Abstract
Introduction
The study sought to assess the feasibility and accuracy of measuring mixed venous oxygen saturation (SvO2) through the left main bronchus (SpO2trachea)
Methods
Twenty hybrid pigs of each sex were studied. After anesthesia, a Robertshaw double-lumen tracheal tube with a single-use pediatric pulse oximeter attached to the left lateral surface was introduced toward the left main bronchus of the pig by means of a fibrobronchoscope. Measurements of SpO2trachea and oxygen saturation from pulmonary artery samples (SvO2blood) were performed with an intracuff pressure of 0 to 60 cmH2O. After equilibration, hemorrhagic shock was induced in these pigs by bleeding to a mean arterial blood pressure of 40 mmHg. With the intracuff pressure maintained at 60 cmH2O, SpO2trachea and SvO2blood were obtained respectively during the pre-shock period, immediately after the onset of shock, 15 and 30 minutes after shock, and 15, 30, and 60 minutes after resuscitation.
Results
SpO2trachea was the same as SvO2blood at an intracuff pressure of 10, 20, 40, and 60 cmH2O, but was reduced when the intracuff pressure was zero (p < 0.001 compared with SvO2blood) in hemodynamically stable states. Changes of SpO2trachea and SvO2blood corresponded with varieties of cardiac output during the hemorrhagic shock period. There was a significant correlation between the two methods at different time points.
Conclusion
Measurement of the left main bronchus SpO2 is feasible and provides similar readings to SvO2blood in hemodynamically stable or in low saturation states. Tracheal oximetry readings are not primarily derived from the tracheal mucosa. The technique merits further evaluation.
Introduction
The saturation of haemoglobin with oxygen in the pulmonary artery is known as the mixed venous oxygen saturation (SvO2), which reflects the balance between the amount of oxygen delivered to the tissues and how much is used. It enables an estimate of the oxygen supply/demand balance to be made and hence enhances our comprehension of physiological concepts of hemodynamics and tissue oxygenation in critically ill patients. However, the routine measurement of SvO2 requires the placement of a pulmonary artery catheter (PAC), which may not always be feasible. Furthermore, a substantial review of literature suggests at present that the use of PAC may lead to an overall increase in morbidity and mortality in critically ill patients [1,2], stimulating the quest for a micro-invasive tool for assessing SvO2.
Pulse oximetry has been widely adopted in anesthesia and critical care medicine to provide noninvasive information about arterial oxygen saturation (SaO2). Several studies have demonstrated that oximeters placed in deep, vessel-rich areas such as the esophagus [3], pharynx [4], and trachea [5] seemed to provide more accurate readings than superficial oximetry. The tissue being sampled was once assumed to be the surrounding mucosa [3], but recent studies have shown that the signals were derived primarily from deeper tissues, such as underlying large vessels around the esophagus and trachea [5,6].
The pulmonary artery lies close to the bronchus, with nothing but some connective tissues between them, raising the possibility that an appropriately located and directed bronchial oximetry probe might be able to derive oximetry readings from mixed venous blood (Figure 1). The present study was undertaken to test the feasibility of measuring SvO2 through the left main bronchus (SpO2trachea), and to compare SpO2trachea with oxygen saturation from pulmonary artery samples (SvO2blood) in a healthy hybrid pig to improve our understanding of the hypothesis that bronchial oximetry readings are derived primarily from the pulmonary artery, not from the tracheal mucosa. Furthermore, the stability and accuracy of SpO2trachea were evaluated by assessing the impact of altered cardiac output on tracheal SpO2 in hemorrhagic shock status.
Figure 1.
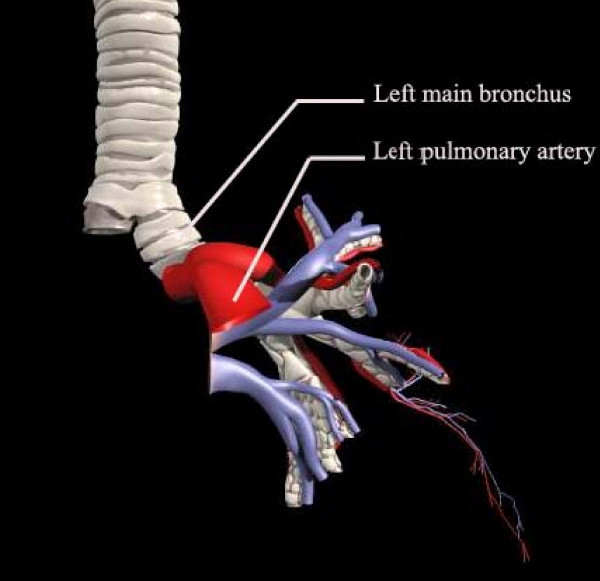
Anatomic relationship between the left main bronchus and the left pulmonary artery.
