Abstract
Viruses and viral components can be potent inducers of alpha/beta interferons (IFN-α/β). In culture, IFN-α/β prime for their own expression, in response to viruses, through interferon regulatory factor 7 (IRF-7) induction. The studies presented here evaluated the requirements for functional IFN receptors and the IFN signaling molecule STAT1 in IFN-α/β induction during infections of mice with lymphocytic choriomeningitis virus (LCMV). At 24 h after infection, levels of induced IFN-α/β in serum were reduced 90 to 95% in IFN-α/β receptor-deficient (IFN-α/βR−/−) and STAT1−/− mice compared to those in wild-type mice. However, at 48 h, these mice showed elevated expression in the serum whereas IFN-α/β levels were still reduced >75% in IFN-α/βγR−/− mice even though the viral burden was heavy. Levels of IFN-β, IFN-α4, and non-IFN-α4 subtype mRNA expression correlated with IFN-α/β bioactivity, and all IFN-α/β subtypes were coincidentally detectable. IRF-7 mRNA was induced under conditions of IFN-α/β production, including late production in IFN-α/βR−/− mice. These data demonstrate that the presence of the virus alone is not sufficient to induce IFN-α/β during LCMV infection in vivo. Instead, autocrine amplification through the IFN-α/βR is necessary for optimal induction. In the absence of a functional IFN-α/βR, however, alternative mechanisms, independent of STAT1 but requiring a functional IFN-γR, take over.
Many viral infections, including those with lymphocytic choriomeningitis virus (LCMV), induce high levels of alpha/beta interferons (IFN-α/β) early (3). The importance of these cytokines for defense goes beyond their antiviral activities and includes many immunoregulatory functions (4, 5, 8, 16). The factors are products of a multigene family including 1 IFN-β gene and at least 12 IFN-α genes. Each gene is regulated by its own distinct promoter, resulting in differential expression of subtypes. Viruses induce transcription of IFN-α/β genes in cells by several different pathways. Some of these involve extracellular interactions between viral glycoproteins and cell surface receptors, whereas others rely on intracellular interactions between viral components and cytoplasmic receptors (6, 15). IFN-α/β can amplify their own expression in vitro through a positive feedback loop (14, 22). This occurs in a two-step fashion, with the subtypes expressed early, IFN-β and IFN-α4, signaling through the IFN-α/β receptor (IFN-α/βR) and STAT1 to induce interferon regulatory factor 7 (IRF-7) expression that subsequently leads to induction of the non-IFN-α4 (IFN-αnon4) subtypes upon interaction with virus (1, 14, 21-23). Cells deficient for the IFN-α/βR or the STAT1 molecule are profoundly inhibited in their expression of IFN-α/β in culture, especially the late subtypes. Furthermore, under particular challenge conditions, cells from mice lacking the gene for IFN-β are inhibited in overall expression of IFN-α/β (9, 11).
Our studies were undertaken to determine whether the bulk of the IFN-α/β produced during in vivo LCMV infection is directly elicited by the virus or depends on the autocrine induction pathway and to identify the first LCMV-activated IFN-α/β target gene. The kinetics and quantity of IFN-α/β expression were examined after infection of wild-type (WT) mice and mice deficient for the IFN-α/βR (IFN-α/βR−/−), the IFN-γR (IFN-γR−/−), both IFN receptors (IFN-α/βγR−/−), or STAT1 (STAT1−/−). Our results demonstrate that the autocrine induction pathway is necessary for an optimal IFN-α/β response in vivo and that a single first target gene cannot be detected. In the absence of a functional IFN-α/βR, however, an alternative pathway is activated, but with delayed kinetics. The alternative pathway is not dependent on the viral burden only and/or on STAT1 but is dependent on the IFN-γR. Collectively, our data indicate that LCMV is a poor direct inducer of IFN-α/β but that the host has mechanisms in place by which to promote cytokine production in response to infection with such an agent.
MATERIALS AND METHODS
Mice.
