Abstract
We developed a system that allows the selection of the reciprocal products resulting from spontaneous mitotic cross-overs in the yeast Saccharomyces cerevisiae. A number of other types of genetic events, including chromosome loss, can be monitored with this system. For a 120-kb chromosome interval on chromosome V (CEN5-CAN1), the rate of mitotic cross-overs was 4 × 10−5 per division, a rate ≈25,000-fold lower than the meiotic rate of cross-overs. We found no suppression of mitotic cross-overs near the centromere of chromosome V, unlike the suppression observed for meiotic exchanges. The rate of reciprocal cross-overs was substantially (38-fold) elevated by treatment of cells with hydroxyurea, a drug that reduces nucleotide pools and slows DNA replication.
Keywords: DNA repair, recombination, yeast
Because of the way in which genetic maps are usually constructed, most geneticists are more concerned with meiotic recombination than mitotic recombination. Mitotic recombination, however, has a number of important roles in eukaryotes, including (i) repairing DNA lesions such as dsDNA breaks (DSBs), (ii) restarting stalled replication forks, (iii) providing an alternative pathway of telomere replication in cells lacking telomerase, and (iv) contributing to the evolution of the genome by generating novel chromosome rearrangements (1, 2). In addition, human cells that are heterozygous for a mutation in a tumor suppressor gene are at risk for developing into a tumor cell as a consequence of loss of the protective WT gene [loss of heterozygosity (LOH); ref. 3]. Although LOH has a variety of causes, about half of the LOH events in one large study of retinoblastomas reflected mitotic recombination (4).
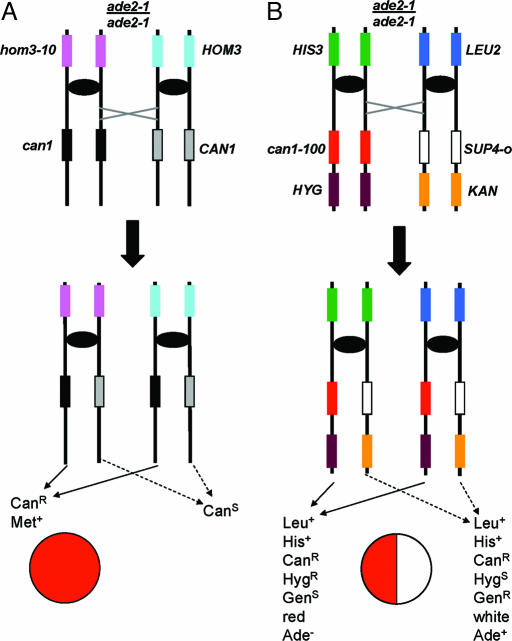
Because mitotic recombination events are rare, a number of selective methods have been developed for their detection. One common method used in Saccharomyces cerevisiae (5) is shown in Fig. 1A. A diploid is constructed that is heterozygous for two recessive mutations on chromosome V, can1 and hom3. The resulting diploid is sensitive to the drug canavanine (CanS) and is a methionine prototroph (Met+). A mitotic cross-over followed by disjunction of the recombined chromatids into different daughter cells results in one cell that is homozygous for can1 and, therefore, canavanine-resistant (CanR), and a second that is homozygous for the WT allele and CanS. The hom3 marker is used to screen for CanR derivatives that reflect loss of the homologue containing the WT CAN1, because such derivatives should be CanR Met−. The rate of mitotic cross-overs was determined to be ≈1.2 × 10−5 per division (5, 6).
Fig. 1.
Two systems for the detection of mitotic recombination and chromosome loss in diploid yeast cells. (A) One commonly used system for detection of mitotic recombination events uses a diploid that is heterozygous for mutations in the can1 and hom3 loci. The starting diploid strain is CanS and Met+. The depicted strain is also homozygous for the ade2-1 mutation that results in cells that are Ade− and form red colonies. Cells are transferred to plates containing Can, and any CanR derivatives are tested for their ability to grow in the absence of methionine. Met− cells represent chromosome loss events, and Met+ cells are assumed to represent mitotic cross-overs. Note that the CAN1/CAN1 product cannot be selected by this system. (B) Selection of both products of an RCO in the diploid MAB6. The starting diploid is phenotypically CanS GenR HygR His+ Leu+ Ade+/− and forms white colonies. An RCO between the centromere and the CAN1 locus will result in a CanR colony with one red and one white sector, resulting from the growth of two CanR cells, one with the genotype can-100/can1-100 and one with the genotype SUP4-o/SUP4-o. HygS, hygromycin-sensitive; GenS, geneticin-sensitive.
