Abstract
Cryopreservation of male germ cells is a strategy to conserve animal species and strains of animals valuable to biomedical research. We tested whether mouse male germ cells could be cryopreserved without cryoprotection by simply freezing epididymides, testes, or whole bodies. The reproductive organs were isolated from killed mice and frozen for 1 week to 1 year at −80°C before spermatozoa and spermatids were collected and injected into mature oocytes. Normal pups were born irrespective of strains tested (ICR and C57BL/6). Epididymides and testes frozen and transported internationally to another laboratory by air could produce pups of inbred C57BL/6 mice. Testicular spermatozoa retrieved from the bodies of male mice (BALB/c nude and C3H/He strains) that had been kept frozen (−20°C) for 15 years could also produce normal offspring by microinsemination. Thus, freezing of either male reproductive organs or whole bodies is the simplest way to preserve male germ cells. Restoration of extinct species could be possible if male individuals are found in permafrost.
Keywords: cryopreservation, gametes, intracytoplasmic sperm injection, microinsemination, mouse
Sperm cryopreservation has been widely used for both human reproduction and animal breeding. Because much of basic research of mammalian genetics and early development has been done by using laboratory mice, and the number of genetically engineered mice (transgenesis, gene targeting, and mutagenesis) is increasing exponentially, development of simple, cost-saving, and space-effective means of mouse sperm preservation is much needed (1). Mouse sperm cryopreservation using raffinose and skim milk as cryoprotectants has been successful, but defrosted spermatozoa of some strains of mice do not fertilize eggs well (2). This is especially true for C57BL/6 (B6) mice, most frequently used for generation of genetically engineered mice. This problem has been overcome by partial zona dissection before in vitro fertilization (3) or intracytoplasmic sperm injection of frozen-thawed or freeze-dried spermatozoa (4–7).
Immature male germ cells such as spermatids and spermatocytes are currently used to produce offspring (8–11) when spermatozoa cannot be obtained due to spermatogenic arrest because of genetic mutations or as the result of in vitro manipulation of germ cells (7, 10, 11). Therefore, cryopreservation of these immature sperm cells is necessary, but they suffer more cryodamage than mature epididymal spermatozoa. A cryopreservation protocol we developed for mouse spermatogenic cells in the past is rather complicated, and therefore the development of simpler techniques is required (12).
Thus far, the simplest method of cryopreserving mouse spermatozoa is to freeze spermatozoa in simple salt solutions without any cryoprotectants (6). Although defrosted spermatozoa are not alive in the conventional sense, they apparently maintain their genetic integrity, because they are able to produce live offspring by microinsemination. Here we report that spermatozoa or spermatids, retrieved from frozen reproductive organs or frozen bodies of mice, can produce offspring by microinsemination.
Results
Reproductive Capacity of Spermatozoa and Spermatids in Frozen Epididymides and Testes.
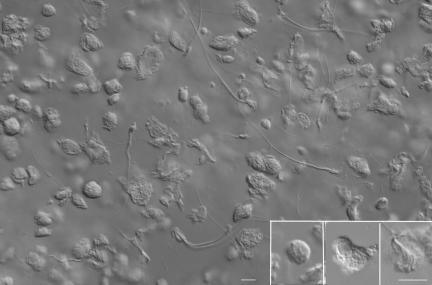
First, we examined whether spermatozoa and spermatids within epididymides and testes withstand freezing and thawing. We used ICR mice, because their spermatozoa are known to be less sensitive to damage by freezing and thawing than those of B6 mice (2). Table 1 summarizes the results of experiments in which isolated epididymides and testes were frozen for 1–8 weeks in liquid nitrogen (−196°C) or in freezers (−80°C) with or without using freezing containers (Fig. 1). Spermatozoa retrieved from the epididymides or testes were completely immotile and “dead,” as confirmed by propidium iodide staining (LIVE/DEAD Sperm Viability Kit, Molecular Probes, Eugene, OR; data not shown). Virtually all round and elongated spermatids had extensively disintegrated cytoplasm or no cytoplasm around their nuclei (Fig. 2). In all experiments, >70% of oocytes survived injection of sperm and round spermatids, and >80% of them developed into two-cell embryos, although those injected with round spermatids required exogenous activation stimulus to develop (see Materials and Methods). Therefore, we performed statistical analysis of surviving fetuses based on the number of two-cell embryos transferred to recipient females. A statistical analysis (two-way ANOVA) revealed that both cell type and freezing protocol for the cooling rate (see Fig. 3) had significant effects on embryo implantation and fetal development (P < 0.05). As seen in Table 1, epididymal spermatozoa produced considerably fewer live offspring than testicular spermatozoa and spermatids [Fisher’s protected least significant difference (PLSD); P < 0.05]. Direct plunging of testes into liquid nitrogen and subsequent recovery and injection of round spermatids also resulted in poor embryo development (Fisher’s PLSD; P < 0.05). There was no significant interaction between the two factors (cell type and freezing protocol) with respect to both implantation rates and birth rates (P > 0.05), indicating that these factors are independent of each other.
