SUMMARY
The widely conserved phage-shock-protein A (pspA) operon encodes an extracytoplasmic stress response system that is essential for virulence in Yersinia enterocolitica, and has been linked to other important phenotypes in Escherichia coli, Salmonella enterica and Shigella flexneri. Regulation of pspA operon expression is mediated through a promoter upstream of pspA that depends on sigma factor RpoN (σ54) and the enhancer binding protein PspF. PspA, PspB and PspC, encoded within the pspA operon, also regulate expression by participating in a putative signal transduction pathway that probably serves to modulate PspF activity. All of this suggests that appropriate expression of the pspA operon is critical. Previous genetic analysis of the Y. enterocolitica pspA operon suggested that an additional level of complexity might be mediated by PspF/RpoN-independent expression of some psp genes. Here, we used an rpoN null mutation and interposon analysis to confirm that PspF/RpoN-independent gene expression does originate within the psp locus. Molecular genetic approaches were used to systematically analyze the two large non-coding regions within the psp locus. Primer extension, control region deletion and site directed mutagenesis experiments led to the identification of RpoN-independent promoters both upstream and downstream of pspA. The precise location of the PspF/RpoN-dependent promoter upstream of pspA was also determined. The discovery of these RpoN-independent promoters reveals yet another level of transcriptional complexity for the Y. enterocolitica pspA operon that may function to allow low-level constitutive expression of psp genes and/or additional regulation under some conditions.
INTRODUCTION
The phage-shock-protein system (Psp) may help the bacterial cell to survive during dissipation of the proton motive force (PMF) and is conserved in many Gram-negative bacteria (for a recent review see Darwin, 2005). It was originally identified in Escherichia coli K-12 (Brissette et al., 1990), where it has been studied extensively. Expression of the E. coli psp regulon (pspABCDE and pspG) occurs in response to several stresses, including heat shock, osmotic shock, ethanol treatment, exposure to proton ionophores, and the overexpression of some cell envelope proteins, most notably secretins (for reviews see Darwin, 2005; Model et al., 1997).
The homologous psp regulon of Yersinia enterocolitica (pspABCDycjXF and pspG) is predicted to have similar inducing signals. Recently, specific induction of Y. enterocolitica pspA operon expression has been observed following overexpression of secretins, some cytoplasmic membrane proteins, and upon disruption of the F0F1-ATPase (Maxson & Darwin, 2004). The Psp system of Y. enterocolitica is also essential for virulence in a mouse model of infection (Darwin & Miller, 1999; Darwin & Miller, 2001). This is probably because Psp is essential during production of the Ysc type III secretion system (Darwin & Miller, 2001). Apparently, the Y. enterocolitica Psp system must respond to a stress caused by mislocalization of the YscC secretin component of the type III secretion system (Darwin & Miller, 2001). Consequently, null mutations of some psp genes cause severe growth defects when yscC is overexpressed (Darwin & Miller, 2001; Green & Darwin, 2004; Maxson & Darwin, 2006).
Regulation of E. coli psp gene expression has been well studied, and much of the current understanding is probably applicable to the homologous Y. enterocolitica psp regulon. The E. coli pspA and pspG control regions each contain an RpoN (σ54)-dependent promoter, as well as binding sites for integration host factor (IHF) and PspF, a member of the enhancer binding protein family (Jovanovic et al., 1996; Lloyd et al., 2004; Weiner et al., 1991; Weiner et al., 1995). Induction of the psp regulon is completely dependent on PspF. Regulation is also mediated by several of the other Psp proteins. The peripheral cytoplasmic membrane protein PspA acts as a negative regulator, by directly interacting with PspF and inhibiting its activity (Dworkin et al., 2000; Elderkin et al., 2002; Elderkin et al., 2005). The integral cytoplasmic membrane proteins PspB and PspC act as positive regulators, presumably by interacting with PspA (Adams et al., 2003) and possibly inhibiting its ability to interfere with PspF.
Previous analysis of Y. enterocolitica pspA expression, together with examination of the DNA sequence of its control region, indicated that it appeared to have a PspF/RpoN-dependent promoter (Darwin & Miller, 2001; Green & Darwin, 2004). Here, we confirm the presence of this promoter and determine its exact location. In addition, earlier genetic experiments raised the possibility of PspF- and, therefore, RpoN-independent expression of at least some of the genes within the pspA operon (Darwin & Miller, 2001). In this study, we strengthened this hypothesis by constructing an rpoN null mutation and using interposon analysis of Φ(pspABC’-lacZY) operon fusion expression. We then identified RpoN-independent transcription initiation sites both upstream and immediately downstream of pspA. These promoters, which are predicted to be (σ70-dependent, offer a plausible explanation for the predicted low-level PspF/RpoN-independent expression of some Y. enterocolitica psp genes.
METHODS
Bacterial strains, plasmids and routine growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1. For routine plasmid manipulations the host strain was E. coli DH5α. Plasmids with an R6K ori were maintained in E. coli CC118 λpir, and conjugated into Y. enterocolitica from either E. coli S17-1 λpir or E. coli SM10 λpir. In most cases E. coli strains were grown at 37°C, and Y. enterocolitica strains were grown at 26°C or 37°C as noted. All strains were grown in Luria-Bertani (LB) broth and on LB agar (Miller, 1972). Antibiotics were used as before (Maxson & Darwin, 2004).