The system shown in Fig. 1A detects only one of the two expected products of the reciprocal cross-over (RCO). The failure to detect both products is a problem because two types of nonreciprocal recombination can also generate a strain homozygous for the can1 mutant allele (Fig. 5, which is published as supporting information on the PNAS web site). In S. cerevisiae, it is likely that most recombination events are initiated by a DSB that can then be repaired in several different ways (1, 7). In two-ended repair, the broken ends form heteroduplexes with the unbroken homologue. If there are mismatches within these heteroduplex regions, repair of these mismatches can result in a gene conversion event. Because the length of the heteroduplexes is usually less than a few kb, the amount of DNA transferred nonreciprocally between the homologues is usually 100 bp to several kb. We will refer to this type of conversion as “local” gene conversion. In a second type of DSB repair, one broken end invades a homologous region, setting up a replication fork that duplicates the entire chromosome from the point of invasion to the telomere; this event has been termed “break-induced replication” (BIR; ref. 7). Both local conversion events and BIR events can produce CanR cells. The distinction between these events and the RCO is that the CanS cell resulting from local conversion or BIR events is can1/CAN1, whereas the CanS cell resulting from the RCO is CAN1/CAN1. Because these two types of CanS cells are nonselectable, the system shown in Fig. 1A cannot distinguish RCOs from various classes of nonreciprocal exchange.
Several nonselective screens for reciprocal events have been done (8–12). One screen is based on constructing diploids that are heterozygous for auxotrophic mutations that are then grown nonselectively on rich growth medium, and then replica-plated to omission medium. In three studies examining different chromosome intervals, the rate of RCOs was <10−4 per division. The fraction of loss of heterozygosity events that were RCOs varied widely in different studies from 0% (10) to 79% (9). Another screen (12) used a diploid with complementing ade2 heteroalleles (ade2-40 and ade2-199). Null ade2 alleles result in red colonies. Because of the complementing alleles, the heteroallelic diploid formed white colonies. An RCO produced a colony with a pink sector (homozygous for ade2-119) next to a red sector (homozygous for ade2-40). Only one such colony was detected among ≈8,000 analyzed.
In this article, we describe a genetic system that allows selection of both products of an RCO. Although our analysis was limited to one genetic interval on chromosome V, the same approach can be extended to any region of the yeast genome.
Results
Description of the System.
The system for the selection of RCOs consists of a diploid strain (MAB6) that has the can1-100 allele (an ochre-suppressible nonsense mutation) on one copy of chromosome V, and the SUP4-o (an ochre suppressor) gene replacing the CAN1 locus on the other copy of chromosome V. The CAN1 gene encodes an arginine permease, and Can is a toxic arginine analogue. Thus, cells with a WT CAN1 gene are sensitive to Can. The MAB6 diploid is sensitive to Can because the can1-100 mutation is suppressed by SUP4-o. In addition, we inserted the dominant drug resistance markers KAN and HYG at allelic positions centromere-distal to CAN1, and the LEU2 and HIS3 markers at allelic positions on the opposite arm of chromosome V. Yeast strains with the KAN and HYG genes are resistant to geneticin (GenR) and hygromycin (HygR), respectively. The MAB6 diploid is also homozygous for the ade2-1 allele, an ochre-suppressible mutation. In the absence of the suppressor, strains carrying this mutation are Ade− and form red colonies. In the presence of the suppressor, the colonies are white and grow slowly on medium lacking adenine (Ade+/− phenotype). In summary, the diploid MAB6 is CanS GenR HygR His+ Leu+ Ade+/− (Fig. 1B Upper).
An RCO between the centromere and the CAN1 locus can produce two CanR cells, one cell homozygous for the can1-100 allele and lacking SUP4-o, and one homozygous for SUP4-o and lacking can1-100 (Fig. 1B Lower). The cell lacking the suppressor will be Ade− and give rise to a red colony or red sector, whereas the cell homozygous for the suppressor will be Ade+ and give rise to a white colony or white sector. An RCO that occurs as the cell is plated onto the Can-containing plate will produce a CanR red/white-sectored colony with sectors of the phenotypes shown in Fig. 1B. These phenotypes are detected by replica-plating colonies grown on medium with Can to omission media or media containing hygromycin or geneticin. This system detects half of the RCOs, because the chromosome disjunction events in which both recombinant products are segregated into one cell and the nonrecombinant products into the second cell are CanS. Chua and Jinks-Robertson (13) showed that these two types of segregation were equally frequent for conversion-associated mitotic recombination in yeast.