Table 1.
Development of embryos after microinsemination of oocytes with male germ cells collected from epididymides and testes that were frozen for 1–8 weeks under various freezing protocols (ICR male germ cells and B6D2F1 oocytes)
| Male germ cells | Freezing temperature, °C | Use of freezing container | No. of two-cell embryos transferred | No. of recipient females |
Total no. of implantation sites (%)‡ | No. of live term fetuses (%) | |
|---|---|---|---|---|---|---|---|
| Total | Pregnant | ||||||
| Epididymal sperm | −196* | − | 42 | 3 | 1 | 5 (11.9) | 1 (2.4) |
| −80 | − | 99 | 7 | 0 | 0 (0.0) | 0 (0.0) | |
| −80 | + | 73 | 6 | 2 | 19 (26.0) | 2 (2.7) | |
| −80 and −196† | + | 63 | 4 | 2 | 8 (12.7) | 2 (3.2) | |
| Testicular sperm | −196* | − | 35 | 3 | 2 | 13 (37.1) | 5 (14.3) |
| −80 | − | 31 | 3 | 3 | 19 (61.3) | 10 (32.3) | |
| −80 | + | 15 | 2 | 2 | 9 (60.0) | 5 (33.3) | |
| −80 and −196† | + | 21 | 2 | 2 | 10 (47.6) | 3 (14.3) | |
| Testicular round spermatids | −196* | − | 55 | 4 | 2 | 2 (3.6) | 1 (1.8) |
| −80 | − | 71 | 5 | 5 | 47 (66.2) | 19 (26.8) | |
| −80 | + | 94 | 7 | 7 | 61 (64.9) | 24 (25.5) | |
| −80 and −196† | + | 65 | 4 | 4 | 48 (73.8) | 17 (26.2) | |
Results of two replicates.
*Direct plunging of epididymides or testes into liquid nitrogen.
†Frozen at −80°C for 1 day, then stored in liquid nitrogen for 1–8 weeks.
‡Live fetuses plus resorption sites.
Fig. 1.
Freezing epididymides and testes. (A) Epididymides (small arrow) and testes (large arrow) removed from adult male mice; two cryotubes (upper left) and a freezing container (Bicell, upper right). (B) Epididymides and testes are put in cryotubes before being placed in a freezing container. (C) Freezing containers stored in a freezer.
Fig. 2.
Spermatozoa and spermatogenic cells collected from a testis frozen at −80°C for 1 month. Haploid germ cells can be identified by their size and nuclear shape, although their plasma membrane is disintegrated extensively. (Insets, from the left) A presumptive round spermatid, an early elongated spermatid, and an elongated spermatid. (Bar, 10 μm.)
