Abstract
Alzheimer's disease is characterized by two primary pathological features: amyloid plaques and neurofibrillary tangles. The interconnection between amyloid and tau aggregates is of intense interest, but mouse models have yet to reveal a direct interrelationship. We now show that NO may be a key factor that connects amyloid and tau pathologies. Genetic removal of NO synthase 2 in mice expressing mutated amyloid precursor protein results in pathological hyperphosphorylation of mouse tau, its redistribution to the somatodendritic compartment in cortical and hippocampal neurons, and aggregate formation. Lack of NO synthase 2 in the amyloid precursor protein Swedish mutant mouse increased insoluble β-amyloid peptide levels, neuronal degeneration, caspase-3 activation, and tau cleavage, suggesting that NO acts at a junction point between β-amyloid peptides, caspase activation, and tau aggregation.
Keywords: amyloid, chronic neurodegeneration, inducible nitric oxide synthase, nitric oxide, tau
In addition to neurodegeneration, Alzheimer's disease (AD) brain pathology includes insoluble amyloid deposits and accumulation of abnormally phosphorylated and aggregated forms of tau, a microtubule binding protein. Attempts to recreate the complete spectrum of AD pathologies in mouse models have had mixed success. Transgenics that overexpress mutated forms of amyloid precursor protein (APP) display amyloid plaques, but fail to demonstrate tau pathology that is fully reminiscent of AD (1–5). Both amyloid and tau pathologies are observed in double/triple transgenic mouse models that express mutated human tau and mutated APP, in the presence or absence of mutated presenilin-1 (6, 7). Although mutated human tau isoforms are observed in dementia, they are typically associated with frontal temporal dementias, whereas tau aggregates in AD are composed of normal tau (8). Capsoni et al. (9) developed a mouse expressing recombinant antibodies that neutralizes nerve growth factor and displays nonmutated tau pathology and amyloid plaques. This model implies a major role for nerve growth factor signaling in the pathophysiology of AD, but is associated with the uncommon presence of brain antibodies. We now report the induction of somatodendritic tau pathology in cortical and hippocampal neurons in a well established mouse model of AD that expresses the Swedish familial AD double mutation K670N-M671L in APP (Tg2576) and that lacks a functional NO synthase (NOS) 2 gene.
The NOS2 gene encodes inducible NOS (iNOS), one of three NOS protein isoforms (iNOS, neuronal NOS, and endothelial NOS) that produce NO in the brain. Although commonly localized to macrophagic immune cells, iNOS is observed primarily in neurons and astrocytes in AD (10–12). Footprints of NO's past presence include observation of nitrated proteins in AD brains, compared with normal age-matched brains (13), suggesting that NO plays a role in the disease process. Our data demonstrating hyperphophorylation of tau at disease-specific sites, redistribution of tau to the somatodendritic compartment of cortical and hippocampal neurons, and tau aggregates in brains of mice expressing the APP Swedish mutation (APPsw) on a NOS2 knockout background strongly indicates that NO may be protective in AD.
Results
Mice expressing APPsw on a NOS2 null background were assessed for the presence of NOS2 mRNA and brain NOS activity. mRNA for NOS2 was observed in WT and APPsw control littermates, but was not found in APPsw/NOS2−/− or NOS2−/− brains (Fig. 1A). To determine whether NOS activity fell when NOS2 was deleted in APPsw/NOS2−/− mice, calcium-independent NOS activity was measured in brain lysates from APPsw/NOS2−/− and APPsw mice by using the arginine-to-citrulline conversion assay. A significant decrease in activity was observed in the APPsw/NOS2−/− lysates compared with APPsw alone (Fig. 1B), indicating that NOS activity and, most likely, NO production was reduced in our bigenic mouse. Quantitative RT-PCR was used to detect compensatory changes in NOS1 and NOS3. NOS1 mRNA fell by 0.64 ± 0.03-fold (n = 5), whereas NOS3 mRNA increased by 1.48 ± 0.13-fold (n = 5) compared with WT littermates. These changes closely mimicked values observed in the NOS2−/− mouse (0.63 ± 0.04 for NOS1 and 1.52 ± 0.23 for NOS3). These data demonstrate that the compensatory changes in NOS1 and NOS3 are characteristic of the NOS2−/− deletion.
Fig. 1.
Loss of NOS2 RNA and activity. Brains from APPsw/NOS2−/− mice were assayed for the expression of NOS2 mRNA (A) and calcium-independent NOS activity (B). Detection of GAPDH mRNA served as a loading control in A. ∗, P = 0.05.