The 129 IFN-γR−/−, IFN-α/βR−/−, or IFN-α/βγR−/− mice were obtained from B&K Universal Limited (North Humberside, United Kingdom) or Joan Durbin, Ohio State University, Columbus (10). STAT1−/− C57BL/6 mice were from Joan Durbin (16). All genetically deficient mice were bred under specific-pathogen-free conditions at Brown University. Age-matched WT C57BL/6 and 129/SvEv mice were purchased from Taconic Laboratory Animals and Services (Germantown, N.Y.). Mice used for experiments were 5 to 10 weeks old. Mice were handled in accordance with institutional guidelines for animal care and use.
Infections and preparation of biological materials.
Mice were either uninfected or infected intraperitoneally at time zero with 2 × 104 PFU of LCMV Armstrong clone E350 or the more aggressive and liver-tropic LCMV WE isolate (17, 19). Mice were anesthetized and bled before sacrifice for organ harvesting. Sera, spleen homogenates, and peritoneal cells were prepared as previously described (8, 10, 16). Viral titers in spleen homogenates were determined by plaque assays on Vero cells (18, 19) and expressed as PFU per gram.
RNA extraction, RT, and PCR.
Total RNA was extracted from spleens and peritoneal cells, DNase treated (Ambion, Inc., Austin, Tex.), and analyzed by reverse transcription (RT)-PCR as previously described (20). Briefly, 1 to 2 μg of RNA was reverse transcribed into cDNA. For relative quantitative PCR, 5 μl of cDNA was used as a template with primers specific for IFN-α4, IFN-αnon4, IFN-β, and IRF-7. Gene-specific primers were identified from publications (9, 14) and synthesized by Operon (Alameda, Calif.). As internal controls for random variations, 18S rRNA primers and competimers (Ambion Inc.) were used (20). Amplifications were carried out in a programmable thermal cycler (PTC-200; MJ Research, Waltham, Mass.) with the following parameters: IFN-α4 and IFN-αnon4, 32 cycles and an annealing temperature of 55°C; IFN-β, 36 cycles and an annealing temperature of 56°C; IRF-7, 30 cycles and an annealing temperature of 57°C. Southern blot hybridization with an internal oligonucleotide psoralen-biotin-labeled probe (Ambion, Inc.) was used to verify the specificity of IFN-α4 and IFN-αnon4 RT-PCR products. The IFN-αnon4 probe detected only IFN-αnon4 PCR products and not IFN-α4 PCR products and vice versa.
Measurement of IFN-α/β levels.
IFN-α/β bioactivity in sera and spleen homogenates was detected by a biological assay for protection against vesicular stomatitis virus (12). The dilution mediating 50% protection was defined as containing 1 U of IFN per ml. Specificity was determined by neutralization with rabbit anti-mouse IFN-α/β (Lee Biomolecular Research Laboratories, San Diego, Calif.). High levels of IFN-γ did not interfere with the assay.
Immunohistochemical analysis for LCMV and IFN-α/β proteins.
Spleens were embedded in O.C.T. (Sekura Finetek, Torrance, Calif.), cut into 5-μm sections with a cryostat (CM3050S; Leica, Deerfield, Ill.), fixed, rehydrated, and quenched for endogenous peroxidase. IFN-α/β was identified with polyclonal rabbit anti-mouse IFN-α/β antibody. Sections were washed and incubated with biotinylated Universal Antibody from a VECTASTAIN Quick Kit (Vector Laboratories, Inc.). LCMV antigen was identified with guinea pig anti-LCMV antibody (from M. J. Buchmeier, Scripps Research Institute, La Jolla, Calif.). Sections were washed and incubated with biotinylated anti-guinea pig immunoglobulin G (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.). Bound antibodies were detected with ABC reagent containing streptavidin-peroxidase or streptavidin-peroxidase from a VECTASTAIN Quick Kit, followed by VECTASTAIN Substrate Kit VIP (Vector Laboratories, Inc.). Specificity was demonstrated by lack of staining in the absence of a primary antibody. The counterstain used was methyl green (Vector Laboratories, Inc.).
RESULTS
Importance of IFN receptors for induction of IFN-α/β cytokines during in vivo infection.
