Abstract
Affecting over 30% of the population, obesity is an epidemic in the United States and is associated with multiple chronic medical problems. Obesity is also associated with numerous hormonal changes, many of which have been implicated in prostate cancer development and progression. Although, on the whole, controversy exists over whether obesity increases the risk of prostate cancer, data strongly suggest that obesity is a significant risk factor for prostate cancer death. In this review, we discuss the epidemiologic data surrounding obesity and prostate cancer. We also discuss some of the sequelae of obesity and their relationships with prostate cancer, including alterations in insulin, the insulin-like growth factor axis, and leptin levels; insulin resistance; and diabetes. Although a complete overview of all the various dietary and lifestyle factors that are associated with obesity and prostate cancer risk is beyond the scope of this review, we discuss data concerning the relationship between a high-fat diet and prostate cancer.
Key words: Body mass index, Dietary fat, Insulin-like growth factor, Leptin, Obesity, Prostate cancer
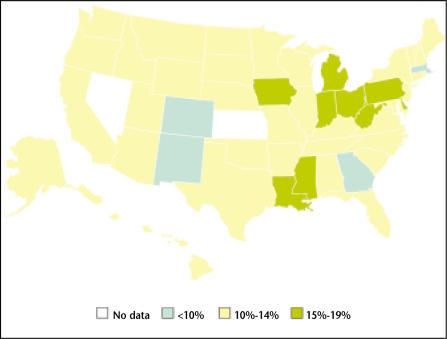
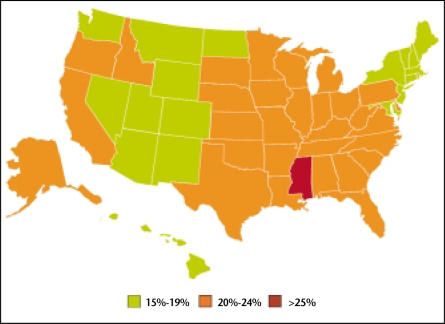
Obesity is a major health problem that affects more than 30% of adults in the United States.1 Every state has seen a dramatic increase in the prevalence of obesity over the past 10 years (Figures 1 and 2).2 Obesity is associated with the development of multiple chronic diseases, including coronary artery disease, hypertension, and diabetes.3–5 Obesity has also been linked to several types of cancer, including that of the breast and colon.6 A recent study of 900,000 persons found that obese patients were more likely to die from a number of cancers, including prostate cancer.7 However, studies of obesity and prostate cancer are complicated by the fact that obesity is associated not only with excess body fat but also with altered serum levels of numerous hormones, including testosterone, estrogen, insulin, insulin-like growth factor (IGF)-1, and leptin, all of which have to some degree been linked to prostate cancer. In addition, obesity is highly correlated with dietary intake in terms of the number of calories as well as the amount of dietary fat, both of which have been linked to cancer.8
Figure 1.
The percentage of US adults aged 20 years or older who are obese (BMI >30 kg/m2) by state in 1991. Data from Ahluwalia IB et al. MMWR Surveill Summ. 2003;52:1–80.2
Figure 2.
The percentage of US adults aged 20 years or older who are obese (BMI >30 kg/m2) by state in 2001. Data from Ahluwalia IB et al. MMWR Surveill Summ. 2003;52:1–80.2
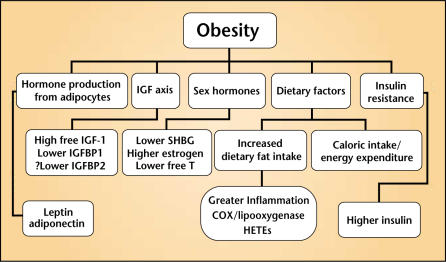
Whereas some studies have examined obesity in and of itself (excess body weight), others have examined various components that are disregulated in obesity. A complete review of all the various sequelae of obesity (Figure 3) and their relationships to prostate cancer is beyond the scope of this review. Therefore, we focus primarily on the data that directly link obesity to prostate cancer. We also briefly describe some of the factors related to obesity and their relationships with prostate cancer, focusing on insulin, the IGF axis, leptin, and dietary fat. More detailed review articles regarding sex hormones (testosterone and estrogen) or hormones in general and their relationships to prostate cancer can be found in the literature.9–11
Figure 3.
Obesity and its related sequelae. IGF, insulin-like growth factor; IGFBP, IGF-binding protein; SHBG, sexhormone-binding globulin; COX, cyclooxygenase; HETE, hydroxyeicosatetraenoic acid.