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype/Features | Source or reference |
|---|---|---|
| Y. enterocolitica strains * | ||
| JB580v | ΔyenR (R−M+) pYV+ | Kinder et al., 1993 |
| AJD432 | ΔrpoN | This study |
| AJD950 | ΔaraGFB::[Φ(pspABC’-lacZY)] | This study |
| AJD952 | ΔaraGFB::[Φ(pspA pspB::Ω-Sp pspC’-lacZY)] | This study |
| AJD953 | ΔaraGFB::[Φ(pspA::Ω-Sp pspBC’-lacZY)] | This study |
| AJD957 | ΔaraGFB::[Φ(pspA-lacZY)] † | This study |
| AJD958 | ΔaraGFB::[Φ(pspB-lacZY)] † | This study |
| AJD960 | ΔaraGFB::[Φ(pspABC’-lacZY)] ΔrpoN | This study |
| AJD962 | ΔaraGFB::[Φ(pspA pspB::Ω-Sp pspC’-lacZY)] ΔrpoN | This study |
| AJD963 | ΔaraGFB::[Φ(pspA::Ω-Sp pspBC’-lacZY)] ΔrpoN | This study |
| AJD1057 | ΔaraGFB::[Φ(pspA-lacZY)] ΔrpoN † | This study |
| Plasmids | ||
| Palter®-1 | Tcr, pUC ori | Promega |
| pBR322 | Apr, Tcr, pMB1 ori | Bolivar et al., 1977 |
| pSL1180 | Apr, super polylinker, pUC18 ori | Pharmacia |
| pVLT33 | Kmr, tacp expression vector, RSF1010 ori | de Lorenzo et al., 1993 |
| pVLT35 | Smr/Spr, tacp expression vector, RSF1010 ori | de Lorenzo et al., 1993 |
| pAJD107 | Apr, super polylinker, pMB1 ori | Darwin & Miller, 2001 |
| pAJD126 | tacp-yscC+ in pVLT35 | Darwin & Miller, 2001 |
| pAJD113 | Cmr, pspFpspABCDycjXF+ in pAJD107 | This study |
| pAJD457 | ~ 2.1 kb pspF’pspABC’ fragment in pBR322 | This study |
| pAJD509 | tacp-yscC+ in pVLT33 | This study |
| pAJD524 | ~ 0.7 kb pspF’-pspA’ KpnI – HindIII fragment in palter®-1 | This study |
| pAJD525 | ~ 0.56 kb pspA’pspB’ HindIII – HpaI fragment in pSL1180 | This study |
| pAJD531 | ~ 0.56 kb pspA’pspB’ KpnI/HindIII fragment in palter®-1 | This study |
| pAJD905 | Cmr, R6K ori, mob+(RP4), sacB1+, lacZY operon fusion vector | Maxson & Darwin, 2005 |
All Y. enterocolitica strains are derivatives of strain JB580v (pYV = virulence plasmid)
The Φ(pspA-lacZ) and Φ(pspB-lacZ) strains listed have the longest control region fragments, Δ278 and Δ270, respectively (see Fig. 2).
Strain constructions
The λ red recombinase gene replacement system (Datsenko & Wanner, 2000), adapted for use in Y. enterocolitica (Maxson & Darwin, 2004) was used to construct an rpoN in frame deletion mutation. Briefly, a ΔrpoN::kan mutation was constructed using Red recombinase-mediated allelic exchange. The kanamycin resistance gene was then removed by FLP recombinase mediated excision and the in frame deletion mutation was confirmed by colony PCR and Southern hybridization analysis (data not shown). The rpoN null mutant was grown in the presence of 5 mM alanyl-glutamine to alleviate a minor growth defect in LB media. The growth and regulatory phenotypes of rpoN deletion mutants could be fully complemented by a plasmid encoding RpoN (data not shown). Single copy lacZ operon fusions were constructed, integrated into the ara locus and confirmed by colony PCR analysis as described previously (Maxson & Darwin, 2005). When necessary, strains were cured of the virulence plasmid as described (Darwin & Miller, 2001).
Interposon analysis of Φ(pspABC’-lacZA)
The pspF’pspABC’ insert fragment of pAJD457 (Table 1) has a unique HindIII site within pspA, and a unique HpaI site within pspB. These sites were used as the insertion point for the Ω-Sp cassette from plasmid pHP45Ω (Fellay et al., 1987). The Ω-Sp cassette was inserted into the HpaI site of pspB as a blunt ended SmaI fragment. To be consistent, the Ω-Sp cassette was also inserted into the HindIII site of pspA as a SmaI fragment. Therefore, pAJD457 was digested with HindIII and treated with DNA polymerase I Large (Klenow) fragment and dNTPs, prior to insertion of the Ω-Sp cassette. For consistency, for both the pspA and pspB insertions, clones were chosen with the Ω-Sp in the same orientation. Following insertion of the Ω-Sp cassette, the pspF’pspA::Ω-Sp pspBpspC’ and pspF’pspApspB:: Ω-Sp pspC’ fragments were cloned into pAJD905 (Table 1). Each operon fusion was integrated into the ara locus of Y. enterocolitica strains and confirmed by colony PCR analysis as described previously (Maxson & Darwin, 2005).
β-Galactosidase assays
The effect of YscC production on the expression of Φ(pspABC’-lacZ) and Φ(pspA-lacZ) operon fusions (Fig. 2 and Table 2) was determined as described previously (Darwin & Miller, 2001). Briefly, saturated cultures were diluted into 5 ml of LB broth containing appropriate antibiotics, in 18 mm diameter test tubes, so that the optical density (600 nm) was approximately 0.04. Cultures were grown on a roller drum at 37°C for two hours. Then 0.2 mM IPTG (final concentration) was added to induce yscC expression. Growth at 37°C continued for two more hours prior to harvest for enzyme assays.
Fig. 2.

Large intergenic regions of the Y. enterocolitica psp locus. (a) Genetic structure of the psp locus. Genes are shown as thick arrows to indicate the direction of transcription. The pspF-pspA and pspA-pspB intergenic regions are labeled by their sizes in base pairs (bp). (b) Nucleotide sequence surrounding the pspF-pspA intergenic region. Numbering is with respect to the RpoN-dependent transcription initiation site (P1), which is also shown in bold face type over-lined with >. RpoN-independent 5′ mRNA ends (P2) are labeled similarly. The pspF and pspA translation initiation codons are underlined and labeled. A putative −10 element for P2 and −24/−12 elements for P1 are also underlined and labeled. Deletion end points of constructs used in this study are indicated. Downward facing arrows and bold face type indicate nucleotide substitutions used in this study. The putative PspF binding region is overlined and labeled (assigned by alignment with the PspF-binding site in the E. coli pspA control region; Jovanovic et al., 1999)). (c) Nucleotide sequence surrounding the pspA-pspB intergenic region. Numbering is with respect to the transcription initiation site (+1), which is also shown in bold face type over-lined with >. The pspA termination codon, pspB translation initiation codon and putative −10 and −35 elements are underlined and labeled. Deletion end points of constructs used in this study are indicated. A tri-nucleotide substitution of the putative −10 element used in this study is indicated by downward facing arrows and bold face type.