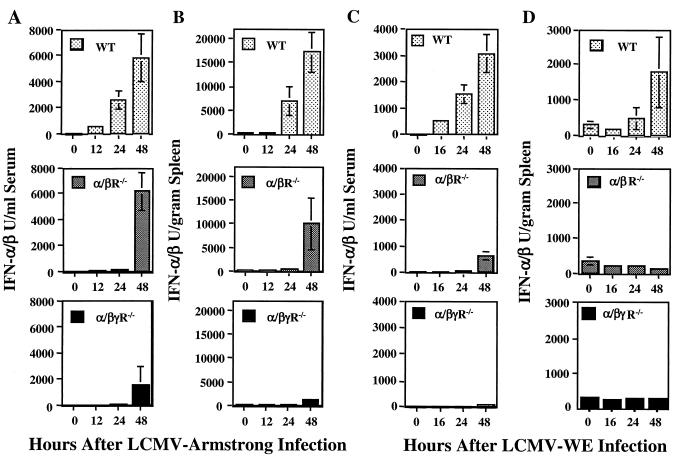
To examine the in vivo importance of the autocrine induction pathway, WT, IFN-α/βR−/−, IFN-γR−/−, or IFN-α/βγR−/− mice were infected with LCMV Armstrong or LCMV WE. The kinetics and magnitudes of the IFN-α/β responses were measured by biological assays. In WT mice, strong induction of bioactive IFN-α/β expression was detected in both sera (Fig. 1A) and spleen homogenates (Fig. 1B) at 24 and 48 h after infection with LCMV Armstrong. The IFN-γR−/− mice showed kinetics similar to those of the WT mice (data not shown). In contrast, IFN-α/βR−/− mice had either no bioactive IFN-α/β or barely detectable levels of bioactive IFN-α/β at 24 h but recovered at 48 h after infection, when levels of IFN-α/β were normal in the serum (Fig. 1A) and increasing in the spleen (Fig. 1B). However, in IFN-α/βγR−/− mice, profoundly reduced IFN-α/β levels were detected at both 24 and 48 h (Fig. 1A and B). Similar trends were observed after infection with LCMV WE, a more aggressive virus replicating to higher titers, but recovery in IFN-α/βR−/− mice was less dramatic (Fig. 1C and D).
FIG. 1.
IFN receptor requirement for expression of IFN-α/β during LCMV infections. WT, IFN-γR−/−, IFN-α/βR−/−, and IFN-α/βγR−/− mice were uninfected or infected with LCMV Armstrong (A and B) or LCMV WE (C and D) for 12, 24, or 48 h. IFN-α/β levels were quantified in serum samples (A and C) and spleen homogenates (B and D) in bioassays. Results are means ± the standard error of the mean of three mice per group and representative of at least two independent experiments.
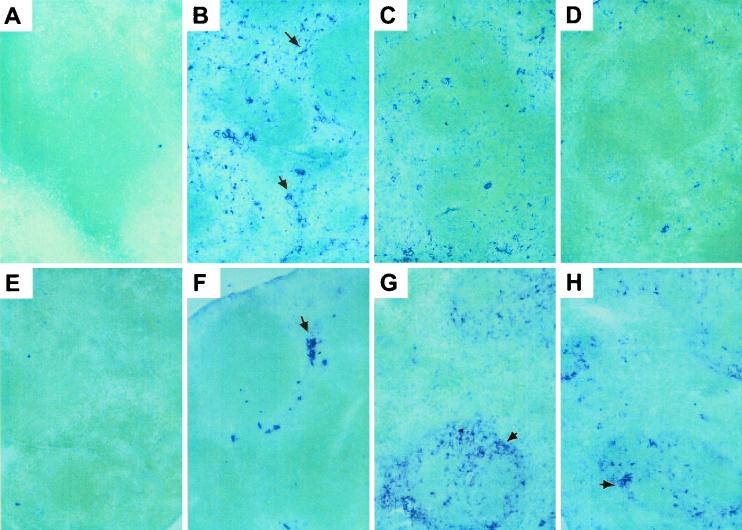
To determine if the delayed IFN-α/β response was directly induced as a result of a heavy viral burden, viral titers were measured in spleen homogenates from mice infected with LCMV Armstrong for 48 h. The titers, expressed as PFU per gram of spleen, were as follows: WT, 5.78 ± 0.65; IFN-γR−/−, 5.97 ± 0.58; IFN-α/βR−/−, 8.30 ± 0.12; IFN-α/βγR−/−, 9.35 ± 0.27. Thus, IFN-α/βγR−/− mice had the highest viral titers but the lowest IFN-α/β levels (Fig. 1B) and viral burdens and IFN-α/β production did not correlate. To localize virus-infected cells and IFN-producing cells, spleen sections from uninfected mice and mice infected with LCMV for 48 h were examined by immunohistochemical analysis. Quantitatively, the IFN-α/β staining reflected the bioassay results (Fig. 1B). No IFN-α/β protein or LCMV antigen could be detected in uninfected animals (Fig. 2A and E). At 48 h after infection, IFN-α/β proteins were abundant in WT mice, with staining localized mainly in red pulp (Fig. 2B). In IFN-α/βR−/− mice, levels of IFN-α/β expression were reduced but the localization pattern was the same as that in WT mice (Fig. 2C). In contrast, staining for IFN-α/β protein was very restricted in IFN-α/βγR−/− mice (Fig. 2D). LCMV antigen was detected in WT mice at 48 h but only as a few localized clusters in red pulp (Fig. 2F). Consistent with the viral titer data, LCMV antigen was much more abundant in the tissues from both receptor-deficient