Abstract
We report a multiresistant Enterobacter cloacae outbreak in an intensive care unit, associated with mattresses and with antibacterial-treated and vapour-permeable polyurethane synthetic mattress covers of therapeutic beds.
An increased risk of infection and pressure sores is associated with contaminated mattresses [1-6]. This is mostly due to disruption of the integrity of the mattress cover surface [7]. Antibacterial-treated and vapour-permeable polyurethane synthetic mattress covers have been developed to reduce bacterial and fungal colonisation of mattresses. These are currently widely used on therapeutic beds. These covers are considered easy to clean and to disinfect.
We report an outbreak associated with recently developed therapeutic beds in an intensive care unit in which there appeared to be satisfactory nursing procedures and in which the mattress covers appeared to be visually intact. Our observations allow us to propose additional measures to the recommended maintenance procedure to avoid this type of hospital infection.
Between 1 February and 30 May 2005, a cluster of 15 patients infected/colonised (12 infected and three colonised) by a third-generation cephalosporin-resistant Enterobacter cloacae was observed in the surgery intensive care unit of the teaching hospital of Tours, France. Molecular typing of E. cloacae isolates identified a clonal strain responsible for seven of the infection/colonisation cases (Fig. 1). A review of the procedures and techniques of the intensive care unit medical staff revealed a common factor among the infected/colonised patients: all patients had been nursed on therapeutic beds. These beds (n = 6) consisted of a soft, dense, modern foam mattress covered with a waterproof antibacterially treated permeable polyurethane cover. The mattresses and covers had been recently acquired (<18 months) and the covers were cleaned daily using an adequate procedure according to manufacturer recommendations and with a common hospital cleaning agent.
Figure 1.
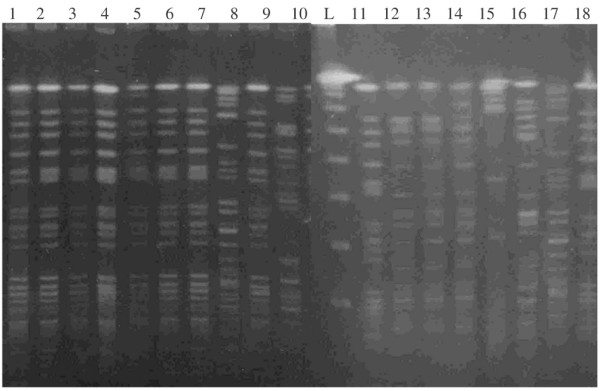
Using pulse field gel electrophoresis, an Enterobacter cloacae clonal type was found with four environmental isolates (lanes 1–4) and six clinical isolates (lanes 5–7, 9, 11 and 18).
As the beds were the only common factor among the infected/colonised patients, we suspected that these beds participated in the outbreak. However, visual inspection of the covers did not reveal any alterations. We therefore removed the covers and noticed that the foam underneath was stained, especially where the patient had been situated (Fig. 2a) and where the seams of the cover were located (Fig. 2b). Microbial swabbing revealed a high level of bacterial contamination of all six permeable polyurethane covers and mattress foams. We found epidemiological links between environmental and clinical strains from 10 patients (Fig. 1), demonstrating that at least three of the mattresses were the probable cause of the outbreak. The outbreak was stopped by discarding the six contaminated mattresses and covers.
Figure 2.
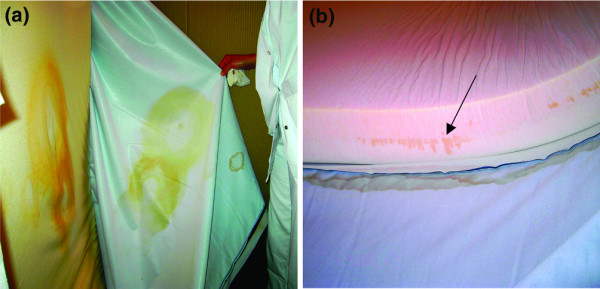
Photographs of a 6-month-old therapeutic bed. (a) Stained mattress foam where the patient was situated. (b) Stained mattress foam where the seams of the cover were situated.
The observation of stained foam where the seams of the covers were situated suggested a fault in the impermeability of the covers at this point and suggested that fluids penetrated the foam of the mattress through the seams. As the cleaning procedure recommends vigorous cleaning around the seams, it is probable that the cleaning procedure altered the cover, causing a loss of impermeability at the seams. This may also explain the observed stained foam where the patient was situated, which is a place that is intensively and frequently cleaned due to urine contamination. As the covers no longer remained an effective impermeable barrier, any spilt body fluids would make the mattress foam wet. The mattresses then acted as a bacterial reservoir capable of sustaining the growth of Enterobacteriaceae because, as previously demonstrated, soluble material within permeable polyurethane can serve as carbon and nitrogen sources for bacterial growth [8]. The weight of the patient then caused bacteria-containing aerosols, which contaminated the patient's surroundings despite daily cleaning of the cover, thus generating transmission.
We believe this study is the first report of mattress contamination associated with recently developed therapeutic beds for decreasing the risk of infection and pressure sores. Our data suggest that the actual recommended maintenance procedure may be ineffective and could be potentially dangerous. We therefore propose stricter conditions for checking of the mattresses, including the systematic removal of the mattress cover once the patient has been discharged. Any stained mattresses should be discarded. Our observations also suggest the need for studying the clinical circumstances leading to the regular replacement of covers to prevent the problems of their rapid loss of impermeability.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
NvdM-M made substantial contributions to the analysis and interpretation of data, and drafted the manuscript. SG carried out the molecular genetic studies. FL, DB, IV and IL made substantial contributions to the acquisition of clinical data. J-MB and RQ were involved in drafting the manuscript and revising it critically for important intellectual content.
References
- Fujita K, Lilly HA, Kidson A, Ayliffe GAJ. Gentamicin-resistant Pseudomonas aeruginosa infection from mattresses in a burn unit. Br Med J. 1981;283:219–220. doi: 10.1136/bmj.283.6285.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndawula EM, Brown L. Mattresses as reservoirs of epidemic MRSA [letter] Lancet. 1991;337:488. doi: 10.1016/0140-6736(91)93420-E. [DOI] [PubMed] [Google Scholar]
- O'Donoghue MAT, Allen KD. Costs of an outbreak of wound infections in an orthopaedic ward. J Hosp Infect. 1992;22:73–79. doi: 10.1016/0195-6701(92)90132-6. [DOI] [PubMed] [Google Scholar]
- Orr KE, Gould FK, Perry JD, Ford M, Morgan S, Sisson PR, Morrison D. Therapeutic beds: the Trojan horses of the 1990s? Lancet. 1994;342:65–66. doi: 10.1016/S0140-6736(94)91092-8. [DOI] [PubMed] [Google Scholar]
- Peto R, Calrow A. An audit of mattresses in one teaching hospital. Professional Nurse. 1996;11:623–626. [PubMed] [Google Scholar]
- Thomas S. Observations on mattress covers: results of a pilot study. J Tissue Viability. 1998;8:5–11. doi: 10.1016/s0965-206x(98)80004-1. [DOI] [PubMed] [Google Scholar]
- Rithalia S. Pressure sores: which foam mattress and why? J Tissue Viability. 1996;6:115–119. [Google Scholar]
- Jenkins RO, Sherburn RE. Growth and survival of bacteria implicated in sudden infant death syndrome on cot mattress materials. J Appl Microbiol. 2005;99:573–579. doi: 10.1111/j.1365-2672.2005.02620.x. [DOI] [PubMed] [Google Scholar]


