Abstract
Since targeting of recombinant adenovirus vectors to defined cell types in vivo is a major challenge in gene therapy and vaccinology, we explored the natural diversity in human adenovirus tissue tropism. Hereto, we constructed a library of Ad5 vectors carrying fibers from other human serotypes. From this library, we identified vectors that efficiently infect human cells that are important for diverse gene therapy approaches and for induction of immunity. For several medical applications (prenatal diagnosis, artificial bone, vaccination, and cardiovascular disease), we demonstrate the applicability of these novel vectors. In addition, screening cell types derived from different species revealed that cellular receptors for human subgroup B adenoviruses are not conserved between rodents and primates. These results provide a rationale for utilizing elements of human adenovirus serotypes to generate chimeric vectors that improve our knowledge concerning adenovirus biology and widen the therapeutic window for vaccination and many different gene transfer applications.
The transfer of genes into mammalian cells potentially offers elegant solutions to a wide variety of medical problems. Adenoviruses are attractive for gene delivery because they efficiently introduce DNA into host cells, are not inactivated by complement in vivo, can be produced to high titers, and are able to transduce terminally differentiated cells. However, many human cells and tissues of therapeutic interest are refractory to Ad2 and Ad5 infection, limiting the applications of these commonly used serotypes. Vectors improved in their ability to infect target cells would principally allow for a significant reduction of the therapeutic or preventive vector dose, resulting in reduced local and disseminated toxicity. To identify vectors superior to Ad2 and Ad5 for infection of therapeutically relevant human cells and tissues, we made use of the observation that human adenovirus serotypes differ in their association with specific subclinical symptoms in humans (7, 14). Such differences might be due to differences in the tropism of human adenovirus serotypes (1, 2, 5, 10, 15, 24, 32, 33). The capsid proteins involved in determining the tropism are known for Ad5 and include both fiber and penton (3, 4, 13, 23, 28). Modification of these capsids demonstrates that vectors with a tropism different from that of Ad5 can be obtained (9, 11, 29, 41).
We exploited the natural complexity of receptor recognition of human adenoviruses by constructing a library of Ad5 vectors carrying the fiber molecules of alternative human serotypes. We demonstrate that fiber-chimeric vectors selected from this library improve the efficiency of gene transfer to, for instance, primary human chorion villus cells, fibroblasts, human bone marrow stroma cells (HBMSCs), dendritic cells (DCs), endothelial cells, and smooth muscle cells (SMCs). These cells are important target cells for diverse medical areas (i.e., prenatal diagnosis, tisue engineering, vaccination, and vein grafting).
MATERIALS AND METHODS
Fiber amplification and generation of fiber-chimeric viruses.
All human wild-type adenoviruses (types 1 to 51) were propagated on PER.C6 cells (8), and viral DNA was isolated as described previously (42). Fiber genes were PCR amplified with distinct sets of “subgroup-specific” oligonucleotides. Fiber sequences were subsequently cloned in an NdeI- and NsiI-digested pBr/Ad.BamRΔFIB plasmid, thus generating a library of plasmids coded pBr/Ad.BamRΔFIBXX (where XX represents the serotype from which the fiber was amplified). Plasmid pBr/Ad.BamRΔFIB has been described previously (29). Briefly, pBr/Ad.BamRΔFIB contains the Ad5 viral genome sequence from the BamHI site (nucleotide [nt] 21562) until the 3′ end of the viral genome. The Ad5 fiber sequence (nt 31042 to 32787) was deleted by PCR, while simultaneously introducing unique restriction sites (NdeI and NsiI) to allow for the insertion of fiber molecules derived from other serotypes. The generation and purification of fiber-chimeric adenoviral vectors on PER.C6 cells have been described previously (11, 29). Briefly, virus produced on four T175 triple-layer tissue culture flasks was purified with a two-step cesium chloride purification protocol. After purification, virus was aliquoted and stored at −80°C. The virus titer expressed in virus particles (vp) per milliliter was determined by high-pressure liquid chromatography (HPLC) (36). Plaque purifications and endpoint titrations were performed as described previously (8, 12).
Cell lines, primary cells, organ cultures, and expression of cell surface molecules.
Cell lines were obtained from the American Type Culture Collection (ATCC) and were routinely cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Zwijndrecht, The Netherlands) or, in some cases, in specific cell medium as suggested in the accompanying information obtained from the ATTC. Adenovirus transduction experiments were performed in triplicate at increasing amounts of vp per cell in 24-well plates with each well containing ± 105 cells. In general, virus exposure was allowed for 1 to 2 h at 37°C unless indicated otherwise. Primary human cell and organ culture experiments were performed as described previously (9, 11, 16, 29, 34, 35). Analyses of coxsackie-adenovirus receptor (CAR) and integrin expression with a flow cytometer (FACScalibur; Becton Dickinson) and antibodies used to detect CAR, αvβ3, or αvβ5 have been reported previously (30). Immunohistochemical detection of desmin and myosin has been described previously (34, 35).
Real-time PCR.
To determine the adenoviral copy number, a multiplex real-time PCR was set up essentially as described by Klein and coworkers (19, 20). Briefly, total DNA of transduced cells was extracted with a DNeasy tissue kit (Qiagen, Venlo, The Netherlands). To amplify the adenoviral DNA, Ad5Clip-F (5′-CGACGGATGTGGCAAAAGT-3′) and Ad5Clip-R (5′-CCTAAAACCGCGCGAAAA-3′) oligonucleotides were designed with Primer Express software (Perkin-Elmer, Foster City, Calif.). A fluorogenic probe (Ad5Clip-Pr; 5′-VIC-CACCGGCGCACACCAAAAACG-TAMRA-3′) was also designed with Primer Express software. To determine the amount of cellular DNA, a second pair of primers and a FAM (6-carboxyfluorescein) probe directed to 18S ribosomal DNA (rDNA) were used (19). The PCR mixture contained 1× buffer A, 3 mM MgCl2, 200 μM deoxynucleoside triphosphates (dNTPs), 90 nM each adenovirus primer, 100 nM each 18S rDNA primer, 200 nM each probe, 0.6 U of AmpliTaq Gold polymerase (all obtained from Perkin-Elmer), and 5 μl of total DNA sample. To determine the amount of adenoviral genomes and cellular DNA, a plasmid containing approximately 5,000 bp of the left part of the Ad5 genome (pAdapt) was mixed with cellular DNA extracted from A549 cells. The PCR was initiated with a hot start at 95°C for 10 min. Amplification occurred during 45 cycles, with each cycle consisting of 15 s at 95°C and 1 min at 60°C.
