Abstract
To determine if any heat shock proteins are incorporated into human immunodeficiency virus type 1 (HIV-1) virions in a manner similar to that of the peptidyl-prolyl isomerase cyclophilin A, we probed purified virions with antibodies against heat shock proteins Hsp27, Hsp40, Hsp60, Hsp70, Hsc70, and Hsp90. Of these proteins, Hsp60, Hsp70, and Hsc70 associated with virions purified based on either particle density or size and were shown to be incorporated within the virion membrane, where they were protected from digestion by exogenous protease. Virion incorporation of Hsp70 was also observed with HIV-2 and with simian immunodeficiency viruses SIVMAC and SIVAGM, but it appears to be specific for primate lentiviruses, since Hsp70 was not detected in association with Moloney murine leukemia virus virions. Of the HIV-1 genes, gag was found to be sufficient for Hsp70 incorporation, though Hsp70 was roughly equimolar with pol-encoded proteins in virions.
The Gag proteins of human immunodeficiency virus type 1 (HIV-1) play numerous roles in the viral life cycle from assembly to early postentry steps (10). By an unknown mechanism which most likely utilizes host factors, the Gag polyprotein is transported to the plasma membrane, where it directs the formation and release of enveloped virions from infected cells. Concurrent with the release of nascent virions, the viral protease cleaves the Gag polyprotein into matrix (MA), capsid (CA), nucleocapsid (NC), and p6 proteins. Upon entry into a new cell, the viral ribonucleoprotein complex is released into the cytoplasm, where gag-encoded proteins participate in reverse transcription, transport of the viral preintegration complex into the nucleus, and possibly establishment of the provirus.
Since the roles played by HIV-1 Gag proteins are numerous and complex, it has been hypothesized that host factors might be required for gag-encoded functions. Many Gag-interacting factors have been identified, including actin (20, 25, 29, 37), ubiquitin (24), calmodulin (28), the motor protein KIF-4 (33), the nuclear transporter karyopherin-alpha (11), the human nuclear shuttling protein VAN (14), translation elongation factor 1-alpha (7), translation initiation factor 2 (38), and the HO3 histidyl-tRNA synthetase (18). Via interaction with the PTAP motif in the p6 domain of Gag, the ubiquitin-conjugating enzyme homologue Tsg101 is targeted to the site of virion budding, where it is required for fission of the nascent virion membrane from the host cell membrane (12, 23, 34). Cyclophilin A (CyPA) is incorporated into HIV-1 virions via interaction with the CA domain of the Gag polyprotein and is required for wild-type viral replication kinetics (5). CyPA is a member of a large family of proteins which catalyze the isomerization of peptidyl-prolyl bonds (13) and protect cells from heat shock (32).
Several stress proteins have been shown to play roles in the life cycle of a variety of RNA and DNA viruses (30), and there is growing evidence that some of these proteins may also be important for HIV-1 replication. Heat shock protein 60 (Hsp60) copurifies with HIV-1 and simian immunodeficiency virus (SIV) virions, though it was not demonstrated that this cellular protein is a bona fide virion component (2). Hsp70 and Hsp27 expression is selectively increased following infection with HIV-1 (35), and it has been suggested that Hsp70 plays a role in the nuclear import of HIV-1 preintegration complexes (1). In addition, Mason-Pfizer monkey virus assembly in tissue culture and HIV-1 assembly in vivo and in vitro are ATP-dependent processes (16, 19, 36), consistent with a requirement for the ATPase activity of a heat shock protein.
