronic viral hepatitis. Dig. Dis. Sci. 38:477-481. [DOI] [PubMed] [Google Scholar]
- 13.Musson, R. A. 1983. Human serum induces maturation of human monocytes in vitro. Changes in cytolytic activity, intracellular lysosomal enzymes, and nonspecific esterase activity. Am. J. Path. 111:331-340. [PMC free article] [PubMed] [Google Scholar]
- 14.Scopes, J., S. Daly, R. Atkinson, S. E. Ball, E. C. Gordon-Smith, and F. M. Gibson. 1996. Aplastic anemia: evidence for dysfunctional bone marrow progenitor cells and the corrective effect of granulocyte colony-stimulating factor in vitro. Blood 87:3179-3185. [PubMed] [Google Scholar]
- 15.Sing, G. K., S. Prior, A. Fernan, and G. Cooksley. 1993. Hepatitis B virus differentially suppresses myelopoiesis and displays tropism for immature hematopoietic cells. J. Virol. 67:3454-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallbracht, A., L. Hofmann, K. G. Wurster, and B. Flehmig. 1984. Persistent infection of human fibroblasts by hepatitis A virus. J. Gen. Virol. 65:609-615. [DOI] [PubMed] [Google Scholar]
- 17.Vallbracht, A., B. Fleischer, and F. W. Busch. 1993. Hepatitis A: hepatotropism and influence on myelopoiesis. Intervirology 35:133-139. [DOI] [PubMed] [Google Scholar]
- 18.Van Furth, R., J. A. Raeburn, and T. L. van Zwet. 1979. Characteristics of human mononuclear phagocytes. Blood 54:485-500. [PubMed] [Google Scholar]
- 19.Van Furth, R. 1981. Current view of the mononuclear phagocyte system. Hämatol. Bluttransfus. 27:3-10. [DOI] [PubMed] [Google Scholar]