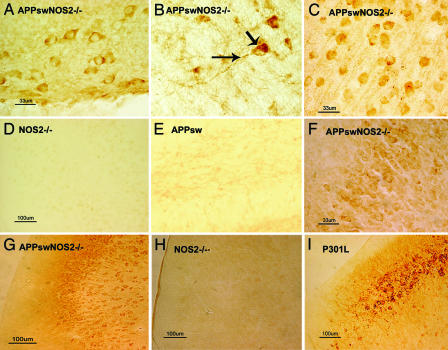
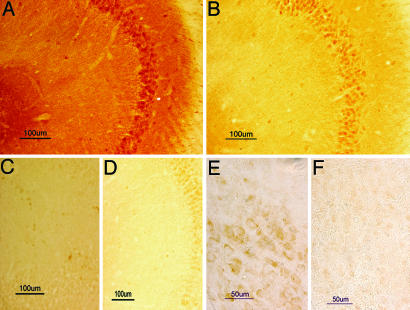
The presence of abnormally phosphorylated tau was detected in APPsw/NOS2−/− mice (n = 5) by using immunocytochemistry on brain sections with AT8, CP13, and AT180 antibodies to specific, disease-associated phosphorylation sites in tau protein (14–16). Immunopositive staining for hyperphosphorylated tau was observed in the somatodendritic compartments of numerous neurons in the hippocampus (Fig. 2 A and F), globus pallidus (Fig. 2B), and frontal cortex (Fig. 2C) in APPsw/NOS2−/− brain. Both AT8 and CP13 produced similar patterns of staining in APPsw/NOS2−/− mice, whereas AT8/CP13 immunostaining was not observed in cortical sections from mNOS2−/− littermates (Fig. 2D) or hippocampal sections from APPsw littermates (Fig. 2E). Immunopositive phospho-tau was also seen in apical dendrites, and intracellular aggregate–like structures were observed in some neurons from the APPsw/NOS2−/− brains (Fig. 2B, arrows).
Fig. 2.
The APPsw/NOS2−/− mouse demonstrates somatodendritic localization of hyperphosphorylated tau. (A–C and F) CA4 hippocampal (A) and globus palladius (B) neurons from an APPsw/NOS2−/− mouse were immunopositive for tau phosphorylated at Ser-202/Thr-205 by using the CP13 antibody or the AT8 antibody in neurons from frontal cortex (C) or hippocampus (F). Note the dense hyperphosphorylated tau immunoreactivity in soma and apical dendrites in B. (D and E) AT8 immunoreactivity was not observed in brain sections from littermate NOS2−/− mice (D; cortex) or APPsw mice (E; hippocampus). (G) Neurons from APPsw/NOS2−/− brain were also immunopositive for tau phosphorylated at Thr-231 by using the AT180 antibody in the cortex. (H) No AT180 staining was observed in littermate NOS2−/− brains. (I) AT180 immunoreactivity in cortical sections from a mouse expressing the P301L human tau mutation was used as a positive control for hyperphosphorylated tau.
A similar immunostaining pattern was observed in brain sections with the AT180 antibody to phosphorylated Ser-231 (Fig. 2 G–I). Phospho-tau immunoreactivity was again observed in the cell somas and apical dendrites of cortical and hippocampal neurons. The neuronal pattern of immunostaining in APPsw/NOS2−/− brains qualitatively resembled the AT180 immunostaining pattern observed in JNPL3 mice with the P301L human tau mutation (Fig. 2I) that express hyperphosphorylated and aggregated tau (17).
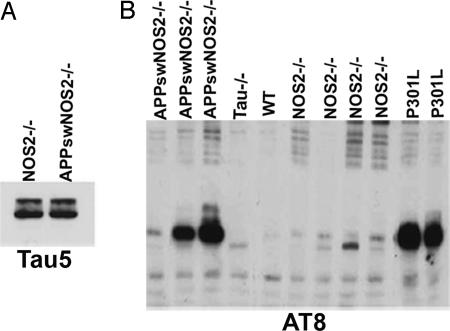
To determine whether tau protein levels were altered in the APPsw/NOS2−/− mice, we compared total tau expression by using Western blots and the Tau5 antibody that detects both phosphorylated and nonphosphorylated forms of tau. No difference in total tau was observed between lysates from APPsw/NOS2−/− and NOS2−/− brains (Fig. 3A). Using the AT8 antibody, we examined brain lysates for the presence of hyperphosphorylated tau (Fig. 3B). Neither WT nor tau knockout mice demonstrated bands corresponding to hyperphosphorylated tau. In contrast, each of three individual APPsw/NOS2−/− brain samples demonstrated AT8 immunoreactivity. Interestingly, low levels of AT8-positive tau were seen in brain lysates from the NOS2−/− littermate controls.
Fig. 3.
Western blot confirms the presence of hyperphosphorylated tau in APPsw/NOS2−/− brains. (A) Total tau (Tau5+) levels are similar in APPsw/NOS2−/− brain compared with littermate NOS2−/− controls. (B) Western blot for AT8 immunoreactive bands in APPsw/NOS2−/−, Tau−/−, P301L human tau mutation, WT, and NOS2−/− mice.