Before undertaking a review of the literature regarding obesity and prostate cancer, the term “obesity” must be defined. Webster’s dictionary defines obesity as “a condition characterized by excessive bodily fat.”12 One of the most common definitions of obesity is an increased body mass index (BMI), which is calculated by dividing weight in kilograms by height in meters squared. Both the World Health Organization and the National Institutes of Health define overweight as a BMI of greater than 25 kg/m2 and obesity as a BMI of greater than 30 kg/m2. Although it is easily determined, BMI has its limitations. For example, body composition, such as whether someone is particularly muscular or thick-boned, is not factored into BMI calculations. Therefore, alternative measurements and definitions of obesity, including waist-to-hip ratio (WHR), percent body fat, skinfold thickness, crude weight, and lean body mass, have been used in various studies.13
Increased BMI and Risk of Prostate Cancer
The epidemiologic evidence linking an increased adult BMI to an increased risk of prostate cancer is controversial. Although several large studies have found an increased BMI in adulthood to be associated with an increased risk of the development of prostate cancer,14–20 others have shown no such association.21–29 Interestingly, a recent study from Norway, which followed 950,000 men for an average of 21 years, found that a BMI of greater than 30 kg/m2 increased the risk of prostate cancer by only 9%.30 However, obese men aged 50 to 59 years at study completion had a 58% increased risk of prostate cancer; no other age group had a statistically significant increased risk. Thus, an interaction between age and obesity could explain why some studies have found an increased risk whereas others have found no relationship between obesity and prostate cancer risk. Moreover, Giovannucci and colleagues,31 using data from the Health Professionals Follow-up Study, an ongoing prospective study of more than 50,000 male US health professionals, which began enrollment in 1986, found that an increased BMI was associated with a decreased risk of prostate cancer among men younger than 60 years or those with a family history of the disease. In this study, obesity was not related to prostate cancer risk among older men. Although the relationship between obesity and prostate cancer risk was opposite from that seen in the Norwegian study, both studies suggest that a significant interaction exists among age, obesity, and prostate cancer risk.
Based on their findings, Giovannucci and colleagues31 argued that tumors in younger men and those with a family history of prostate cancer may be driven by increased androgens and, thus, the lower androgen levels seen in men with obesity may have been protective against prostate cancer.32 Interestingly, whereas the authors found that obesity was protective against prostate cancer in general, they found no relationship between obesity and advanced disease, suggesting that, although obesity protects against early disease, it provides no protection against advanced disease. Similarly, several of the studies that did find a significant association between increased BMI and risk of prostate cancer also found that the risk of advanced disease was even stronger than the risk of prostate cancer in general.15,17
Given the protracted course of prostate cancer, it is thought that events that occur earlier in life may predispose to prostate cancer later in life. Thus, examination of adulthood BMI may miss the window during which increased BMI and its sequelae affect prostate cancer risk. To address this issue, several studies examined the relationship between prostate cancer risk and obesity earlier in life (ages 10–20 years). These studies, like those examining adulthood BMI, have demonstrated mixed results: some studies have found a direct relationship between BMI early in life and the risk of prostate cancer,22 whereas others have found increased BMI to be protective against prostate cancer.21
Increased BMI and Oncologic Outcomes After Primary Therapy
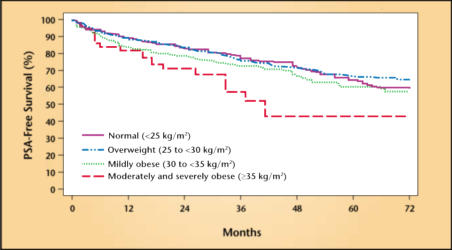
Several studies have found that, at the time of radical prostatectomy, men with an increased BMI had higher-grade and/or higher-stage disease.33–35 Two recent studies utilized large multi-institutional databases of multi-ethnic patients to address whether increased BMI was associated with higher biochemical failure rates following radical prostatectomy (Figure 4).36,37 Both studies concluded that obese men were at increased risk of biochemical failure. Interestingly, both studies found that black men were more likely to be obese, which may explain, in part, the higher rate of mortality from prostate cancer among black men.38 Further studies are needed to determine whether obese men are at increased risk for failure following other forms of primary therapy, such as external-beam radiation therapy and brachytherapy.
Figure 4.
Actuarial risk of biochemical recurrence following radical prostatectomy segregated by body mass index. PSA, prostate-specific antigen. Reproduced, with permission, from Freedland SJ et al. J Clin Oncol. 2004;22:446–453.36
Increased BMI and Risk of Dying of Prostate Cancer
The majority of the epidemiologic literature suggests that, although obesity may be related to the risk of prostate cancer, it is clearly associated with an increased risk of dying from prostate cancer. Although results from small studies have been conflicting,39–41 data from larger studies have consistently demonstrated an association between increased BMI and risk of dying from prostate cancer.7,15,42,43 Two large prospective studies deserve particular attention. In 1959 and again in 1982, the American Cancer Society enrolled a cohort of patients for longitudinal studies on cancer, known as the Cancer Prevention Study (CPS) I and II, respectively. Men were then followed for 13 years in CPS-I and 14 years in CPS-II.43 Together, these studies followed 816,268 men, among whom there were 5212 prostate cancer deaths. Both CPS-I and CPS-II reported that obese men (BMI >30 kg/m2) were significantly more likely to die of prostate cancer: 27% increased risk of prostate cancer death in CPS-I and 21% increased risk in CPS-II.43 More data from CPS-II were recently published showing that severely obese men (BMI >35 kg/m2) were at even greater risk for prostate cancer death, having a 34% higher risk relative to normal- weight men.7 Moreover, a recent study found that obesity in adolescence also increases the risk of dying of prostate cancer, suggesting that events involved in prostate carcinogenesis and progression may occur early in life.44
WHR and Prostate Cancer
WHR, the ratio of waist-to-hip circumference, provides an estimate of abdominal obesity. In general, a WHR value greater than 0.9 is considered obese.13 The significance of abdominal obesity is that it more closely correlates with various hormonal and metabolic sequelae of obesity, as well as the risk of other comorbid diseases, such as heart disease and diabetes.45–47 Few studies have examined WHR in terms of its relationship to prostate cancer. One study among men in China found that those in the highest quartile of WHR had an almost 3-fold increased risk of prostate cancer.24 Interestingly, despite a significant relationship between WHR and prostate cancer risk, the authors found no association between BMI and prostate cancer risk, suggesting that abdominal obesity as measured by WHR may be more closely related to the development of prostate cancer than other more general measurements of obesity.