Materials and methods
Anesthesia and surgical preparation
The study was approved by the rules of Veterinary Medicine and Animal Care. After 12 hours of fasting, 20 Shanghai hybrid pigs (Shanghai University, Shanghai, China) of both sexes, weighing 50.7 ± 3.2 kg, were premedicated intramuscularly with ketamine (20 mg kg-1) and atropine (0.04 mg kg-1). Anesthesia was maintained by the intermittent application of pentothal sodium (2.5%) and diazepam. After endotracheal intubation of a Robertshaw double-lumen tracheal tube (details are given in the section on fabrication of the measuring catheter and intubation), the animals were ventilated mechanically with oxygen. The ventilation rate was 16 breaths min-1, and the respiratory tidal volume was set to 10 to 15 ml kg-1 body weight to adjust the end-expiratory partial pressure of CO2 to 4.5 to 6.0 kPa. The Inspire:Expire (I:E) ratio was 1:2. Respiratory rates, tidal volume and concentrations of oxygen and carbon dioxide were adjusted in accordance with periodic blood gas analysis to keep adequate blood pH. The right femoral artery was cannulated with a 22-gauge catheter connected to a pressure sensor to measure the mean artery pressure. The left femoral vein was cannulated with a 7F Swan–Ganz catheter, which was positioned according to the wave form, for intermittent sampling of pulmonary arterial blood for blood gas analysis. The right internal jugular vein was cannulated with a catheter to provide a venous line for infusion and anesthesia. Throughout the experiments, all animals received a Ringer lactate solution infusion at a rate of 10 ml kg-1 h-1. Electrocardiograph, heart rate and mean artery pressure were monitored continuously.
Refitting the oximetry probe, and stability test
Because a pulse oximeter stops working when in contact with water or another fluid, it should be waterproofed before use. The processing of disposable single-use pediatric pulse oximeters (Datex Medical Instrumentation, Helsinki, Finland) adopted in our experiments was as follows. First the fixed membrane was removed, the light emitter and sensor were exposed, then a surface coat of medical silica gel (provided by Shanghai Latex Institute) was applied, leaving it to solidify at normal temperature for 72 hours. Medical silica gel is made from pure silica gel with very thin texture. It is capable of forming a fine surface coating and can withstand a certain level of friction and tension after full solidification at normal temperature. Pulse oximetry of the tongue was obtained with both the refitted oximetry probe and the original probe. The readings were compared to test the stability and accuracy of the refitted probe.
Fabrication of the measuring catheter, and intubation
After inflation of the left lateral cuff portion of a Robertshaw double-lumen tracheal tube (37F), the light emitter and sensor of the waterproof oximeter were fixed along the longitudinal axis of the tracheal tube, and the infrared probe of the light emitter and the light-sensitive surface of the light sensor were faced in the same direction. The sensor was wrapped with copper foil except for a small window to expose the light-sensitive plate. A distance of 1 cm was left between the two terminals. Then the oximeter probe was fixed to the tube with a medical membrane, with two holes in the position of the light emitter and sensor to avoid any possible interference, as shown in Figure 2.
Figure 2.
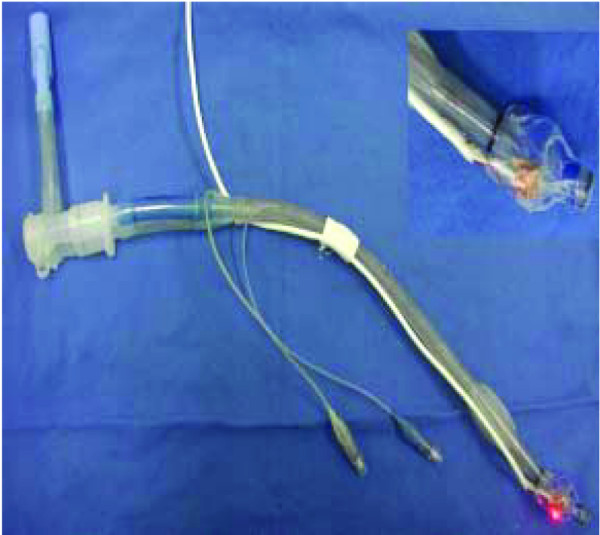
The Robertshaw double-lumen tracheal tube attached to a single-use pediatric pulse oximeter.