RESULTS AND DISCUSSION
Generation and validation of fiber-chimeric Ad5 vectors.
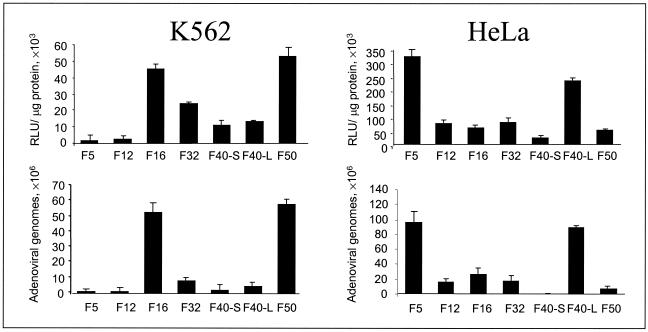
With the exception of serotypes 1, 4, 6, 18, and 26, fibers from all human adenovirus serotypes were amplified by PCR (two amplified products from each fiber) and sequenced to confirm the existence of an open reading frame. Phylogenetic analysis at the DNA and amino acid level of the fibers demonstrated that all of the amplified sequences represented genuine adenovirus fiber molecules (Fig. 1). The PCR-amplified fiber sequences were cloned into the pBr/Ad.BamRΔFIB plasmid (11, 29), thus generating a library of 47 pBr/Ad.BamRΔFIB constructs consisting of 45 different serotypes, plus the long (L) and short (S) fibers of serotype 40 (18). Using these constructs, 26 recombinant Ad5-based fiber-chimeric viruses have been produced with marker genes such as luciferase, green fluorescent protein (GFP), or LacZ. The fiber-chimeric vectors are produced on the PER.C6 adenoviral packaging cell line (8) with yields similar to yields obtained with recombinant Ad5 vectors (data not shown). Vector identity, i.e., the presence of the correct fiber, was confirmed by subgroup-specific fiber PCR (data not shown) and hemagglutination (HA) assays with human, rat, monkey, and mouse erythrocytes (Table 1). To select for improved vectors, we tested whether increased levels of transgene expression after infection is a valid criterion. Hereto, human erythroid leukemia (K562) and cervix carcinoma (HeLa) cells, which are refractory and sensitive to Ad5 infection, respectively, were infected with a selected panel of the fiber-chimeric vector library. A good correlation between transgene expression levels and Ad genome copy numbers was observed 48 h after infection (Fig. 2). Thus, increased levels of transgene expression are due to an increased efficiency of vector binding and are less influenced by differences in intracellular virus stability or trafficking, two other processes in which fibers might be involved (26). These results also demonstrate that Ad5 and Ad5.Fib12 are unable to infect K562 cells, whereas Ad5.Fib16 and Ad5.Fib50 (both B group), Ad5.Fib28 and Ad5.Fib32 (both D group), and Ad5.Fib40-S and Ad5.Fib40.L (both F group) show variable levels of transgene expression. Thus, viruses derived from subgroups B, D, and F are able to enter cells via receptors different from Ad5 and other than CAR, which extends the observations reported by Roelvink et al., who demonstrated that CAR functions as an attachment molecule for serotypes derived from subgroups A, C, D, E, and F (33). Since the profile of sensitivity to a panel of fiber-chimeric viruses expressing either luciferase or GFP on human A549 cells proved identical for both marker genes, we excluded that differences in transgene expression are due to variations in the quality of the virus preparations (data not shown).
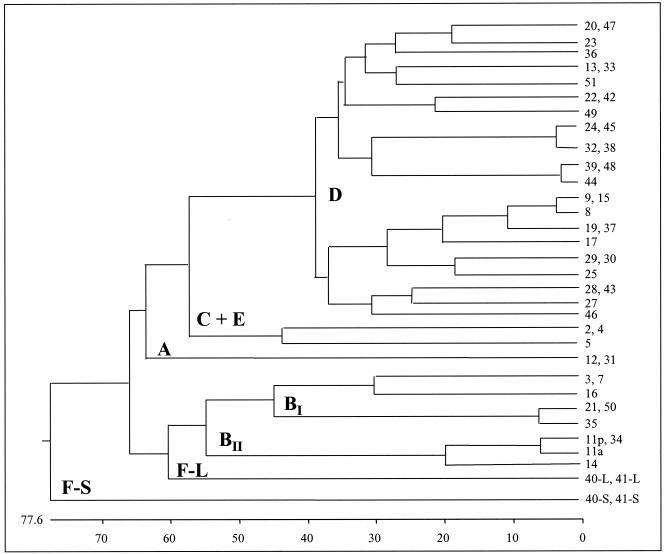
FIG. 1.
Phylogenetic tree generated by parsimony analysis of fiber knob amino acid sequences of fiber derived from human adenovirus serotypes. The fiber's origin from the wild-type serotype is indicated by the number to the right. Designations A, BI, BII, C, D, E, FL (long fiber of subgroup F virus), and FS (short fiber of subgroup F virus) correspond to subgroup determination based on classical subtyping assays (i.e., HA inhibition assays and cross-neutralization). Amino acid diversity is shown below the tree.
TABLE 1.