Insulin
Obesity is associated with insulin resistance and non-insulin-dependent diabetes mellitus.5,48 It has been hypothesized that insulin resistance may be related to prostate cancer.49 Studies examining the relationship between insulin resistance and/or diabetes and prostate cancer risk have shown mixed results. For example, using data from CPS-I, Will and colleagues50 found no relationship between diabetes and the risk of prostate cancer development, except among men who had diabetes for 5 years or longer.
On the other hand, Giovannucci and colleagues,51 using data from the Health Professionals Follow-up Study, found that diabetes was associated with a decreased risk of prostate cancer and that the longer one had diabetes, the greater the risk reduction. Other studies found that insulin resistance, diabetes, and/or higher insulin/glucose levels either increase,52–55 decrease,56–58 or have no effect59,60 on the risk of prostate cancer. Interestingly, certain polymorphisms in the insulin gene have been associated with an increased risk of prostate cancer.61 Given the amount of conflicting data in the literature, it is difficult to determine the true impact of insulin resistance and/or diabetes on prostate cancer.
IGF Axis
IGF-I is a peptide growth factor and a potent mitogen for the growth of androgen-responsive and androgen-independent human prostate cancer cell lines.62 IGF-1 activity is modulated by high-affinity IGF-binding proteins (IGFBPs 1–6).63 The IGFBPs collectively bind and inactivate IGF-1, resulting in lower levels of free (bioavailable) IGF-1. Circulating levels of IGFBP-1 and -2 are nutritionally regulated.64 Obesity is associated with increased free or bioactive IGF-1.11,65 IGF-1 plays a pivotal role in stimulating cell proliferation, regulating differentiation, and reducing apoptosis.64,66 Tissue levels of IGF-1 appear to be a critically important factor during initiation and progression of prostate cancer.64,67
Multiple epidemiologic studies have found a direct correlation between serum IGF-1 levels and the risk of prostate cancer.68–71 A recent meta-analysis of studies examining hormonal influences on the risk of prostate cancer concluded that higher serum IGF-1 levels were significantly associated with an increased prostate cancer risk.72 However, it should be noted that the majority of studies that found this association were performed either before widespread PSA screening,68 during periods of widespread PSA screening but with a high PSA cutoff point for biopsy (>10 ng/mL),71 or in countries in which PSA screening is not particularly common.69,70 Thus, men in these studies had tumors detected at more advanced stages than is commonly seen in the United States today. Indeed, a follow-up investigation of participants from the Physicians’ Health Study, a prospective study of nearly 15,000 male physicians in the United States, found that IGF-1 and IGFBP-3 levels correlated with advanced-stage prostate cancer but not with early, clinically localized prostate cancer.73
These findings raise the possibility that the majority of men in these studies who later received a diagnosis of prostate cancer already had prostate cancer at the time of serum collection and, thus, higher IGF-1 levels may not be etiologic in prostate cancer but rather may function as a tumor marker. This hypothesis was supported by a recent investigation within a case-control study of men in the United States. The authors found no relationship between IGF-1 or IGFBP-3 levels and prostate cancer risk but did find that, among cases, but not controls, serum IGF-1 levels steadily increased over time.74 Some authors have even examined whether IGF-1 levels can be used, along with PSA measurement, to help diagnose prostate cancer; results have been mixed.75,76 However, the fact that IGF-1 levels increase after radical prostatectomy77 argues against IGF-1 as a useful tumor marker. Further studies are needed to determine whether elevated IGF-1 levels are etiologic in prostate cancer development or merely function as a tumor marker.
Free IGF-1 levels are regulated by serum levels of the IGFBPs, of which IGFBP-3 predominates. Whereas most studies that examined the IGF axis and prostate cancer risk found an increased IGF-1 level to be associated with prostate cancer, the results when examining serum IGFBP-3 levels are less clear. Using data from the Physicians’ Health Study, Chan and colleagues68 found that IGFBP-3 level was not associated with prostate cancer risk. However, after adjustment for IGF-1 levels, higher IGFBP-3 levels, and thus lower free IGF-1 levels, were associated with a decreased risk of prostate cancer. This is in contrast to a nested case-control study within the Northern Sweden Health and Disease Cohort Study, which found that higher IGFBP-3 levels were associated with an increased risk of prostate cancer. After controlling for IGF-1 levels, however, no significant relationship was seen.69
Leptin
Leptin is a polypeptide hormone produced by adipocytes. In general, increased numbers of adipocytes are associated with higher serum leptin levels. The normal physiologic role of leptin is in appetite control.78 A direct link between leptin and obesity exists in that mice lacking or with mutations in either the leptin or leptin receptor genes are phenotypically obese.78 In humans, mutations in the leptin receptor and leptin genes have been described in some obese patients, although most obese persons have elevated leptin levels in line with their degree of adiposity.79
The link between leptin and body weight was further demonstrated when a double-blind, randomized, controlled trial found that administration of recombinant leptin resulted in greater weight loss than did placebo among obese patients.80 In vitro, leptin stimulates growth of the androgen-independent cell lines DU145 and PC-3, but not the androgen-sensitive cell line LNCaP.81,82 In addition, human prostate cancers express the leptin receptor.83 Studies examining the relationship between serum leptin levels and risk of prostate cancer have produced mixed results. Some have found a positive correlation between serum leptin levels and prostate cancer risk,83,84 whereas others have found no association.54,85,86 Interestingly, the few studies that examined leptin levels among men with prostate cancer found that higher leptin levels were associated with larger tumors, higher-grade tumors, and more advanced tumors.84,87 Thus, leptin may be more useful as a prognostic marker among men with prostate cancer than as a predictor of who will develop prostate cancer; however, more studies are needed to confirm these findings.
