Abstract
After loss of a particular sensory channel, the deprived cortex can be activated by inputs from other sensory modalities. It is not known whether activation of the rewired cortex evokes subjective experiences characteristic of that cortex or consistent with the rerouted sensory information. In a previous study, blind subjects were trained to perform visual tasks with a tongue display unit, a sensory substitution device that translates visual displays into electrotactile tongue stimulation. This cross-modal sensory stimulation activated their visual cortices. We now extend this finding by using transcranial magnetic stimulation to examine the perceptual correlates of training-induced plastic responses. We find that blind subjects proficient with the use of the tongue display unit report somatopically organized tactile sensations that are referred to the tongue when transcranial magnetic stimulation is applied over the occipital cortex. No such sensations were evoked in trained, blindfolded, seeing control subjects who performed the sensory substitution task equally well. These data show that the perceptual correlate of activity in a given cortical area reflects the characteristics of its novel sensory input source.
Keywords: blindness, cross-modal plasticity, rewiring, sensation, vision
The human cortex has a remarkable capacity for plasticity and reorganization (1–2). After loss of a particular sense, input from other modalities invades the cortical area that is deprived of its normal inputs (3–7). Animal studies have shown that, if brain damage occurs during development, abnormal connectivity patterns can be produced. For instance, after lesions of central retinal targets in mammals, new and permanent retinofugal projections to nonvisual thalamic sites are induced (3–4; 6). These new retinal projections are retinotopically organized and make functional synaptic contacts. Neurons in the auditory cortex of animals with these ectopic retinal projections have visual response properties that resemble those of cells in the visual cortices of normal animals (8–9). Behavioral studies have further shown that such animals use the “rewired” auditory cortex to perform visual discrimination tasks, and that lesions of the nominally auditory cortex abolish this capacity (5, 7). Although these studies reveal the rewired cortex to be functional in the visual domain, the subjective character of this activation remains to be elucidated.
In humans deprived since birth of a particular sensory modality, the deprived cortex can be recruited by other sensory modalities (reviewed in ref. 10), supporting the concept of cross-modal plasticity. For example, the auditory cortices of deaf subjects are activated by (visual) sign language (11). Furthermore, Braille reading by blind subjects proficient in Braille activates their visual cortices (12–15). The role of the visual cortex in cross-modal plasticity was further substantiated by results of transcranial magnetic stimulation (TMS) studies showing that functional blockade of the rewired cortex interferes with task performance (16).
More recently, we used positron-emission tomography (PET) (17–18) to investigate changes in regional cerebral blood flow (rCBF) in congenitally blind and normally seeing control (SC) subjects when using the tongue display unit (TDU), a tactile vision sensory substitution system (19). PET recordings with [15O]water were obtained before and after a 1-week training period, during which time the subjects had to learn to detect the orientation of visual stimuli presented through the TDU. Both the blind and seeing subjects learned equally well to use the TDU after this brief training. Whereas no task-evoked rCBF increase was detected in the visual cortex of either group before training, tongue stimulation after training significantly activated CBF in the visual cortex of the blind, but not of the sighted control, subjects. We concluded that, in congenital blindness, there was a training-induced rerouting of tactile information to the visual cortex, possibly involving strengthened or unmasked parietooccipital connections (17–18). In this study, we exploit this model of sensory substitution to examine the subjective character of experience associated with the activation of the occipital cortex before and after the establishment of cross-modal plasticity. More specifically, we wanted to test the possibility whether stimulation of the new input can induce qualia. An advantage of the TDU model is that it uses the tongue instead of the fingertips, which makes blind and SC subjects equally naïve with respect to the behavioral task, allowing the dissociation of group or group × time effects from simple performance effects. We used TMS to stimulate the visual cortex before and after TDU training in a group of early blind (EB), late blind (LB), and blindfolded SC subjects while noting the sensory experiences reported by the subjects. Our results show that TMS to the occipital cortex in trained blind subjects induced tactile sensations in the tongue that were somatotopically organized. In sharp contrast, TMS of the occipital cortex in trained blindfolded SC subjects evoked only visual phosphenes. Our results indicate that the subjective character of the percept depends on the stimulated sensory channel (e.g., somesthesis) and not on the activated cortex (e.g., visual).