mice. Here, the infected cells localized mainly in the marginal zone and the proximal white pulp (Fig. 2G and H). Thus, there was no staining for IFN-α/β in cell populations where LCMV antigen could be detected. Taken together, these data demonstrate that virus infection is not sufficient to induce IFN-α/β by itself and that the IFN-α/βR is necessary for normal induction of IFN-α/β expression early during an in vivo infection. They also show a delayed induction mechanism in the absence of a functional IFN-α/βR, which is not a result of an increased viral burden alone but is dependent on the presence of a functional IFN-γR.
FIG. 2.
Localization of IFN-α/β protein and LCMV antigen in spleens. Immunohistochemical analysis was performed on spleen sections from uninfected WT mice (A and E) or WT (B and F), IFN-α/βR−/− (C and G), and IFN-α/βγR−/− (D and H) mice infected with LCMV Armstrong for 48 h with an antibody specific for IFN-α/β (A to D) or LCMV antigen (E to H). The arrow in panel B points to IFN-α/β-expressing cells in red pulp, the arrow in panel F points to LCMV-infected cells in red pulp, and the arrows in panels G and H point to LCMV-infected cells in marginal zones.
Characterization of IFN-α/β subtype expression.
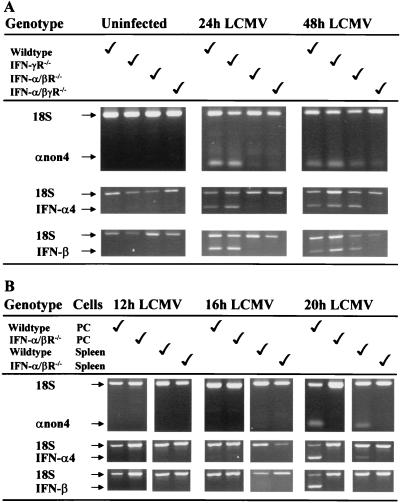
To examine kinetics of subtype IFN-α/β mRNA expression, splenic RNA was extracted from LCMV-infected WT, IFN-γR−/−, IFN-α/βR−/−, or IFN-α/βγR−/− mice and analyzed by relative quantitative RT-PCR. Uninfected mice did not show any expression of IFN-α/β mRNA. At 24 h after infection, mRNAs for the IFN-α4, IFN-β, and IFN-αnon4 subtypes were strongly expressed in WT and IFN-γR−/− mice but not in IFN-α/βR−/− or IFN-α/βγR−/− mice (Fig. 3A). However, at 48 h, strong expression of mRNAs for the three different IFN-α/β subtypes was observed in WT, IFN-γR−/−, and IFN-α/βR−/− mice whereas only weak expression was detected in IFN-α/βγR−/− mice (Fig. 3A). To determine whether LCMV could directly induce early IFN-α/β expression independently of the amplification pathway, differential expression of the subtypes was examined at earlier times after infection of WT and IFN-α/βR−/− mice. As infections were done intraperitoneally, RNA was extracted from both spleen and peritoneal cells. At 12 h after infection, no IFN-α/β message was detectable in spleen or peritoneal cells (Fig. 3B). At 16 h, weak expression of IFN-αnon4 was sometimes detected in the spleens of WT mice. Only at 20 h after infection was strong and consistent expression of IFN-α/β mRNA observed. Even though the spleen expressed mainly IFN-αnon4 and IFN-α4 at this time point, all three IFN subtypes were detected in the peritoneum (Fig. 3B). No initial expression of IFN-α/β was detected in IFNα/βR−/− mice at 12 to 20 h after infection. These data demonstrate that all three IFN-α/β subtypes are expressed in WT, IFN-γR−/−, and IFN-α/βR−/− mice. They also suggest that although they are delayed in IFN-α/βR−/− mice, all three subtypes are expressed coincidentally. Thus, there is no detectable single direct IFN gene target for LCMV.