After anesthesia, the head and neck of the pig were positioned in the midline, with the occiput on a pillow 7 cm in height. The tracheal tube was inserted into the left main bronchus under the guidance of a pediatric fibrobronchoscope, and positioned at adequate depth and in an appropriate direction (the pilot open chest study had proved that a depth of 2 to 3 cm was adequate and that an appropriate direction was 15 to 20° left-leaning to the midline) to ensure that it was on the opposite side of the left pulmonary artery. Then the oximeter was connected to a monitor (Datex AS/3; Datex Medical Instrumentation) that had been previously checked and calibrated to ensure that it gave the same reading when attached to the same probe. The tracheal tube was fixed once the oxygen saturation curve had become a sine wave, and the position of the oximeter was confirmed by ultrasound and chest radiology (Figure 3).
Figure 3.
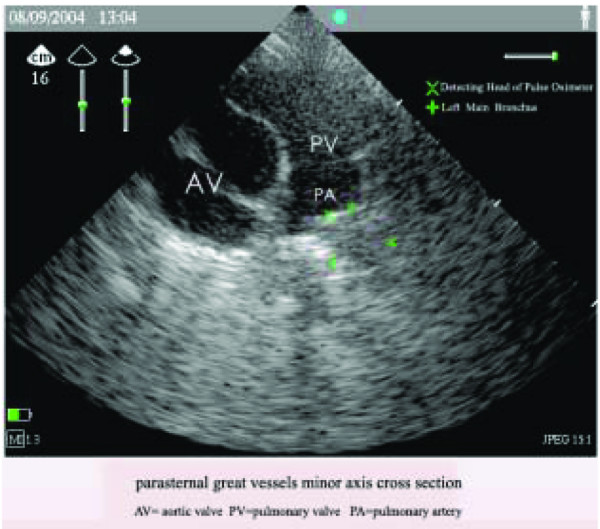
The position of the oximeter confirmed by ultrasound. A minor-axis cross-section of parasternal great vessels is shown, and is representative of 20 subjects. AV, aortic valve; PA, pulmonary artery; PV, pulmonary vein.
Changes in SvO2 with intracuff pressure
SpO2trachea was measured during a hemodynamically stable period of anesthesia. Readings were allowed to stabilize for two minutes before they were recorded. At the same time pulmonary arterial blood was collected and analyzed to measure SvO2blood (Serie 800; Chiron Diagnostics GmbH, Salzburg, Austria). The arterial blood gas monitor was accurate to 0.01% (SaO2) and calibrated before each case. Readings were taken with an intracuff pressure of 0, 10, 20, 40, and 60 cmH2O. The intracuff pressure was set with a digital cuff pressure monitor (Digital P-V Gauge™; Mallinckrodt Medical). One set of observations was obtained in each animal at each cuff pressure. All observations were made in a hemodynamically stable period.
Changes in SvO2 in hemorrhagic shock status
The same 20 pigs were used in the present study. After instrumentation, pigs were allowed to equilibrate for 30 minutes; they then underwent a standardized controlled hemorrhage to a mean artery pressure of 40 mmHg and were maintained at this level for 60 minutes. During hemorrhage, the blood was stored in a closed reservoir primed with sodium citrate and pig heparin to inhibit clot formation. At the end of 60 minutes, animals were resuscitated with the preserved shed blood, which was withdrawn from the pig to induce hypotension, and an equal volume of lactated Ringers to restore the baseline mean artery pressure. Cardiac output was assessed by the thermal dilution method during the procedure. The intracuff pressure was kept at 60 cmH2O. SpO2trachea and SvO2blood were measured at the pre-shock period, immediately after the onset of shock, 15 and 30 minutes after shock, and 15, 30 and 60 minutes after resuscitation.
Statistical analysis
Results are reported as means ± SEM and analyzed with a pair-matching t test and linear regression. To compare the accuracy of the new method, Bland–Altman plots were used. p < 0.05 was considered statistically significant.