Dietary Fat
Significant preclinical data have linked dietary fat to prostate cancer. The fatty acid consumed in the greatest quantity in the Western diet is linoleic acid (omega-6 polyunsaturated fatty acid), which is a known growth factor for androgen-dependent and androgen-independent prostate cancer cell lines.88,89 In human xenograft models, a low-fat diet results in slower androgen-sensitive prostate cancer growth and can delay progression from androgen-sensitive to androgen-insensitive growth.89–91 Changes in dietary fat intake have been linked to changes in IGF-1 levels, suggesting one possible mechanism for the relationship between dietary fat and prostate cancer.92 Two genes consistently found to be upregulated in human prostate cancer relative to normal states, fatty acid synthase and alpha-methylacyl-CoA racemase, are involved in fatty acid production and beta-oxidation, respectively.93–96
Globally, prostate cancer incidence and mortality rates are associated with a Western lifestyle and diet; however, whether this reflects increased fat intake or decreased intake of various protective products, such as soy and lycopene, is unclear.97 Comparison of diets between countries with low and high incidences of prostate cancer suggests that one of the strongest dietary risk factors for the development of prostate cancer is intake of animal products.98–100 Moreover, as men in underdeveloped nations have adopted a more Western lifestyle and diet, the incidence and mortality of prostate cancer in those areas has increased.97
Many epidemiologic studies have investigated the relationship between dietary fat intake and human prostate cancer. A complete review of the epidemiologic literature regarding the relationship between prostate cancer and all the various components of dietary fat—animal versus vegetable, unsaturated versus saturated, omega-3 versus omega-6, monounsaturated versus polyunsaturated—is beyond the scope of this review. More details regarding dietary fat and prostate cancer can be found in 2 excellent reviews by Kolonel and colleagues101 and Moyad.102 Here, we focus on total and animal fat intake.
Most case-control studies have demonstrated a positive relationship between dietary fat intake and prostate cancer risk.102 However, case-control studies have the limitation of recall bias: dietary recall can result in inaccurate information regarding true exposure history.103,104 Therefore, more accurate information regarding the role of dietary fat in prostate cancer risk can be obtained from prospective cohort studies in which dietary information is assessed at study entry and/or longitudinally. The majority of large prospective cohort studies have shown no association between dietary total fat intake and prostate cancer risk.18,105–109 However, some studies have found a positive but not statistically significant association between animal fat intake and prostate cancer risk.110 Others have found a statistically significant relationship between the two.111 Interestingly, one study found that animal fat intake, but not total fat intake, was correlated with prostate cancer risk.112 This suggests that it may not be the fat itself but rather the preparation of the food and the toxins formed during the cooking process that are involved in carcinogenesis.113,114 Several studies found that animal and/or total fat intake was related to the risk of prostate cancer death.42,115 Taken together, these data relating dietary fat and prostate cancer suggest a possible relationship with prostate cancer development but a stronger relationship with advanced disease and risk of prostate cancer death.
Conclusion
Obesity is clearly an epidemic in American society and is linked to numerous chronic medical conditions. The relationship among obesity, its physiologic sequelae, and the risk of prostate cancer is unclear. What is clear is that obese men are at significantly greater risk for dying of prostate cancer. Attempts to reduce obesity in the general population are necessary. Whether lifestyle alterations after the diagnosis of prostate cancer can alter the natural history of the disease remains to be determined.116 Further studies are needed to determine the molecular basis for increased prostate cancer mortality among obese men.
Main Points.
Results of studies examining body mass index (BMI) and prostate cancer risk are conflicting.
Larger studies, notably the Cancer Prevention Studies of the American Cancer Society, have consistently demonstrated that obese men have a significantly greater chance of dying of prostate cancer than non-obese men.
The data on the impact of insulin resistance and diabetes on prostate cancer are conflicting: some studies have found that insulin resistance, diabetes, and/or higher insulin/glucose levels increase the risk of prostate cancer, whereas others have reported a decreased risk or no effect on risk at all.
Although many epidemiologic studies have found a direct correlation between higher serum insulin-like growth factor (IGF)-1 levels and increased prostate cancer risk, most such studies were performed before prostate-specific antigen screening was widespread or in places in which it is not common; thus, the participants may have already had cancer during sampling. The resulting hypothesis that high IGF-1 level may be a tumor marker rather than an etiologic factor has been supported by some authors, but more research is necessary.