Conformation of fiber origin on fiber-chimeric Ad5 vector via HA assay
| Virus | HA assay result with serotypea
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 8 | 9 | 10 | 11 | 12 | 13 | 16 | 17 | 24 | 27 | 28 | 30 | 32 | 33 | 35 | 38 | 40-S | 40-L | 45 | 47 | 49 | 50 | |
| Human | |||||||||||||||||||||||
| Wild type | − | ND | + | − | − | − | + | − | − | − | + | − | − | ND | − | − | + | ND | ND | − | − | − | − |
| Fiber-chimeric | − | + | + | − | − | − | + | − | − | − | + | ND | − | + | − | − | + | − | − | − | − | − | − |
| Rhesus | |||||||||||||||||||||||
| Wild type | − | ND | − | − | + | − | + | + | − | − | − | − | − | ND | − | + | + | ND | ND | − | − | − | + |
| Fiber-chimeric | − | − | − | − | + | − | + | + | − | − | − | ND | − | − | − | + | + | − | − | − | − | − | + |
| Rat | |||||||||||||||||||||||
| Wild type | + | ND | + | + | − | + | + | − | + | + | + | + | + | ND | + | − | + | ND | ND | + | + | + | + |
| Fiber-chimeric | + | + | + | + | − | + | + | − | + | + | + | ND | + | + | + | − | + | − | + | + | + | + | + |
| Mouse | |||||||||||||||||||||||
| Wild type | − | ND | + | + | − | − | − | − | + | + | − | − | + | ND | ND | − | − | ND | ND | + | + | + | − |
| Fiber-chimeric | − | + | + | + | − | − | − | − | + | + | − | ND | + | + | − | − | − | − | − | + | + | + | − |
Both wild-type adenovirus serotype and corresponding fiber-chimeric vector were tested with erythrocytes derived from human, rhesus, mouse, and rat for the HA profile. +, HA-positive viruses;−, HA-negative viruses; ND, not determined. Readout of the HA profile was performed 1 h after addition of 50 μl of 0.3% erythrocyte solution to 109 vp of cesium chloride-purified wild-type or fiber-chimeric virus (incubation performed at 37°C).
FIG. 2.
Correlation between transgene expression levels and Ad genomes. Human K562 and HeLa cells were exposed for 2 h to 1,000 vp of different fiber-chimeric vectors per cell. Virus was then discarded by washing the cells with fresh medium, and the cells were harvested 48 h later. Half of the cells were used for luciferase transgene activity measurements (upper graphs), and the other half was used to extract DNA. DNA was used in real-time PCR to determine the number of adenovirus copies present in the cell population (lower graphs). Luciferase activity is expressed in relative light units (RLU) per microgram of total cellular protein.
Diversity in fiber-chimeric adenovector tropism.
A panel of human established cancer cell lines and primary cells were tested for sensitivity toward fiber-chimeric vectors (Table 2). The expression of molecules known to be involved in Ad5 infection (i.e., CAR, αvβ3, and αvβ5) and the efficiency with which Ad5 infects these cells were also monitored. From these experiments, several conclusions were drawn. (i) Apparently Ad5 can recognize receptors other than its primary receptor CAR, since transgene expression levels do not correlate with expression levels of CAR and/or integrins, as witnessed on U87, Capan-1, and Mz-Cha-1 cells, as well as synoviocytes and amniocytes. It is noteworthy that although CAR could never be detected on certain cell types, the ability of Ad5 to infect cells drastically varied between individual donors, resulting in a relatively high standard error of the mean observed in experiments with primary cells. (ii) From 17 established cancer cell lines tested, we identified an improved vector for 10 (59%), which in 80% of the cases proved a vector carrying a fiber derived from subgroup B adenoviruses (Ad5.Fib16, Ad5.Fib35, and Ad5.Fib50). (iii) With the exception of human hepatocytes, an improved fiber-chimeric vector could be identified on all human primary cell types tested, which in all cases carried a fiber derived from the subgroup B viruses. (iv) Cell lines representing a particular human tumor type (i.e., pancreatic cancer) differ strikingly in expression patterns of CAR and integrins, indicating that primary tumor cells should be used to determine the sensitivity toward adenoviruses. The panel of human primary cells tested includes cells that are important target cells for diverse medical intervention strategies, i.e., cardiovascular disease, rheumatoid arthritis, prenatal diagnosis, tissue engineering (bone, skin, and cartilage), and vaccination.
TABLE 2.
Sensitivity of established cancer cells and primary cells toward fiber-chimeric vectors
| Cell type | Tissue | Flow cytometry resulta
|
Luciferase activity with Ad5 (RLU/μg of protein)b | Best vector | Fold luciferase increase (n)d | ||
|---|---|---|---|---|---|---|---|
| CAR | α1β3 | α1β5 | |||||
| Cancer lines | |||||||
| A549 | Lung | ++ | + | ++ | 106 | Ad5c | − (12) |
| K562 | Erythroid | − | + | + | 102 | Ad5.Fib16c | 814 ± 176 (4) |
| TF-1 | Erythroid | − | ++ | + | 104 | Ad5.Fib35c | 60 ± 18 (3) |
| CEM | Lymphoid | − | − | − | 102 | Ad5.Fib16c | 11 (1) |
| SupT1 | Lymphoid | ++ | − | − | 105 | Ad5c | (3) |