Table 2.
Effects of control region alterations on Φ(pspA-lacZ) expression
| β-Galactosidase specific activity *
|
|||||
|---|---|---|---|---|---|
|
rpoN+
|
ΔrpoN
|
||||
| Promoter † | Mutation ‡ | − YscC # | + YscC | − YscC | + YscC |
| Δ278 | None | 57 ± 3 | 180 ± 11 | 29 ± 1 | 31 ± 1 |
| Δ252 | None | 55 ± 1 | 180 ± 10 | 29 ± 1 | 29 ± 1 |
| Δ132 | None | 52 ± 4 | 130 ± 2 | 34 ± 1 | 32 ± 2 |
| Δ71 | None | 52 ± 2 | 54 ± 1 | 78 ± 3 | 78 ± 2 |
| Δ39 | None | 8 ± 1 | 8 ± 1 | 8 ± 1 | 8 ± 1 |
| Δ12 | None | 8 ± 1 | 9 ± 1 | 9 ± 1 | 9 ± 1 |
| Δ278 | P1 (−12/−24) | 34 ± 1 | 35 ± 1 | 30 ± 2 | 32 ± 1 |
| Δ278 | P2 (−10) | 34 ± 1 | 130 ± 9 | 10 ± 1 | 10 ± 1 |
Determined as described in the Methods and expressed in arbitrary (Miller) units as the means of three assays ± standard deviation.
Each strain has a Φ(pspA-lacZY) fusion integrated on the chromosome with differing amounts of DNA upstream of the RpoN-dependent transcription initiation site (P1) as shown in Fig. 2.
Each control region fragment has either the wild type sequence (none), or mutations within the putative RpoN-binding site of P1 (−12/−24) or the putative −10 motif of P2 (−10) as indicated in Fig. 2.
Strains with the tacp vector pVLT35 (−YscC) or the tacp-yscC+ expression plasmid pAJD126 (+YscC) were grown as described in the Methods.
To monitor expression of the Φ(pspB-lacZ) operon fusion (Table 3) saturated cultures were diluted into 5 ml of LB broth, in 18 mm diameter test tubes, so that the optical density (600 nm) was approximately 0.06. The cultures were grown on a roller drum at 26°C for four hours, prior to harvest for enzyme assays.
Table 3.
Effects of control region alterations on Φ(pspB-lacZ) expression
| Promoter * | Mutation † | β-Galactosidase specific activity ‡ |
|---|---|---|
| Δ270 | None | 180 ± 2 |
| Δ177 | None | 180 ± 13 |
| Δ115 | None | 190 ± 7 |
| Δ55 | None | 200 ± 12 |
| Δ17 | None | 71 ± 1 |
| Δ270 | −10 | 32 ± 2 |
Each strain has a Φ(pspB-lacZY) fusion integrated on the chromosome with differing amounts of DNA upstream of the transcription initiation site as shown in Fig. 2.
Each control region fragment has either the wild type sequence (none), or a TAT to GCG mutation within the putative −10 motif (−10) as indicated in Fig. 2.
Determined as described in the Methods and expressed in arbitrary (Miller) units as the means of three assays ± standard deviation.
β-Galactosidase enzyme activity was determined at room temperature (approximately 22°C) in permeabilized cells as described previously (Maloy et al., 1996). Activities are expressed in arbitrary units, which were determined according to the formula of Miller (Miller, 1972). Individual cultures were assayed in duplicate, and values were averaged from at least three independent cultures. Data were rounded to two significant figures.
RNA isolation and primer extension analysis
Total RNA was isolated from Y. enterocolitica rpoN+ or ΔrpoN strains containing the multicopy psp locus plasmid pAJD113 and the tacp-yscC expression plasmid pAJD126. Cultures were grown as described above for the β-galactosidase assay experiments. RNA was isolated with the RNeasy mini kit (QIAGEN Inc.) according to the manufacturer’s instructions. End labeling of oligonucleotides and primer extension reactions were done with the Primer Extension System – AMV Reverse Transcriptase (Promega Corp.). For analysis of the region upstream from pspA the primer 5′-CTGTGGATCTTCAGCTTTATCCAG corresponds to pspA codons 20 to 26 in the template strand. For the region downstream from pspA the primer 5′-CGTGCATCGTCAGTTAACTGCGAT corresponds to pspB codons 45 to 52 in the template strand. The primers were labeled at the 5′ end with [γ-32P]-ATP and used in extension reactions containing 5 μg of RNA. To generate size markers the same primers were used in DNA sequencing reactions of the pAJD113 template using the fmol® DNA Cycle Sequencing System (Promega Corp.). Samples were resolved by denaturing 8% polyacrylamide-urea electrophoresis and visualized by autoradiography.
Promoter deletion analysis
Truncated control region fragments were generated by PCR using a common downstream primer and upstream primers that annealed at various distances upstream. The fragments were cloned into the lacZ operon fusion plasmid pAJD905 and the DNA sequences were confirmed. The operon fusions were integrated into the ara locus and confirmed by colony PCR analysis as described previously (Maxson & Darwin, 2005).