Results
Behavior.
Before training with the TDU, performance on the motion-direction task was at the chance level in all groups. After the 1-week training period, all but 3 of the 23 subjects (1 EB, 1 LB, and 1 SC) successfully performed the task.
Phosphenes.
Two LB subjects reported phosphenes during occipital TMS (Table 1). These were described as fugitive central sparks, as reported by others (20). None of the EBs reported phosphenes. In contrast, 9 of 10 SC subjects described phosphenes after occipital TMS (mean intensity threshold, 69 ± 12% of maximal stimulator intensity). Attribution of phosphene locations varied with stimulation sites, in accordance with other studies (20, 21).
Table 1.
Characteristics of the blind subjects
| Group | Subject | Age | Sex | Blindness |
Dexterity |
TMS-induced subjective sensations |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset | Cause | Visual perception | L/R* | LQ† | Visual | Before |
After |
||||
| Tactile | |||||||||||
| EB | LP | 37 | M | Birth | Retinopathy of prematurity | None | R | 64 | − | − | − |
| EB | VL | 36 | M | Birth | Retinopathy of prematurity | None | Amb | 25 | − | − | − |
| EB | AD | 27 | M | Birth | Retinopathy of prematurity | None | Amb | 40 | − | − | − |
| EB | OB | 43 | M | Birth | Retinopathy of prematurity | None | L | –80 | − | − | + |
| EB | KT | 35 | M | Birth | Retinopathy of prematurity | Bright lights | R | 100 | − | − | − |
| EB | SN | 37 | F | 7 | Leber’s congenital disease | None | R | 100 | − | − | + |
| EB | BF | 41 | F | Birth | Retinopathy of prematurity | None | R | 100 | − | − | − |
| EB | DI | 36 | M | 7 | Leber’s congenital disease | None | Amb | –20 | − | − | + |
| LB | AN | 35 | F | 18 | Aniridia, glaucoma | None | R | 100 | − | − | + |
| LB | MA | 38 | M | 9 | Glaucoma | None | Amb | 45 | − | − | − |
| LB | CL | 26 | M | 19 | Leber’s congenital disease | None | R | 100 | + | − | − |
| LB | GJL | 47 | M | 40 | Aniridia, glaucoma | Bright lights | L | –69 | − | − | − |
| LB | WF | 39 | M | 18 | Retinitis pigmentosa | Bright lights | R | 100 | + | − | − |
*L, left handed; R, right handed; Amb, ambidextrous.
†LQ, laterality quotient.
Tactile Sensations in Blind Subjects.
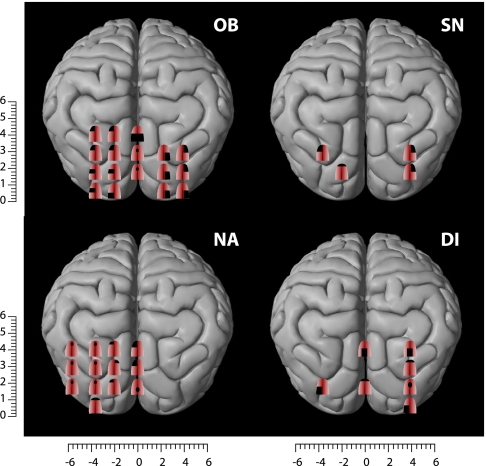
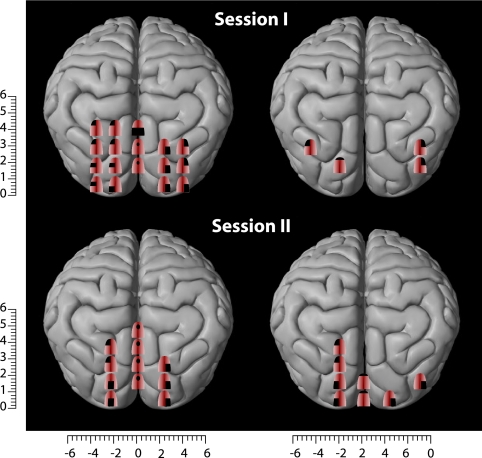
Before training, no subjective sensations were evoked when applying TMS over the visual cortex of blind subjects, with the exception of three subjects who reported tactile sensations in the fingertips. After training, three EB and one LB subject reported tactile sensations on the tongue after occipital TMS (mean stimulation intensity, 87 ± 10%). These sensations were described as short-lasting experiences of distinct tingling, varying in intensity, extent, and topography depending on the locus of the occipital cortex that was stimulated (Fig. 1). To test for the reproducibility of the responses, some occipital positions were randomly selected and stimulated again at the end of the session, resulting in very similar results. Two of the blind subjects (OB and SN) were tested a second time, 2 months after the initial study. Roughly similar results were obtained upon reexamination, although there were some differences between the sensory maps obtained in the two sessions. For instance, the number of sites from which tactile sensations could be induced decreased from 17 to 11 for subject OB and increased from 4 to 8 in subject SN (Fig. 2).