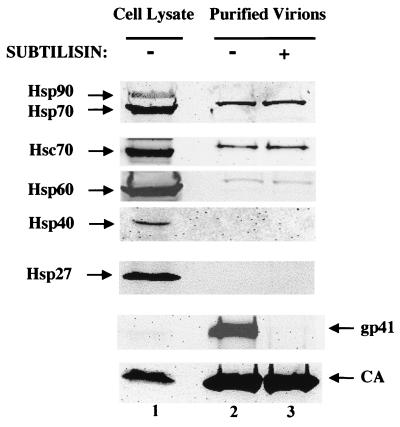
To find out whether any heat shock proteins other than CyPA are incorporated into HIV-1 virions, we transfected 293T cells with the proviral DNA-containing plasmid pNL4-3 and harvested and purified virions from the culture supernatant by sedimentation through 25% sucrose cushions as previously described (4). Proteins associated with the purified virions were then probed in Western blots with antibodies specific for Hsp27, Hsp40, Hsp60, Hsc70, Hsp70, and Hsp90 (all from Santa Cruz Biotechnology, Santa Cruz, Calif.) as well as for CA (National Institutes of Health AIDS Reagent Program no. 740) and gp41 (NEN Life Science Inc., Boston, Mass.). Using the subtilisin protease protection assay (26), we demonstrated that, of these proteins, Hsp60, Hsc70, and Hsp70 were incorporated within the virion membrane (Fig. 1). Since the Western blot signal was strongest with the anti-Hsp70 antibody, we concentrated on this protein for our subsequent experiments.
FIG. 1.
Presence of heat shock proteins in HIV-1 virions. Virions produced by 293T cells transfected with HIV-1NL4-3 proviral DNA were purified by centrifugation through a 25% sucrose cushion. The purified virions were either mock treated (lane 2) or treated with 0.2 mg of subtilisin/ml (lane 3). Subsequently, the transfected cell lysates (lane 1) and virions (lanes 2 and 3) were analyzed by Western blotting with antibodies against gp41, HIV-1 CA, and the indicated heat shock proteins.
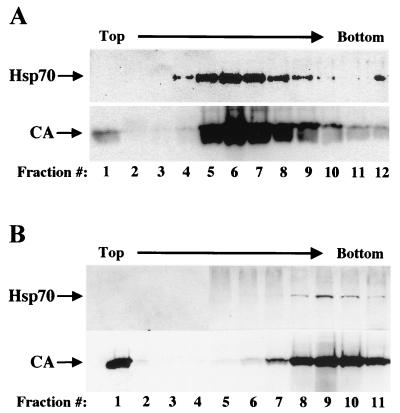
To provide further evidence that Hsp70 is a bona fide virion protein, we examined its localization with respect to a virion marker (CA) on a 20 to 60% linear sucrose density gradient as previously described (9). Gradient fractions were collected and probed by immunoblotting, and it was found that Hsp70 cosedimented with CA (Fig. 2A). Nonetheless, cell membranemicrovesicles are of comparable density to virions and may contaminate HIV-1 particles purified on linear sucrose density gradients (3). To determine whether the Hsp70 signal detected in the density gradient fractions reflected contamination with microvesicles, we performed a velocity sedimentation gradient with 6 to 18% iodixanol (OptiPrep; Invitrogen), which separates HIV-1 virions from the majority of contaminating microvesicles (9). Once again, both Hsp70 and CA cosedimented in the same fractions, providing further evidence for the specific incorporation of Hsp70 into the virions (Fig. 2B).
FIG. 2.
Hsp70 copurifies with HIV-1 virions after purification based on either density or particle size. Virions in culture supernatant from HIV-1NL4-3 proviral DNA-transfected 293T cells were resuspended after sedimentation through a 25% sucrose cushion and then layered onto either of two gradients as follows: (A) 20 to 60% linear sucrose gradient accelerated to equilibrium (16 h at 100,000 × g) to monitor particle density, or (B) 6 to 18% linear iodixanol gradient accelerated for a shorter duration (250,000 × g for 1.5 h) to monitor velocity of particle sedimentation. Fractions were collected from the top of each gradient (as indicated by numbers across the bottom of each pair of panels) and analyzed by immunoblotting with anti-Hsp70 and anti-CA antibodies (as indicated).