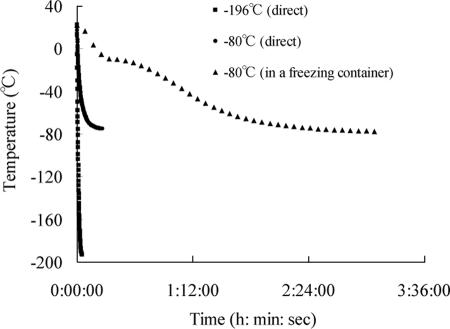
Fig. 3.
Temperature changes inside cryotubes in different freezing protocols. Temperature declines most slowly when the cryotube is placed in a freezing container and is fastest when a cryotube is plunged directly into liquid nitrogen (LN2).
Second, we studied whether spermatozoa and spermatids within epididymides and testes of B6 males withstand freezing and thawing. We used freezing containers for this experiment, because they gave good results in the previous experiments (Table 1). Oocytes were collected from B6 females to maintain inbred background of offspring. There was a significant effect of cell type on both implantation and birth rates (P < 0.05; one-way ANOVA). Results, summarized in Table 2, indicated that spermatozoa collected from frozen cauda epididymis produced live offspring when defrosted spermatozoa were suspended in K+-rich nucleus isolation medium (NIM) (13), not conventional GL-PBS medium (Dulbecco’s PBS supplemented with 5.6 mM glucose/5.4 mM sodium lactate/5 mg/ml BSA/0.01% polyvinyl alcohol; ref. 12), before injection into oocytes (P < 0.05; vs. suspension in GL-PBS). Spermatozoa and round spermatids collected from frozen testes were able to produce offspring without using NIM.
Table 2.
Development of embryos after microinsemination of oocytes with male germ cells collected from caudae epididymides and testes that were frozen at −80°C for 5–7 months (B6 male germ cells and B6 oocytes)
| Male germ cells (postthawing medium) | No. of two-cell embryos transferred | No. of recipient females |
Total no. of implantation sites (%) | No. of normal term fetuses (%) | |
|---|---|---|---|---|---|
| Total | Pregnant | ||||
| Epididymal sperm (GL-PBS) | 63 | 5 | 0 | 0 (0.0)* | 0 (0.0)† |
| Epididymal sperm (NIM) | 73 | 8 | 6 | 43 (58.9)** | 17 (23.3)†† |
| Testicular sperm (GL-PBS) | 26 | 2 | 2 | 10 (38.5) | 5 (19.2)†† |
| Testicular round spermatids (GL-PBS) | 50 | 5 | 4 | 26 (52.0)** | 13 (26.0)†† |
Results of two replicates. ∗ vs. ∗∗ and † vs. ††, P < 0.05; Fisher’s protected least significant difference test.
International Transportation of Frozen Testes.
We tested whether frozen epididymides and testes of B6 mice can be transported internationally by shipping frozen samples with dry ice from the United Kingdom to Japan by air. Spermatozoa and elongated spermatids released from defrosted epididymides and testes into GL-PBS or NIM were injected into oocytes. As shown in Table 3, normal live B6 offspring were obtained in all test groups except for epididymal spermatozoa suspended in GL-PBS. NIM always gave better results than GL-PBS.
Table 3.
Development of embryos after microinsemination of oocytes with spermatozoa and spermatids from frozen epididymides and testes, transported internationally (B6 male germ cells and B6 oocytes)
| Male germ cells | Cells suspension medium | No. of two-cell embryos transferred | No. of recipient females |
Total no. of implantation sites (%) | No. of normal term fetuses (%) | |
|---|---|---|---|---|---|---|
| Total | Pregnant | |||||
| Epididymal sperm | GL-PBS | 63 | 5 | 0 | 0 (0.0)* | 0 (0.0)‡ |
| NIM | 38 | 3 | 2 | 17 (44.7)** | 9 (23.7)‡‡ | |
| Testicular sperm | GL-PBS | 50 | 4 | 4 | 34 (68.0)† | 11 (22.0)§ |
| NIM | 20 | 2 | 2 | 19 (95.0)†† | 13 (65.0)§§ | |
| Testicular elongated spermatids | GL-PBS | 10 | 1 | 1 | 3 (30.0) | 1 (10.0) |
| NIM | 39 | 4 | 3 | 12 (30.8) | 7 (17.9) | |
∗, ∗∗, P < 5 × 10−8. †, ††, P < 0.05. ‡, ‡‡, P < 1 × 10−4. §, §§, P < 1 × 10−3 (Fisher’s exact probability test). ‡, ‡‡, P < 1 × 10−4. §, §§, P < 1 × 10−3 (Fisher’s exact probability test).
