Abstract
The intended therapeutic effect of gonadotropin-releasing hormone (GnRH) agonists is hypogonadism, which is a leading cause of osteoporosis in men. Consistent with this observation, GnRH agonists decrease bone mineral density and increase fracture risk in men with prostate cancer. GnRH agonists markedly decrease serum levels of both testosterone and estrogen. Estrogens play a central role in homeostasis of the normal male skeleton, and the available evidence suggests that estrogen deficiency rather than testosterone deficiency accounts for the adverse skeletal effects of GnRH agonists. The central role of estrogens in male bone metabolism provides a strong rationale to evaluate selective estrogen receptor modulators for prevention of treatment-related osteoporosis in men with prostate cancer. Preliminary evidence suggests that both raloxifene and toremifene increase bone mineral density in GnRH agonist-treated men. An ongoing pivotal study will evaluate the effects of toremifene on fractures and other complications of GnRH agonists in men with prostate cancer.
Key words: Bone mineral density, Gonadotropin-releasing hormone agonists, Osteoporosis, Prostate cancer, Raloxifene, Toremifene
Osteoporosis is often regarded as a disease of aging women, although it is also common in older men. In the United States, osteoporosis is prevalent in more than 2 million men, and another 12 million men are at risk (Table 1).1 Men experience one third of all hip fractures, and mortality after hip fractures is higher in men than in women.2
Table 1.
Osteoporosis in US Men
|
Data from National Osteoporosis Foundation.1
Alcohol abuse, chronic glucocorticoid therapy, and hypogonadism are the major causes of acquired osteoporosis in men.3 These 3 causes account for approximately one half of all cases of male osteoporosis. Hyperparathyroidism and hyperthyroidism are less common causes of osteoporosis in men. Smoking, low dietary calcium intake, vitamin D deficiency, and a sedentary lifestyle contribute to risk for osteoporosis.4
Hormone therapy for prostate cancer is a major cause of male hypogonadism. Gonadotropin-releasing hormone (GnRH) agonists are the mainstay of treatment for metastatic prostate cancer and a routine part of management for many men with locally advanced or recurrent non-metastatic prostate cancer. About 500,000 men are treated with a GnRH agonist annually in the United States.
Definition of Osteoporosis
Low bone mineral density correlates with fracture risk in men and women. A working group of the World Health Organization (WHO) defined osteopenia and osteoporosis in women based on bone mineral density compared with values in young adults (Table 2).5 Osteopenia is defined as bone mineral density between 1.0 and 2.5 standard deviations (SD) below the mean for young adults (T-score between −1.0 and −2.5). Osteoporosis is defined as bone mineral density more than 2.5 SD below the mean for young adults (T-score < −2.5). The fracture risk for women with osteoporosis is at least 5-fold greater than for women with normal bone mineral density.6 WHO definitions are routinely applied to men; however, application of the same limits for men is controversial because these definitions were developed from data in women, and men have larger bones and a higher peak bone mass than women.3
Table 2.
World Health Organization Classification of Osteoporosis
| Classification | Bone Mineral Density* |
|---|---|
| Normal | -1.0 SD and above |
| Osteopenia | Between -1.0 SD and -2.5 SD |
| Osteoporosis | -2.5 SD and below |
| Severe osteoporosis | -2.5 SD and below with fragility fractures |
Standard deviations (SD) are derived from the mean peak value in young adults.
Data from Kanis JA et al.5
Diagnosis
Bone mineral density can be measured by 3 noninvasive methods: dual-energy x-ray absorptiometry (DEXA), quantitative computed tomography (CT), and ultrasound.6
Dual-energy x-ray absorptiometry is the favored method to measure bone mineral density in most cases. DEXA can easily and precisely measure bone mineral density at several skeletal sites with nominal radiation exposure. The hip is the preferred skeletal site to screen men for osteoporosis using DEXA. Average bone mineral density of the posteroanterior lumbar spine by DEXA increases in men after age 55 years because of development of osteoarthritis in the posterior spinous elements. Accordingly, DEXA may overestimate real bone mineral density of the spine in older men.
Quantitative CT is a more sensitive but less precise method than DEXA to detect osteoporosis in men. Quantitative CT of the spine evaluates trabecular bone mineral density in the central region of the vertebral bodies and thus avoids the potential problems related to osteoarthritis. However, quantitative CT is not widely available.
Ultrasound is a portable and relatively inexpensive method to measure bone mineral density of the heel, finger, tibia, and patella. Ultrasound measurements of bone mineral density are less precise than other methods. This imaging technique evaluates skeletal sites that are unresponsive to treatment.