| MCF-7 | Breast | ND | ND | ND | 106 | Ad5.Fib50c | 42 (2) |
| U87 | Astroglia | − | − | ++ | 105 | Ad5c | (2) |
| HeLa | Cervix | ++ | + | + | 106 | Ad5c | (4) |
| HepG2 | Liver | ++ | − | + | 107 | Ad5c | (3) |
| SK-N-MC | Neuronal | ++ | − | + | 106 | Ad5c | (1) |
| NRK-52E | Kidney | ND | ND | ND | 104 | Ad5c | (1) |
| MIA-PaCa-2 | Pancreas | + | − | + | 107 | Ad5.Fib35 | 2 (3) |
| HS766T | Pancreas | + | ++ | + | 106 | Ad5.Fib16 | 2 (2) |
| BxPC3 | Pancreas | + | + | − | 106 | Ad5.Fib50 | 4 (2) |
| CAPAN-1 | Pancreas | − | − | + | 106 | Ad5.Fib16 | 5 (3) |
| Sk-Cha-1 | Cholangio | + | − | + | 106 | Ad5.Fib32 | 14 (1) |
| Mz-Cha-1 | Cholangio | − | − | + | 106 | Ad5.Fib50 | 107 (1) |
| Primary cells | |||||||
| Endothelial cells | Umbilical vein | + | ++ | + | 105 | Ad5.Fib16c | 8 ± 3 (9) |
| Smooth muscle cells | Umbilical vein | − | ++ | ++ | 103 | Ad5.Fib16 | 64 ± 20 (8) |
| Synoviocytes | Synovium | − | + | + | 105 | Ad5.Fib16 | 154 ± 68 (4) |
| Fibroblasts | Skin | − | ++ | − | 103 | Ad5.Fib16 | 64 ± 58 (4) |
| Amniocytes/chorion villus cells | Amniotic fluid | − | ++ | + | 105 | Ad5.Fib50 | 7 ± 3 (1) |
| Hepatocytes | Liver | ND | ND | ND | 107 | Ad5 | (2) |
| Mature DCs | Hemopoietic | − | − | + | 103 | Ad5.Fib35 | 21 ± 5 (5) |
| Immature DCs | Hemopoietic | − | − | + | 103 | Ad5.Fib35 | 9 ± 5 (3) |
| Bone marrow stroma cells | Hemopoietic | − | + | + | 104 | Ad5.Fib16 | 138 ± 86 (3) |
| Chondrocytes | Hemopoietic | − | + | + | 104 | Ad5.Fib16 | 13 ± 5 (3) |
| Myoblasts | Skeletal muscle | ND | ND | ND | 105 | Ad5.Fib50 | 18 ± 8 (3) |
| Melanocytes | Skin | ND | ND | ND | 105 | Ad5.Fib35 | 17 (1) |
| Follicle dermal papilla cells | Skin | ND | ND | ND | 105 | Ad5.Fib35 | 36 (1) |
| Hemopoietic stem cells | Hemopoietic | − | − | + | ND | Ad5.Fib50 | Determined with GFP only |
Expression of CAR and integrins was monitored by flow cytometry. Cells were scored as −, +, or ++, respectively, when <2%, between 2 and 50%, or >50% of the cell population express the molecule. ND, not determined.
Range of luciferase activity in relative light units (RLU) per microgram of total cellular protein as measured 48 h after a 2-h vector exposure of 1,000 vp/cell.
A panel of eight fiber-chimeric vectors was tested representing five of the six subgroups. On all other cell lines, the entire fiber-chimeric vector library was tested.
Fold luciferase increase is defined as luciferase activity obtained with the best vector divided by luciferase activity obtained with Ad5. The number (n) of independent experiments performed (each in triplicate) is listed in parentheses. Values are averages±standard deviations.
Potential therapeutic applications for fiber-chimeric vectors.
Taken together, the results obtained so far with the fiber-chimeric vector library demonstrate that human adenoviruses can recognize distinct cellular attachment molecules and that exploration of this diversity leads to vectors better suited to infect cells of therapeutic interest without compromising the currently available production platform technology for recombinant Ad5 vectors. Having identified several fiber-chimeric vectors that, based on the levels of luciferase expression, are superior to Ad5, we next set out to further evaluate the usefulness and potency of these vectors in four distinct medical applications, i.e., prenatal diagnosis, tissue engineering, vaccination, and vein grafting.
Prenatal diagnosis of genetic diseases.
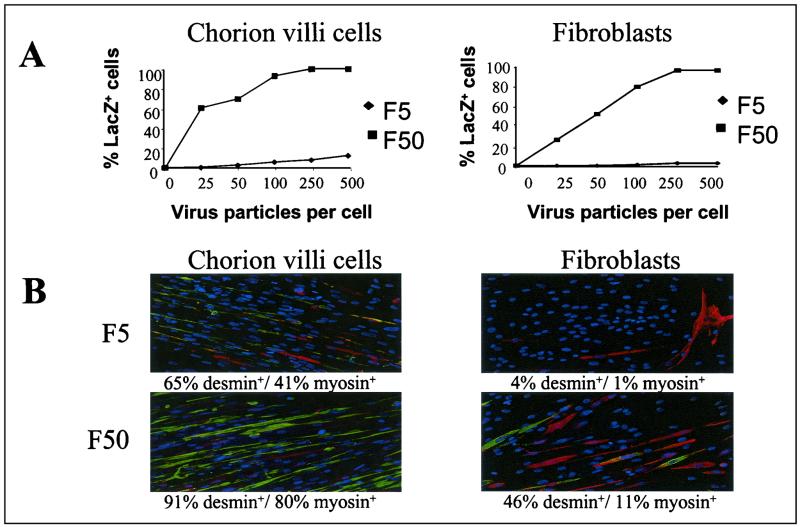
Gene transfer to chorion villus cells, fibroblasts, and amniocytes cultured from an amniotic fluid biopsy is used for prenatal diagnosis of genetic diseases, such as Duchenne muscular dystrophy (DMD) (34, 35). In short, the strategy aims at transfer of the MyoD gene (35) that results in redifferentiation of chorion villus cells to myogenic cells. The cells can then be investigated for correct expression of proteins known to be involved in genetic diseases, such as dystrophin in DMD (34). Although elegant, this strategy has been hampered by the low efficiency of gene transfer and vector-mediated toxicity of viral vectors like retrovirus and adenovirus to chorion villus cells, fibroblasts, and amniocytes. From the library, we have identified Ad5.Fib50 as the most potent vector for transduction of cells derived from amniotic fluid (Table 2 and Fig. 3A). Next, we constructed Ad5 and Ad5.Fib50 viruses carrying the MyoD gene. Results with these viruses on transduced chorion villus cells and fibroblasts induced to form myotubes via serum deprivation show that, upon transduction, a significantly higher number of cells differentiated toward skeletal muscle cells with Ad5.Fib50 as witnessed by desmin (early differentiation marker) and myosin (late differentiation marker) expression, which are myogenic markers (Fig. 3B). Thus, Ad5.Fib50 offers a potent tool and is now routinely used in our laboratory to diagnose DMD in utero.