To confirm the presence of aggregated tau, we used a filter assay (18) that traps protein aggregates present in brain lysates on a cellulose filter. Tau5+ staining of trapped aggregates was observed with brain filtrates from NOS2−/−, APPsw/NOS2−/−, and P301L mice, whereas no staining was observed with brain filtrates from WT or tau−/− mice (Fig. 4A). AT8-immunoreactive hyperphosphorylated tau was found in filter-trapped aggregates from APPsw/NOS2−/− and P301L brains, with only slight immunoreactivity observed from the NOS2−/− filtrate (Fig. 4B).
Fig. 4.
Aggregated tau proteins are observed in APPsw/NOS2−/− brain. (A and B) Trapped aggregates from whole brain filtrates were immunoreactive to Tau5 (A) and AT8 (B). APPsw/Tau−/− or Tau−/− brains served as negative controls, and P301L mouse brain served as positive control for the presence of tau aggregates. AT8 did not cross-react with Aβ aggregates formed by the addition of preaggregated Aβ42 to brain lysates and then filtered. Filter-trapped Aβ aggregates were detected by 4G8, an antibody against Aβ peptide (data not shown). (C) Aggregates (Tau5+) were also detected by using scanning EM. Tau5+ aggregates were observed in lysates from APPsw/NOS2−/−, P301L mice, and autopsied AD brain samples. (D) Intracellular tau aggregates were detected by using thioflavin S histochemistry. Fluorescent particles were observed in neuronal somas from APPsw/NOS2−/− brains, but not littermate controls.
Tau aggregation was further confirmed with scanning EM and thioflavin S histochemistry. Filters containing trapped tau aggregates were immunoreacted with Tau5 antibody, followed by an immunogold secondary antibody and silver enhancement to improve detection. Tau aggregates were clearly observed in brain filtrates from APPsw/NOS2−/− mice (Fig. 4C) and were comparable to immunoreactive tau aggregates prepared from brains of human AD or JNPL3 mice. Thioflavin S-positive aggregates were observed within the cell bodies of cortical neurons in the APPsw/NOS2−/− mice, but were not observed in NOS2−/− littermates (Fig. 4D).
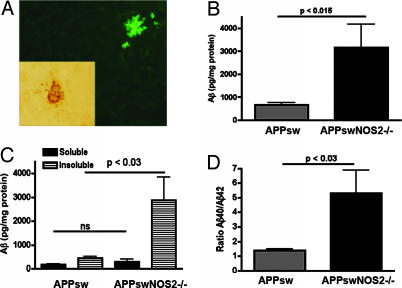
In addition to tau pathology, amyloid plaque-like pathology was observed in APPsw/NOS2−/− brains. Amyloid deposits could be detected by using 4G8, an antibody that reacts with human β-amyloid (Aβ) peptides, or thioflavin S, a fluorescent indicator for β-pleated sheet structures (Fig. 5A). To compare APPsw/NOS2−/− with APPsw littermates, we directly measured soluble and insoluble Aβ40 and Aβ42 levels in brain lysates by using a quantitative ELISA (19, 20). Total brain Aβ levels were significantly greater in APPsw/NOS2−/− mice compared with APPsw littermate controls (Fig. 5B). This increase was caused by a significant increase in insoluble Aβ peptides, resulting in an increased Aβ40/Aβ42 ratio (Fig. 5 C and D).
Fig. 5.
Total and insoluble Aβ is increased in the APPsw/NOS2−/− brain. (A) Amyloid deposits in brain sections from APPsw/NOS2−/− mice were detected by using thioflavin S staining and immunoreactivity to the 4G8 antibody (Inset). (B–D) Aβ levels in brain lysates of the APPsw/NOS2−/− mice were compared with APPsw littermate controls by using an ELISA. Average values (± SEM) for the ratio of Aβ40 to Aβ42 (D), soluble and insoluble Aβ levels (C), and total Aβ levels (B) are shown. ns, no significant difference.
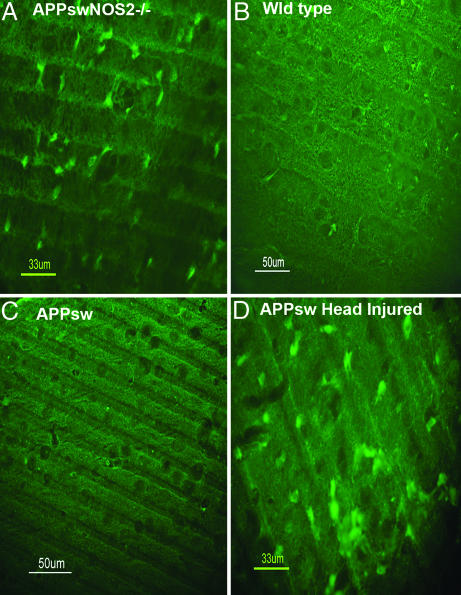
Although neuronal loss is not common in APPsw mice, we used Fluorojade B to identify degenerating neurons in the APPsw/NOS2−/− brains (21). Widespread cortical neuronal damage was observed in three of four APPsw/NOS2−/− mice compared with no apparent damage in either APPsw or WT brains (Fig. 6). Head-injured APPsw mice served as a positive control and displayed numerous degenerating neurons.