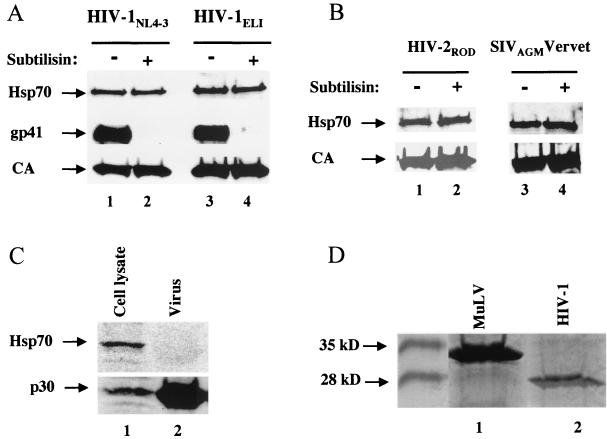
We next examined two other common HIV-1 laboratory strains (HIV-1LAI and HIV-1HXB2), as well as a more distantly related, primary HIV-1 isolate (HIV-1ELI), and showed that virions encoded by these viruses also incorporate Hsp70-family members (Fig. 3A and data not shown). Hsp70 incorporation into HIV-1 was not specific to the virions produced by 293T cells, since virions produced by transfected HeLa cells or Jurkat T cells harboring a spreading infection gave an equally strong signal for Hsp70 (data not shown). Thus, virions produced by T cells also contain Hsp70. We also checked several proviral clones from different subgroups of primate lentiviruses and found that HIV-2ROD, SIVMAC239, SIVAGMVervet, and SIVAGMGrivet incorporated Hsp70 with roughly the same efficiency as HIV-1 (Fig. 3B and data not shown).
FIG. 3.
Hsp70 is incorporated into virions produced by three different subgroups of primate lentiviruses. Virions were purified from the supernatant of 293T cells transfected with the following indicated proviral DNAs: (A) HIV-1NL4-3 or HIV-1ELI or (B) HIV-2ROD or SIVAGMVervet. After subtilisin treatment, Western blot analysis was performed using antibodies against Hsp70 or CA (A and B) or HIV-1 gp41 (A). (C) MLV virions were harvested from the supernatant of chronically infected Rat-2 cells. Immunoblot analysis was performed on the infected cell lysate and purified virions with antibodies against Hsp70 and MLV p30 CA. (D) The same amounts of HIV-1NL4-3 and MLV virion samples as used in the gels shown in panels A and C, respectively, were processed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were visualized with Coomassie blue to directly compare the relative amounts of the two viral CAs. The arrows point at the molecular mass standards.
To determine whether Hsp70 packaging into virions is specific to HIV-1 and related primate lentiviruses, we examined Moloney murine leukemia virus (MLV) virions purified from the supernatant of chronically infected Rat-2 cells. Immunoblot analysis was performed using anti-Hsp70 antibody (catalogue no. sc-1060, human and rat cross-reactive; Santa Cruz) along with an antibody that recognizes MLV CA (79S-804; National Cancer Institute). Unlike the results of our experiments with primate lentiviruses, we were unable to detect Hsp70 in association with MLV virions (Fig. 3C), despite the fact that our MLV virion preparation was three to four times more concentrated than our HIV-1 virion preparation (compare lanes 1 and 2 in Fig. 3D).