Fig. 1.
Somatotopically organized tactile sensations in the tongue induced by TMS over the occipital cortices in four blind subjects who were trained to use their tongues to perform a visual motion-direction task. Shown are the areas of the tongue where tactile sensations were felt after TMS stimulation of the visual cortex. The numbers on the scales refer to the distance (in cm) from the inion.
Fig. 2.
Comparison of the TMS-induced tactile paresthesiae in two subjects who were tested twice at an interval of 2 months. As shown, a high degree of similarity was observed when subjects were retested.
Although blindfolded controls matched the blind in terms of hours of training with the TDU and also in performance, TMS of occipital cortex before and after training induced only phosphenes, but not tactile sensations, in these subjects. In none of the groups were subjective sensations produced by TMS over the primary sensory cortex (S1), either before or after training.
Discussion
These data constitute a demonstration that the subjective experience, or qualia, of activity in the visual cortex after sensory remapping is tactile and not visual. Our data confirm and extend an earlier anecdotal observation of occipital TMS-induced somatosensory distortions during Braille reading (16). In contrast to that report, where tactile input from the fingertips was present, we now show that TMS of the occipital cortex can induce tactile sensations in the absence of any direct tactile input. Tactile sensations were evoked from both lower-tier visual cortex and higher-level visual cortex. In subject OB, tactile sensations were induced from 17 sites in both hemispheres, extending from the ”inion“ to locations 4 cm more dorsal. Tactile sensations were also evoked by application of TMS over a wide medial–lateral range, from the midline to 6 cm lateral. When stimulating these more lateral sites, there is a risk of stimulating the cranial portion of the facial nerve, especially in a subject with a small head. Because stimulation of the facial nerve in the infraauricular region, below the stylomastoid foramen, induces a facial contraction, results were discarded whenever we observed a motor response. Nevertheless, it is still possible that lower intensities might be sufficient to stimulate sensory fibers of the facial nerve. Not all blind subjects reported tactile sensations after training with the TDU. Although we have no definitive explanation for this intersubject variability, this finding was in agreement with results in the PET study, in which four of the present TMS subjects had earlier participated. Of these, the two subjects reporting TMS-induced tactile sensations were the same subjects showing the largest increase in rCBF during tasks involving the TDU (Fig. 3, which is published as supporting information on the PNAS web site). In the present group, only two LB subjects experienced phosphenes during occipital TMS. This proportion is lower than in a previous study (21), perhaps because of the fact the earlier study used trains of TMS pulses (15 Hz) instead of single pulses. In strong contrast with the results obtained in blind subjects, and despite equal task performance on the motion direction task, the SC subjects reported only TMS-induced phosphenes but no tactile sensations on the tongue or elsewhere. There are strong parallels between the current results and the results of our earlier PET study in which only blind, but not the equally well performing SC subjects, showed increased rCBF in the occipital cortex during an orientation discrimination task using the TDU (17).
We have used the same criterion as in most TMS studies of the motor and visual system (21, 22) for mapping TMS-induced subjective sensations. We considered that a tactile sensation was present when it could be reliably evoked in at least three of five TMS pulses applied over the same spot. In other words, each location was stimulated a minimum of three times to ascertain the presence or absence of a tactile sensation. In addition, some randomly selected spots were stimulated for a second time at the end of each mapping session. For 85% of the stimulated sites, subjects reported sensations in the same somatotopic areas when stimulation was repeated within the session.