Site directed mutagenesis
The Altered Sites® II in vitro Mutagenesis System (Promega Corp.) was used. The starting point for these constructions was plasmid pAJD457, which has a pspF’pspABC’ insert (Table 1). For mutagenesis of the region upstream of pspA, a 0.7 kb pspF’-pspA’ KpnI – HindIII fragment of pAJD457 was cloned into pALTER®-1. For mutagenesis of the region downstream from pspA, a 0.56 kb ‘pspApspB’ HindIII – HpaI fragment was cloned into pSL1180 (Table 1), and then transferred to pALTER®-1 as a HindIII – KpnI fragment. The oligonucleotides for mutagenesis were designed according to the manufacturers instructions, and incorporate mismatches as indicated in Fig. 2. After mutagenesis, the DNA sequence of the insert fragment was determined to confirm the desired mutations, and to ensure that no spurious mutations were introduced. Mutagenic fragments were then cloned into the lacZ operon fusion plasmid pAJD905 and the operon fusions were integrated into the ara locus and confirmed by colony PCR analysis exactly as described previously (Maxson & Darwin, 2005). Colony PCR and DNA sequence analysis was used to verify the presence of each site directed mutation on the chromosome.
RESULTS
Interposon analysis indicates a complex pspA operon structure
A Y. enterocolitica pspC null mutant has a growth defect when the YscC secretin is overproduced and mislocalized (Darwin & Miller, 2001). pspABCDycjXF operon expression depends on the enhancer binding protein PspF, which is predicted to activate an RpoN-dependent promoter upstream of pspA (Green & Darwin, 2004). However, a pspF null mutation causes a less severe YscC-induced growth defect than a pspC null mutation, which led to a prediction of some PspF-independent expression of pspC (Darwin & Miller, 2001). Consistent with this, we have found that an rpoN null mutant also has a less severe YscC-induced growth defect than a pspC null mutant and that the YscC-related growth phenotypes of pspF and rpoN null strains are indistinguishable (data not shown). A likely location for a RpoN/PspF-independent promoter driving some pspC expression was thought to be the 161 bp non-coding region separating pspA and pspB (Darwin & Miller, 2001). We used insertional mutagenesis with the Ω interposon (a strong transcriptional terminator cassette; Fellay et al., 1987) to begin to address this hypothesis.
Merodiploid strains were constructed with a Φ(pspABC’-lacZ) operon fusion integrated into the ara locus (Maxson & Darwin, 2005) and an intact pspF-pspABCDycjXF locus. Therefore, polar effects of Ω-Sp interposon insertions on lacZ expression were determined in strains that retained intact copies of all psp genes. Derivatives were constructed with a Ω-Sp transcriptional terminator cassette inserted into the pspA or pspB genes of the Φ(pspABC’-lacZ) fusion and β-galactosidase activites were determined in the presence or absence of YscC overexpression (Fig. 1). The hypothesis predicts that the Ω-Sp insertion in pspA should abolish lacZ expression from the RpoN-dependent pspA promoter. The Ω-Sp insertion in pspB should abolish lacZ expression from both the RpoN-dependent pspA promoter, and the putative promoter in the pspA-pspB intergenic region.
Fig. 1.

Interposon analysis of psp gene expression. The diagrams on the left represent the location of the Ω-Sp insertions and their predicted effects on transcription (thin arrows indicate predicted transcripts). The lacZY operon fusions were integrated into the ara locus as described in the Methods (i.e. the native pspA operon of each strain is unaffected). The data on the right are from β-galactosidase assays of each fusion construct in rpoN+ or ΔrpoN mutant strains (determined as described in the Methods and expressed in arbitrary Miller units as the means of three assays ± standard deviation). Strains with the tacp vector pVLT33 (−YscC) or the tacp-yscC+ expression plasmid pAJD509 (+YscC) were grown in the presence of 0.2 mM IPTG, as described in the Methods. Note that a strain with a promoterless lacZY operon fusion integrated into the chromosome had approximately 5–10 Miller units of endogenous β-galactosidase activity.
In an rpoN+ strain, Φ(pspABC’-lacZ) expression was increased by approximately 50% when YscC was produced (Fig. 1). As expected, the pspA::Ω-Sp insertion reduced Φ(pspABC’-lacZ) expression and abolished YscC-dependent induction. The pspB::Ω-Sp insertion reduced expression to a greater extent. These data support the prediction that some lacZ expression originates from downstream of the Ω-Sp insertion site within pspA.
When the operon fusions were analyzed in an rpoN null mutant the interpretation became more complex. An rpoN null mutation abolished YscC-dependent induction of Φ(pspABC’-lacZ) expression, which is consistent with the predicted RpoN-dependence of the pspA promoter. However, the effect of the ΔrpoN mutation on Φ(pspABC’-lacZ) was much less than that of the pspA::Ω-Sp insertion in the rpoN+ strain (Fig. 1). Furthermore, even in the rpoN null strain the pspA:: Ω-Sp insertion reduced Φ(pspABC’-lacZ) expression by 50%. Taken together all of these data suggest the following. First, RpoN-dependent expression originates only from upstream of the Ω-Sp insertion site within pspA. Second, significant RpoN-independent expression originates both upstream and downstream of the Ω-Sp insertion site within pspA. Therefore, we next analyzed the non-coding regions upstream and downstream of pspA (Fig. 2) for the presence of RpoN-dependent and RpoN-independent transcription initiation sites.
Identification of RpoN-dependent and RpoN-independent 5′ mRNA ends expressed from the pspA control region
Interposon analysis had suggested that both RpoN-dependent and RpoN-independent transcription initiation might originate upstream from pspA (Fig. 1). Therefore, we used primer extension analysis to identify 5′ mRNA ends originating from the pspF-pspA intergenic region of the intact psp locus. RNA was isolated from both rpoN+ and ΔrpoN strains and analyzed with a primer complementary to the 5′ end of pspA (see Methods). The most abundant mRNA molecule isolated from the rpoN+ strain had a 5′ end at the expected position downstream of the predicted RpoN-binding site (P1 in Fig. 3). This confirms the location of the RpoN-dependent promoter. As expected, this mRNA was undetectable in the rpoN null mutant (Fig. 3). We also identified two additional 5′ mRNA ends separated by 2 nucleotides, which mapped a short distance upstream of P1 (P2 in Fig. 3), indicating that they did not arise from processing of the RpoN-dependent transcript. Furthermore, these mRNAs were detected in both rpoN+ and rpoN null strains. Sequences similar to the −10 element of a (σ70-dependent promoter were identified upstream (Figs. 2 and 3). All of these results were also confirmed by 5′ RACE analysis of mRNA initiated from a different template (a multicopy Φ(pspA-lacZ) fusion plasmid; data not shown).