FIG. 3.
Expression of IFN-α/β mRNA subtypes. WT, IFN-α/βR−/−, and IFN-α/βγR−/− mice were uninfected or infected with LCMV Armstrong for 24 or 48 h before total splenic RNA was extracted (A). WT and IFN-α/βR−/− mice were infected with LCMV Armstrong for 12, 16, or 20 h before total RNA was extracted from spleen or peritoneal cells (PC) (B). RNA preparations were amplified by RT-PCR with primers specific for the IFN-αnon4, IFN-α4, and IFN-β subtypes. For semiquantitation and as internal controls, the primer-competimer system for 18S rRNA was included in each sample. The data shown are representative of at least two independent experiments.
IFN receptor requirements for expression of IRF-7.
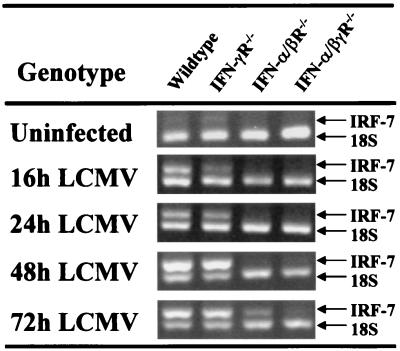
As IRF-7 can be a transcription factor for the autocrine amplification loop, IRF-7 expression in the spleens of mice was analyzed by RT-PCR. Weak constitutive expression was observed in samples from WT and especially IFN-γR−/− mice. In these mice, strong IRF-7 expression was induced already at 16 h after infection with LCMV and continued through 72 h postinfection (Fig. 4). In the IFN-α/βR−/− mice, IRF-7 expression was observed but not until 72 h after infection. It was, however, observed at the earlier 48-h time point in samples prepared from other compartments (data not shown). In IFN-α/βγR−/− mice, only weak IRF-7 expression was observed in the time frame studied. Hence, IRF-7 is induced early during LCMV infection of WT and IFN-γR−/− mice, whereas IFN-α/βR−/− mice only express IRF-7 at later times. The induction of IRF-7 in IFN-α/βR−/− mice demonstrates that IRF-7 is not a specific target for a functional IFN-α/βR. The lack of IRF-7 induction in IFN-α/βγR−/− mice suggests that the IFN-γR is necessary for induction in the absence of IFN-α/β functions.
FIG. 4.
IRF-7 expression in spleens of mice deficient for one or both IFN receptors. WT, IFN-α/βR−/−, and IFN-α/βγR−/− mice were infected with LCMV Armstrong for 16, 24, 48,or 72 h. Total splenic RNA was extracted and amplified by RT-PCR with primers specific for IRF-7. The primer-competimer system for 18S rRNA was included in each sample. The data shown are representative of at least two independent experiments.