A direct link exists between leptin level and obesity, but the results are mixed as to leptin’s link to prostate cancer risk and its usefulness as a predictor. However, a few studies have shown higher leptin levels to be associated with larger tumors, higher-grade tumors, and more advanced tumors, making leptin a potential prognostic marker in patients with prostate cancer.
Although the Western diet appears to have a role in prostate cancer globally, the mechanism behind the relationship is unclear; the evidence suggests that dietary fat has a possible link to prostate cancer development but a stronger link with advanced disease and risk of prostate cancer death.
Footnotes
Supported by the Department of Veterans Affairs (WJA), National Institutes of Health R01CA100938 (WJA), Grant CA42710 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), the Department of Defense, Prostate Cancer Research Program, PC030666 (SJF). Views and opinions of and endorsements by the author(s) do not reflect those of the U.S. Army or the Department of Defense.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia IB, Mack KA, Murphy W, et al. State-specific prevalence of selected chronic disease-related characteristics—Behavioral Risk Factor Surveillance System, 2001. MMWR Surveill Summ. 2003;52(8):1–80. [PubMed] [Google Scholar]
- 3.Criqui MH, Mebane I, Wallace RB, et al. Multivariate correlates of adult blood pressures in nine North American populations: The Lipid Research Clinics Prevalence Study. Prev Med. 1982;11:391–402. doi: 10.1016/0091-7435(82)90043-3. [DOI] [PubMed] [Google Scholar]
- 4.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA. The underlying basis for obesity: relationship to cancer. J Nutr. 2002;132(11 suppl):3451S–3455S. doi: 10.1093/jn/132.11.3451S. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 8.Satia-Abouta J, Patterson RE, Schiller RN, Kristal AR. Energy from fat is associated with obesity in US men: results from the Prostate Cancer Prevention Trial. Prev Med. 2002;34:493–501. doi: 10.1006/pmed.2002.1018. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths K. Estrogens and prostatic disease. International Prostate Health Council Study Group. Prostate. 2000;45:87–100. doi: 10.1002/1097-0045(20001001)45:2<87::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002;52:213–235. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]
- 11.Kaaks R, Lukanova A, Sommersberg B. Plasma androgens, IGF-1, body size, and prostate cancer risk: a synthetic review. Prostate Cancer Prostatic Dis. 2000;3:157–172. doi: 10.1038/sj.pcan.4500421. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed February 22];Merriam-Webster Online. 2004 Available at: http://www.m-w.com.
- 13.Moyad MA. Is obesity a risk factor for prostate cancer, and does it even matter? A hypothesis and different perspective. Urology. 2002;59(4) suppl 1:41–50. doi: 10.1016/s0090-4295(01)01175-x. [DOI] [PubMed] [Google Scholar]
- 14.Aziz NM, Hartman T, Barrett M, Albanes D. Weight and prostate cancer in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Trial. ASCO. 2000;19:647a. [Google Scholar]
- 15.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135,006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 16.Severson RK, Grove JS, Nomura AM, Stemmermann GN. Body mass and prostatic cancer: a prospective study. BMJ. 1989;297:713–715. doi: 10.1136/bmj.297.6650.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putnam SD, Cerhan JR, Parker AS, et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol. 2000;10:361–369. doi: 10.1016/s1047-2797(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 18.Veierod MB, Laake P, Thelle DS. Dietary fat intake and risk of prostate cancer: a prospective study of 25,708 Norwegian men. Int J Cancer. 1997;73:634–638. doi: 10.1002/(sici)1097-0215(19971127)73:5<634::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Sung JF, Lin RS, Pu YS, et al. Risk factors for prostate carcinoma in Taiwan: a case-control study in a Chinese population. Cancer. 1999;86:484–491. [PubMed] [Google Scholar]
- 20.Gronberg H, Damber L, Damber JE. Total food consumption and body mass index in relation to prostate cancer risk: a case-control study in Sweden with prospectively collected exposure data. J Urol. 1996;155:969–974. [PubMed] [Google Scholar]
- 21.Giovannucci E, Rimm EB, Stampfer MJ, et al. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:557–563. [PubMed] [Google Scholar]
- 22.Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–549. doi: 10.1093/oxfordjournals.aje.a010241. [DOI] [PubMed] [Google Scholar]
- 23.Whittemore AS, Kolonel LN, Wu AH, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995;87:652–661. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 24.Hsing AW, Deng J, Sesterhenn IA, et al. Body size and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomarkers Prev. 2000;9:1335–1341. [PubMed] [Google Scholar]
- 25.Lee IM, Sesso HD, Paffenbarger RS., Jr A prospective cohort study of physical activity and body size in relation to prostate cancer risk (United States) Cancer Causes Control. 2001;12:187–193. doi: 10.1023/a:1008952528771. [DOI] [PubMed] [Google Scholar]