Fig. 3.
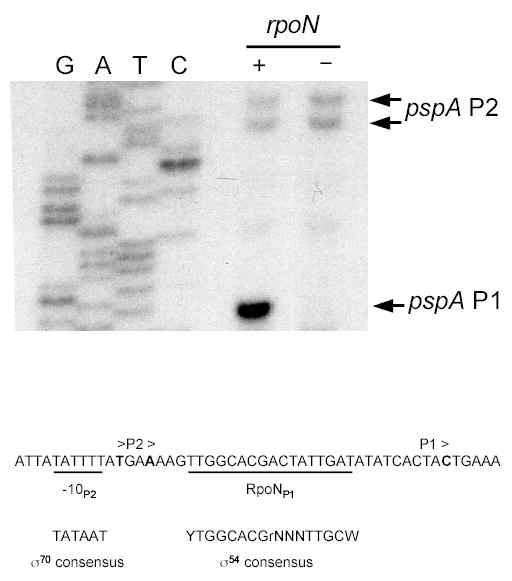
Primer extension analysis of the pspA control region. RNA was extracted from rpoN+ or rpoN null strains containing the multicopy psp locus plasmid pAJD113 and the tacp-yscC expression plasmid pAJD126. Arrows indicate the major primer extension products. G, A, T, C indicate the DNA sequencing reactions. The nucleotide sequence surrounding the putative transcription initiation sites is shown at the bottom, with a predicted RpoN-binding site for P1 and −10 element for P2 underlined. Consensus sequences for the −10 element of an E. coli σ70-dependent promoter and for an RpoN-binding site are shown for comparison. The gel lane images have been rearranged from the original order, but the data are from the same gel and a single experiment.
5′ deletion analysis of the pspA control region
The primer extension analysis had identified RpoN-dependent and RpoN-independent 5′ mRNA ends within the pspA control region (Fig. 3). Next we undertook a 5′ deletion analysis in order to identify how much upstream DNA was required for RpoN-dependent and RpoN-independent expression of a Φ(pspA-lacZ) operon fusion made with the isolated pspA control region. A set of single copy Φ(pspA-lacZ) operon fusion strains was constructed with different 5′ deletions of the pspA control region fragment (Fig. 2). Strains were grown under both non-Psp-inducing (−YscC) and Psp-inducing (+YscC) conditions and β-galactosidase activities were determined (Table 2). YscC overproduction does not induce Φ(pspA-lacZ) expression as much as some other proteins (Maxson & Darwin, 2004; Maxson & Darwin, 2005; Maxson & Darwin, 2006). However, it does cause a severe growth defect in some psp null mutants (Darwin & Miller, 2001; Green & Darwin, 2004; Maxson & Darwin, 2006) and so it’s effect on pspA operon expression is probably physiologically significant.
In an rpoN+ strain the β-galactosidase activities of deletion constructs Δ252, Δ132 and Δ71 were indistinguishable from that of the original construct (Δ278) under uninduced conditions (−YscC). However, the YscC-induced activities of constructs Δ132 and Δ71 were slightly reduced and completely abolished, respectively (Table 2). This shows that DNA upstream of position −71 is needed for regulated expression of Φ(pspA-lacZ). PspF is required for YscC-dependent induction. In E. coli the PspF binding site overlaps the pspF translation initiation codon (Jovanovic et al., 1999). Sequence alignment suggests that the same is probably true in Y. enterocolitica (Fig. 2 and data not shown). Therefore, the PspF binding site is likely to have been compromised in construct Δ132 and completely removed in construct Δ71 (Fig. 2). All Φ(pspA-lacZ) expression was completely abolished in deletion constructs Δ39 and Δ12 (Table 2). The remaining 8–9 Miller units can be attributed to endogenous Y. enterocolitica β-galactosidase activity and basal expression from an empty Φ(-lacZ) operon fusion (Maxson & Darwin, 2005 and data not shown). From this it can be concluded that the region upstream of position −39 is required for all basal Φ(pspA-lacZ) expression.
The major goal of these experiments was to characterize RpoN-independent Φ (pspA-lacZ) expression. To more clearly identify the region required specifically for RpoN-independent expression the deletion constructs were analyzed in an rpoN null strain (Table 2). The results confirmed that the region upstream of position −39 is required for RpoN-independent expression of Φ(pspA-lacZ). This region includes almost the entire DNA region upstream from where the RpoN-independent 5′ mRNA ends were mapped (Figs. 2 and 3). Furthermore, the Δ39 construct removes part of a putative −10 element (Fig. 2). Together, all of these data suggest that one or both of the 5′ mRNA ends represents an RpoN-independent transcription initiation site. Finally, in the rpoN null strain only, we noticed that Φ(pspA-lacZ) expression was elevated in the Δ71 construct (Table 2). We do not yet understand the explanation for this, and do not draw any conclusions from it. It may be an artifact resulting from the relative positioning of vector sequences upstream of the control region end point in this particular deletion construct.
Site directed mutagenesis of the pspA control region
To complete the analysis of the pspA control region, putative promoter elements were targeted by site directed mutagenesis. Mutations were introduced into the original Δ278 Φ(pspA-lacZ) construct and β-galactosidase activities were determined (Table 2). First, the highly conserved −24 and −12 dinucleotides of the predicted RpoN-binding site were disrupted (Fig. 2). In an rpoN+ strain this mutation abolished YscC-dependent induction and reduced basal expression by almost 50% (Table 2). This effect was indistinguishable from that of an rpoN null mutation on expression of the original Φ(pspA-lacZ) construct with the wild type control region sequence. In an rpoN null strain the −24/−12 mutation had no effect on Φ(pspA-lacZ) expression (Table 2). Therefore, as expected the mutation only affected RpoN-dependent expression.