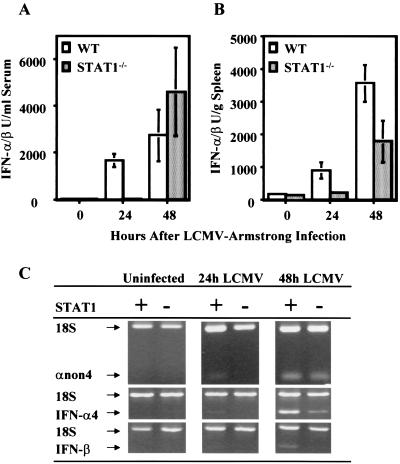
Role of STAT1 during early and delayed IFN-α/β responses.
To determine the role of STAT1 in the different pathways of induction, IFN-α/β expression was examined in STAT1−/− mice infected with LCMV. The kinetics of IFN-α/β induction in the sera (Fig. 5A) and spleens (Fig. 5B) of STAT1−/− mice was delayed by 24 h compared to that in WT mice, similar to results obtained with IFN-α/βR−/− mice. IFN-α/β message was detected in the spleens of WT but not STAT1−/− mice after 24 h of infection, although at a low level (Fig. 5C). However, at 48 h after infection, clear expression was observed in both types of mice. Thus, the early induction pathway for IFN-α/β is STAT1 dependent but the alternative delayed-induction pathway is not.
FIG. 5.
Requirement of the STAT1 molecule for expression of IFN-α/β. WT or STAT1−/− mice were infected with LCMV Armstrong for 12, 24, or 48 h. Levels of IFN-α/β were determined in sera (A) and spleen homogenates (B) with a bioassay. Results are means ± the standard error of the mean of three mice per group. (C) Total splenic RNA was extracted and amplified by RT-PCR with primers specific for the IFN subtypes. The primer-competimer system for 18S rRNA was included in each sample. The data shown are representative of at least two independent experiments.
DISCUSSION
These studies demonstrate that production of IFN-α/β early during LCMV infection of mice is dependent upon the IFN-α/βR and the STAT1 molecule. Although this autocrine induction pathway is of major importance early, alternative mechanisms, independent of the IFN-α/βR and STAT1 but dependent on a functional IFN-γR, can be activated. As the amounts of IFN-α/β produced do not correlate with viral titers but require endogenous amplification pathways, LCMV cannot be effective in directly inducing expression of IFN-α/β. Taken together, the results show that the host has evolved a variety of mechanisms by which to facilitate IFN-α/β induction in response to infections with viral agents with a poor ability to directly activate expression of the factors.
To our knowledge, this is the first report directly demonstrating function of the IFN-α/β amplification pathway in vivo. The results are consistent with and extend earlier in vitro studies demonstrating that IFN-α/β can promote its own expression through induction of transcription factors not expressed constitutively, i.e., IRF-7 (14, 21, 22). In contrast to the in vitro experiments, however, we had two surprising in vivo observations. First, the studies showed that without the assistance of endogenous amplification pathways, LCMV is a poor direct inducer of IFN-α/β expression. Thus, this virus is different from the potent IFN-α/β-inducing agents New Castle disease virus and Sendai virus, which appear to directly activate at least a subset of IFN-α/β genes. As early IFN-α/β expression is dependent on activation of the constitutively expressed transcription factor IRF-3 (13, 23), the poor ability of LCMV to induce IFN-α/β may result from ineffective activation of IRF-3. The potent induction of IFN-α/β in response to LCMV infection in WT mice may be a result of low-level background endogenous IFN-α/β production and signaling to induce the alternative transcription factor activating expression of a broader range of IFN-α/β gene targets, IRF-7, and activation of IRF-7 by LCMV. As other members of the IRF family promoting IFN-α/β expression may also be endogenously regulated through endogenous IFN functions (1, 2), other possibilities exist. However, in agreement with at least one other report showing endogenous IRF-7 expression in mouse embryo fibroblasts (21), we observed low-level expression of IRF-7 in the spleens (Fig. 4) and livers (data not shown) of uninfected WT and IFN-γR−/− mice. This IRF-7 expression was not detectable in IFN-α/βR−/− or IFN-α/βγR−/− mice (Fig. 4). Thus, our results indicate that amplification pathways for IFN-α/β expression are in place not only to facilitate production of high levels of the factors but also to make it possible, under certain viral infection conditions, to induce these cytokines at all. Background induction of IRF-7 may be one such mechanism.