Long-Term Storage of Male Germ Cells in Frozen Testes or Whole Bodies.
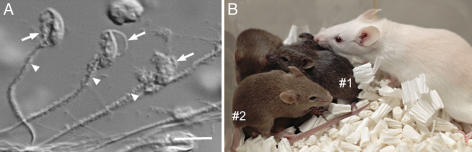
In the last series of experiments, we tested the feasibility of long-term storage of frozen testes or frozen bodies. When ICR male germ cells were retrieved from testes frozen at −80°C for 1 year, suspended in NIM, and injected into oocytes, normal pups were born at moderate rates (11.5–22.6%; Table 4). Although the spermatozoa retrieved from mice frozen for 15 years looked severely damaged (Fig. 4A), heads could be separated from tails as easily as those of fresh live spermatozoa. Within 24 h after sperm injection, >80% of oocytes developed into two-cell embryos, and apparently normal pups were born after embryo transfer in two strains of mice (BALB/c-nude and C3H/He) at fairly high rates (Table 5). Two BALB/c pups died shortly after Caesarian section due to respiratory failure but others grew normally (Fig. 4B) and were proven to be fertile when they matured (at least 19 of 20 mice tested).
Table 4.
Development of embryos after microinsemination of oocytes with male germ cells collected from the testes kept at −80°C for 1 year (ICR male germ cells and B6D2F1 oocytes)
| Male germ cells | No. of two-cell embryos transferred | No. of recipient females |
Total no. of implantation sites (%) | No. of normal term fetuses (%)* | |
|---|---|---|---|---|---|
| Total | Pregnant | ||||
| Testicular sperm | 31 | 2 | 2 | 16 (51.6) | 7 (22.6) |
| Elongated spermatids | 32 | 2 | 2 | 9 (28.1) | 6 (18.8) |
| Round spermatids | 61 | 3 | 3 | 26 (42.6) | 7 (11.5) |
*The results within the same column were not significantly different (P > 0.05, Fisher’s exact probability test).
Fig. 4.
Production of mice using spermatozoa retrieved from testes of male mice frozen for 15 years. (A) Spermatozoa collected from seminiferous tubules show severe structural damages. The head (arrows) and midpiece (arrowheads) have cellular debris. (B) Pups born after injection of frozen spermatozoa (BALB/c-nude) into B6D2F1 oocytes. Because BALB/c and B6D2F1 have the coat-color genotype of A/A b/b c/c D/D and A/a B/b C/C D/D, respectively, the offspring are agouti (heterozygous for all loci; #1) or brown (homologous for the b locus; #2). The albino mouse is their foster mother.
Table 5.
Development of embryos after microinsemination with spermatozoa collected from testes of mice frozen at −20°C for 15 years (B6D2F1 oocytes)
| Strain of male mouse | No. of oocytes that survived injection | No. of oocytes cleaved (%) | No. of two-cell embryos transferred | No. of recipient females |
Total no. of implantation sites, (%) | No. of normal term fetuses (%) | No. weaned (%) | |
|---|---|---|---|---|---|---|---|---|
| Total | Pregnant | |||||||
| BALB/c-nude | 106 | 81 (76.4) | 81 | 6 | 4 | 42 (51.9) | 17 (21.0) | 15 (88.2) |
| C3H/He | 108 | 97 (89.8) | 97 | 7 | 4 | 34 (35.1) | 12 (12.4) | 12 (100.0) |
We could not use elongated and round spermatids from testes because of the complete degeneration of these cells.