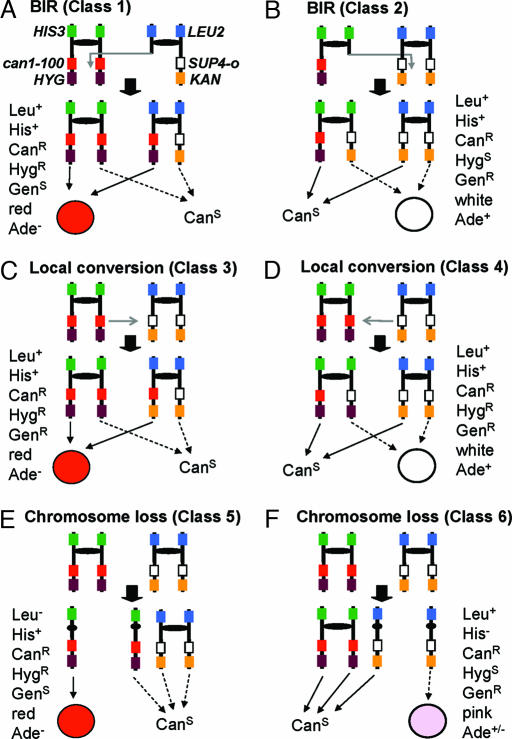
In addition to the red/white-sectored CanR colonies resulting from RCOs, six different phenotypic classes of unsectored CanR colonies were observed (Fig. 2). Class 1 colonies are likely to reflect two types of genetic events: (i) a BIR event in which the initiating DSB was on the chromosome with the SUP4-o gene, and (ii) one of the two types of cells produced by an RCO before the plating of cells on Can-containing medium. Class 2 white colonies represent the comparable classes: a BIR event initiated with a DSB on the other homologue, and the other product of the reciprocal exchange. Class 3 colonies represent local gene conversion events (unassociated with a cross-over) in which the can1-100 gene replaces the SUP4-o gene, whereas class 4 colonies represent conversion events in which the SUP4-o gene replaces the can1-100 gene. Alternatively, class 3 and class 4 colonies could be a consequence of additional mutations in the SUP4-o or can1-100 genes, respectively. Class 5 colonies reflect loss of the SUP4-o-containing homologue, and class 6 events reflect loss of the can1-100-containing homologue.
Fig. 2.
Phenotypic classes of unsectored CanR colonies (derived from MAB6) resulting from nonreciprocal mitotic recombination or chromosome loss events. (A) Class 1: A BIR event initiated in the SUP4-o-containing chromosome will give rise to one CanR cell and one CanS cell. It is also possible that class 1 events could reflect an RCO that occurred in the culture, before the plating of the cells on medium containing Can. (B) Class 2: These BIR events are comparable to class 1, except that the event initiates by breakage of the can1-100-containing homologue. (C) Class 3: A local gene conversion (unassociated with a cross-over) in which SUP4-o is converted to the can1-100 allele will produce a CanR HygR GenR Leu+ His+ Ade− red colony. The same phenotype can be produced by a new mutation within SUP4-o. These two possibilities can be distinguished by PCR (as described in Supporting Text). (D) Class 4: This class is similar to class 3 except can1-100 is converted to SUP4-o. (E) Class 5: This class results from loss of the chromosome containing SUP4-o by nondisjunction. (F) Class 6: This class results from loss of the chromosome containing can1-100.
Rates of Mitotic Recombination and Chromosome Loss.
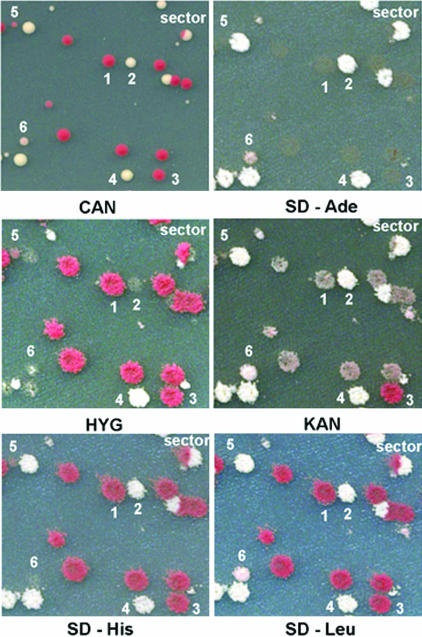
To measure the rates of the various mitotic events, we plated cells from multiple (≈40) independent cultures on Can-containing medium (to measure the frequencies of the various Can-resistant phenotypes) and nonselective medium (to measure the number of cells in the culture). Colonies formed on the Can-containing plate were then replica-plated to five different types of diagnostic media: those lacking histidine, leucine, or adenine, and those containing geneticin or hygromycin. Photographs of colonies formed on a Can-containing plate and replica-plated to the various diagnostic plates are shown in Fig. 3. A red/white-sectored colony and all six classes of unsectored colonies are shown in Fig. 3.
Fig. 3.
Photographs of the different classes of CanR colonies. Cells of the MAB6 strain were allowed to form colonies on Can-containing medium and were then replica-plated to five different types of diagnostic media; those containing hygromycin or geneticin and those lacking adenine, histidine, or leucine. The colony marked “sector” reflects an RCO, and the numbers represent class 1–6 colonies. The sizes of colonies in the photograph are about the same as the sizes on the plates.
The data for all classes of events for all experiments are shown in Fig. 4A and Table 1, which is published as supporting information on the PNAS web site. Rates were calculated in two different ways. Because a sectored colony requires the event to occur at the time the cell is plated on Can-containing medium, the frequency of such colonies is the same as the rate. The average rate of sectored colonies (40 cultures in two experiments) was 2 × 10−5 per division. As discussed, because we detect only half of the RCOs, we conclude that the rate of reciprocal exchange between CEN5 and CAN1 is 4 × 10−5 per division.