- 26.Jonsson F, Wolk A, Pedersen NL, et al. Obesity and hormone-dependent tumors: cohort and co-twin control studies based on the Swedish Twin Registry. Int J Cancer. 2003;106:594–599. doi: 10.1002/ijc.11266. [DOI] [PubMed] [Google Scholar]
- 27.Nilsen TI, Vatten LJ. Anthropometry and prostate cancer risk: a prospective study of 22,248 Norwegian men. Cancer Causes Control. 1999;10:269–275. doi: 10.1023/a:1008967330619. [DOI] [PubMed] [Google Scholar]
- 28.Habel LA, Van Den Eeden SK, Friedman GD. Body size, age at shaving initiation, and prostate cancer in a large, multiracial cohort. Prostate. 2000;43:136–143. doi: 10.1002/(sici)1097-0045(20000501)43:2<136::aid-pros8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Nomura A, Heilbrun LK, Stemmermann GN. Body mass index as a predictor of cancer in men. J Natl Cancer Inst. 1985;74:319–323. [PubMed] [Google Scholar]
- 30.Engeland A, Tretli S, Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950,000 Norwegian men. Br J Cancer. 2003;89:1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003;95:1240–1244. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 32.Pasquali R, Casimirri F, Cantobelli S, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40:101–104. doi: 10.1016/0026-0495(91)90199-7. [DOI] [PubMed] [Google Scholar]
- 33.Amling CL, Kane CJ, Riffenburgh RH, et al. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001;58:723–728. doi: 10.1016/s0090-4295(01)01373-5. [DOI] [PubMed] [Google Scholar]
- 34.Rohrmann S, Roberts WW, Walsh PC, Platz EA. Family history of prostate cancer and obesity in relation to high-grade disease and extraprostatic extension in young men with prostate cancer. Prostate. 2003;55:140–146. doi: 10.1002/pros.10211. [DOI] [PubMed] [Google Scholar]
- 35.Mydlo JH, Tieng NL, Volpe MA, et al. A pilot study analyzing PSA, serum testosterone, lipid profile, body mass index and race in a small sample of patients with and without carcinoma of the prostate. Prostate Cancer Prostatic Dis. 2001;4:101–105. doi: 10.1038/sj.pcan.4500514. [DOI] [PubMed] [Google Scholar]
- 36.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 37.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 38.Stanford JL, Stephenson RA, Coyle LM, et al. Prostate Cancer Trends 1973–1995. Bethesda, Md: SEER Program, National Cancer Institute; 1999. NIH publication 99-4543. [Google Scholar]
- 39.Daniell HW. A better prognosis for obese men with prostate cancer. J Urol. 1996;155:220–225. [PubMed] [Google Scholar]
- 40.Furuya Y, Akimoto S, Akakura K, Ito H. Smoking and obesity in relation to the etiology and disease progression of prostate cancer in Japan. Int J Urol. 1998;5:134–137. doi: 10.1111/j.1442-2042.1998.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 41.Oefelein MG, Ricchiuti VS, Conrad PW, et al. Clinical predictors of androgen-independent prostate cancer and survival in the prostate-specific antigen era. Urology. 2002;60:120–124. doi: 10.1016/s0090-4295(02)01633-3. [DOI] [PubMed] [Google Scholar]
- 42.Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984;120:244–250. doi: 10.1093/oxfordjournals.aje.a113886. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez C, Patel AV, Calle EE, et al. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–353. [PubMed] [Google Scholar]
- 44.Okasha M, McCarron P, McEwen J, Smith GD. Body mass index in young adulthood and cancer mortality: a retrospective cohort study. J Epidemiol Community Health. 2002;56:780–784. doi: 10.1136/jech.56.10.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women: importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon CG, Manson JE. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66(4 suppl):1044S–1050S. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- 47.Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women: The Nurses’ Health Study. Am J Epidemiol. 1997;145:614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 48.Shuldiner AR, Yang R, Gong DW. Resistin, obesity and insulin resistance—the emerging role of the adipocyte as an endocrine organ. N Engl J Med. 2001;345:1345–1346. doi: 10.1056/NEJM200111013451814. [DOI] [PubMed] [Google Scholar]
- 49.Barnard RJ, Aronson WJ, Tymchuk CN, Ngo TH. Prostate cancer: another aspect of the insulin-resistance syndrome? Obes Rev. 2002;3:303–308. doi: 10.1046/j.1467-789x.2002.00081.x. [DOI] [PubMed] [Google Scholar]
- 50.Will JC, Vinicor F, Calle EE. Is diabetes mellitus associated with prostate cancer incidence and survival? Epidemiology. 1999;10:313–318. [PubMed] [Google Scholar]
- 51.Giovannucci E, Rimm EB, Stampfer MJ, et al. Diabetes mellitus and risk of prostate cancer (United States) Cancer Causes Control. 1998;9:3–9. doi: 10.1023/a:1008822917449. [DOI] [PubMed] [Google Scholar]
- 52.Hsing AW, Gao YT, Chua S, Jr, et al. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95:67–71. doi: 10.1093/jnci/95.1.67. [DOI] [PubMed] [Google Scholar]
- 53.Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol Biomarkers Prev. 1995;4:807–811. [PubMed] [Google Scholar]
- 54.Hsing AW, Chua S, Jr, Gao YT, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001;93:783–789. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 55.Tulinius H, Sigfusson N, Sigvaldason H, et al. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6:863–873. [PubMed] [Google Scholar]