Discussion
We reported here that the spermatozoa and spermatids collected from frozen epididymis and testis or from frozen bodies of mice were able to produce normal offspring after injection into oocytes. Obviously, those frozen spermatozoa and spermatids were all “dead” in the conventional sense, as confirmed by propidium iodide staining. Their plasma membranes were severely damaged. Motionless spermatozoa had no chance whatsoever of fertilizing oocytes in vivo or in vitro. Nevertheless, some of these spermatozoa and spermatids, if not all, were “alive” or “genomically intact,” because they were able to produce apparently normal offspring.
Freezing protocols used here are very simple. Simple freezing of animal bodies, using ordinary freezers, is perhaps the simplest procedure we can think of, and it can be done in any laboratory. Isolation and freezing of testes and epididymides are also simple and can be done in most laboratories or even in the field. Our protocols require a set of micromanipulators to generate offspring, but microsurgical injection of spermatozoa (or spermatogenic cells) into oocytes is no longer a special procedure. In fact, thousands of infertility clinics throughout the world routinely perform microsurgical sperm injection to overcome various types of male infertility (14, 15). Normal births after microinsemination have been reported in 14 mammalian species, as of today (11). Laboratories that do not have microsurgical facilities or experience may keep the frozen testis/epididymis or entire bodies in an ordinary freezer (without an automatic defrosting cycle) until they are ready to send the specimens to other laboratories, where microinsemination is routinely performed. This would be particularly pertinent when precious male animals died unexpectedly.
Of all mouse strains, C57BL/6 (B6) would receive the most benefit from this freezing procedure. B6 mice have been most extensively used as a standard strain of mouse for genetic studies. Many genetically modified mice have a B6 background. Difficulty in cryopreserving their spermatozoa has been a major obstacle in mouse genetics (2, 16). Here we demonstrated that B6 mice can be produced by using spermatozoa retrieved from testes frozen for 5–7 months or air-transported with dry ice. The freezing protocols reported here are simple and cost-effective. They would enhance exchange of mouse genetic resources among many laboratories around the world.
It was rather unexpected that spermatozoa in the testes would withstand freezing better than those in the epididymis (Table 3), because nuclei of epididymal spermatozoa are known to be much more stable, both physically and chemically, than testicular spermatozoa because of extensive crosslinkings of nuclear protamines by disulfide bonds (17, 18). Our two-factorial statistical analysis, consisting of three germ cell types and four freezing protocols (Table 1), revealed that epididymal spermatozoa were most sensitive to freezing of all germ cells examined. The reason for this is not clear, but luminal fluid in the seminiferous tubule and/or some materials from Sertoli or spermatogenic cells might have contributed to alleviating sperm damage by freezing. Although round spermatids recovered from defrosted testes can be used to produce live offspring (Tables, 1, 2, and 4), selective identification of these cells from other cells could be rather difficult for inexperienced researchers. Elongated spermatids or spermatozoa are obviously much easier to identify (Fig. 2).
One thing to be stressed here is that the type of medium used for suspension of defrosted spermatozoa and spermatids makes a difference in the outcome of the experiments: the birth of normal offspring. Potassium-rich Ca2+- and Mg2+-free NIM medium (13, 19) always gave better results (Tables 2 and 3). Ca2+-containing ordinary cell culture media like PBS may activate endogenous nucleases (20), which attack the DNAs of these spermatozoa and spermatids with broken plasma membranes. Cauda epididymal spermatozoa may have a higher nuclease activity than testicular spermatozoa and spermatids, and this may make the former more sensitive to freezing and thawing than the latter.
The present study has shown that spermatozoa and spermatids can retain their fertilizing ability in frozen reproductive organs or whole bodies for longer than we anticipated. We found that the spermatozoa retrieved from the testes of mice frozen at −20°C for 15 years were able to produce normal offspring by microinsemination. It would be interesting to know the optimal temperature to use for whole-body freezing and how long male germ cells can retain their fertilizing ability. Accelerated degradation kinetics that have been applied to estimate the maximum storage period of freeze-dried mouse spermatozoa (21) may be applicable to answer this question. If spermatozoa of extinct mammalian species (e.g., woolly mammoth) can be retrieved from animal bodies that were kept frozen for millions of years in permanent frost, live animals might be restored by injecting them into oocytes from females of closely related species.