Results
Stability and accuracy of the refitted oximetry probe
Pulse oximetry of the tongue was obtained with both the refitted oximetry probe (SpO2refit) and the original probe (SpO2origin) to test the stability and accuracy of the refitted probe. SpO2refit was similar to SpO2origin when the probe contacted tightly with the tongue (p > 0.05). The readings did not vary with changing intracuff pressure, and there was significant correlation between the two kinds of probe (p < 0.01; Table 1). However, SpO2refit was significantly lower than SpO2origin if there were spaces between the probe and the tongue (p < 0.001).
Table 1.
Comparisons of pulse oximetry measurements on the tongue with the original and refitted oximetry probes
| Concentration of inspiratory oxygen (%) | n | Oxygen saturation (%) | Correlation coefficient (r) | |
| SpO2refit | SpO2origin | |||
| 100 | 10 | 100 | 100 | 1.0 |
| 21 | 10 | 93.2 ± 2.4 (92–96) | 93.4 ± 2.7 (91–96) | 0.95 |
| 10 | 10 | 81.5 ± 2.2 (77–84) | 81.1 ± 2.5 (78–85) | 0.94 |
Values are means ± SEM (range). SpO2origin, pulse oximetry obtained with the original oximetry probe; SpO2refit, pulse oximetry obtained with the refitted oximetry probe.
Correlations between SpO2trachea and the intracuff pressure in normal situation
The age and weight ranges of the pigs were 6–8 months and 45–55 kg, respectively. The male:female ratio was 8:12. The mean (range) core temperature during the readings was 36.4°C (36.0 to 36.9°C) with the room temperature maintained at 21°C. SpO2trachea was the same as SvO2blood at an intracuff pressure of 10 to 60 cmH2O with no significant differences (p > 0.05) but significant correlations (p < 0.01) between each other (Tables 2 and 3). Values of SvO2blood did not vary with changing intracuff pressure, but SpO2trachea was lower when intracuff pressure was zero. There were significant differences between them (p < 0.001; Tables 2 and 3). Bland–Altman graphs for SpO2trachea versus SvO2blood are presented in Figure 4.
Table 2.
Oxygen saturation measurements in physiological states
| Intracuff pressure (cmH2O) | n | Oxygen saturation (%) | |
| SpO2trachea | SvO2blood | ||
| 0 | 20 | 70.2 ± 6.2 (57–76) | 74.4 ± 4.3 (62.6–76.4) |
| 10 | 20 | 74.2 ± 4.7 (62–77) | 74.4 ± 4.4 (62.5–76.9) |
| 20 | 20 | 74.2 ± 4.8 (62–77) | 74.3 ± 4.3 (62.4–76.7) |
| 40 | 20 | 74.2 ± 4.6 (61–76) | 74.4 ± 4.3 (62.3–76.9) |
| 60 | 20 | 74.2 ± 4.6 (62–77) | 74.3 ± 4.4 (62.5–77.1) |
| Overall | 100 | 72.5 ± 6.8 (57–77) | 74.4 ± 6.3 (61.9–77.2) |
| Overall excluding 0 cmH2O | 80 | 74.2 ± 4.2 (61–77) | 74.4 ± 4.3 (61.2–77.6) |
Values are means ± SEM (range). SpO2trachea, mixed venous oxygen saturation measured through the left main bronchus; SvO2blood, oxygen saturation from pulmonary artery samples.
Table 3.
Between-method statistical comparisons for the oxygen saturation measurement (SpO2trachea versus SvO2blood)
| Intracuff pressure (cmH2O) | n | MD (%) | SD | SEM | LOA | SEL |
| 0 | 20 | 4.87 | 3.10 | 0.73 | -1.33 to 11.07 | 1.201 |
| 10 | 20 | 0.25 | 0.97 | 0.21 | -1.69 to 2.19 | 0.376 |
| 20 | 20 | 0.22 | 0.89 | 0.19 | -1.56 to 2.00 | 0.345 |
| 40 | 20 | 0.31 | 0.66 | 0.14 | -1.01 to 1.63 | 0.256 |
| 60 | 20 | 0.17 | 0.74 | 0.18 | -1.31 to 1.65 | 0.287 |
| Overall | 100 | 1.26 | 2.39 | 0.25 | -3.52 to 6.04 | 0.414 |
| Overall excluding 0 cmH2O | 80 | 0.24 | 0.68 | 0.17 | -1.12 to 1.6 | 0.132 |
LOA, limits of agreement (MD ± 1.96SD); MD, mean difference; SD, standard deviation of the difference; SEL, standard error of limit; SEM, standard error of the mean difference; SpO2trachea, mixed venous oxygen saturation measured through the left main bronchus; SvO2blood, oxygen saturation from pulmonary artery samples.