We used a figure-eight-shaped coil with a 7-cm diameter, which had been used in many previous TMS studies. Although the use of a smaller coil might have improved precision, a recent paper showed that a high spatial precision can be obtained by using the standard coils (23). The observation that our subjects reported tactile sensations in a different part of the tongue when the coil was moved 1 cm in the dorsoventral or mediolateral plane suggests that we achieved a good spatial resolution of our somatotopic maps, further underscored by the results of the retesting in two subjects, which revealed roughly similar somatotopic maps when testing was repeated 2 months later.
Our results provide insight into the long established scientific debate on cortical dominance or deference (24). What is the experience of a subject in whom areas of cortex receive input from sensory sources not normally projected to those areas? Our data show that the qualitative character of the subject’s experience is not determined by the area of cortex that is active (cortical dominance) but by the source of input to it (cortical deference). Our results are also in line with recent evidence that sensory cortical areas receive input from multiple sensory modalities early in development (25–27). Tactile sensations of the tongue were obtained only after training with the TDU, in accordance with the dynamic sensorimotor hypothesis; different sensory modalities are governed by highly specific learned dynamic interaction patterns between sensory stimulation and active movement (28). The pathway mediating the induction of phantom tactile sensations in the blind may involve either a reorganization of corticocortical pathways (17, 29, 30), or a rerouting at the thalamic level, as revealed by anatomical findings in animals (6, 31).
Training-Induced Unmasking and Strengthening of Existing Connections.
In this and in our previous studies, cross-modal responses were already observed after <1 week of training with the TDU (17–18). The rapid onset of cross-modal responses excludes the possibility of mediation by the establishment of new anatomical connections. What might therefore be the basis of this plasticity? One possibility is that training unmasks and strengthens preexisting connections between the parietal and the occipital cortices. There is electrophysiological (32) and anatomical (26, 27) evidence that the primary visual cortex in normal mammals receives input not only from the visual thalamus but also from somatosensory and auditory modalities. Single-unit recordings in the visual cortex in unanesthetized cats have shown that neurons in areas 17 and 18 receive both visual and auditory input. For many of these bimodal cells, receptive fields in the acoustic and visual domains overlapped spatially (32). Anatomical tracing studies have further shown that there are direct projections from the auditory cortex and from the polysensory area of the temporal lobe to area 17 of the macaque monkey (26). In addition, direct projections from parietal association areas to areas V1 and V2 in the calcarine fissure have also been described (27). These nonvisual inputs conveying tactile and auditory inputs to the occipital cortex may modulate the processing of visual information (33), although they do not give rise to subjective nonvisual sensations under normal circumstances because of masking by the dominant visual input. Thus, in our trained control subjects, TMS over the occipital cortex produced only visual (phosphenes), without tactile, sensations. However, under certain circumstances, nonvisual processing in the occipital cortex can be strengthened or unmasked. Empirical support for this hypothesis comes from an experiment showing activation of the visual cortex by tactile stimulation after prolonged blindfolding in sighted subjects (cited in ref. 34). Therefore, we hypothesize that cross-modal tactile rerouting to the visual cortex was already present in our blind subjects before the TDU experience (see below). However, these cross-modal responses have been shaped mainly from tactile input from the fingers as the result of an increased use of fingers during Braille reading and haptic exploration of the environment. In line with the dynamic sensorimotor hypothesis, training with the TDU device results in new highly specific learned dynamic-interaction patterns between sensory stimulation and active movement (28), thereby further strengthening and unmasking existing connections between the parietal and occipital cortices.
Long-Term Cross-Modal Changes.