The region immediately upstream of the putative RpoN-independent transcription initiation sites has several overlapping sequences that might represent −10 elements (CATTATATTTT; Fig. 5), although there is no recognizable −35 element. To test the functionality of the putative −10 elements, and to distinguish between them, we made three different trinucleotide substitutions. Two of these (CATTAT to GCGTAT) and (TATATT to CCAATT) did not reduce RpoN-independent expression of Φ(pspA-lacZ) (data not shown). However, a TATTTT to TTGGTT mutation (Fig. 2) essentially abolished Φ(pspA-lacZ) expression in an rpoN null strain and also reduced expression in an rpoN+ strain (Table 2). Therefore, we tentatively assigned the TATTTT motif as a −10 element. The TATATT to CCAATT mutation, that also disrupts the first position of this −10 element, did not affect expression possibly because it leaves the most highly conserved positions intact (Harley & Reynolds, 1987; Hawley & McClure, 1983). It also remains possible that multiple sequences serve as a −10 elements. Nevertheless, when taken together the primer extension, 5′ deletion and site directed mutagenesis strongly suggest the presence of at least one RpoN-independent transcription initiation site upstream of pspA.
Identification of 5′ mRNA ends expressed from the pspA-pspB intergenic region
Interposon analysis had also suggested that some RpoN-independent transcription initiation might originate downstream from pspA (Fig. 1). Therefore, we also used primer extension analysis to identify 5′ mRNA ends originating from the pspA-pspB intergenic region of the intact psp locus. RNA was analyzed by primer extension analysis with an oligonucleotide complementary to the 5′ end of pspB (see Methods). Surprisingly, the 5′ ends of mRNA molecules mapped to a single C residue that was just within the pspB gene (Fig. 4). We are confident that the pspB translation initiation codon has been correctly identified. It has a Shine-Dalgarno (SD) motif, there are no alternative downstream ATG or GTG codons with SD motifs, and the predicted PspB amino terminus is conserved between different species (data not shown). We also confirmed the primer extension result by 5′ RACE analysis of mRNA initiated from a different template (a multicopy Φ(pspB-lacZ) fusion plasmid; data not shown). This 5′ mRNA end may be generated from a σ70-dependent promoter because putative −35 and −10 elements were identified upstream (Fig. 4).
Fig. 4.

Primer extension analysis of the pspA-pspB intergenic region. RNA was extracted from a rpoN+ strain containing the multicopy psp locus plasmid pAJD113 and the tacp-yscC expression plasmid pAJD126. An arrow indicates the primer extension (PE) product. G, A, T, C indicate the DNA sequencing reactions. The nucleotide sequence surrounding the putative transcription initiation site is shown at the bottom. The pspB translation initiation codon is double underlined and possible −10 and −35 elements are single underlined. The arrangement of a consensus E. coli σ70-dependent promoter (−35 and −10) is also shown for comparison. The gel lane images have been rearranged from the original order, but the data are from the same gel and a single experiment.
5′ deletion analysis and site directed mutagenesis of the pspA-pspB intergenic region
To investigate the function of the putative promoter identified by mRNA analysis we first constructed a single copy Φ(pspB-lacZ) operon fusion strain. The fragment used for this fusion encompassed the 3′ end of pspA (position −270 in Fig. 2) to 63 bp within the 5′ end of pspB. The single copy Φ(pspB-lacZ) fusion strain produced 180 Miller units of β-galactosidase activity when grown to mid-exponential phase at 26°C in LB broth (Table 3). This compares to only 5–10 Miller units due to endogenous Y. enterocolitica β-galactosidase activity and basal expression of an empty Φ(-lacZ) operon fusion (Maxson & Darwin, 2005 and data not shown). Therefore, these results support the hypothesis that the ‘pspA-pspB’ fragment contains an active promoter. Φ(pspB-lacZ) expression was not affected by rpoN and pspF null mutations, or by yscC overexpression (data not shown).
Next we did a 5′ deletion analysis of the ‘pspA-pspB’ fragment to determine how much upstream DNA was important for Φ(pspB-lacZ) expression. For this we constructed a set of single copy Φ(pspB-lacZ) operon fusion strains with different 5′ deletions of the ‘pspA-pspB’ fragment (Fig. 2). The strains were grown to mid-exponential phase at 26°C in LB broth and β-galactosidase activities were determined (Table 3). Deletion constructs Δ177, Δ115 and Δ55 expressed β-galactosidase activities that were similar to that of the original construct (Δ270). This suggests that DNA upstream of position −55 is not required for Φ(pspB-lacZ) expression, making it unlikely as a target for a regulatory protein under the growth conditions we used. However, the β-galactosidase activity of deletion construct Δ17 was reduced by 60% in comparison to the full-length fragment (Δ270). This may be because this deletion removed a putative −35 element (Fig. 2).
Finally, if the putative promoter within pspB is authentic then disruption of the predicted −10 element should reduce expression. Therefore, we tested whether Φ(pspB-lacZ) expression was affected by a trinucleotide substitution in the −10 region of the full-length Δ270 construct (Fig. 2). This mutation reduced Φ(pspB-lacZ) expression by over 80% (Table 3), which further corroborates the identification of a putative (σ70-dependent promoter.
DISCUSSION
This work was motivated by data from a previous study that suggested some PspF-independent expression of pspC (Darwin & Miller, 2001). The phenotypes of an rpoN null mutant further supported this hypothesis (Fig. 1 and data not shown). The pspF-pspABCDycjXF locus contains two large non-coding regions that we decided to focus on for this study (Fig. 2): the region containing promoters for the divergently transcribed pspF and pspA genes (187 bp) and the pspA-pspB intergenic region (161 bp). RpoN-independent promoters were identified both upstream and downstream of pspA. The expected location of the PspF/RpoN-dependent pspA promoter was also confirmed.