Materials and Methods
Preparation of Mouse Oocytes.
Female B6D2F1 and C57BL/6 (B6) mice (7–10 weeks old) were each injected with 7.5 units of equine chorionic gonadotropin followed by injection of 7.5 units of human chorionic gonadotropin (hCG) 48 h later. Mature oocytes were collected from oviducts 15–17 h after hCG injection and were freed from cumulus cells by a 3-min treatment with 0.1% hyaluronidase in Chatot, Ziomet, and Bavister (CZB) medium (22). The oocytes were transferred to fresh CZB medium and incubated in it at 37°C in an atmosphere of 5% CO2 in air for up to 90 min before micromanipulation.
Cryopreservation of Isolated Epididymides and Testes.
Caudae epididymides and testes were isolated from sexually mature male ICR and B6 mice (2–6 months of age), and each was placed at the bottom of a 2-ml polypropylene cryotube (12.5 × 48 mm; MS-4503, Sumitomo Bakelite, Tokyo, Japan; Fig. 1A). Some tubes were plunged into liquid nitrogen and kept there. Other tubes were transferred into a deep freezer (−80°C) with or without a freezing container (Bicell; Nihon Freezer, Osaka, Japan; Fig. 1 B and C). Some tubes, frozen in a freezing container, were transferred to and kept in liquid nitrogen. After storage for 1 week to 1 year, the cryotubes with frozen epididymides and testes were put in an 1-liter water bath (25°C) for ≈2 min. It was important to collect spermatozoa and spermatids immediately after defrosting.
Collection of Spermatozoa and Spermatogenic Cells from Defrosted Epididymis and Testis.
A defrosted cauda epididymis was placed under mineral oil in a Petri dish and punctured with a 26-gauge needle to release spermatozoa. Using a 200-μl micropipette, a sperm mass was transferred to the bottom of a 1.5-ml plastic centrifuge tube and gently covered with 200 μl of GL-PBS (12). After gentle pipetting, the sperm suspension was kept at 4°C. In some experiments, spermatozoa were suspended in K+-rich NIM medium. Testicular spermatozoa and spermatids were mechanically isolated from defrosted testes, as described for hamsters (23). Defrosted testes were placed in erythrocyte-lysing buffer (155 mM NH4Cl/10 mM KHCO3/2 mM EDTA, pH 7.2), and the tunica albuginea were removed. Seminiferous tubules were transferred into cold (4°C) GL-PBS and cut into small pieces using a pair of fine scissors. They were gently pipetted to allow spermatogenic cells to disperse into the medium. The cell suspension was filtered through a 38-μm nylon mesh and washed twice by centrifugation (200 × g for 4 min). After gentle washings, cells were resuspended in GL-PBS and were stored at 4°C for up to 120 min.
Cryopreservation and Thawing of Whole Mouse Bodies.
Mature males of BALB/c-nude and C3H/He strains (8 weeks of age) were killed by overdose of pentobarbital on February 20, 1991 and March 8, 1991, respectively, and kept in a −20°C freezer without automatic defrost cycle. The body of a BALB/c-nude mouse was thawed on February 6, 2006, and that of a C3H/He mouse on February 13, 2006, by putting them in a large water bath (>3 liters of water at 25°C) for 5 min until the outer surface of the body was softened. The body was then removed from the water, and the testes were isolated through an abdominal incision. Germ cells were released from the testes, as already described.
International Transport of Frozen Epididymides and Testes.
Epididymides and testes were collected from B6 mice (3 months old), and each was placed in a cryotube. Several cryotubes, placed in a freezing container, were stored in a deep freezer (−80°C) for 1 day. Immediately before shipment, cryotubes were put in a styrene foam box filled with dry ice and sent by air from Oxfordshire, U.K., to Tsukuba, Japan. After 3 days of air transportation, the cryotubes were transferred to and kept in a deep freezer (−80°C) for ≈1 month before thawing.