FIG. 3.
Ad5- and Ad5.Fib50-mediated gene transfer to chorion villus cells and fibroblasts. (A) Freshly isolated chorion villus cells or fibroblasts were exposed to an increasing dose of either Ad5 or Ad5.Fib50-LacZ. Cells were stained 48 h later, and β-galactosidase-positive cells were scored. (B) Chorion villus cells and fibroblasts were exposed to 100 vp (per cell) of Ad5 or Ad5.Fib50 carrying MyoD. To determine the percentage of cells expressing myosin (green) or desmin (red), the nuclei of cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue).
Tissue engineering of bone.
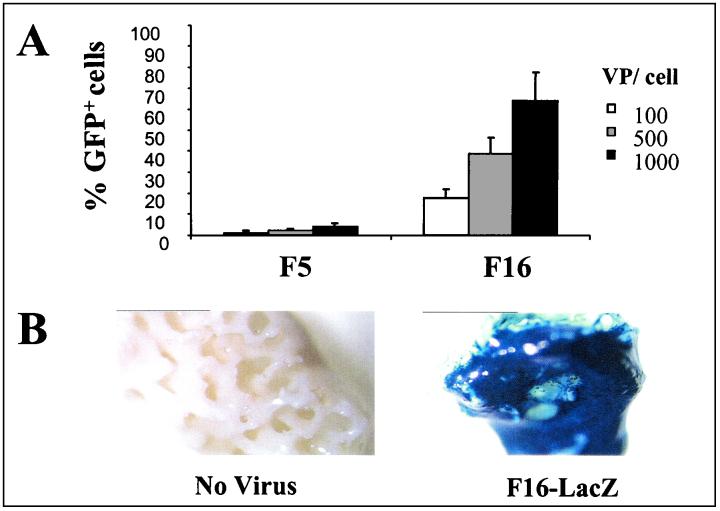
There is a substantial medical need for tissues such as skin, cartilage, and bone due to the increasingly aging population and the resulting wear of body parts (6, 21, 22, 25, 27, 31). For bone replacement surgery, such as revision hip arthroplasty, spine fusions, and jaw augmentation, the currently used bone grafts are mainly from allogeneic or autologous origin. The risk of disease transfer and immunological responses arising from allografts and the limited availability and necessity for a second surgical procedure for autografts with associated donor site morbidity make these grafts far from optimal. A tissue engineering approach with which autologous bone tissue can be cultured would be a suitable alternative for the traditional bone graft therapies. The HBMSC population contains progenitor cells that are capable of differentiating to various mesenchymal tissues, such as bone. Expanding these cells in vitro and seeding on a suitable biomaterial scaffold followed by osteogenic differentiation will allow the generation of large amounts of living autologous bone graft equivalents. Ectopic implantation of such tissue-engineered bone grafts in nude mice has shown the feasibility of this approach (6, 25). Improvements could be made in the reproducibility of this technique by using bone marrow cells from especially elderly patients. The availability of a gene therapy approach with which, e.g., bone growth factors could be delivered at the defect site would further enhance the success rate of such a tissue engineering approach. Therefore, we have initiated gene transfer studies to HBMSCs of genes that are either related to osteogenic differentiation or angiogenesis, in an attempt to enhance the amount of bone formed. A prerequisite for these studies is the availability of a vector to effectively transfer genes into HBMSCs without compromising cell viability. A systematic screening of the fiber-chimeric vector library identified Ad5.Fib16 as a potent vector for the transduction of HBMSCs (Table 2 and Fig. 4A). The potency of this vector was tested in a “scaffold-seeding” experiment. The results of this experiment are shown in Fig. 4B, demonstrating efficient lacZ gene transfer to HBMSCs when using Ad5.Fib16. The number of copies of Ad5.Fib16 present in the nuclei of transduced HBMSCs is sufficient to warrant 100% marker gene-positive cells even after several rounds of cell doubling without compromising the proliferation rate and cell viability (data not shown). The identification of a vector that efficiently infects HBMSCs is a first prerequisite to start studies with the aim of optimizing both the quantity and quality of tissue-engineered human bone.
FIG. 4.
Ad5.Fib16-mediated gene transfer to HBMSCs. (A) HBMSCs were exposed for 2 h to 100, 500, or 1,000 vp (per cell) of either Ad5 or Ad5.Fib16-GFP (n = 3). Forty-eight hours after virus exposure, cells were scored for GFP expression with a flow cytometer. (B) HBMSCs were exposed to 1,000 vp of Ad5.Fib16 carrying LacZ per cell. Subsequently, cells were seeded in the calcium phosphate support. Forty-eight hours later, LacZ expression was determined. As a control for LacZ staining, nontransduced cells were seeded and stained.
Vaccination.