Fig. 4.
Comparisons of rates of mitotic recombination and chromosome loss and rates of mitotic and meiotic recombination. (A) Rates of mitotic recombination and chromosome loss in MAB6 (MATa/MATα), MAB35 (MATa/matαΔ), and MAB38 (mataΔ/MATα). In addition, we examined MAB6 pregrown in medium containing 100 mM HU. Error bars indicate 95% confidence limits. (B) Comparisons of the physical and genetic distances for two intervals on chromosome V, CEN5-URA3 and URA3-CAN1. For each interval, the data are shown as a percentage of the distance between CEN5 and CAN1. The physical distances for CEN5-URA3 and URA3-CAN1 are 36 and 84 kb, respectively. The meiotic recombination distances (95% confidence limits shown in parentheses) for these same two intervals are 8 cM (7.5–8.4 cM) and 42 cM (41–44 cM) for >1,500 tetrads in the Saccharomyces Genome Database (SGD), and 7 cM (5–9 cM) and 41 cM (37–45 cM) based on 360 tetrads in our study. The meiotic data shown use the SGD data. The mitotic distances were derived from our analysis of sectors in MAB13 as described in Results. The 95% confidence limits are indicated for the meiotic and mitotic intervals.
The rates for all classes of unsectored colonies were determined by fluctuation analysis (14). The rates of class 1 and 2 events (2 × 10−5 per division and 2.2 × 10−5 per division, respectively) were about the same as observed for the rate of RCOs. The simplest interpretation of this result is that most of the class 1 and 2 events represent RCOs that occurred before plating, rather than BIR events. Because the 95% confidence limits on the rates of RCOs and class 1 and 2 events are rather large (Table 1), however, our data do not provide unambiguous evidence for or against the existence of spontaneous BIR events.
The rates of local gene conversions (class 3 and 4) were ≈3.5 × 10−6 per division, 10-fold less frequent than RCOs. As described above, class 3 and 4 colonies could also be generated by additional mutations in the SUP4-o and can1-100 genes. A class 3 event resulting from conversion would be homozygous for the can1-100 gene (Fig. 2C), whereas a class 3 event resulting from mutation in SUP4-o would retain SUP4-o sequences. These two types of class 3 events can be readily distinguished by PCR analysis (see Supporting Text, which is published as supporting information on the PNAS web site). Of 128 class 3 events analyzed, 90 were local conversion events and 38 were additional mutations within SUP4-o. The rates of class 3 events shown in Table 1 were adjusted to exclude those derivatives with additional mutations in SUP4-o.
The rates of chromosome loss (class 5 and 6) were ≈0.8 × 10−5 per cell division. To confirm that these classes represent chromosome loss rather than a BIR event that covers all three heterozygous markers, we sporulated and dissected two independent class 5 and six independent class 6 strains. In all eight strains, the majority of the tetrads had two viable spores or less, as expected for chromosome V monosomic strains. We also analyzed two class 5 and two class 6 strains by using microarrays containing all of the yeast genes (described in Supporting Text). All four strains had one copy of chromosome V and two copies of all other chromosomes (Fig. 6, which is published as supporting information on the PNAS web site). Chromosome losses can reflect either nondisjunction events as shown in Fig. 2 or failure to replicate one of the homologues. Because a chromosome V trisomic strain (the other expected product of a nondisjunction event) is expected to produce a CanS cell, we cannot distinguish these possibilities with our system.
In addition to the classes described above, there are several other types of genetic events that could produce CanR colonies. Cross-overs associated with a local conversion event could contribute to classes 1 and 2. Because less than half of mitotic gene conversion events are associated with cross-overs (13) and local gene conversion events unassociated with cross-overs are 10-fold less frequent than the RCO class, this type of event is unlikely to contribute significantly to these classes. Two further points should be made. First, our data do not allow an accurate comparison of the relative rates of local gene conversion and cross-overs, because the rate of conversion is determined at a single site, whereas the cross-over rate is assayed in a 120-kb interval. In addition, the conversion event involves a sequence heterology that could reduce rates. Second, because local conversions associated with cross-overs produce the same colony phenotypes as BIR events and RCO events that occur before the plating of cells on Can-containing medium, we cannot determine the fraction of conversion events that are associated with cross-overs.
Another type of mitotic event that would be expected to be infrequent is a two-strand double RCO event, with one cross-over between CEN5 and can1-100/SUP4-o and a second cross-over between can1-100/SUP4-o and the drug resistance markers. This type of exchange would produce a sectored colony with the sector phenotypes: CanR GenR HygR His+ Leu+ Ade− Red and CanR GenR HygR His+ Leu+ Ade+ White. Of 135 red/white-sectored colonies examined, only two had these phenotypes.