Fig. 6.
Degenerating neurons are observed in the APPsw/NOS2−/− brain. Fluorojade B was used to detect degenerating neurons as described by Schmued et al. (21). (A) Degenerating neurons were observed in cortical sections from APPsw/NOS2−/− mouse brains. (B and C) No degenerating neurons were observed with fluorojade B staining in brain sections from WT (B) or APPsw (C) brains. (D) Intense staining was observed in a head-injured APPsw control mouse brain.
The mechanism of cell injury was further explored by evaluating markers for apoptosis. The activated form of caspase-3, a known executioner caspase involved in apoptotic cell death (22), was detected by using immunocytochemistry. Activated caspase-3 was observed in cell bodies and apical dendrites in cortical and hippocampal neurons in APPsw/NOS2−/− brains (Fig. 7A). Slight, but observable, activated caspase-3 immunoreactivity was found in NOS2−/− brains versus background staining in APPsw or WT littermates. To detect whether activated caspase cleaved tau, we immunostained APPsw/NOS2−/− brain sections with an antibody that recognizes tau truncated at Asp-421 (TauC3) (23). TauC3-positive staining was observed in cell bodies and dendrites of cortical neurons in APPsw/NOS2−/− mice (Fig. 7E) compared with APPsw control brains (Fig. 7F).
Fig. 7.
Immunoreactivity for cleaved caspase-3 is increased in APPsw/NOS2−/− brain. (A–D) Cleaved caspase-3 immunoreactivity was increased in hippocampal neurons and their processes in the APPsw/NOS2−/− brain (A) compared with NOS2−/− (B), APPsw (C), and WT (D) control mice. (C) NOS2−/− mice demonstrated low, but clearly observable, cleaved caspase immunoreactivity, confirming published data demonstrating increased caspase-3 activity in the NOS2−/− mouse brain (59, 60). (E and F) Neurons in the APPsw/NOS2−/− brain also demonstrated immunoreactivity for caspase-cleaved (truncated) tau by using the TauC3 antibody (E) compared with APPsw (F) brains.
Discussion
The amyloid and tau pathologies that characterize AD brain lesions were simultaneously observed in the APPsw/NOS2−/− mouse brain. Unlike other common mouse models for amyloid deposition, redistribution of normal mouse tau to the somatodendritic region of cortical and hippocampal neurons, hyperphosphorylation of mouse tau at multiple, disease-associated residues, and mouse tau aggregates were observed. These changes occurred in the presence of nonmutated mouse tau and are associated with neuronal degeneration in the cortex. Amyloid plaque morphology and distribution in the APPsw/NOS2−/− brain was visually similar to that observed in APPsw littermates. However, we observed a significant increase in total brain Aβ peptides, which were primarily in the insoluble form. Increased levels of total Aβ peptides and altered ratios of Aβ40 to Aβ42 in the APPsw/NOS2−/− brain suggest that NO acts on Aβ generation or clearance, although the mechanism of interaction is unknown.
Increased tau pathology and increased Aβ levels in our APPsw/NOS2−/− mice were in contrast to the report of Nathan et al. (24). In that study, trigenic mice with the APP Swedish double mutation (K670N, M671L), a mutated human presenilin gene (hPS1-A246E mutation), and a NOS2−/− background had an increased life span, decreased microglial activation, and an age-dependent decrease in amyloid plaque burden, suggesting that NO is detrimental in these trigenics. However, the addition of a mutated presenilin gene by Nathan et al. is a significant difference from our APPsw/NOS2−/− mice and may account for the opposing results. Hashimoto et al. (25) demonstrated that the PS-1 A246E mutation promotes NO-mediated toxicity. As a consequence, removal of NO in PS-1 A246E mice would be predicted to decrease cell death and alter gamma secretase function to potentially reduce amyloid deposition. The subtype of mutation thus may dictate differing requirements for NO. Furthermore, expression of mutated PS-1 in immune cells is associated with a greatly enhanced inflammatory response that is not typical of sporadic AD (26). Because the immune response and the disease progression changes with age in AD (27), the brain's requirement for NO may also fluctuate, enabling NO function at specific disease stages to result in differing outcomes.