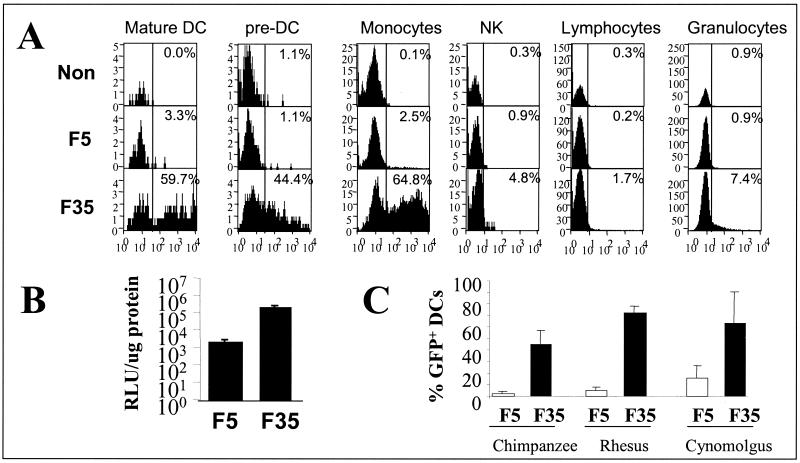
Recombinant adenoviruses are used as vaccine carriers to induce protective immunity against viral infections or to treat cancer (37, 39, 40). The target cells for efficient vaccination are DCs that are CAR negative (30). Human DCs are difficult to transduce with recombinant Ad5 vectors unless high dosages are used. From the library of fiber-chimeric vectors, Ad5.Fib35 was identified as a potent vector for the genetic modification of human monocyte-derived mature and immature DCs (Table 2), and the increased infection with this vector resulted in a better T-cell activation as compared to Ad5 (29). For direct in vivo application in humans, the ideal adenovirus vaccine should, besides its capability to infect antigen-presenting cells, infect skeletal muscle cells for efficient cross-priming and should not infect hemopoietic cells other than DCs and precursors of DCs, such as monocytes. To test to what extent the Ad5.Fib35 vector adheres to these characteristics, the specificity of Ad5.Fib35 was tested in peripheral blood mononuclear cells (PBMCs). Hereto, PBMCs were exposed to Ad5-GFP or Ad5.Fib35-GFP for 2 h, and 24 h later, cells were stained with CD14, CD16, and CD33 to visualize mature and immature DCs residing in the blood. By using flow cytometry, we demonstrate that Ad5.Fib35 efficiently infects monocytes and mature and immature DCs, whereas with Ad5, no transgene expression could be observed in any of the hemopoietic lineages at the low vector dose (100 vp/cell) used (Fig. 5A). The ability of Ad5.Fib35 to genetically modify skeletal muscle cells efficiently as compared to Ad5 was demonstrated on skeletal muscle samples obtained postmortem (Fig. 5B). Finally, preclinical testing of emerging vaccines is usually performed in nonhuman primates. Therefore, we investigated the sensitivity of monocyte-derived DCs cultured from chimpanzees, rhesus monkeys, and cynomolgus monkeys for Ad5.Fib35. Levels of marker gene expression obtained after transduction of these DCs showed that in all cases Ad5.Fib35 is superior to Ad5 (Fig. 5C). These studies suggest that Ad5.Fib35 is likely to be superior compared to Ad5 in in vivo protocols aimed at evoking an immune response against desired target antigens.
FIG. 5.
Infection of antigen-presenting cells by Ad5.Fib35. (A) PBMCs were exposed to 100 vp (per cell) of either Ad5 or Ad5.Fib35 carrying GFP. Twenty-four hours after virus exposure, cells were stained with CD14, CD16, and CD33 to visualize different hemopoietic lineages. Nontransduced cells were used to set the flow cytometric gates at a background level of 1% or less (vertical line). Percentages of cells scored positive are indicated in the upper right corner of each histogram. (B) Skeletal muscle biopies (1 cm3) were obtained ±7 h postmortem. Samples were exposed to 1010 vp of Ad5 or Ad5.Fib35 carrying luciferase. Forty-eight hours later, samples were minced, and luciferase activity was determined (expressed as relative light units [RLU] per microgram of protein). (C) Monocyte-derived DCs from nonhuman primates were obtained by a protocol identical to that used for the isolation of human monocyte-derived DCs. Cells were exposed to 1,000 vp of either Ad5 or Ad5.Fib35 carrying GFP. Forty-eight hours after virus exposure, the percentage of cells positive for GFP was scored with a flow cytometer.
Vascular SMC proliferation.
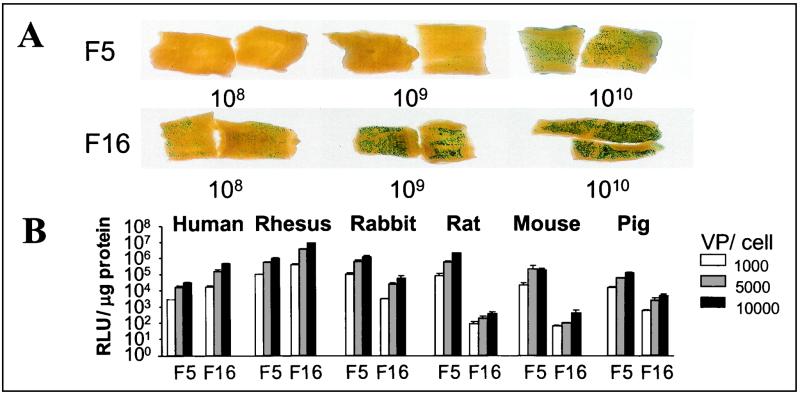
Extensive SMC proliferation that occurs during vascular remodelling is thought to be the major cause of intimal hyperplasia after artery bypass surgery, ultimately resulting in vascular occlusion (17, 38). As a consequence, strategies to inhibit SMC proliferation via gene transfer, using genes like nitric oxide synthase, are considered to circumvent vascular occlusion in response to injury after, for instance, vein grafting (38). However, the genetic modification of human SMCs with Ad5 is cumbersome, requiring high viral loads to obtain a level of infection that compromises cell viability. Ad5.Fib16 has been identified from the library (Table 2) as the best vector for the genetic modification of human SMCs and endothelial cells (11). In a dose response experiment with freshly isolated human saphenous vein sections, it was shown that 108 vp of Ad5.Fib16 results in LacZ expression comparable to 1010 vp of Ad5 (Fig. 6A). Such a reduction in the therapeutic vector dose will result in significant less toxicity. In addition, we have tested SMCs derived from the carotid artery of different species that are frequently used as animal models to test intervention strategies for cardiovascular diseases. From these experiments, it was concluded that, as opposed to humans and rhesus monkeys, pig, rat, rabbit, and mouse SMCs are less susceptible to Ad5.Fib16 than Ad5 (Fig. 6B). The latter findings demonstrate that the cellular attachment molecule(s) used by, at least some, human subgroup B adenoviruses are not conserved between species and identify the nonhuman primate animal model as the only available in vivo model to test the superiority of the novel Ad5.Fib16 and Ad5.Fib35 fiber-chimeric vectors.
FIG. 6.