Three 5′ mRNA ends were identified upstream of pspA (Fig. 3). The most abundant was expressed from the RpoN/PspF-dependent promoter and served to confirm its anticipated location. This RpoN-dependent promoter is apparently highly conserved upstream of pspA in different bacteria (Green & Darwin, 2004). Two RpoN-independent 5′ mRNA ends were also identified, separated by only 2 nucleotides, and mapping approximately 30 bp upstream of the RpoN-dependent transcription initiation site. These two mRNA ends might represent two different promoters or alternative transcription initiation sites for the same promoter. Another possibility is that there is a single initiation site and the shorter transcript arose from a processing event. Multiple putative −10 elements were found upstream, but only one mutation reduced RpoN-independent Φ(pspA-lacZ) expression (Table 2 and data not shown). Although a −35 element could not be identified we are confident that at least one of the 5′ mRNA ends is indicative of a promoter. First, RpoN-independent Φ(pspA-lacZ) expression was abolished by deletion of upstream DNA (Table 2). Second, RpoN-independent Φ(pspA-lacZ) expression was also abolished by the −10 mutation (Table 2). RpoN-independent pspA transcription initiation sites were not detected in E. coli by ribonuclease protection assays (Weiner et al., 1991). It is possible that the sensitivity was insufficient. E. coli pspA primer extension experiments were also only reported to have identified the RpoN-dependent 5′ mRNA end, but the data were not shown (Weiner et al., 1991). Either RpoN-independent expression upstream of pspA is unique to Y. enterocolitica, or also occurs in E. coli but was below the limit of detection in the published experiments.
The close proximity of RpoN-dependent and RpoN-independent promoters upstream of pspA (Figs. 2 and 3) raises the question of whether they can be occupied (active) simultaneously. Mutation of the putative −10 element of the RpoN-independent promoter reduced Φ(pspA-lacZ) expression in both rpoN+ and ΔrpoN strains (Table 2). This suggests that this promoter does contribute to Φ(pspA-lacZ) expression when the RpoN-dependent promoter is active. However, it is also possible that this mutation independently compromises both promoters. Alternatively, only one promoter may be occupied at any one time and the total β-galactosidase activity measured reflects the average of the population of cells. It might be interesting to investigate this question in future experiments, particularly with an in vitro transcription system.
The literature contains numerous examples of bacterial genes with multiple upstream promoters, including the E. coli rpoE operon which, like the pspA operon, is involved in extracytoplasmic stress response (Raina et al., 1995). In many cases the precise physiological function of the multiple promoters is unknown and this is certainly true for RpoN-independent expression of the Y. enterocolitica pspA operon. RpoN-independent expression from upstream of pspA might ensure low-level constitutive expression of some psp genes or regulation under unknown conditions. Constitutive expression might indicate that low levels of some Psp proteins are critical under non-inducing conditions, perhaps to facilitate a fast response to inducing stress conditions.
Another RpoN-independent promoter was identified downstream from pspA. Unexpectedly the promoter overlaps the 5′ end of pspB (Fig. 2). There are many examples of promoters within bacterial genes, but the location of this one at the extreme 5′ end of pspB was surprising. Nevertheless, we are confident of the veracity of this promoter. The 5′ mRNA end was mapped from different templates by both primer extension analysis (Fig. 2) and 5′ RACE (data not shown). Putative −35 and −10 elements were identified at the appropriate locations and their disruption by deletion (−35) or mutation (−10) significantly reduced Φ(pspB-lacZ) expression (Table 3). Expression of Φ(pspB-lacZ) was not completely abolished by these disruptions. However, it is not unprecedented for control region mutations in −35 and −10 elements to leave some promoter function intact. The location of this promoter indicates that it can only control expression of pspC and the downstream genes. Its activity was unaffected by pspF and rpoN null mutations, by YscC overproduction or by heat or osmotic shock (Fig. 1 and data not shown). Therefore, the promoter may drive low-level constitutive expression of pspC and downstream genes. Alternatively, it could be responsible for regulated expression under as yet unidentified conditions.
Examination of the genome sequences from closely related Enterobacteriaceae suggests that the relatively large size of the pspA-pspB intergenic region is a signature of Yersinia species. It occurs in Y. enterocolitica, Y. pseudotuberculosis and Y. pestis (data not shown). Furthermore, the −10 element of the promoter is perfectly conserved in all three of these species. In contrast, the pspA-pspB intergenic region is approximately 100 bp shorter in both E. coli and Salmonella enterica serovar Typhimurium. Therefore, the RpoN-independent promoter and any upstream control elements identified here may not be present in all species. However, it is possible that different internal promoters are present in the psp loci of other species. For example, northern hybridization analysis of the E. coli pspABCDE operon detected a constitutively expressed transcript encoding only pspBCDE, although its origin was not determined (Brissette et al., 1991).
Appropriate expression of the Psp response system is probably critical. Indeed, pspA operon expression is highly regulated in both E. coli and Y. enterocolitica. This regulation is mediated entirely by a conserved RpoN/PspF-depdendent promoter upstream of pspA. In addition, the PspA, PspB and PspC proteins all modulate PspF activity via a putative signal transduction pathway, which further increases the complexity of pspA operon regulation. This study has revealed yet another level of transcriptional complexity within the Y. enterocolitica pspA operon. RpoN-independent promoters are present both upstream and downstream of pspA. These promoters may only be responsible for a relatively small amount of the total level of psp gene expression in comparison to the highly inducible PspF/RpoN-independent promoter (e.g. see Maxson & Darwin, 2004). Nevertheless, a goal for future experiments will be to investigate role of these promoters and whether there are any conditions under which their activity is altered.
Acknowledgments
This study was supported by Public Health Service grant AI-052148 from the National Institute of Allergy and Infectious Diseases, and by a grant from the Speaker’s Fund for Biomedical Research: Toward the Science of Patient Care, awarded by the City of New York. We thank Heran Darwin for critically reviewing a draft version of the manuscript.