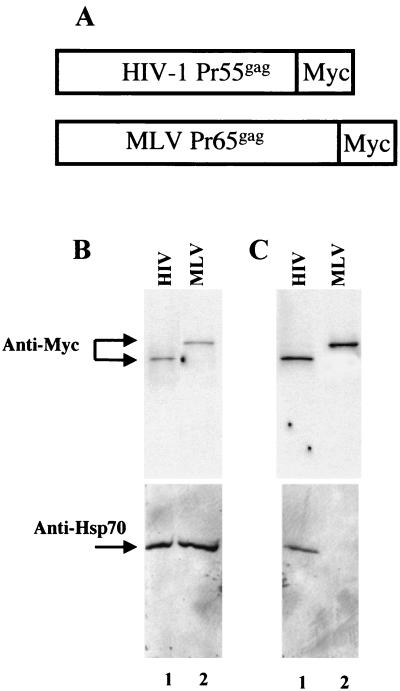
Expression of the HIV-1 Gag polyprotein is sufficient for the assembly and release of virus-like particles (VLPs) from the plasma membrane. To check whether VLPs formed by HIV-1 Gag incorporate Hsp70 in the absence of other viral proteins, we PCR amplified and cloned a previously described gag cDNA (that was modified to be Rev independent) (31) into mammalian expression vector pEF /myc/cyto (Invitrogen) such that it was in-frame with a myc tag at the carboxyl terminus. Since MLV does not incorporate Hsp70, we also cloned MLV gag into the same expression vector as a negative control. 293T cells were transfected with these two constructs, and VLPs were purified from the supernatant through a 25% sucrose cushion. The cell lysates and purified VLPs were analyzed by Western blotting with antibodies against the myc tag (Santa Cruz) and Hsp70. We found that HIV-1 Gag is sufficient for the incorporation of Hsp70 (Fig. 4C, lane 1). Even though MLV Gag is well expressed and forms VLPs just as efficiently as HIV-1 Gag, it does not incorporate Hsp70 (Fig. 4C, lane 2), consistent with the fact that infectious MLV virions do not incorporate Hsp70 (Fig. 3C).
FIG. 4.
Gag is sufficient for Hsp70 incorporation into HIV-1 virions. (A) Schematic representation of the HIV-1 and MLV Gag coding constructs. Both constructs were fused to a myc tag to permit normalization of the purified VLPs using the same antibody. (B and C) 293T cells were transfected with the Gag-expression constructs shown in panel A. Cell lysates (B) and purified VLPs produced by these cells (C) were analyzed by Western blotting by using anti-myc (top panels) and anti-Hsp70 antibodies (bottom panels).
Finally, we determined the molar ratio of Hsp70 to CA in a purified, subtilisin-treated, HIV-1NL4-3 virion preparation. Virion-associated Hsp70 and CA were quantitated by comparing Western blot signal intensities to the intensities obtained by serial dilution of purified Hsp70 (Stressgen, Victoria, Canada) and CA (Intracell Corp.) standards. The concentration and purity of the protein standards were confirmed by using Pierce BCA protein assay reagent (Pierce Chemical, Rockford, Ill.) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis, respectively. Using Kodak Image Station 440CF with 1D image analysis software to quantitate the signal intensities, we estimated that the molar ratio of Hsp70 to CA is in the 1:25 to 1:30 range (data not shown). This indicates that the amount of incorporated Hsp70 is similar to the amount of viral pol protein that is incorporated into HIV-1 virions.
Hsp70 protein family members, by controlled binding and release, facilitate the folding, oligomeric assembly-disassembly, and intracellular transport of protein complexes (15). Both the stress-inducible protein Hsp70 and its constitutive form, Hsc70, interact with various viral proteins and may be involved in the assembly of adenovirus (21), enterovirus (22), and polyomavirus capsid protein complexes (8). Hence, Hsp70 and Hsc70 could bind to nascent HIV-1 Gag polyprotein chains and hold them in an assembly-competent conformation during transport to the plasma membrane. Alternatively, upon entry into susceptible target cells, virion-associated Hsp70 might participate in early events of infection. For example, Hsp70 might actively uncoat the viral capsid in a manner similar to its role in the uncoating of clathrin cages (6). Hsp60, Hsp70, and Hsp90 have been shown to interact with hepatitis B virus reverse transcriptase and to facilitate the initiation of viral DNA synthesis from hepatitis B virus pregenomic RNA (17, 27). Thus, the Hsp70, Hsc70, and Hsp60 proteins in HIV-1 virions might serve a similar function in the initiation of HIV-1 cDNA synthesis. Finally, the Hsp70 and Hsc70 proteins might target the HIV-1 viral preintegration complex to the nuclear pore complexes, as has been suggested by others (1).