In addition to the training-induced unmasking and strengthening of existing connections, long-term cross-modal neuroplastic changes are also likely to have been involved in the occipital TMS-induced tactile sensations. Electrophysiological recording studies in awake monkeys after early visual deprivation showed that 18% of the recorded neurons in visual cortical area 19 responded to somatic inputs, such as manipulating the experimenter’s hand to search for food (35). This was in sharp contrast to the findings obtained in normally seeing animals, in which 100% of the neurons recorded in area 19 responded exclusively to visual inputs. This difference implies that, after early visual deprivation, tactile information reaches the visual cortex. This claim is largely supported by results of brain-imaging studies showing activation of the visual cortex in EB subjects during Braille reading (12–15) and other forms of tactile stimulation (17, 36). The importance of deprivation early in life is further underscored by the observation that brain-activity patterns in the occipital cortex evoked by tactile stimulation are significantly stronger in EB compared with LB subjects (14, 37). Together, these findings suggest that, when the brain is deprived of visual input at an early age, tactile (and other nonvisual) information is rerouted to the visual cortex.
Which Pathways Mediate the Cross-Modal Responses?
The rerouting of tactile information to the visual cortex may be accomplished either by the formation of new thalamocortical pathways or through strengthening of existing corticocortical pathways. Although the latter possibility cannot be excluded with certainty and finds some support in animal studies (6, 31), the available data in man suggest that tactile information reaches the occipital cortex via existing corticocortical pathways. In our previous PET study (17), we showed that functional connectivity between the multimodal dorsal intraparietal sulcal area and the cuneus was significantly increased after 1 week of training with the TDU. In a recent study (unpublished data), we recorded somatosensory evoked potentials induced by electrical stimulation of the tongue before and after a 1-week training period with the TDU. In naïve nontrained blind subjects, an N1–P1 complex was measured over the parietal cortex with a latency of ≈13–18 ms. After the 1-week training with the TDU, a second peak appeared with a latency of ≈48–60 ms, suggesting mediation by a corticocortical pathway (Fig. 4, which is published as supporting information on the PNAS web site). Additional evidence for increased connectivity between the parietal and visual cortices in EB subjects comes from a study that combined TMS and PET to study functional connectivity (29). It was found that TMS of the primary somatosensory cortex induced a significant rCBF increase in the occipital cortex in the congenitally blind but not in blindfolded control subjects. A recent diffusion-tensor imaging and diffusion-tensor tractography study showed evidence of atrophy of the geniculocortical tract in EB subjects but preservation of connections between the visual cortex and prefrontal and temporal cortices (30). Because no additional tracts were observed in the EB group, the data suggest that cross-modal functionality of the visual cortex in early blindness is primarily mediated by strengthened corticocortical instead of thalamocortical connections.
TMS over the Parietal Cortex.
In neither the blind nor normally SCs did we succeed in provoking subjective sensations by stimulating the tongue area of the S1, not even when stimulating at maximum stimulator output. We claim that we have stimulated the correct somatotopic (tongue) area of S1, because we searched by stimulating the motor cortex until the subjects reported a twitch of the tongue. Having found this location, we moved the TMS coil gradually to a more posterior position to selectively stimulate the S1. However, we were not able to induce pure sensory (tactile) responses dissociated from motor responses in the tongue. Furthermore, stimulation of the S1 tongue representation at high intensities is described as very unpleasant by most subjects. To the best of our knowledge, there have been only two reports in the literature describing TMS-induced pure tactile sensations, produced by either stimulating the primary motor cortex (M1) (38) or S1 region (39). It has been argued that excitation of the postcentral gyrus requires prolonged repetitive stimulation for accessing the perceptual system (40).
Our results are consistent with a report by Cohen and colleagues (16), who found that TMS applied over the occipital cortex during Braille reading occasionally elicited distorted somatosensory perceptions. However, such sensations were never reported when TMS was applied over the S1 during Braille reading.
Conclusion
The present data show that tactile sensations can be induced by stimulating the visual cortex in the congenitally blind, adding further evidence for the development of cross-modal plasticity after deprivation of a particular sensory input. These data show that the perceptual correlate of activity in a given cortical area reflects the characteristics of the sensory input to it, thereby lending empirical support for the theory of cortical deference.
Methods
Subjects.