One final class of CanR colony had the same phenotypes for all markers on chromosome V as the parental MAB6 strain except that the colonies were pink instead of white. These colonies, which appeared at a rate of ≈0.7 × 10−5 per cell division, grew slowly, similar to the growth rates of the monosomic class 5 and 6 strains. We sporulated and dissected four of these strains, and three of these strains segregated two live to two dead spores. This pattern of spore viability is expected for a recessive lethal or a monosomic strain. Microarray analysis on the four strains showed that these strains were monosomic for chromosome XVI. This result suggests that there is a gene (or genes) on XVI that positively regulates the expression or function of the Can1p and, because of the low expression of Can1p in MAB6, loss of one of the copies of these genes in the diploid results in a CanR colony. Because this class is not relevant to the recombination and chromosome loss events involving chromosome V, it will not be discussed further.
Mitotic Crossing-Over in an Interval Near the Centromere of Chromosome V.
In many organisms, including S. cerevisiae, meiotic recombination is reduced near the centromere (15). According to the genetic and physical maps in the Saccharomyces Genome Database (www.yeastgenome.org), the 36-kb CEN5-URA3 interval is 8 cM, whereas the 84-kb URA3-CAN1 interval is 42 cM. Thus, there are 0.22 cM/kb in the first interval and 0.5 cM/kb in the second, indicating substantial suppression of meiotic recombination near CEN5. We measured meiotic recombination in these same two intervals in MAB54, an isogenic derivative of MAB6 that was heterozygous for mutations at the URA3 and CAN1 loci, in addition to being heterozygous at the centromere-linked TRP1 locus. From analysis of 360 tetrads, we measured the CEN5-URA3 distance to be 7 cM and the URA3-CAN1 distance to be 41 cM.
To investigate whether this suppression near the centromere was also seen in mitotic recombination, we constructed a strain (MAB13) that was isogenic with MAB6 except that it was also heterozygous at the URA3 locus. In MAB13, the WT URA3 allele was on the homologue with the can1-100 and HYG markers, and the mutant allele was on the homologue with the SUP4-o and KAN markers. Strains with a WT URA3 allele are sensitive to 5-fluoroorotic acid, whereas cells harboring only the mutant allele are resistant (16). An RCO in MAB13 between CEN5 and the URA3 locus would be expected to produce a red Ura+/white Ura−-sectored CanR colony (Fig. 7A, which is published as supporting information on the PNAS web site). An RCO between URA3 and CAN1 would yield a red/white CanR colony in which both sectors are Ura+ (Fig. 7B).
The average rate (60 independent cultures) of CanR red/white sectored colonies in MAB13 was 1.3 × 10−5 per cell division, similar to the rate observed in MAB6. The rate of RCO between CEN5 and URA3 was 0.4 × 10−5, and the rate of RCO between URA3 and CAN1 was 0.9 × 10−5. Thus, the ratio of cross-overs in these two intervals is 0.44, similar to the ratio of the physical distances of the intervals (0.42). Thus, mitotic cross-overs, unlike meiotic cross-overs, are not significantly suppressed near CEN5 (Fig. 4B). Of 176 red/white-sectored colonies observed, 171 had the phenotypes expected for single cross-over events, and five had the phenotypes expected for double cross-over events (one between URA3 and can1-100/SUP4-o, and one between can1-100/SUP4-o and the drug resistance markers).
Effect of Mating-Type Heterozygosity on the Rate of Mitotic Recombination and Chromosome Loss.
Diploid yeast strains that are heterozygous at the mating-type locus are more resistant to ionizing radiation and, in some assays, have a higher rate of x-ray-induced and UV-induced mitotic recombination events than diploid cells that express only MATa or MATα information (7). We examined the effect of mating type on spontaneous mitotic recombination by constructing two diploids that were isogenic with MAB6, except that one of them was deleted for the MATα locus (MAB35) and one was deleted for the MATa locus (MAB38). As shown in Fig. 4A and Table 1, MAB35 and MAB38 had about the same rates of RCOs and class 1–4 events as we observed in MAB6, demonstrating that heterozygosity at the mating locus does not affect spontaneous mitotic recombination. Chromosome loss, however, was elevated 3- to 5-fold in MAB35 and MAB38.
Stimulation of RCOs and Other Mitotic Recombination Events by Hydroxyurea (HU).
In S. cerevisiae, deoxyribonucleotide synthesis depends on ribonucleotide reductase (17). HU inhibits ribonucleotide reductase (18), leading to a reduction in dNTPs (19) and slow progression of DNA replication forks (20, 21). Yeast cells exposed to HU have elevated levels of gene conversion, deletions, and chromosome loss (22, 23). Using a nonselective assay for cross-overs, Mayer et al. (22) also reported that HU induced reciprocal exchange, although the level of induction was not statistically significant.