The second surprising observation from our studies is that an alternative delayed pathway for induction of IFN-α/β exists in IFN-α/βR−/− or STAT1−/− mice but is absent in IFN-α/βγR−/− mice. The absence of IFN-α/β expression in mice deficient for both IFN receptors suggests that IFN-γ is involved in the alternative induction pathway. Earlier work from our laboratory has shown that WT mice infected with LCMV have no early IFN-γ response because of negative regulation by IFN-α/β. Conversely, in mice deficient for the IFN-α/βR or the STAT1 molecule, LCMV is able to induce IFN-γ expression early (8, 16). In fact, the IFN-γ response in IFN-α/βR−/− mice correlated kinetically with the delayed expression of IFN-α/β. However, attempts to conclusively show that IFN-γ substitutes for IFN-α/β in promoting the delayed expression of IFN-α/β have been inconclusive (data not shown). IFN-γR-deficient and IFN-γ-deficient conditions can be different (7). Therefore, it is possible that the effect is mediated through the receptor rather than IFN-γ. Because the rescued IFN-α/β production in the spleens of IFN-α/βR−/− mice (Fig. 4) can be detected before IRF-7 expression in this compartment, alternative IRF-7-independent induction pathways may exist. IRF-5 has been implicated in the induction of genes for IFN-α (2), but other, unidentified, factors may also contribute. However, IRF-7 induction could be detected at times overlapping with rescued IFN-α/β production in other compartments (data not shown). Therefore, the levels of expression may have simply been too low to detect in the spleen.
The compartmental differences observed between the serum and spleen with respect to the late alternative induction of IFN-α/β in mice deficient for the IFN-α/βR (Fig. 1) or STAT1 (Fig. 5) are interesting. The IFN-α/β levels were most normalized in the serum after 48 h of infection. These results suggest that IFN-α/β in serum originates from compartments other than, or in addition to, the spleen and that the alternative induction pathway is not fully functional in the spleen. Other work in our laboratory has found other compartmental differences in cytokine production and function (19, 20).
Our immunohistochemical analyses of spleens from WT mice demonstrate that IFN-α/β is expressed by cells that fail to produce detectable levels of LCMV antigen (Fig. 2). It is not clear whether this is indicative of populations challenged with LCMV but blocked for LCMV replication and antigen expression as a result of IFN-α/β antiviral effects or of virus-independent mechanisms for IFN-α/β induction. In agreement with earlier studies (24), LCMV localization in the spleen suggests that the virus only effectively infects cells in the red pulp of normal mice. Conversely, LCMV antigen is much more widely distributed in IFN-α/βR−/− mice. Thus, the lack of viral antigen expression in WT mice may not indicate the lack of a virus challenge. On the other hand, the apparent shift in viral tropism or viral spreading in IFN-α/βR-deficient mice may result in infection of another subset of cells that produce IFN-α/β independently of autocrine induction pathways. The latter possibility is not likely because infected IFN-α/βγR−/− mice also show exaggerated virus spreading but poor IFN induction. Studies are under way to define the specific subset of cells producing IFN-α/β and to analyze whether these cells are identical to those productively infected with LCMV.
Taken together, these results demonstrate the plasticity available in vivo for IFN-α/β induction during viral infections. Given the important antiviral and immunoregulatory functions activated by these factors, the physiological role of the various induction pathways could be to ensure a protective IFN-α/β response even during infections with viruses that are poor IFN inducers. The alternative induction pathway might secure high-level expression of IFN-α/β under conditions in which the cell is short of STAT1 or others factors needed for the IFN-α/βR-dependent induction pathway. Given the complexity of the genes for IFN-α/β and their promoters, a variety of mechanisms are likely to be in place to access these cytokines in vivo.
Acknowledgments
We thank K. B. Nguyen, M. Dalod, G. Pien, and R. Salomon for help with experiments and stimulating discussions; Joan Durbin for insightful discussions; and Mike Buchmeier for generously providing the anti-LCMV antibody.