Eight EB, five LB, and eight age-matched blindfolded SC subjects participated in this study. The average age of the subject groups were 36.5 ± 4.7 y (EB), 37.0 ± 7.6 y (LB), and 34.5 ± 11 y (SC). Blindness was of peripheral origin (Table 1). All SC had normal visual acuity. Subjects were asked to fill in the Edinburgh Handedness Inventory to obtain a lateralization quotient. The study protocol was approved by the local ethics committees and all subjects provided written informed consent.
Experimental Procedures
TDU.
Subjects were trained daily in the use of TDU to perform a visual motion-orientation task (17). In brief, moving dots are presented on a laptop screen connected to a TDU unit. The TDU translates the visual image into electrotactile stimulation, which is delivered to the tongue via a 3- × 3-cm electrode array (19). Subjects had to learn to discriminate the movement direction of a random dot pattern. The performance criterion for successful learning was set at a minimum of 85% correct responses on two consecutive testing days.
TMS.
We applied single-pulse TMS with a Magstim rapid magnetic stimulator (Magstim, Whitland, Dyfed, U.K.) connected to a double 7-cm figure-eight-shaped coil with a maximum stimulator output of 1.2 T. We started with the assessment of the motor and phosphene thresholds, followed by mapping of TMS-induced subjective sensations.
Determination of Motor and Phosphene Thresholds.
To determine phosphene thresholds, the coil was placed in a vertical position (handle pointing upward) on the inion–nasion line, with its inferior limit 1 cm above the inion. Stimulation was initially applied at 40% of maximum stimulator output and increased in 5% increments until the subject reported phosphenes. The threshold was then finely determined, by changing the intensity in 1% increments. With the coil placed at the optimal position over the left motor area, the motor threshold was defined as the lowest intensity able to produce, at rest, an electromyographic response in the first dorsal interosseus muscle of the right hand of >50-μV peak-to-peak amplitude, in at least 5 of 10 trials. Stimulation intensity was initially 40% of maximal output and increased in 5% increments. The threshold was then finely determined by changing the intensity in 1% increments.
Mapping of TMS-Induced Subjective Sensations.
Mapping was done before and after training with the TDU. For mapping TMS-induced subjective sensations, single TMS pulses were applied as follows. The coil was moved in a pseudorandomized manner over the entire occipital and adjoining occipitoparietal and occipitotemporal association cortices. In addition, S1 was also stimulated. After each magnetic pulse (single TMS pulses of 100 μs), subjects were asked to report any subjective sensation associated with the pulse. No suggestions were given that might bias reports. Scalp coordinates for stimulation were determined on a tightly fitting rubber swimming cap placed over the subject’s head. In the mediolateral direction, markings were made every 2 cm from the midline extending to 6 cm lateral. In the dorsoventral direction, stimulation points were made at the level of the inion and 1.25, 2.5, 4, and 6 cm more dorsal. To discard potential effects produced by stimulation of the facial nerve in the infraauricular region when stimulating at lateral locations, results were discarded whenever a facial motor response was elicited. If a phosphene was induced, subjects were asked to point in its direction. If subjects reported a phantom tactile sensation, they were asked to describe it in greater detail. In case it was referred to the tongue, they were given a three-dimensional model of the tongue and asked to indicate where exactly they had experienced the sensation. We considered that a tactile phantom sensation was present when it could be reliably evoked in at least three of five pulses applied to the same spot. Sham stimulation was produced by placing the lateral part of the coil on the skull while a TMS pulse was delivered in the air.
TMS stimulation of the tongue area of the primary somatosensory cortex was obtained as follows. We first mapped the optimal coil position to produce a motor response of the tongue muscle (41). The coil was oriented in such a way that the induced electric field was aimed toward the motor cortex, anterior to the central sulcus. Once the M1 tongue “hot spot” was found, the TMS coil was rotated 180°, so that the electric field was directed posterior to the central sulcus. The coil was then gradually moved in the posterior direction to stimulate selectively the tongue area of the S1.
Supplementary Material
Acknowledgments
We thank Denis Latendresse (Université de Montréal) for expert graphical help with the figures, Damien Debatisse (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland) for help with the electrophysiological experiments, Professor Poul Erik Buchholtz Hansen (Risskov Psychiatric Hospital, Aarhus, Denmark) for providing unlimited access to the TMS Facilities, and Dr. Paul Cumming (Aarhus University) and professor Jens-Bo Nielsen (Panum Institute, Copenhagen, Denmark) for critical comments on an earlier version of this manuscript. This work was supported by a grant from the Danish Medical Research Council (to R.K., A.G., and M.P.), the Svend Andersen Foundation (R.K.), and by Belgian Fund for Medical Research Grant 3.4523.00 (to J.S.).