Acknowledgments
We thank Vanessa Hirsch and Keith Peden for proviral clones and David Ott and Markus Dettenhofer for technical advice.
This work was supported by grant AI 41857 (J.L.) and by shared core facilities of the Columbia-Rockefeller Center for AIDS Research (P30 AI42848), both from the U.S. National Institutes of Health.
REFERENCES
- 1.Agostini, I., S. Popov, J. Li, L. Dubrovsky, T. Hao, and M. Bukrinsky. 2000. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex. Exp. Cell Res. 259:398-403. [DOI] [PubMed] [Google Scholar]
- 2.Bartz, S. R., C. D. Pauza, J. Ivanyi, S. Jindal, W. J. Welch, and M. Malkovsky. 1994. An Hsp60 related protein is associated with purified HIV and SIV. J. Med. Primatol. 23:151-154. [DOI] [PubMed] [Google Scholar]
- 3.Bess, J. W., Jr., R. J. Gorelick, W. J. Bosche, L. E. Henderson, and L. O. Arthur. 1997. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology 230:134-144. [DOI] [PubMed] [Google Scholar]
- 4.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 70:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braaten, D., and J. Luban. 2001. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20:1300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell, T. G., W. J. Welch, D. M. Schlossman, K. B. Palter, M. J. Schlesinger, and J. E. Rothman. 1986. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell 45:3-13. [DOI] [PubMed] [Google Scholar]
- 7.Cimarelli, A., and J. Luban. 1999. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 73:5388-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cripe, T. P., S. E. Delos, P. A. Estes, and R. L. Garcea. 1995. In vivo and in vitro association of hsc70 with polyomavirus capsid proteins. J. Virol. 69:7807-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dettenhofer, M., and X. F. Yu. 1999. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J. Virol. 73:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freed, E. O. 1998. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. [DOI] [PubMed] [Google Scholar]
- 11.Gallay, P., V. Stitt, C. Mundy, M. Oettinger, and D. Trono. 1996. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J. Virol. 70:1027-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 13.Gething, M. J., and J. Sambrook. 1992. Protein folding in the cell. Nature 355:33-45. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, K., D. Ott, T. J. Hope, R. F. Siliciano, and J. D. Boeke. 2000. A human nuclear shuttling protein that interacts with human immunodeficiency virus type 1 matrix is packaged into virions. J. Virol. 74:11811-11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-579. [DOI] [PubMed] [Google Scholar]
- 16.Hong, S., G. Choi, S. Park, A. S. Chung, E. Hunter, and S. S. Rhee. 2001. Type D retrovirus Gag polyprotein interacts with the cytosolic chaperonin TRiC. J. Virol. 75:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu, J., and C. Seeger. 1996. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 93:1060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lama, J., and D. Trono. 1998. Human immunodeficiency virus type 1 matrix protein interacts with cellular protein HO3. J. Virol. 72:1671-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingappa, J. R., R. L. Hill, M. L. Wong, and R. S. Hegde. 1997. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell-free system. J. Cell Biol. 136:567-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, B., R. Dai, C. J. Tian, L. Dawson, R. Gorelick, and X. F. Yu. 1999. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J. Virol. 73:2901-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macejak, D. G., and R. B. Luftig. 1991. Association of HSP70 with the adenovirus type 5 fiber protein in infected HEp-2 cells. Virology 180:120-125. [DOI] [PubMed] [Google Scholar]