Clinical Manifestations
Compression fractures of the spine are the most common clinical manifestation of osteoporosis. Vertebral body compression fractures may result from bending, lifting, or other minimal stress. Pain results from collapse of vertebral bodies and may be worsened by standing or abrupt movements. Multiple vertebral fractures may cause loss of height, dorsal kyphosis, and cervical lordosis.
Compression fractures of the spine may be mistaken for bone metastases in men with prostate cancer. Patients with bone metastases and benign compression fractures often present with back pain. They also share radiographic findings, including tracer accumulation by bone scan.7 Because osteoporosis is common in men with prostate cancer, fractures should be included in the differential diagnosis when men present with bone pain.
Hip fractures are the gravest manifestation of osteoporosis. About one fifth of elderly individuals with hip fracture die from thromboembolic events, infection, and other complications. About one third of elderly hip fracture victims need long-term nursing home care.
GnRH Agonists and Osteoporosis
Fracture Risk
Retrospective studies have consistently reported high rates of clinical fracture in GnRH agonist-treated men with prostate cancer.8–11 In a large population-based cohort study, GnRH agonists significantly increased the risk of any clinical fractures, vertebral fractures, and hip fractures in men with nonmetastatic prostate cancer.12 Bilateral orchiectomies are also associated with increased fracture risk in men with prostate cancer.13,14
Bone Mineral Density
GnRH agonists significantly decrease bone mineral density in men with prostate cancer.15–20 Most studies report rates of bone loss similar to those associated with early menopause in women.6 Some, but not all, men develop osteoporosis during long-term hormone therapy. Pretreatment bone mineral density varies among men because of individual differences in peak bone mass and the rates of adult bone loss. Accordingly, men begin hormone therapy with different relative risks for developing osteoporosis. Rates of treatment-related bone loss also vary among individuals.
Mechanisms of Treatment-Related Osteoporosis
GnRH agonists increase bone turnover in men with prostate cancer.16,18 Biochemical markers of osteoclast and osteoblast activity rise progressively after treatment with a GnRH agonist and appear to reach a plateau after approximately 6 months.18 GnRH agonists increase parathyroid hormone-mediated osteoclast activation,21 suggesting that changes in skeletal sensitivity to parathyroid hormone play an important role in the pathogenesis of hypogonadal bone loss.
Estrogens play an important role in skeletal homeostasis in men. Estrogen receptors are expressed in osteoblasts and osteoclasts. In older men, serum estradiol levels are better predictors of bone mineral density and fracture risk than are serum testosterone levels.22–24 Estrogens contribute to the regulation of both bone formation and bone resorption in normal men.25,26 In addition, medical castration with estrogens does not decrease bone mineral density27 or increase biochemical markers of osteoclast activity28 in men with prostate cancer.
The central role of estrogens in male skeletal homeostasis suggests that estrogen deficiency rather than testosterone deficiency accounts for the adverse skeletal effects of GnRH agonists in men. These observations also provide a strong rationale for evaluating estrogen and selective estrogen receptor modulators (SERMs) for prevention of treatment-related osteoporosis in men with prostate cancer.
Estrogens to Prevent Treatment-Related Osteoporosis
In contrast to bilateral orchiectomy or treatment with a GnRH agonist, medical castration with estrogen is not associated with treatment-related osteoporosis. In a small nonrandomized study of men with prostate cancer, changes in bone mineral density were compared in men who underwent bilateral orchiectomy versus those who were treated with estrogen.27 Hip bone mineral density decreased by 10% after 1 year in men who underwent bilateral orchiectomy compared with only 1% in men treated with estrogen.
Estrogen replacement therapy may prevent osteoporosis in GnRH agonist-treated men, although information about the efficacy and safety of estrogens in castrate men with prostate cancer is limited. In a randomized, controlled trial of 25 castrate men with nonmetastatic prostate cancer, estradiol (1 mg/d) significantly decreased biochemical markers of osteoclast activity after 9 weeks.29 A small cross-sectional study reached similar conclusions.28
To date, no controlled trial has assessed the effects of medical castration with estrogen on bone mineral density or fracture risk in men with prostate cancer. Similarly, there have been no controlled trials to assess estrogen replacement therapy on clinical outcomes in castrate men with prostate cancer.
Selective Estrogen Receptor Modulators to Prevent Treatment-Related Osteoporosis
Raloxifene is a SERM approved to prevent and treat osteoporosis in women. Raloxifene mimics the beneficial effects of estrogen in bone without stimulatory effects in most other tissues.30 Raloxifene prevents early postmenopausal bone loss in women and reduces the rate of vertebral fractures in women with postmenopausal osteoporosis.31,32 Less is known about the effects of raloxifene in men. In a 12-month open-label study, men with non-metastatic prostate cancer who were receiving a GnRH agonist (n = 48) were assigned randomly to raloxifene (60 mg/d) or no raloxifene (Table 3).33 Bone mineral density of the posteroanterior lumbar spine and proximal femur were measured by DEXA. Raloxifene significantly increased bone mineral density of the hip and tended to increase bone mineral density of the spine. Raloxifene decreased biochemical markers of bone turnover, suggesting that raloxifene increases bone mineral density by similar mechanism(s) in postmenopausal women and hypogonadal men.