To examine the effect of HU in more detail, we grew MAB6 cells in medium containing 100 mM HU and then plated the treated cells on Can-containing medium. As shown in Fig. 4A and Table 1, HU treatment stimulated reciprocal mitotic cross-overs ≈40-fold and classes 1–6 ≈10- to 20-fold. These results argue that HU treatment leads to recombinogenic DNA lesions that are often repaired to generate an RCO. The elevated chromosome loss in HU-treated cells is likely to reflect either chromosome loss associated with unrepaired DNA lesions or incomplete chromosome replication.
Although MAB6 and related strains allow us to determine unambiguously the rate of RCOs, we cannot directly determine the rate of spontaneous BIR events, because class 1 and 2 events represent either BIR events or RCOs that occurred before the plating of cells on Can-containing medium. Because of the large increase in mitotic recombination events in HU-treated cells, we were able to use nonselective methods in HU-treated cells to detect BIR events. Colonies grown on HU-containing plates for 3 days were suspended in water, diluted, and plated on nonselective medium (SD-arginine + 10 μg/ml adenine). We screened the resulting colonies for those that had red/white sectors, and then checked the phenotypes of the sectors to determine whether they represented RCOs or BIR events. Class 1, but not class 2, BIR events would produce a red/white-sectored colony. In examining 66,464 colonies, we found 67 sectors, 26 with the phenotypes expected for an RCO (3.9 × 10−4 per division) and 41 with the phenotypes expected for a BIR event (6.1 × 10−4 per division).
For the RCO, both red and white sectors have phenotypes different from the parental MAB6 strain. For the BIR event, however, one of the expected sectors has exactly the same phenotype as the parental strain. Consequently, a sectored colony indicative of a class 1 BIR event could be a false sector resulting from a cell of the CanR GenS HygR His+ Leu+ Ade− red phenotype (reflecting a previous RCO) being adjacent to a WT MAB6 cell at the time of plating. Experiments in which we mixed cells of the Ade− red phenotype with cells of the Ade+ white phenotype indicate that most of the sectors indicative of a class 1 event are not false sectors (data not shown). In summary, we conclude that HU treatment stimulates both RCOs and BIR events.
Analysis of Meiotic Events: An Alternative to Tetrad Dissection.
Much of what we know about recombination is based on tetrad analysis in fungi. The diploid MAB6 allows a method of diagnosing the segregation of markers into the four meiotic products without tetrad dissection, because all four spores are CanR. We sporulated MAB6 and then plated the resulting tetrads, at low cell density, on solid medium containing Can. Some of the resulting colonies had four sectors (Fig. 8A, which is published as supporting information on the PNAS web site). We purified cells from each sector on rich growth medium, and then checked the segregation of heterozygous markers (Fig. 8B), including mating type (Fig. 8C). The 2:2 segregation of all markers demonstrates that the four sectors represent the four haploid meiotic products of a single tetrad. Not all of the tetrad-derived CanR colonies had four sectors and, in some of the colonies, the cells purified from the sectors were diploid, rather than haploid, indicating mating between haploid cells of opposite mating type occurred during growth of the CanR colony. Thus, this method needs to be improved to be a practical alternative to tetrad dissection.
Discussion
The system that we have described allows an accurate measurement of the rate of spontaneous mitotic RCOs. For the 120-kb CEN5-CAN1 interval, this rate is 4 × 10−5 per cell division. Assuming this rate is the average for the genome, we calculate that the chance of an RCO within the 14-Mb yeast genome is ≈0.5% per cell division. Thus, one would expect that genetic variants that arise in diploid cells to become homozygous fairly quickly. The meiotic genetic distance between CEN5 and CAN1 is 51 cM, indicating approximately one cross-over per meiotic cell. For the same interval, therefore, meiotic cross-overs are 25,000-fold more frequent per division than mitotic cross-overs.
Our data do not provide unambiguous evidence for spontaneous BIR events, but we cannot rule out the possibility that some of the class 1 and 2 events reflect BIR. McMurray and Gottschling (9) found that most mitotic recombination events observed in nonselected “young” diploid cells were reciprocal, and our data are consistent with this conclusion. McMurray and Gottschling (9) also found that the frequency of nonreciprocal recombination events increased in old cells (cells that had undergone >20 divisions). Because our experiments involve exponentially growing cultures, almost all of the cells in our experiments are young.
Meiotic recombination events are distributed nonrandomly along the chromosomes (24). Recombination rates are controlled both regionally (suppression of recombination at the telomeres and centromeres) and locally (for example, by local G-C content and transcription factor binding). Although no detailed mitotic recombination maps have been constructed in yeast or any other eukaryote, a number of factors have been associated with elevated levels of mitotic recombination, including high levels of transcription, stalled DNA replication forks, and inverted repeated DNA sequences capable of forming secondary structures (cruciforms and “hairpins”) (25–27).