Microinsemination.
Intracytoplasmic injection of spermatozoa and spermatids was performed by using a micropipette attached to a Piezo-electric actuator (Prime Tech, Ibaraki, Japan), as described (24, 25). The cover of a plastic dish (50 × 3 mm; Falcon no. 1006; Becton Dickinson, Franklin Lakes, NJ) was used as a microinjection chamber. Several small drops (≈2 μl) of Hepes-CZB with or without 10% polyvinylpirrolidone (PVP) were placed on the bottom and covered with mineral oil. Spermatozoa and spermatogenic cells were placed in one of the PVP droplets. A single spermatozoon was sucked, tail first, into an injection pipette, and the head was separated from the tail by applying a few Piezo pulses to the head–tail junction. The isolated sperm head was injected into an oocyte in Hepes-buffered CZB (24). Before injection of the nuclei of round spermatids, oocytes were activated by treatment with Ca2+-free CZB medium containing 2.5 mM SrCl2 for 20 min at 37°C. The oocytes, at 45–90 min after onset of activation treatment (reaching telophase II), were each injected with a round spermatid. Operated oocytes were kept in Hepes-CZB at room temperature (25°C) for ≈10 min before culture in CZB at 37°C under 5% CO2 in air.
Embryo Culture and Transfer.
Embryos that reached the two-cell stage by 24 h of culture in CZB were transferred into the oviducts of pseudopregnant ICR females (8–12 weeks old) on the day after mating (day 0.5). On day 19.5, recipient females were killed, and their uteri were examined for live term fetuses. In some experiments, live fetuses were nursed by lactating ICR females to see whether they could survive until weaning.
Care and Use of Animals.
All procedures described here were reviewed and approved by the Animal Experimental Committee at the RIKEN Institute and were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Statistical Analysis.
The efficiencies of embryo development were analyzed by using arcsine transformation, followed by two-way ANOVA analysis. A post hoc procedure using Fisher’s protected least significant difference test was adopted for multiple comparisons where appropriate. Results were evaluated by using Fisher’s exact probability test when each experimental group consisted of a single experiment (Tables 3 and 4). A P value <0.05 was considered statistically significant.
Acknowledgments
We thank Akie Shinmen and Mika Ohkawa for technical help. This research was supported by grants from Ministry of Education, Culture, Sports, Science, and Technology; the Ministry of Health, Labour, and Welfare; Core Research for Evolutional Science and Technology; and the Human Science Foundation (all from Japan).
Glossary
Abbreviations:
- NIM
nucleus isolation medium
- CZB medium
Chatot, Ziomet, and Bavister medium.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Knight J., Abbott A. Nature. 2002;417:785–786. doi: 10.1038/417785a. [DOI] [PubMed] [Google Scholar]
- 2.Nakagata N. Mamm. Genome. 2000;11:572–576. doi: 10.1007/s003350010109. [DOI] [PubMed] [Google Scholar]
- 3.Nakagata N., Okamoto M., Ueda O., Suzuki H. Biol. Reprod. 1997;57:1050–1055. doi: 10.1095/biolreprod57.5.1050. [DOI] [PubMed] [Google Scholar]
- 4.Szczygiel M. A., Kusakabe H., Yanagimachi R., Whittingham D. G. Biol. Reprod. 2002;67:1278–1284. doi: 10.1095/biolreprod67.4.1278. [DOI] [PubMed] [Google Scholar]