Abbreviations
- CBF
cerebral blood flow
- EB
early blind
- LB
late blind
- PET
positron- emission tomography
- rCBF
regional CBF
- S1
primary sensory cortex
- SC
seeing control
- TDU
tongue display unit
- TMS
transcranial magnetic stimulation
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pascual-Leone A., Amedi A., Fregni F., Merabet L. B. Annu. Rev. Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 2.Kaas J. H. Prog. Brain Res. 2002;138:167–176. doi: 10.1016/S0079-6123(02)38077-4. [DOI] [PubMed] [Google Scholar]
- 3.Frost D. O., Metin C. Nature. 1985;317:162–164. doi: 10.1038/317162a0. [DOI] [PubMed] [Google Scholar]
- 4.Metin C., Frost D. O. Proc. Natl. Acad. Sci. USA. 1989;86:357–361. doi: 10.1073/pnas.86.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Melchner L., Pallas S. L., Sur M. Nature. 2000;404:871–876. doi: 10.1038/35009102. [DOI] [PubMed] [Google Scholar]
- 6.Sur M., Garraghty P. E., Roe A. W. Science. 1988;242:1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- 7.Frost D. O., Boire D, Gingras G., Ptito M. Proc. Natl. Acad. Sci. USA. 2000;97:11068–11073. doi: 10.1073/pnas.190179997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ptito M., Giguere J. F., Boire D., Frost D. O., Casanova C. Prog. Brain Res. 2001;134:447–458. doi: 10.1016/s0079-6123(01)34029-3. [DOI] [PubMed] [Google Scholar]
- 9.Sharma J., Angelucci A., Sur M. Nature. 2000;404:841–847. doi: 10.1038/35009043. [DOI] [PubMed] [Google Scholar]
- 10.Merabet L. B., Rizzo J. F., Amedi A., Somers D. C., Pascual-Leone A. Nat. Rev. Neurosci. 2005;6:71–77. doi: 10.1038/nrn1586. [DOI] [PubMed] [Google Scholar]
- 11.Finney E. M., Fine I., Dobkins K. R. Nat. Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- 12.Sadato N., Pascual-Leone A., Grafman J., Ibanez V., Deiber M. P., Dold G., Hallett M. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- 13.Buchel C., Price C., Frackowiak R. S., Friston K. Brain. 1998;121:409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- 14.Burton H., Snyder A. Z., Conturo T. E., Akbudak E., Ollinger J. M., Raichle M. E. J. Neurophysiol. 2002;87:589–607. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gizewski E. R., Gasser T., de Greiff A., Boehm A., Forsting M. NeuroImage. 2003;19:968–975. doi: 10.1016/s1053-8119(03)00114-9. [DOI] [PubMed] [Google Scholar]
- 16.Cohen L. G., Celnik P., Pascual-Leone A., Corwell B., Falz L., Dambrosia J., Honda M., Sadata N., Gerloff C., Catala M. D., Hallett M. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 17.Ptito M., Moesgaard S., Gjedde A., Kupers R. Brain. 2005;128:606–614. doi: 10.1093/brain/awh380. [DOI] [PubMed] [Google Scholar]
- 18.Ptito M., Kupers R. J. Integr. Neurosci. 2005;4:479–488. doi: 10.1142/s0219635205000951. [DOI] [PubMed] [Google Scholar]
- 19.Bach-y-Rita P., Kercel S. Trends Cogn. Sci. 2003;7:541–546. doi: 10.1016/j.tics.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Cowey A., Walsh V. NeuroReport. 2000;11:3269–3273. doi: 10.1097/00001756-200009280-00044. [DOI] [PubMed] [Google Scholar]
- 21.Gothe J., Brandt S. A., Irlbacher K., Roricht S., Sabel B. A., Meyer B. U. Brain. 2002;125:479–490. doi: 10.1093/brain/awf045. [DOI] [PubMed] [Google Scholar]