Although NO is generally viewed as detrimental to neurons (28), mounting evidence indicates a protective role for NO in the brain (10, 29, 30). For example, Sinz et al. (31) have shown that cognitive behavioral responses after head injury are worse in iNOS knockout mice than in WT controls. Although these data support a beneficial function of iNOS in head trauma, exogenous Abeta peptide-mediated synaptic damage in hippocampal slices is significantly reduced when NO is removed (32). Resolution of these disparate views about NO's actions in the brain may reside in understanding specific concentration and temporal profiles of NO release and the intracellular signaling pathways initiated by individual profiles. Low levels of NO (<100 nM) promote progrowth and antiapoptotic pathways (33). For example, activation of guanyl cyclase by NO and the subsequent phosphorylation of cGMP-dependent protein kinases such as Ras-Raf-extracellular regulated kinase and phosphatidylinositol 3-kinase-Akt (33, 34) play a critical role in neuronal survival (35, 36). Neuronal survival is also mediated by NO's inhibition of executioner caspase-3 and caspase-6 (37) where NO can block the conversion of the proenzymes via Akt-mediated phosphorylation or chemically modify and decrease enzyme activity (38, 39). Higher levels of NO (>400 nM), as observed in eradication of pathogens during an acute immune response, have pathophysiological consequences. At pathogenic levels, p53 activation and the induction of apoptosis can occur (33, 37). Taken together, the temporal and spatial distribution of NO plays a vital role in controlling cell functions.
Our data suggest that maintenance of a critical level of NO at the neuron is essential to the survival programs that depend on NO signaling pathways. The source of NO may not be as important as the actual level of NO. In fact, changes in both NOS enzymes and NO scavengers were observed in AD. Fernandez-Vizarra et al. (40) have shown that neuronal NOS protein levels aberrantly increase in AD neurons, whereas neuronal NOS activity in brain lysates is reduced. Increased expression of endothelial NOS in AD is also observed, but is primarily localized to the dystrophic neurites and astrocytes that surround amyloid plaques (11, 41). Our quantitative PCR data support the idea that changes in constitutive NOS isoforms are unlikely to adequately compensate for the genetic loss of iNOS. A fall in NO may be caused, in part, by a parallel increase in scavenging mechanisms for NO. For example, redox active iron accumulates in neurons in AD (42) and contributes to nitration of proteins such as Aβ (43). The process of nitration, in turn, represents conditions that reduce NO bioavailability and alter cell signaling pathways initiated by specific levels of NO.
Interestingly, the changes in neuronal NOS and endothelial NOS levels and activities as AD progresses are associated with ectopic expression of iNOS within neurons (40). iNOS serves as a widely diffusible and long-lasting source of NO that is most commonly associated with activated immune cells such as microglia and astrocytes. Although contradictory data exist (44), it has been generally believed that Aβ peptides induce iNOS that increases NO and kills surrounding neurons (45). Microglia and astrocytes in AD brain, however, may not produce those pathological levels of NO associated with LPS injection into the brain or with acute immune stimulation (29, 46, 47). Neuronal expression of iNOS may then serve to compensate for the integrated loss of NO in an attempt to retain NO's protection in AD.
Our data clearly indicate that genetic reduction in iNOS activates caspase-3 in neurons in vivo. Although Aβ peptide-mediated activation of caspase-3 and caspase-6 has been directly related to neuronal apoptosis in AD (48, 49), the lack of neuronal death in mouse models that overexpress APP and Aβ strongly suggest that natural inhibitors of apoptosis must also be present. In addition to other apoptosis inhibitors found in neurons (49), NO may inhibit apoptosis. Caspase-3 activation may also directly impact tau pathology, because increased caspase-3 activation in the APPsw/NOS2−/− brain is associated with tau truncation. This truncated form of tau appears to promote neurofibrillary tangle formation (23, 48, 50), and loss of NO may enhance this truncation/aggregation mechanism.
NO's biological activity is diverse, and alternative mechanisms of tangle formation may also exist. Reynolds et al. (51) reported that specific N-terminal tyrosine residues of tau are nitrated, that these nitrotyrosines inhibit tau aggregation, and thus may protect against pathological tau deposition (50). By this mechanism, the decreased NO in APPsw/NOS2−/− mice would reduce tau nitration and promote tau aggregation (52, 53). Alternatively, the activity of glycogen synthase kinase 3β (GSK-3β) that phosphorylates tau is regulated by Akt-mediated signaling, which is regulated by NO, potentially via soluble guanylate cyclase activation and cGMP. NO increases the activity of Akt, which in turn inhibits the phosphorylation of GSK-3β. When NO is reduced, the inhibitory pathway over kinase function is also reduced. As a principal kinase involved in tau hyperphosphorylation (8, 54), loss of this regulatory effect of NO on GSK-3β activity may promote tau pathology. Other kinases involved in tau phosphorylation may also be affected by NO-mediated events.