Ad5.Fib16-mediated gene transfer to cardiovascular cells and tissues. (A) Dose response with Ad5 and Ad5.Fib16 carrying LacZ on samples of human saphenous vein. The indicated virus dose was added to the samples for 1 h, and LacZ expression was investigated 48 h later. (B) Side-by-side comparison of the ability of Ad5 and Ad5.Fib16 to genetically modify SMCs cultured from the carotid artery of different species. Hereto, cells were exposed to 1,000, 5,000, or 10,000 vp/cell for 2 h (n = 3 per dose). Forty-eight hours later, luciferase activity (relative light units [RLU] per microgram of protein) was determined.
In summary, a library of 26 different E1-deleted fiber-chimeric adenoviral vectors has been generated, and these vectors are produced on PER.C6 adenovirus packaging cells to titers comparable with standard Ad5 recombinant viruses. This library continues to serve as a platform to identify vectors better suited than Ad5 to genetically modify cells that are considered important target cells in preventive or therapeutic gene transfer strategies. In four distinct medical areas, the increased ability of gene transfer and the potential of the fiber-chimeric vectors are demonstrated. In vivo proof of principle for many of these vectors has been delayed by the finding that not all species are suitable to test for safety, toxicity, and potency of the improved fiber-chimeric vectors due to either a lack in receptor conservation or differences in expression patterns. However, the observed improvement in the efficiency of gene transfer obtained with the novel vectors in their respective area is expected to result in a broader therapeutic window for each of the medical areas described and thus in the clinical applicability of recombinant adenoviruses as gene delivery vehicles.
Acknowledgments
We thank J. G. Fitz (University of Colorado Health Sciences Center, Denver) for cell line Mz-Cha-1 and J. M. Bergelson (Harvard Medical School, Boston, Mass.) for providing the anti-CAR antibody. J. A. Bruijn (Department of Pathology, Leiden University Medical Center, Leiden, The Netherlands) is acknowledged for providing human tissue samples.
REFERENCES
- 1.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74:42-48. [PMC free article] [PubMed] [Google Scholar]
- 2.Arnberg, N., A. H. Kidd, K. Edlund, F. Olfat, and G. Wadell. 2000. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus αV integrins. J. Virol. 74:7691-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson, J. M. 1999. Receptors mediating adenovirus attachment and internalisation. Biochem. Pharmacol. 57:975-979. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B virus and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Chu, Y., D. Heistad, M. I. Cybulsky, and B. L. Davidson. 2001. Vascular cell adhesion molecule-1 augments adenovirus-mediated gene transfer. Arterioscler. Thromb. Vasc. Biol. 21:238-242. [DOI] [PubMed] [Google Scholar]
- 6.De Bruijn, J. D., I. van den Brink, S. Mendes, R. Dekker, Y. P. Bovell, and C. A. van Blitterswijk. 1999. Bone induction by implants coated with cultured osteogenic bone marrow cells. Adv. Dent. Res. 13:74-81. [DOI] [PubMed] [Google Scholar]
- 7.De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. W. Slaterus, P. Wertheim-Van Dillen, G. J. J. Van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallaux, F. J., A. Bout, I. van der Velde, D. J. van den Wollenberg, K. M. Hehir, J. Keegan, C. Auger, S. J. Cramer, H. van Ormondt, A. J. van der Eb, D. Valerio, and R. C. Hoeben. 1998. New helper cells and matched E1-deleted adenovirus vectors prevent generation of replication competent adenoviruses. Hum. Gene Ther. 9:1909-1917. [DOI] [PubMed] [Google Scholar]
- 9.Goossens, P. H., M. J. Havenga, E. Pieterman, A. A. Lemckert, F. C. Breedveld, A. Bout, and T. W. Huizinga. 2001. Infection efficiency of type 5 adenoviral vectors in synovial tissue can be enhanced with a type 16 fiber. Arthritis Rheum. 44:570-577. [DOI] [PubMed] [Google Scholar]
- 10.Hautala, T., T. Grunst, A. Fabrega, P. Freimuth, and W. C. Welsh. 1998. An interaction between penton-base and alpha-v-integrins plays a minimal role in the adenovirus mediated gene transfer to hepatocytes in vitro and in vivo. Gene Ther. 5:1259-1264. [DOI] [PubMed] [Google Scholar]
- 11.Havenga, M. J. E., A. A. C. Lemckert, J. M. Grimbergen, R. Vogels, L. G. M. Huisman, D. Valerio, A. Bout, and P. H. A. Quax. 2001. Improved adenovirus vectors for infection of cardiovascular tissues. J. Virol. 75:3335-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hehir, K. M., D. Armentano, L. M. Cardoza, T. L. Choquette, P. B. Berthelette, G. A. White, L. A. Couture, M. B. Everton, J. Keegan, J. M. Martin, D. A. Pratt, M. P. Smith, A. E. Smith, and S. C. Wadsworth. 1996. Molecular characterization of replication-competent variants of adenovirus vectors and genome modifications to prevent their occurrence. J. Virol. 70:8459-8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidaka, C., E. Milano, P. L. Leopold, J. M. Bergelson, N. R. Hackett, R. W. Finberg, T. J. Wickham, I. Kovesdi, P. Roelvink, and R. G. Crystal. 1999. CAR-dependent and CAR-independent pathways of adenovirus-mediated gene transfer and expression in human fibroblasts. J. Clin. Investig. 103:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hierholzer, J. C. 1992. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 5:262-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong, S. S., L. Karayan, J. Tournier, D. T. Curiel, and P. A. Boulanger. 1997. Adenovirus type 5 fiber knob binds to MHC class I alpha 2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 16:2294-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssens, S., D. Flaherty, Z. Nong, O. Varenne, N. van Pelt, C. Haustermans, P. Zoldhelyi, R. Gerard, and D. Collen. 1998. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation 97:1274-1281. [DOI] [PubMed] [Google Scholar]
- 18.Kidd, A. H., J. Chroboczek, S. Cusack, and R. W. Ruigrok. 1993. Adenovirus type 40 virions contain two distinct fibers. Virology 192:73-84. [DOI] [PubMed] [Google Scholar]
- 19.Klein, D., B. Bugl, W. W. Gunzburg, and B. Salmons. 2000. Accurate estimation of transduction necessitates a multiplex real-time PCR. Gene Ther. 7:458-461. [DOI] [PubMed] [Google Scholar]
- 20.Klein, D., P. Janda, R. Steinborn, M. Muller, B. Salmons, and W. H. Gunzburg. 1999. Pro-viral load determination of different feline immunodeficiency virus isolates using real-time polymerase chain reaction: influence of mismatches on quantitation. Electrophoresis 20:291-295. [DOI] [PubMed] [Google Scholar]
- 21.Lamme, E. N., R. T. van Leeuwen, K. Brandsma, K. Van Marle, and E. Middelkoop. 2000. Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J. Pathol. 190:595-603. [DOI] [PubMed] [Google Scholar]
- 22.Lamme, E. N., R. T. van Leeuwen, A. Jonker, J. van Marle, and E. Middelkoop. 1998. Living skin substitutes: survival and function of fibroblasts seeded in a dermal substitute in experimental wounds. J. Investig. Dermatol. 111:989-995. [DOI] [PubMed] [Google Scholar]
- 23.Mathias, P., T. Wickham, M. Moore, and G. Nemerow. 1994. Multiple adenovirus serotypes use αv integrins for infection. J. Virol. 68:6811-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei, Y. F., and G. Wadell. 1993. Hemagglutination properties and nucleotide sequence analysis of the fiber gene of adenovirus genome types 11p and 11a. Virology 194:453-462. [DOI] [PubMed] [Google Scholar]
- 25.Mendes, S. C. 2000. Human bone marrow stromal cells for bone tissue engineering: in vitro and in vivo characterization, p. 505-515. In J. E. Davies (ed.), Bone engineering. EM-squared, Inc., Toronto, Ontario, Canada.