Our method can be used for any chromosome and any interval by constructing strains in which the can1-100, SUP4-o, and the drug resistance markers are inserted into the appropriate positions. By using two parental haploids that have sufficient sequence divergence to provide polymorphisms at ≈1-kb intervals, a fine-structure mitotic cross-over map could be constructed. Such maps are likely to be informative about the mechanisms of mitotic cross-over. For example, one could determine whether “hotspots” for mitotic cross-overs correlated with highly expressed genes, inverted repeats, or regions with stalled replication forks.
We found that heterozygosity at the mating-type locus had no effect on spontaneous mitotic recombination events. Although a number of studies show that MATa/MATα diploids have better survival in the presence of DNA-damaging agents than MATa/MATa or MATα/MATα diploids (7), the data on the effects of heterozygosity at the mating-type locus on recombination rates are much less clear. Some studies find that heterozygous diploids have several-fold more recombination than hemizygous diploids (6, 28), whereas similar studies fail to find a significant effect (5). It is unclear at present why different experiments produce such different results.
The observed increase in chromosome loss in MATa/matαΔ and mataΔ/MATα diploids is consistent with the observation that the stability of centromere-containing plasmids is higher in diploids that are heterozygous at the mating-type locus than in homozygous diploids (29). Because the heterozygous diploids are less sensitive to microtubule-depolymerizing drugs than the homozygous diploids, Steinberg-Neifach and Eshel (29) argue that heterozygous diploids have more stable microtubules, leading to lower rates of chromosome loss. Our results are consistent with this possibility.
HU-treated cells had elevated rates of mitotic cross-overs, local gene conversion and BIR events, and chromosome loss (Fig. 4A). Because HU treatment leads to slow progression of DNA replication forks (20, 21) and HU-stimulated increases in recombination are observed in cycling but not arrested yeast cells (23), it is likely that HU treatment leads to stalled replication forks that are susceptible to DSBs. Some of the resulting DSBs are repaired by using both broken ends to generate a local gene conversion event or an RCO, whereas others are repaired by using only a single end, resulting in a BIR event. Although it is clear that DSBs stimulate mitotic recombination (7), it should be emphasized that the DNA lesions responsible for spontaneous mitotic events and HU-induced events have not been demonstrated to be DSBs. Fabre et al. (30) have argued that mitotic recombination events are frequently initiated by a ssDNA gap rather than a DSB.
In summary, the system that we have developed should be a useful tool for investigating the mechanisms involved in mitotic recombination and the repair of DSBs.
Materials and Methods
Genetic Analysis and Media.
The rich growth medium (yeast extract/peptone/dextrose, YPD), sporulation medium, and various types of omission media were standard (31). Strains were grown at 30°C unless otherwise noted. Mating, transformation, and tetrad dissection procedures were also standard.
Strain Construction.
Strains in this study were isogenic with W303a (a leu2-3,112 his3-11,15 ura3-1 ade2-1 trp1-1 can1-100 rad5-535; ref. 32) except for changes introduced by transformation or crosses with isogenic strains. All strains were RAD5. Details of strain constructions and genotypes of all strains are in Supporting Text and Tables 2–4, which are published as supporting information on the PNAS web site.
Detection of Mitotic Recombination Events in MAB6 and Related Strains.
Diploid cells were streaked for single colonies on rich growth medium (YPD) and incubated at 30°C. After 2 days, independent colonies were picked, resuspended in water, and plated on solid medium lacking arginine (SD-Arg) or SD-Arg with 120 μg/ml Can. Four days after plating, the CanR colonies were replica-plated to SD-Arg media containing Can and lacking histidine or leucine, SD-Arg media containing Can and, in addition, containing hygromycin (300 μg/ml) or geneticin (200 μg/ml), and to SD-adenine media. All omission media (except media lacking adenine completely) had 10 μg/ml adenine (which is 2-fold less than the standard omission media). Further details concerning the detection of the mitotic recombination events are given in Supporting Text.
Statistical Analysis.
Rate calculations for classes 1–6 were done by using the method of the median (14), and 95% confidence limits for these rates were calculated as described (33). Calculations of 95% confidence limits on proportions were done by using VassarStats (http://faculty.vassar.edu/lowry/VassarStats.html), and calculations of 95% confidence limits on the rates of red/white sectors were done with an Excel spreadsheet.
Supplementary Material
Acknowledgments
We thank M. Dominska and P. Greenwell for help with tetrad analysis and/or strain construction; S. Jinks-Robertson, H. Klein, W.-D. Heyer, D. Gottschling, A. Aguilera, and all members of the Petes laboratory for useful suggestions; and P. Detloff (University of Alabama, Birmingham, AL) for suggesting the use of MAB6 for meiotic studies. This research was supported by National Institutes of Health Grant GM24110 (to T.D.P.). M.A.B. was partly supported by the Medical Foundation Genetics Curriculum of the University of North Carolina.