In summary, APPsw/NOS2−/− mice provide clear genetic data that removal of a major synthetic source of NO over a lifetime of exposure to Aβ peptides promotes tau pathology in the brain. Our data also suggest that Aβ, in the presence of reduced NO, may be instrumental in the production of hyperphosphorylated and aggregated tau, thereby regulating the merger of the two pathologies into the amyloid cascade hypothesis of Selkoe and coworkers (55, 56). The potential for NO to act as an inhibitory modulator of caspase activity places NO at a junction point between Aβ peptides, caspase cleavage of tau, and tau aggregation. At the least, the APPsw/NOS2−/− mouse provides a tool to further our understanding of the role of NO-mediated events in AD.
Methods
Mouse Strains.
A bigenic mouse was produced by crossing Tg (HuAPP695.K670N-M671L)2576 mice with NOS2−/− (B6 129P2NOS2tau1Lau/J) (Jackson Laboratory, Bar Harbor, ME) mice. Phenotypes of APPsw and NOS2−/− mice have been described (1, 57). Tg2576 mice were a generous gift of K. Hsaio-Ashe (University of Minnesota, Minneapolis, MN). Littermate controls were generated from the backcrossed strain (≈75% C57BL/6 and 25% SJL/129 SVJ). JNPL3 (TAU-P301L) mice were a generous gift from J. Lewis and M. Hutton (Mayo Clinic, Jacksonville, FL). All mice were genotyped by using standard procedures.
Immunocytochemistry Antibodies.
Hyperphosphorylated tau was detected with the following antibodies: AT8-phospho-Ser-202/Thr-205 (1:500; Pierce Biotechnology, Rockford, IL), CP13-phospo-Ser-202/Thr-205 (1:600; a gift of Peter Davies, Albert Einstein College of Medicine, Bronx, NY), and AT180- phosphor-Thr-231 (1:500; Pierce Biotechnology). Total phosphorylated and nonphosphorylated tau was detected with Tau5 (1:3,000; Calbiochem, San Diego, CA), and Aβ/amyloid deposits were detected with 4G8 (1:1,000; Senetek, Napa, CA). Activated caspase-3 was detected by using anti-activeR caspase-3 (1:50; Cell Signaling Technology, Beverly, MA). Truncated tau was detected with TauC3 antibody [a generous gift of L. I. Binder (Northwestern University, Evanston, IL)].
Quantitative RT-PCR.
Mouse brain RNA was extracted with the Versagene RNA Purification system (Gentra Systems, Minneapolis, MI) and converted to cDNA by using a High-Capacity cDNA archive kit (Applied Biosystems, Foster City, CA). NOS2 mRNA (GenBank accession no. NM_010927) expression was identified with primer a (5′-GCATCCCAAGTACGAGTGGT-3′, spanning the exon9/exon 10 boundary to ensure no amplification of genomic DNA) and primer b (5′-ATTCTGCCAGATGTGGGTCTTCCA-3′).
NOS Activity.
NOS enzyme activity was measured by the conversion of l-arginine to l-citrulline as described (58) and expressed as pmol of l-14C-citrulline produced per mg of protein.
Aggregate Filter Assay.
Aggregate levels were measured with a filter retardation assay (18) and immunodetection for either total tau (Tau5) or hyperphosphorylated tau (AT8).
Scanning EM.
Brain aggregates retained on filters as described were immunostained with Tau5 antibody and detected with goat anti-mouse IgG conjugated with 40 nM gold particles (1:20; Ted Pella, Redding, CA) and a silver enhancing kit (Ted Pella). A Phillips KL 30 environmental scanning electron microscope at the Duke Biological Science Environmental Scanning Electron Microscope facility was used for imaging with the kind assistance of Leslie Eibest.
Detection of Soluble and Insoluble Aβ.
Soluble and insoluble pools of Aβ40 and Aβ42 were measured with a specific ELISA and differential brain extractions as described (19, 20).
Statistics.
Average values ± SEM were calculated for quantitative PCR and ELISA data (n = 3–7 animals per group). Statistical significance was calculated by using the unpaired Student's t test with the Prism 3.02 program (GraphPad, San Diego, CA).
Acknowledgments
We thank Dr. C. M. Hulette and J. Ervin of the Kathleen Bryan Brain Bank at Duke University for AD and normal control brain tissues. This work was supported by National Institutes of Health Grants AG19780 (to M.P.V.) and AG19740 (to C.A.C.).