- 5.Wakayama T., Yanagimachi R. Nat. Biotechnol. 1998;16:639–641. doi: 10.1038/nbt0798-639. [DOI] [PubMed] [Google Scholar]
- 6.Wakayama T., Whittingham D. G., Yanagimachi R. J. Reprod. Fertil. 1998;112:11–17. doi: 10.1530/jrf.0.1120011. [DOI] [PubMed] [Google Scholar]
- 7.Yanagimachi R., Wakayama T., Kishikawa H., Fimia G. M., Monaco L., Sassone Corsi P. Proc. Natl. Acad. Sci. USA. 2004;101:1691–1695. doi: 10.1073/pnas.0307832100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogura A., Matsuda J., Yanagimachi R. Proc. Natl. Acad. Sci. USA. 1994;91:7460–7462. doi: 10.1073/pnas.91.16.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogura A., Suzuki O., Tanemura K., Mochida K., Kobayashi Y., Matsuda J. Proc. Natl. Acad. Sci. USA. 1998;95:5611–5615. doi: 10.1073/pnas.95.10.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanagimachi R. Reprod. Biomed. Online. 2005;10:247–288. doi: 10.1016/s1472-6483(10)60947-9. [DOI] [PubMed] [Google Scholar]
- 11.Ogura A., Ogonuki N., Miki H., Inoue K. Int. Rev. Cytol. 2005;246:189–229. doi: 10.1016/S0074-7696(05)46005-2. [DOI] [PubMed] [Google Scholar]
- 12.Ogura A., Matsuda J., Asano T., Suzuki O., Yanagimachi R. J. Assist. Reprod. Genet. 1996;13:431–434. doi: 10.1007/BF02066177. [DOI] [PubMed] [Google Scholar]
- 13.Kuretake S., Kimura Y., Hoshi K., Yanagimachi R. Biol. Reprod. 1996;55:789–795. doi: 10.1095/biolreprod55.4.789. [DOI] [PubMed] [Google Scholar]
- 14.Nagy Z. P., Liu J., Joris H., Verheyen G., Tournaye H., Camus M., Derde M.-P., Devroey P., Van Steirteghem A. C. Hum. Reprod. 1995;10:1123–1129. doi: 10.1093/oxfordjournals.humrep.a136104. [DOI] [PubMed] [Google Scholar]
- 15.Svalander P., Forsberg A. S., Jakobsson A. H., Wikland M. Fertil. Steril. 1995;63:828–837. doi: 10.1016/s0015-0282(16)57489-5. [DOI] [PubMed] [Google Scholar]
- 16.Nishizono H., Shioda M., Takeo T., Irie T., Nakagata N. Biol. Reprod. 2004;71:973–978. doi: 10.1095/biolreprod.103.024422. [DOI] [PubMed] [Google Scholar]
- 17.Bedford J. M., Calvin H. I. J. Exp. Zool. 1974;188:137–155. doi: 10.1002/jez.1401880203. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko T., Whittingham D. G., Overstreet J. W., Yanagimachi R. Biol. Reprod. 2003;69:1859–1862. doi: 10.1095/biolreprod.103.019729. [DOI] [PubMed] [Google Scholar]
- 19.Tateno H., Kimura Y., Yanagimachi R. Bio. Reprod. 2000;63:341–346. doi: 10.1095/biolreprod63.1.341. [DOI] [PubMed] [Google Scholar]
- 20.Sotolongo B., Huang T. T., Isenberger E., Ward W. S. J. Androl. 2005;26:272–280. doi: 10.1002/j.1939-4640.2005.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawase Y., Araya H., Kamada N., Jishage K., Suzuki H. Biol. Reprod. 2005;72:568–573. doi: 10.1095/biolreprod.104.035279. [DOI] [PubMed] [Google Scholar]
- 22.Chatot C. L., Lewis J. L., Torres I., Ziomek A. Biol. Reprod. 1990;42:432–440. doi: 10.1095/biolreprod42.3.432. [DOI] [PubMed] [Google Scholar]
- 23.Ogura A., Yanagimachi R. Biol. Reprod. 1993;48:219–225. doi: 10.1095/biolreprod48.2.219. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y., Yanagimachi R. Biol. Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 25.Kimura Y., Yanagimachi R. Development (Cambridge, U.K.) 1995;121:2397–2405. doi: 10.1242/dev.121.8.2397. [DOI] [PubMed] [Google Scholar]