Abbreviations
- DSB
dsDNA break
- Can
canavanine
- CanS
Can-sensitive
- CanR
Can-resistant
- RCO
reciprocal cross-over
- BIR
break-induced replication
- HygR
hygromycin-resistant
- HygS
hygromycin-sensitive
- GenR
Gen-resistant
- GenS
Gen-sensitive
- HU
hydroxyurea
- YPD
yeast extract/peptone/dextrose.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Symington L. S. Microbiol. Mol. Biol. Rev. 2002;66:630–670. doi: 10.1128/MMBR.66.4.630-670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helleday T. Mutat. Res. 2003;532:103–115. doi: 10.1016/j.mrfmmm.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Knudson A. G. Proc. Natl. Acad. Sci. USA. 1993;90:10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagstrom S., Dryja T. P. Proc. Natl. Acad. Sci. USA. 1999;96:2952–2957. doi: 10.1073/pnas.96.6.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartwell L. H., Smith D. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein H. L. Genetics. 2001;159:1501–1509. doi: 10.1093/genetics/159.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paques F., Haber J. E. Microbiol. Mol. Biol. Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston J. R. Genet. Res. Camb. 1971;18:179–184. doi: 10.1017/s0016672300012581. [DOI] [PubMed] [Google Scholar]
- 9.McMurray M. A., Gottschling D. E. Science. 2003;301:1908–1911. doi: 10.1126/science.1087706. [DOI] [PubMed] [Google Scholar]
- 10.Schmuckli-Maurer J., Rolfsmeier M., Nguyen H., Heyer W.-D. Nucleic Acids Res. 2003;31:1013–1023. doi: 10.1093/nar/gkg190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman H. Israel J. Med. Sci. 1973;9:669–678. [PubMed] [Google Scholar]
- 12.Zimmermann F. K. Mutat. Res. 1973;21:263–269. [PubMed] [Google Scholar]
- 13.Chua P., Jinks-Robertson S. Genetics. 1991;129:359–369. doi: 10.1093/genetics/129.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lea D. E., Coulson C. A. J. Genet. 1949;49:264–284. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 15.Lambie E. J., Roeder G. S. Cell. 1988;52:863–873. doi: 10.1016/0092-8674(88)90428-x. [DOI] [PubMed] [Google Scholar]
- 16.Boeke J. D., Lacroute F., Fink G. R. Mol. Gen. Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 17.Thelander L., Reichard P. Annu. Rev. Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- 18.Atkin C. L., Thelander L., Reichard P., Lang G. J. Biol. Chem. 1973;248:7464–7472. [PubMed] [Google Scholar]
- 19.Koc A., Wheeler L. J., Mathews C. K., Merrill G. F. J. Biol. Chem. 2004;279:223–230. doi: 10.1074/jbc.M303952200. [DOI] [PubMed] [Google Scholar]
- 20.Santocanale C., Diffley J. F. Nature. 1998;395:615–618. doi: 10.1038/27001. [DOI] [PubMed] [Google Scholar]
- 21.Vassilev L., Russev G. Biochim. Biophys. Acta. 1984;781:39–44. doi: 10.1016/0167-4781(84)90121-0. [DOI] [PubMed] [Google Scholar]
- 22.Mayer V. W., Goin C. J., Zimmermann F. K. Mutat. Res. 1986;160:19–26. doi: 10.1016/s0027-5107(96)90004-4. [DOI] [PubMed] [Google Scholar]
- 23.Galli A., Schiestl R. H. Mutat. Res. 1996;354:69–75. doi: 10.1016/0027-5107(96)00037-1. [DOI] [PubMed] [Google Scholar]
- 24.Petes T. D. Nat. Rev. Genet. 2001;2:360–369. doi: 10.1038/35072078. [DOI] [PubMed] [Google Scholar]
- 25.Aguilera A. EMBO J. 2002;21:195–201. doi: 10.1093/emboj/21.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobachev K. S., Gordenin D. A., Resnick M. A. Cell. 2002;108:183–193. doi: 10.1016/s0092-8674(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 27.Freudenreich C. H., Gentrow S. M., Zakian V. A. Science. 1998;279:853–856. doi: 10.1126/science.279.5352.853. [DOI] [PubMed] [Google Scholar]
- 28.Fasullo M., Bennett T., Dave P. Mutat. Res. 1999;433:33–44. doi: 10.1016/s0921-8777(98)00059-7. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg-Neifach O., Eshel D. Biol. Cell. 2002;94:147–156. doi: 10.1016/s0248-4900(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 30.Fabre F., Chan A., Heyer W.-D., Gangloff S. Proc. Natl. Acad. Sci. USA. 2002;99:16887–16892. doi: 10.1073/pnas.252652399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guthrie C., Fink G. R. Guide to Yeast Genetics and Molecular Biology. San Diego: Academic; 1991. [Google Scholar]
- 32.Thomas B. J., Rothstein R. Genetics. 1989;123:725–738. doi: 10.1093/genetics/123.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wierdl M., Greene C. N., Datta A., Jinks-Robertson S., Petes T. D. Genetics. 1996;143:713–721. doi: 10.1093/genetics/143.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.