Abbreviations
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- APPsw
APP Swedish mutant
- Aβ
β-amyloid
- NOS
NO synthase
- iNOS
inducible NOS.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki A., Shoji M., Harigaya Y., Kawarabayashi T., Ikeda M., Naito M., Matsubara E., Abe K., Nakazato Y. Virchows Arch. 2002;441:358–367. doi: 10.1007/s00428-002-0643-8. [DOI] [PubMed] [Google Scholar]
- 3.Tomidokoro Y., Ishiguro K., Harigaya Y., Matsubara E., Ikeda M. Neurosci. Lett. 2001;299:169–172. doi: 10.1016/s0304-3940(00)01767-5. [DOI] [PubMed] [Google Scholar]
- 4.Sturchler-Pierrat C., Abramowski D., Duke M., Wiederhold K., Mistl C., Rothacher S., Ledermann B., Burki K., Frey P., Paganetti P., et al. Proc. Natl. Acad. Sci. USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwaab C., Hosokawa M., McGeer P. Exp. Neurol. 2004;188:52–64. doi: 10.1016/j.expneurol.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Oddo S., Caccamo A., Shepherd J., Murphy P., Golde T., Kayed R., Metherate R., Mattson M., Akbari Y., LaFerla F. M. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 7.Lewis J., Dickson D., Lin W., Chisholm L., Corral A., Jones G., Yen S., Sahara N., Skipper L., Yager D., et al. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal K., Alonso Adel C., Chen S., Chohan M., El-Akkad E., Gong C., Khatoon S., Li B., Liu F., Rahman A., et al. Biochim. Biophys. Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Capsoni S., Ugolini G., Comparini A., Ruberti F., Berardi N., Cattaneo A. Proc. Natl. Acad. Sci. USA. 2000;97:6826–6831. doi: 10.1073/pnas.97.12.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigo J., Fernandez-Vizarra P., Castro-Blanco S., Bentura M., Nieto M., Gomez-Isla T., Martinez-Murillo R., MartInez A., Serrano J., Fernandez A. Neuroscience. 2004;128:73–89. doi: 10.1016/j.neuroscience.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Luth H., Munch G., Arendt T. Brain Res. 2002;953:135–138. doi: 10.1016/s0006-8993(02)03280-8. [DOI] [PubMed] [Google Scholar]
- 12.Lee S., Zhao M., Hirano A., Dickson D. J. Neuropathol. Exp. Neurol. 1999;58:1163–1169. doi: 10.1097/00005072-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Smith M., Richey Harris P., Sayre L., Beckman J., Perry G. J. Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver C., Espinoza M., Kress Y., Davies P. Neurobiol. Aging. 2000;21:719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 15.Duff K., Knight H., Refolo L., Sanders S., Yu X., Picciano M., Malester B., Hutton M., Adamson J., Goedert M., et al. Neurobiol. Dis. 2000;7:87–98. doi: 10.1006/nbdi.1999.0279. [DOI] [PubMed] [Google Scholar]
- 16.Goedert M., Jakes R., Vanmechelen E. Neurosci. Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- 17.Lewis J., McGowan E., Rockwood J., Melrose H., Nacharaju P., Van Slegtenhorst M., Gwinn-Hardy K., Paul-Murphy P., Baker M., Yu X., et al. Nat. Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 18.Heiser V., Engemann S., Brocker W., Dunkel I., Boeddrich A., Waelter S., Nordhoff E., Lurz R., Schugardt N., Rautenberg S., et al. Proc. Natl. Acad. Sci. USA. 2002;99:16400–16406. doi: 10.1073/pnas.182426599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao J., Vitek M., Xu F., Previti M., Davis J., Van Nostrand W. J. Neurosci. 2005;25:6271–6627. doi: 10.1523/JNEUROSCI.1306-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt S., Nixon R., Matthews P. Methods Mol. Biol. 2005;299:279–298. doi: 10.1385/1-59259-874-9:279. [DOI] [PubMed] [Google Scholar]
- 21.Schmued L., Stowers C., Scallet A., Xu L. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 22.Friedlander R. N. Engl. J. Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 23.Gamblin T., Chen F., Zambrano A., Abraha A., Lagalwar S., Guillozet A., Lu M., Fu Y., Garcia-Sierra F., LaPointe N., et al. Proc. Natl. Acad. Sci. USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan C., Calingasan N., Nezexon J., Ding A., Lucia S., Perle K., Fuortes M., Lin M., Ehert S., Kwon N., et al. J. Exp. Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto Y., Tsukamoto E., Niikura T., Yamagishi Y., Ishizaka M., Aiso S., Takashima A., Nishimoto I. J. Neurosci. Res. 2004;75:417–428. doi: 10.1002/jnr.10861. [DOI] [PubMed] [Google Scholar]
- 26.Lee J., Chan S., Mattson M. Neuromol. Med. 2002;2:29–45. doi: 10.1385/NMM:2:1:29. [DOI] [PubMed] [Google Scholar]
- 27.Eikelenboom P., Rosemuller J. M., Van Muiswinkel F. Exp. Neurol. 1998;154:89–98. doi: 10.1006/exnr.1998.6920. [DOI] [PubMed] [Google Scholar]
- 28.Giasson B., Ischiropoulous H., Lee V., Trojanowski J. Free Radical Biol. Med. 2002;32:1264–1275. doi: 10.1016/s0891-5849(02)00804-3. [DOI] [PubMed] [Google Scholar]