Footnotes
Publisher's Disclaimer: This is an un-copy-edited author manuscript that has been accepted for publication in Microbiology, copyright Society for General Microbiology. Cite this article as appearing in Microbiology. This version of the manuscript may not be duplicated or reproduced, other than for personal use or within the rule of 'Fair Use of Copyrighted Materials' (section 17, Title 17, US Code), without permission from the copyright owner, Society for General Microbiology. The final copy-edited article, which is the version of record, can be found at http://mic.sgmjournals.org, and is freely available without a subscription 24 months after publication. The Society for General Microbiology disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- Adams H, Teertstra W, Demmers J, Boesten R, Tommassen J. Interactions between phage-shock proteins in Escherichia coli. J Bacteriol. 2003;185:1174–1180. doi: 10.1128/JB.185.4.1174-1180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- Brissette JL, Russel M, Weiner L, Model P. Phage shock protein, a stress protein of Escherichia coli. Proc Natl Acad Sci USA. 1990;87:862–866. doi: 10.1073/pnas.87.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette JL, Weiner L, Ripmaster TL, Model P. Characterization and sequence of the Escherichia coli stress-induced psp operon. J Mol Biol. 1991;220:35–48. doi: 10.1016/0022-2836(91)90379-k. [DOI] [PubMed] [Google Scholar]
- Darwin AJ, Miller VL. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol Microbiol. 1999;32:51–62. doi: 10.1046/j.1365-2958.1999.01324.x. [DOI] [PubMed] [Google Scholar]
- Darwin AJ, Miller VL. The psp locus of Yersinia enterocolitica is required for virulence and for growth in vitro when the Ysc type III secretion system is produced. Mol Microbiol. 2001;39:429–444. doi: 10.1046/j.1365-2958.2001.02235.x. [DOI] [PubMed] [Google Scholar]
- Darwin AJ. The phage-shock-protein-response. Mol Microbiol. 2005;57:621–628. doi: 10.1111/j.1365-2958.2005.04694.x. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V, Eltis L, Kessler B, Timmis KN. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- Dworkin J, Jovanovic G, Model P. The PspA protein of Escherichia coli is a negative regulator of σ54-dependent transcription. J Bacteriol. 2000;182:311–319. doi: 10.1128/jb.182.2.311-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin S, Jones S, Schumacher J, Studholme D, Buck M. Mechanism of action of the Escherichia coli phage shock protein PspA in repression of the AAA family transcription factor PspF. J Mol Biol. 2002;320:23–37. doi: 10.1016/S0022-2836(02)00404-7. [DOI] [PubMed] [Google Scholar]
- Elderkin S, Bordes P, Jones S, Rappas M, Buck M. Molecular determinants for PspA-mediated repression of the transcriptional activator PspF. J Bacteriol. 2005;187:3238–3248. doi: 10.1128/JB.187.9.3238-3248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Green RC, Darwin AJ. PspG, a new member of the Yersinia enterocolitica phage shock protein regulon. J Bacteriol. 2004;186:4910–4920. doi: 10.1128/JB.186.15.4910-4920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Reynolds RP. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987;15:2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley DK, McClure WR. Compilation and analysis of Escherichia coli promoter sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic G, Weiner L, Model P. Identification, nucleotide sequence, and characterization of PspF, the transcriptional activator of the Escherichia coli stress-induced psp operon. J Bacteriol. 1996;178:1936–1945. doi: 10.1128/jb.178.7.1936-1945.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic G, Rakonjac J, Model P. In vivo and in vitro activities of the Escherichia coli σ54 transcription activator, PspF, and its DNA-binding mutant, PspFΔHTH. J Mol Biol. 1999;285:469–483. doi: 10.1006/jmbi.1998.2263. [DOI] [PubMed] [Google Scholar]
- Kinder SA, Badger JL, Bryant GO, Pepe JC, Miller VL. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O:8 and construction of a transformable R−M+ mutant. Gene. 1993;136:271–275. doi: 10.1016/0378-1119(93)90478-l. [DOI] [PubMed] [Google Scholar]
- Lloyd LJ, Jones SE, Jovanovic G, Gyaneshwar P, Rolfe MD, Thompson A, Hinton JC, Buck M. Identification of a new member of the phage shock protein response in Escherichia coli, the phage shock protein G (PspG) J Biol Chem. 2004;279:55707–55714. doi: 10.1074/jbc.M408994200. [DOI] [PubMed] [Google Scholar]
- Maloy, S. R., Stewart, V. J. & Taylor, R. K. (1996). Genetic analysis of pathogenic bacteria. Plainview, NY: Cold Spring Harbor Laboratory Press.
- Maxson ME, Darwin AJ. Identification of inducers of the Yersinia enterocolitica phage shock protein system and comparison to the regulation of the RpoE and Cpx extracytoplasmic stress responses. J Bacteriol. 2004;186:4199–4208. doi: 10.1128/JB.186.13.4199-4208.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson ME, Darwin AJ. Improved system for construction and analysis of single-copy β-galactosidase operon fusions in Yersinia enterocolitica. Appl Environ Microbiol. 2005;71:5614–5618. doi: 10.1128/AEM.71.9.5614-5618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson, M. E. & Darwin, A. J. (2006). PspB and PspC of Yersinia enterocolitica are dual function proteins: regulators and effectors of the phage-shock-protein response. Mol Microbiol In press, 10.1111/j.1365–2958.2006.05047.x. [DOI] [PubMed]
- Miller, J. H. (1972). Experiments in molecular genetics. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory.
- Model P, Jovanovic G, Dworkin J. The Escherichia coli phage-shock-protein (psp) operon. Mol Microbiol. 1997;24:255–261. doi: 10.1046/j.1365-2958.1997.3481712.x. [DOI] [PubMed] [Google Scholar]
- Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the sigma E (sigma 24) heat shock sigma factor of Escherichia coli. EMBO J. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L, Brissette JL, Model P. Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on σ54 and modulated by positive and negative feedback mechanisms. Genes Dev. 1991;5:1912–1923. doi: 10.1101/gad.5.10.1912. [DOI] [PubMed] [Google Scholar]
- Weiner L, Brissette JL, Ramani N, Model P. Analysis of the proteins and cis-acting elements regulating the stress-induced phage shock protein operon. Nucleic Acids Res. 1995;23:2030–2036. doi: 10.1093/nar/23.11.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]