- 26.Miyazawa, N., R. G. Crystal, and P. L. Leopold. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moutsatsos, I. K., G. Turgeman, S. Zhou, B. G. Kurkalli, G. Pelled, L. Tzur, P. Kelley, N. Stumm, S. Mi, R. Muller, Y. Zilberman, and D. Gazit. 2001. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol. Ther. 3:449-461. [DOI] [PubMed] [Google Scholar]
- 28.Pearson, A. S., P. E. Koch, N. Atkinson, M. Xiong, R. W. Finberg, J. A. Roth, and B. Fang. 1999. Factors limiting adenovirus-mediated gene transfer to human lung and pancreatic cancer cell lines. Clin. Cancer Res. 5:4208-4213. [PubMed] [Google Scholar]
- 29.Rea, D., M. J. Havenga, M. van Den Assem, R. P. Sutmuller, A. Lemckert, R. C. Hoeben, A. Bout, C. J. Melief, and R. Offringa. 2001. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T-cells. J. Immunol. 166:5236-5244. [DOI] [PubMed] [Google Scholar]
- 30.Rea, D., F. H. E. Schagen, R. C. Hoeben, M. Mehtali, M. J. E. Havenga, R. E. M. Toes, C. J. M. Melief, and R. Offringa. 1999. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J. Virol. 73:10245-10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riesle, J., A. P. Hollander, R. Langer, L. E. Freed, and G. Vunjak-Novakovic. 1998. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J. Cell. Biochem. 71:313-327. [DOI] [PubMed] [Google Scholar]
- 32.Roelvink, P. W., I. Kovesdi, and T. J. Wickham. 1996. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J. Virol. 70:7614-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roelvink, P. W., A. Lizonova, J. G. M. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roest, P. A., E. Bakker, F. J. Fallaux, C. Verellen-Dumoulin, C. E. Murry, and J. T. den Dunnen. 1999. New possibilities for prenatal diagnosis of muscular dystrophies: forced myogenesis with an adenoviral MyoD-vector. Lancet 353:727-728. [DOI] [PubMed] [Google Scholar]
- 35.Roest, P. A., A. C. van der Tuijn, H. B. Ginjaar, R. C. Hoeben, F. B. Hoger-Vorst, E. Bakker, J. T. den Dunnen, and G. J. van Ommen. 1996. Application of in vitro Myo-differentiation of non-muscle cells to enhance gene expression and facilitate analysis of muscle proteins. Neuromuscul. Disord. 6:195-202. [DOI] [PubMed] [Google Scholar]
- 36.Shabram, P. W., D. D. Giroux, A. M. Goudreau, R. J. Gregory, M. T. Horn, B. G. Huyghe, X. Liu, M. H. Nunnally, B. J. Sugarman, and S. Sutjipto. 1997. Analytical anion-exchange HPLC of recombinant type-5 adenoviral particles. Hum. Gene. Ther. 8:453-465. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 38.Varenne, O., S. Pislaru, H. Gillijns, N. Van Pelt, R. D. Gerard, P. Zoldhelyi, F. Van de Werf, D. Collen, and S. P. Janssens. 1998. Local adenovirus-mediated transfer of human endothelial nitric oxide synthase reduces luminal narrowing after coronary angioplasty in pigs. Circulation 98:919-926. [DOI] [PubMed] [Google Scholar]
- 39.Wan, Y., P. Emtage, Q. Zhu, R. Foley, A. Pilon, B. Roberts, and J. Gauldie. 1999. Enhanced immune response to the melanoma antigen gp100 using recombinant adenovirus-transduced dendritic cells. Cell. Immunol. 198:131-138. [DOI] [PubMed] [Google Scholar]
- 40.Xianh, Z.-Q., Y. Yang, J. M. Wilson, and C. J. Ertl. 1996. A replication deficient adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219:220-227. [DOI] [PubMed] [Google Scholar]
- 41.Yotnda, P., H. Onishi, H. E. Heslop, D. Shayakhmetov, A. Lieber, M. Brenner, and A. Davis. 2001. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther. 8:930-937. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, W. W., P. E. Koch, and J. A. Roth. 1995. Detection of wildtype contamination in a recombinant adenoviral preparation by PCR. BioTechniques 18:444-446. [PubMed] [Google Scholar]