- 56.Rosenberg DJ, Neugut AI, Ahsan H, Shea S. Diabetes mellitus and the risk of prostate cancer. Cancer Invest. 2002;20:157–165. doi: 10.1081/cnv-120001141. [DOI] [PubMed] [Google Scholar]
- 57.Thompson MM, Garland C, Barrett-Connor E, et al. Heart disease risk factors, diabetes, and prostatic cancer in an adult community. Am J Epidemiol. 1989;129:511–517. doi: 10.1093/oxfordjournals.aje.a115162. [DOI] [PubMed] [Google Scholar]
- 58.Weiderpass E, Ye W, Vainio H, et al. Reduced risk of prostate cancer among patients with diabetes mellitus. Int J Cancer. 2002;102:258–261. doi: 10.1002/ijc.10685. [DOI] [PubMed] [Google Scholar]
- 59.Tavani A, Gallus S, Bosetti C, et al. Diabetes and the risk of prostate cancer. Eur J Cancer Prev. 2002;11:125–128. doi: 10.1097/00008469-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Stattin P, Kaaks R. Re: Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95:1086–1087. doi: 10.1093/jnci/95.14.1086. [DOI] [PubMed] [Google Scholar]
- 61.Ho GY, Melman A, Liu SM, et al. Polymorphism of the insulin gene is associated with increased prostate cancer risk. Br J Cancer. 2003;88:263–269. doi: 10.1038/sj.bjc.6600747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iwamura M, Sluss PM, Casamento JB, Cockett AT. Insulin-like growth factor I: action and receptor characterization in human prostate cancer cell lines. Prostate. 1993;22:243–252. doi: 10.1002/pros.2990220307. [DOI] [PubMed] [Google Scholar]
- 63.Ferry RJ, Jr, Katz LE, Grimberg A, et al. Cellular actions of insulin-like growth factor binding proteins. Horm Metab Res. 1999;31:192–202. doi: 10.1055/s-2007-978719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 65.Maccario M, Tassone F, Grottoli S, et al. Neuroendocrine and metabolic determinants of the adaptation of GH/IGF-I axis to obesity. Ann Endocrinol (Paris) 2002;63(2 pt 1):140–144. [PubMed] [Google Scholar]
- 66.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60:91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 67.Kaplan PJ, Mohan S, Cohen P, et al. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res. 1999;59:2203–2209. [PubMed] [Google Scholar]
- 68.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 69.Stattin P, Bylund A, Rinaldi S, et al. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92:1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 70.Mantzoros CS, Tzonou A, Signorello LB, et al. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. Br J Cancer. 1997;76:1115–1118. doi: 10.1038/bjc.1997.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolk A, Mantzoros CS, Andersson SO, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 72.Shaneyfelt T, Husein R, Bubley G, Mantzoros CS. Hormonal predictors of prostate cancer: a meta-analysis. J Clin Oncol. 2000;18:847–853. doi: 10.1200/JCO.2000.18.4.847. [DOI] [PubMed] [Google Scholar]
- 73.Chan JM, Stampfer MJ, Ma J, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–1106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 74.Woodson K, Tangrea JA, Pollak M, et al. Serum insulin-like growth factor I: tumor marker or etiologic factor? A prospective study of prostate cancer among Finnish men. Cancer Res. 2003;63:3991–3994. [PubMed] [Google Scholar]
- 75.Ismail HA, Pollak M, Behlouli H, et al. Serum insulin-like growth factor (IGF)-1 and IGF-binding protein-3 do not correlate with Gleason score or quantity of prostate cancer in biopsy samples. BJU Int. 2003;92:699–702. doi: 10.1046/j.1464-410x.2003.04084.x. [DOI] [PubMed] [Google Scholar]
- 76.Scorilas A, Plebani M, Mazza S, et al. Serum human glandular kallikrein (hK2) and insulin-like growth factor 1 (IGF-1) improve the discrimination between prostate cancer and benign prostatic hyperplasia in combination with total and %free PSA. Prostate. 2003;54:220–229. doi: 10.1002/pros.10186. [DOI] [PubMed] [Google Scholar]
- 77.Bubley GJ, Balk SP, Regan MM, et al. Serum levels of insulin-like growth factor-1 and insulin-like growth factor-1 binding proteins after radical prostatectomy. J Urol. 2002;168:2249–2252. doi: 10.1016/S0022-5347(05)64365-0. [DOI] [PubMed] [Google Scholar]
- 78.Yanovski JA, Yanovski SZ. Recent advances in basic obesity research. JAMA. 1999;282:1504–1506. doi: 10.1001/jama.282.16.1504. [DOI] [PubMed] [Google Scholar]
- 79.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 80.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 81.Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003;278:42,660–42,667. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- 82.Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. J Surg Res. 2003;113:50–55. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 83.Stattin P, Soderberg S, Hallmans G, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86:1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 84.Saglam K, Aydur E, Yilmaz M, Goktas S. Leptin influences cellular differentiation and progression in prostate cancer. J Urol. 2003;169:1308–1311. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]
- 85.Stattin P, Kaaks R, Johansson R, et al. Plasma leptin is not associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:474–475. [PubMed] [Google Scholar]