- 22.Rossini P. M., Barker A. T., Berardelli A., Caramia M. D., Caruso G., Cracco R. Q., Dimitrijevic M. R., Hallett M., Katayama Y., Lucking C. H., et al. Electroencephalogr. Clin. Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 23.Hannula H., Ylioja S., Pertovaara A., Korvenoja A., Ruohonen J., Ilmoniemi R. J., Carlson S. Hum. Brain Mapp. 2005;26:100–109. doi: 10.1002/hbm.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James W. Principles of Psychology. New York: Dover; 1980. [Google Scholar]
- 25.Wallace M. T., Ramachandran R., Stein B. Proc. Natl. Acad. Sci. USA. 2004;101:2167–2172. doi: 10.1073/pnas.0305697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falchier A., Clavagnier S., Barone P., Kennedy H. J. Neurosci. 2002;22:5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockland K. S., Ojima H. Int. J. Psychophysiol. 2003;50:19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 28.O’Regan J. K., Noe A. Behav. Brain Sci. 2001;24:939–973. doi: 10.1017/s0140525x01000115. [DOI] [PubMed] [Google Scholar]
- 29.Wittenberg G. F., Werhahn K. J., Wassermann E. M., Herscovitch P., Cohen L. G. Eur. J. Neurosci. 2004;20:1923–1927. doi: 10.1111/j.1460-9568.2004.03630.x. [DOI] [PubMed] [Google Scholar]
- 30.Shimony J. S., Burton H., Epstein A. A., McLaren D. G., Sun S. W., Snyder A. Z. Cereb. Cortex. 2005 doi: 10.1093/cercor/bhj102. 0: bhj102v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brett-Green B., Fifkova E., Larue D. T., Winer J. A., Barth D. S. J. Comp. Neurol. 2003;460:223–237. doi: 10.1002/cne.10637. [DOI] [PubMed] [Google Scholar]
- 32.Fishman M. C., Michael P. Vision Res. 1973;13:1415–1419. doi: 10.1016/0042-6989(73)90002-3. [DOI] [PubMed] [Google Scholar]
- 33.Macaluso E., Frith C. D., Driver J. Science. 2000;289:1206–1208. doi: 10.1126/science.289.5482.1206. [DOI] [PubMed] [Google Scholar]
- 34.Pascual-Leone A., Hamilton R. Prog. Brain Res. 2001;134:427–455. doi: 10.1016/s0079-6123(01)34028-1. [DOI] [PubMed] [Google Scholar]
- 35.Hyvarinen J., Carlson S., Hyvarinen L. Neurosci. Lett. 1981;26:239–243. doi: 10.1016/0304-3940(81)90139-7. [DOI] [PubMed] [Google Scholar]
- 36.Burton H., Sinclair R. J., McLaren D. G. Hum. Brain Mapp. 2004;23:210–228. doi: 10.1002/hbm.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen L. G., Weeks R. A., Sadato N., Celnik P., Ishii K., Hallett M. Ann. Neurol. 1999;45:451–460. doi: 10.1002/1531-8249(199904)45:4<451::aid-ana6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Amassian V. E., Somasundaram M., Rothwell J. C., Britton T., Cracco J. B., Cracco R. Q., Maccabee P. J., Day B. L. Brain. 1991;114:2505–2520. doi: 10.1093/brain/114.6.2505. [DOI] [PubMed] [Google Scholar]
- 39.Sugishita M., Takayama Y. NeuroReport. 1993;4:569–570. doi: 10.1097/00001756-199305000-00027. [DOI] [PubMed] [Google Scholar]
- 40.Libet B., Alberts W. W., Wright E. W., Jr., Delattre L. D., Levin G., Feinstein B. J. Neurophysiol. 1964;27:546–578. doi: 10.1152/jn.1964.27.4.546. [DOI] [PubMed] [Google Scholar]
- 41.Meyer B. U., Liebsch R., Roricht S. Electroencephalogr. Clin. Neurophysiol. 1997;105:15–23. doi: 10.1016/s0924-980x(96)96598-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.