- 29.Hartlage Rubsamen M., Apelt J., Schliebs R. Neurosci. Lett. 2001;302:73–76. doi: 10.1016/s0304-3940(01)01652-4. [DOI] [PubMed] [Google Scholar]
- 30.Guix F., Uribesalgo I., Coma M., Munoz F. Prog. Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Sinz E., Kochanek P., Dixon C., Clark R., Carcillo J., Schiding J., Chen M., Wieniewski S., Carlos T., Williams C., et al. J. Clin. Invest. 1999;104:647–656. doi: 10.1172/JCI6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q., Rowan M., Anwyl R. J. Neurosci. 2004;24:6049–6056. doi: 10.1523/JNEUROSCI.0233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas D., Espey M., Ridnour L., Hofseth L., Manardi D., Harris C., Wink D. Proc. Natl. Acad. Sci. USA. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isenberg J., Ridnour L., Perruccio E., Espey M., Wink D., Roberts D. Proc. Natl. Acad. Sci. USA. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puzzo D., Vitolo O., Trinchese F., Jacob J., Palmeri A., Arancio O. J. Neurosci. 2005;25:6887–6897. doi: 10.1523/JNEUROSCI.5291-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keynes R. G., Garthwaite J. Curr. Mol. Med. 2004;4:179–191. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]
- 37.Brune B. Antiox. Redox. Signal. 2005;7:497–507. doi: 10.1089/ars.2005.7.497. [DOI] [PubMed] [Google Scholar]
- 38.Mannick J., Schonhoff C., Papeta N., Gharfourifar P., Szibor M., Fang K., Gaston B. J. Cell Biol. 2001;154:1111–1116. doi: 10.1083/jcb.200104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y., Talanian R., Billiar T. J. Biol. Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Vizarra P., Fernandez A., Castro-Blanco S., Encinas J., Serrano J., Bentura M., Munoz P., Martinez-Murioll R., Rodrigo R. Neurobiol. Dis. 2004;15:287–305. doi: 10.1016/j.nbd.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 41.de la Monte S., Lu B., Sohn Y., Etienne D., Kraft J., Ganju N., Wands J. Neurobiol. Aging. 2000;21:309–319. doi: 10.1016/s0197-4580(99)00108-6. [DOI] [PubMed] [Google Scholar]
- 42.Sayre L., Perry G., Harris P., Liu Y., Schubert K., Smith M. J. Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- 43.Thomas D., Espey M. G., Vitek M. P., Miranda K., Wink D. Proc. Natl. Acad. Sci. USA. 2002;99:12691–12696. doi: 10.1073/pnas.202312699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitek M., Snell J., Chernyshev O., Colton C. Biophys. Biochem. Res. Commun. 1997;240:391–394. doi: 10.1006/bbrc.1997.7408. [DOI] [PubMed] [Google Scholar]
- 45.Meda L., Cassatella M., Szendrel G., Otvos L., Baron P., Villalba M., Ferrari D., Rossi F. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 46.Duport S., Garthwaite J. Neuroscience. 2005;135:1155–1166. doi: 10.1016/j.neuroscience.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 47.Brannan C., Roberts M. Glia. 2004;48:120–131. doi: 10.1002/glia.20066. [DOI] [PubMed] [Google Scholar]
- 48.Rissman R., Poon W., Blurton-Jones M., Torp R., Vitek M., LaFerla F., Rohn T., Cotman C. J. Clin. Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeBlanc A. Prog. Neurol. Psychopharmacol. Biol. Psychiatry. 2003;27:215–229. doi: 10.1016/S0278-5846(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 50.Binder L., Guillozet-Bongaarts A., Garcia-Sierra F., Berry R. Biochem. Biophys. Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds M., Berry R., Binder L. Biochemistry. 2005;44:1690–1700. doi: 10.1021/bi047982v. [DOI] [PubMed] [Google Scholar]
- 52.Bush A. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 53.Colton C., Chernyshev O., Gilbert D., Vitek M. Ann. N.Y. Acad. Sci. 2000;899:292–307. doi: 10.1111/j.1749-6632.2000.tb06195.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen F., David D., Ferrari A., Gotz J. Curr. Drug Topics. 2004;5:503–515. doi: 10.2174/1389450043345236. [DOI] [PubMed] [Google Scholar]
- 55.Selkoe D., Schenk D. Annu. Rev. Pharmacol. Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 56.Hardy J., Selkoe D. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 57.Laubach V., Shesely E., Smithies O., Sherman P. Proc. Natl. Acad. Sci. USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinberg B., Misukonis M., Shami P., Mason S., Sauls D., Dittman W., Wood E., Smith G., McDonald B., Bachus K. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 59.Cho S., Park E., Zhou P., Frys K., Ross M., Iadecola C. J. Cereb. Blood Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- 60.Zhou P., Qian L., Iadecola C. J. Cereb. Blood Flow Metab. 2005;25:348–357. doi: 10.1038/sj.jcbfm.9600036. [DOI] [PubMed] [Google Scholar]