- 22.Macejak, D. G., and P. Sarnow. 1992. Association of heat shock protein 70 with enterovirus capsid precursor P1 in infected human cells. J. Virol. 66:1520-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 24.Ott, D. E., L. V. Coren, T. D. Copeland, B. P. Kane, D. G. Johnson, R. C. Sowder II, Y. Yoshinaka, S. Oroszlan, L. O. Arthur, and L. E. Henderson. 1998. Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus. J. Virol. 72:2962-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ott, D. E., L. V. Coren, D. G. Johnson, B. P. Kane, R. C. Sowder II, Y. D. Kim, R. J. Fisher, X. Z. Zhou, K. P. Lu, and L. E. Henderson. 2000. Actin-binding cellular proteins inside human immunodeficiency virus type 1. Virology 266:42-51. [DOI] [PubMed] [Google Scholar]
- 26.Ott, D. E., L. V. Coren, D. G. Johnson, R. C. Sowder II, L. O. Arthur, and L. E. Henderson. 1995. Analysis and localization of cyclophilin A found in the virions of human immunodeficiency virus type 1 MN strain. AIDS Res. Hum. Retrovir. 11:1003-1006. [DOI] [PubMed] [Google Scholar]
- 27.Park, S. G., and G. Jung. 2001. Human hepatitis B virus polymerase interacts with the molecular chaperonin Hsp60. J. Virol. 75:6962-6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radding, W., J. P. Williams, M. A. McKenna, R. Tummala, E. Hunter, E. M. Tytler, and J. M. McDonald. 2000. Calmodulin and HIV type 1: interactions with Gag and Gag products. AIDS Res. Hum. Retrovir. 16:1519-1525. [DOI] [PubMed] [Google Scholar]
- 29.Rey, O., J. Canon, and P. Krogstad. 1996. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology 220:530-534. [DOI] [PubMed] [Google Scholar]
- 30.Santoro, M. G. 1994. Heat shock proteins and virus replication: hsp70s as mediators of the antiviral effects of prostaglandins. Experientia 50:1039-1047. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz, S., M. Campbell, G. Nasioulas, J. Harrison, B. K. Felber, and G. N. Pavlakis. 1992. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J. Virol. 66:7176-7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sykes, K., M. J. Gething, and J. Sambrook. 1993. Proline isomerases function during heat shock. Proc. Natl. Acad. Sci. USA 90:5853-5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang, Y., U. Winkler, E. O. Freed, T. A. Torrey, W. Kim, H. Li, S. P. Goff, and H. C. Morse III. 1999. Cellular motor protein KIF-4 associates with retroviral Gag. J. Virol. 73:10508-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VerPlank, L., F. Bouamr, T. J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C. A. Carter. 2001. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag). Proc. Natl. Acad. Sci. USA 98:7724-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wainberg, Z., M. Oliveira, S. Lerner, Y. Tao, and B. G. Brenner. 1997. Modulation of stress protein (hsp27 and hsp70) expression in CD4+ lymphocytic cells following acute infection with human immunodeficiency virus type-1. Virology 233:364-373. [DOI] [PubMed] [Google Scholar]
- 36.Weldon, R. A., Jr., W. B. Parker, M. Sakalian, and E. Hunter. 1998. Type D retrovirus capsid assembly and release are active events requiring ATP. J. Virol. 72:3098-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilk, T., B. Gowen, and S. D. Fuller. 1999. Actin associates with the nucleocapsid domain of the human immunodeficiency virus Gag polyprotein. J. Virol. 73:1931-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson, S. A., C. Sieiro-Vazquez, N. J. Edwards, O. Iourin, E. D. Byles, E. Kotsopoulou, C. S. Adamson, S. M. Kingsman, A. J. Kingsman, and E. Martin-Rendon. 1999. Cloning and characterization of hIF2, a human homologue of bacterial translation initiation factor 2, and its interaction with HIV-1 matrix. Biochem. J. 342:97-103. [PMC free article] [PubMed] [Google Scholar]