- 86.Lagiou P, Signorello LB, Trichopoulos D, et al. Leptin in relation to prostate cancer and benign prostatic hyperplasia. Int J Cancer. 1998;76:25–28. doi: 10.1002/(sici)1097-0215(19980330)76:1<25::aid-ijc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 87.Chang S, Hursting SD, Contois JH, et al. Leptin and prostate cancer. Prostate. 2001;46:62–67. doi: 10.1002/1097-0045(200101)46:1<62::aid-pros1009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 88.Pandalai PK, Pilat MJ, Yamazaki K, et al. The effects of omega-3 and omega-6 fatty acids on in vitro prostate cancer growth. Anticancer Res. 1996;16:815–820. [PubMed] [Google Scholar]
- 89.Wang Y, Corr JG, Thaler HT, et al. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995;87:1456–1462. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 90.Ngo TH, Barnard RJ, Cohen P, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734–2743. [PubMed] [Google Scholar]
- 91.Ngo TH, Barnard RJ, Anton T, et al. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer Res. 2004;64:1252–1254. doi: 10.1158/0008-5472.can-03-3830. [DOI] [PubMed] [Google Scholar]
- 92.Barnard RJ, Ngo TH, Leung PS, et al. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate. 2003;5:201–206. doi: 10.1002/pros.10251. [DOI] [PubMed] [Google Scholar]
- 93.Rubin MA, Zhou M, Dhanasekaran SM, et al. α-Methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA. 2002;287:1662–1670. doi: 10.1001/jama.287.13.1662. [DOI] [PubMed] [Google Scholar]
- 94.Luo J, Zha S, Gage WR, et al. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002;62:2220–2226. [PubMed] [Google Scholar]
- 95.Swinnen JV, Roskams T, Joniau S, et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 96.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: A metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 97.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 98.Grant WB. A multicountry ecologic study of risk and risk reduction factors for prostate cancer mortality. Eur Urol. 2004;45:271–279. doi: 10.1016/j.eururo.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 99.Hursting SD, Thornquist M, Henderson MM. Types of dietary fat and the incidence of cancer at five sites. Prev Med. 1990;19:242–253. doi: 10.1016/0091-7435(90)90025-f. [DOI] [PubMed] [Google Scholar]
- 100.Hebert JR, Hurley TG, Olendzki BC, et al. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 101.Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91:414–428. doi: 10.1093/jnci/91.5.414. [DOI] [PubMed] [Google Scholar]
- 102.Moyad MA. Dietary fat reduction to reduce prostate cancer risk: controlled enthusiasm, learning a lesson from breast or other cancers, and the big picture. Urology. 2002;59(4) suppl 1:51–62. doi: 10.1016/s0090-4295(01)01176-1. [DOI] [PubMed] [Google Scholar]
- 103.Wilkens LR, Hankin JH, Yoshizawa CN, et al. Comparison of long-term dietary recall between cancer cases and noncases. Am J Epidemiol. 1992;136:825–835. doi: 10.1093/aje/136.7.825. [DOI] [PubMed] [Google Scholar]
- 104.Hebert JR, Miller DR. Methodologic considerations for investigating the diet-cancer link. Am J Clin Nutr. 1988;47:1068–1077. doi: 10.1093/ajcn/47.6.1068. [DOI] [PubMed] [Google Scholar]
- 105.Hirayama T. Epidemiology of prostate cancer with special reference to the role of diet. Natl Cancer Inst Monogr. 1979:149–155. [PubMed] [Google Scholar]
- 106.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49:1857–1860. [PubMed] [Google Scholar]
- 107.Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64:598–604. doi: 10.1002/1097-0142(19890801)64:3<598::aid-cncr2820640306>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 108.Hsing AW, McLaughlin JK, Schuman LM, et al. Diet, tobacco use, and fatal prostate cancer: results from the Lutheran Brotherhood Cohort Study. Cancer Res. 1990;50:6836–6840. [PubMed] [Google Scholar]
- 109.Schuurman AG, van den Brandt PA, Dorant E, et al. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86:1019–1027. [PubMed] [Google Scholar]
- 110.Gann PH, Hennekens CH, Sacks FM, et al. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86:281–286. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 111.Le Marchand L, Kolonel LN, Wilkens LR, et al. Animal fat consumption and prostate cancer: a prospective study in Hawaii. Epidemiology. 1994;5:276–282. doi: 10.1097/00001648-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Giovannucci E, Rimm EB, Colditz GA, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 113.Shirai T, Asamoto M, Takahashi S, Imaida K. Diet and prostate cancer. Toxicology. 2002;181–182:89–94. doi: 10.1016/s0300-483x(02)00260-3. [DOI] [PubMed] [Google Scholar]
- 114.Shirai T, Sano M, Tamano S, et al. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–198. [PubMed] [Google Scholar]
- 115.Meyer F, Bairati I, Shadmani R, et al. Dietary fat and prostate cancer survival. Cancer Causes Control. 1999;10:245–251. doi: 10.1023/a:1008913307947. [DOI] [PubMed] [Google Scholar]
- 116.Demark-Wahnefried W, Clipp EC, McBride C, et al. Design of FRESH START: a randomized trial of exercise and diet among cancer survivors. Med Sci Sports Exerc. 2003;35:415–424. doi: 10.1249/01.MSS.0000053704.28156.0F. [DOI] [PubMed] [Google Scholar]