Table 3.
Randomized Trials of Selective Estrogen Receptor Modulators to Prevent Bone Loss During Gonadotropin-Releasing Hormone Agonist Therapy
| Study | n | Treatment | Outcomes |
|---|---|---|---|
| Smith et al33 | 48 | Raloxifene vs | Increased BMD of the |
| no raloxifene | hip and spine | ||
| Steiner et al34 | 46 | Toremifene vs | Increased BMD |
| placebo | Reduced hot flashes |
BMD, bone mineral density.
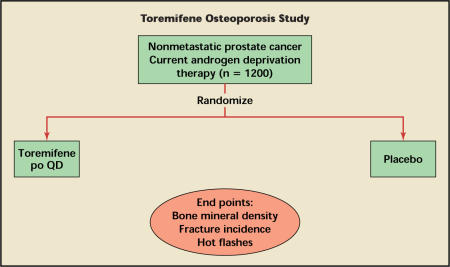
Toremifene is a SERM approved for the treatment of advanced breast cancer. It is also being developed for treatment of osteoporosis and other complications associated with hormone therapy for prostate cancer. In a 6-month placebo-controlled study, 46 men with prostate cancer who were receiving a GnRH agonist were assigned randomly to either toremifene or placebo (Table 3).34 Study outcomes included bone mineral density and hot-flash scores. Toremifene (60 mg/d) significantly increased bone mineral density and reduced hot flashes. In an ongoing pivotal phase III study, 1200 men who are receiving a GnRH agonist for prostate cancer will be randomly assigned to either toremifene or placebo. Study outcomes include fractures, bone mineral density, and hot flashes (Figure 1).
Figure 1.
Design of the ongoing study of toremifene in osteoporosis in nonmetastatic prostate cancer. po, by mouth; QD, every day.
Conclusions
GnRH agonists increase bone turn-over, decrease bone mineral density, and increase fracture risk in men with prostate cancer. Estrogen deficiency rather than testosterone deficiency appears to account for the adverse skeletal effects of GnRH agonists. Preliminary evidence suggests that SERMs decrease bone turnover and increase bone mineral density in GnRH agonist-treated men. An ongoing pivotal study will evaluate the effects of toremifene on fractures and other complications of GnRH agonists in men with prostate cancer.
Main Points.
World Health Organization definitions for osteopenia (T-score between −1.0 and −2.5) and osteoporosis (T-score < 2.5) are routinely applied to men; however, application of the same limits for men is controversial because these definitions were developed from data in women, and men have larger bones and a higher peak bone mass than women.
Compression fractures of the spine are the most common clinical manifestation of osteoporosis, but these may be mistaken for bone metastases in men with prostate cancer. Because osteoporosis is common in men with prostate cancer, fractures should be included in the differential diagnosis when men present with bone pain.
In a large population-based cohort study, gonadotropin-releasing hormone (GnRH) agonists significantly increased the risk of any clinical fractures, vertebral fractures, and hip fractures in men with nonmetastatic prostate cancer. Other studies have shown that GnRH agonists decrease bone mineral density and increase bone turnover.
The central role of estrogens in male skeletal homeostasis suggests that estrogen deficiency rather than testosterone deficiency accounts for the adverse skeletal effects of GnRH agonists in men. These observations also provide a strong rationale to evaluate estrogen and selective estrogen receptor modulators (SERMs) for prevention of treatment-related osteoporosis in men with prostate cancer.
Estrogen replacement therapy may prevent osteoporosis in GnRH agonist-treated men, although information about the efficacy and safety of estrogens in castrate men with prostate cancer is limited.
Preliminary evidence suggests that SERMs decrease bone turnover and increase bone mineral density in GnRH agonist-treated men.