This work was supported by NIH grants RO1-CA41268 and KO1-CA79076 and fellowships from the Faculty of Health Sciences, Aarhus University, Aarhus, Denmark; The Danish Medical Research Council; and The Andreassens and Hougaards Foundation.
REFERENCES
- 1.Au, W. C., P. A. Moore, D. W. LaFleur, B. Tombal, and P. M. Pitha. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210-29217. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, B. J., P. A. Moore, and P. M. Pitha. 2001. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J. Biol. Chem. 276:23382-23390. [DOI] [PubMed] [Google Scholar]
- 3.Biron, C. A. 1998. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10:383-390. [DOI] [PubMed] [Google Scholar]
- 4.Biron, C. A. 2001. Interferons alpha and beta as immune regulators--a new look. Immunity 14:661-664. [DOI] [PubMed] [Google Scholar]
- 5.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-351. In D. Knipe, P. Howley, D. Griffin, R. Lamb, M. Martin, and S. Strans (ed.), Fields virology, 4th edition. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 6.Biron, C. A., M. Dalod, and T. P. Salazar-Mather. 2002. Innate immunity and viral infections, p. 139-160. In S. H. E. Kaufmann, A. Sherr, and R. Ahmed (ed.), Immunology of infectious diseases. ASM Press, Washington, D.C.
- 7.Cantin, E., B. Tanamachi, H. Openshaw, J. Mann, and K. Clarke. 1999. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J. Virol. 73:5196-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousens, L. P., J. S. Orange, H. C. Su, and C. A. Biron. 1997. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. USA 94:634-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deonarain, R., A. Alcami, M. Alexiou, M. J. Dallman, D. R. Gewert, and A. C. Porter. 2000. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J. Virol. 74:3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doughty, L. A., K. B. Nguyen, J. E. Durbin, and C. A. Biron. 2001. A role for interferon-alpha/beta in virus infection-induced sensitization to endotoxin. J. Immunol. 166:2658-2664. [DOI] [PubMed] [Google Scholar]
- 11.Erlandsson, L., R. Blumenthal, M. L. Eloranta, H. Engel, G. Alm, S. Weiss, and T. Leanderson. 1998. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr. Biol. 8:223-226. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa, R., and C. A. Biron. 1993. IFN induction and associated changes in splenic leukocyte distribution. J. Immunol. 150:3713-3727. [PubMed] [Google Scholar]
- 13.Juang, Y., W. Lowther, M. Kellum, W. C. Au, R. Lin, J. Hiscott, and P. M. Pitha. 1998. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. USA 95:9837-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogensen, T. H., and S. R. Paludan. 2001. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 65:131-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen, K. B., L. P. Cousens, L. A. Doughty, G. C. Pien, J. E. Durbin, and C. A. Biron. 2000. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat. Immunol. 1:70-76. [DOI] [PubMed] [Google Scholar]
- 17.Orange, J. S., and C. A. Biron. 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J. Immunol. 156:1138-1142. [PubMed] [Google Scholar]
- 18.Orange, J. S., S. F. Wolf, and C. A. Biron. 1994. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 152:1253-1264. [PubMed] [Google Scholar]
- 19.Pien, G. C., and C. A. Biron. 2000. Compartmental differences in NK cell responsiveness to IL-12 during lymphocytic choriomeningitis virus infection. J. Immunol. 164:994-1001. [DOI] [PubMed] [Google Scholar]
- 20.Salazar-Mather, T. P., T. A. Hamilton, and C. A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Investig. 105:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 22.Sato, M., N. Hata, M. Asagiri, T. Nakaya, T. Taniguchi, and N. Tanaka. 1998. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441:106-110. [DOI] [PubMed] [Google Scholar]
- 23.Sato, M., N. Tanaka, N. Hata, E. Oda, and T. Taniguchi. 1998. Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-beta gene. FEBS Lett. 425:112-116. [DOI] [PubMed] [Google Scholar]
- 24.Smelt, S. C., P. Borrow, S. Kunz, W. Cao, A. Tishon, H. Lewicki, K. P. Campbell, and M. B. A. Oldstone. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor α-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75:448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]