References
- 1.National Osteoporosis Foundation, authors. [Accessed December 6, 2004];
- 2.Seeman E. The structural basis of bone fragility in men. Bone. 1999;25:143–147. doi: 10.1016/s8756-3282(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 3.Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab. 1999;84:3431–3434. doi: 10.1210/jcem.84.10.6060. [DOI] [PubMed] [Google Scholar]
- 4.Orwoll ES. Osteoporosis in men. Endocrinol Metab Clin North Am. 1998;27:349–367. doi: 10.1016/s0889-8529(05)70009-8. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, Melton LJ, 3d, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 6.Eastell R. Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736–746. doi: 10.1056/NEJM199803123381107. [DOI] [PubMed] [Google Scholar]
- 7.Schutte HE, Park WM. The diagnostic value of bone scintigraphy in patients with low back pain. Skeletal Radiol. 1983;10:1–4. doi: 10.1007/BF00355381. [DOI] [PubMed] [Google Scholar]
- 8.Daniell HW. Osteoporosis after orchiectomy for prostate cancer. J Urol. 1997;157:439–444. [see comments] [PubMed] [Google Scholar]
- 9.Townsend MF, Sanders WH, Northway RO, Graham SD., Jr Bone fractures associated with luteinizing hormone-releasing hormone agonists used in the treatment of prostate carcinoma. Cancer. 1997;79:545–550. doi: 10.1002/(sici)1097-0142(19970201)79:3<545::aid-cncr17>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Hatano T, Oishi Y, Furuta A, et al. Incidence of bone fracture in patients receiving luteinizing hormone-releasing hormone agonists for prostate cancer. BJU Int. 2000;86:449–452. doi: 10.1046/j.1464-410x.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- 11.Oefelein MG, Ricchuiti V, Conrad W, et al. Skeletal fracture associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patients with prostate cancer. J Urol. 2001;166:1724–1728. doi: 10.1016/s0022-5347(05)65661-3. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Lee W, Krupski T, et al. Association between androgen deprivation therapy and fracture risk: a population-based cohort study in men with nonmetastatic prostate cancer. Paper presented at: the 40th American Society of Clinical Oncology Annual Meeting; June 5–8, 2004; New Orleans, La. [Google Scholar]
- 13.Melton LJ, 3d, Alothman KI, Khosla S, et al. Fracture risk following bilateral orchiectomy. J Urol. 2003;169:1747–1750. doi: 10.1097/01.ju.0000059281.67667.97. [DOI] [PubMed] [Google Scholar]
- 14.Dickman PW, Adolfsson J, Astrom K, Steineck G. Hip fractures in men with prostate cancer treated with orchiectomy. J Urol. 2004;172:2208–2212. doi: 10.1097/01.ju.0000143930.73016.c6. [DOI] [PubMed] [Google Scholar]
- 15.Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998;83:1561–1566. [PubMed] [Google Scholar]
- 16.Maillefert JF, Sibilia J, Michel F, et al. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–1222. [PubMed] [Google Scholar]
- 17.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 18.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss in men receiving gonadotropin releasing hormone agonist therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 19.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. [PubMed] [Google Scholar]
- 20.Smith MR, Eastham J, Gleason D, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men undergoing androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 21.Leder BZ, Smith MR, Fallon MA, et al. Effects of gonadal steroid suppression on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab. 2001;86:511–516. doi: 10.1210/jcem.86.2.7177. [DOI] [PubMed] [Google Scholar]
- 22.Slemenda CW, Longcope C, Zhou L, et al. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. J Clin Invest. 1997;100:1755–1759. doi: 10.1172/JCI119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khosla S, Melton LJ, 3d, Atkinson EJ, et al. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 24.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res. 1997;12:1833–1843. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- 25.Falahati-Nini A, Riggs BL, Atkinson EJ, et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leder BZ, LeBlanc KM, Schoenfeld DA, et al. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–210. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson S, Eriksson A, Stege R, Carlstrom K. Bone mineral density in patients with prostatic cancer treated with orchidectomy and with estrogens. Calcif Tissue Int. 1995;57:97–99. doi: 10.1007/BF00298427. [DOI] [PubMed] [Google Scholar]
- 28.Scherr D, Pitts WR, Jr, Vaughn ED., Jr Diethylstilbesterol revisited: androgen deprivation, osteoporosis and prostate cancer. J Urol. 2002;167:535–538. doi: 10.1016/S0022-5347(01)69080-3. [DOI] [PubMed] [Google Scholar]
- 29.Taxel P, Fall PM, Albertsen PC, et al. The effect of micronized estradiol on bone turnover and calciotropic hormones in older men receiving hormonal suppression therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:4907–4913. doi: 10.1210/jc.2002-020539. [DOI] [PubMed] [Google Scholar]
- 30.Balfour JA, Goa KL. Raloxifene. Drugs Aging. 1998;12:335–341. doi: 10.2165/00002512-199812040-00006. [DOI] [PubMed] [Google Scholar]
- 31.Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 32.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 33.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 34.Steiner MS, Patterson A, Israeli R, et al. Toremifene citrate versus placebo for treatment of bone loss and other complications of androgen deprivation therapy in patients with prostate cancer. Paper presented at: the 40th American Society of Clinical Oncology Annual Meeting; June 5–8, 2004; New Orleans, La. [Google Scholar]