Figure 4.
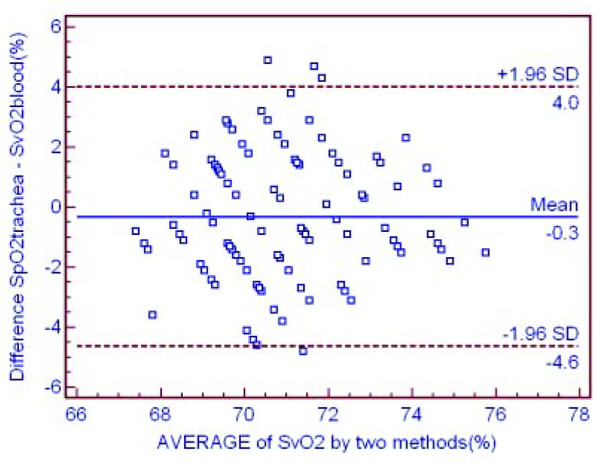
The accuracy of the new method in hemodynamically stable status. Shown is a Bland–Altman graph comparing the difference between mixed venous oxygen saturation through the left main bronchus (SpO2trachea) and oxygen saturation from pulmonary artery samples (SvO2blood) versus the mean oxygen saturation by the 'gold standard' and the new method in hemodynamically stable status.
Changes in SpO2trachea in hemorrhagic shock status and correlations between SpO2trachea and SvO2blood
With the intracuff pressure maintained at 60 cmH2O, changes in SpO2trachea and SvO2blood were due to variations in cardiac output during the hemorrhagic shock period (Table 4). There was significant correlation between SpO2trachea and SvO2blood (p < 0.01; Table 5). Bland–Altman analysis revealed excellent accordance between the two methods, with only few points located outside the 'limits of agreement' area (Figure 5).
Table 4.
Changes in SpO2trachea and SvO2blood in hemorrhagic shock status
| Time | n | Oxygen saturation (%) | |
| SpO2trachea | SvO2blood | ||
| Pre-shock period | 20 | 74.6 ± 4.5 (62–78) | 74.3 ± 4.7 (62.6–76.8) |
| Immediately after onset of shock | 20 | 74.2 ± 4.3 (60–78) | 74.8 ± 4.6 (61.9–77.2) |
| 15 min after shock | 20 | 61.2 ± 4.8 (52–67) | 61.7 ± 4.3 (52.4–68.2) |
| 30 min after shock | 20 | 42.2 ± 4.6 (41–54) | 42.8 ± 4.7 (41.3–55.9) |
| 15 min after resuscitation | 20 | 51.8 ± 4.6 (49–63) | 51.3 ± 4.4 (49.5–62.6) |
| 30 min after resuscitation | 20 | 64.5 ± 6.8 (57–77) | 64.2 ± 6.3 (57.9–77.2) |
| 60 min after resuscitation | 20 | 74.2 ± 4.2 (61–77) | 74.4 ± 4.3 (61.2–77.6) |
Values are means ± SEM (range). SpO2trachea, mixed venous oxygen saturation measured through the left main bronchus; SvO2blood, oxygen saturation from pulmonary artery samples.
Table 5.
Between-method statistical comparisons for oxygen saturation measurements in hemorrhagic shock status (SpO2trachea versus SvO2blood)
| Time | n | MD (%) | SD | SEM | LOA | SEL |
| Pre-shock period | 20 | -0.845 | 3.065 | 0.685 | -6.975 to 5.285 | 1.187 |
| Immediately after onset of shock | 20 | 0.495 | 3.014 | 0.674 | -5.533 to 6.523 | 1.167 |
| 15 min after shock | 20 | -0.165 | 3.210 | 0.718 | -6.585 to 6.255 | 1.243 |
| 30 min after shock | 20 | -1.275 | 2.759 | 0.617 | -6.793 to 4.243 | 1.069 |
| 15 min after resuscitation | 20 | -0.315 | 1.509 | 0.3374 | -3.333 to 2.703 | 0.584 |
| 30 min after resuscitation | 20 | 0.460 | 2.463 | 0.551 | -4.466 to 5.386 | 0.954 |
| 60 min after resuscitation | 20 | 1.865 | 2.844 | 0.636 | -3.823 to 7.553 | 1.101 |
LOA, limits of agreement (MD ± 1.96SD); MD, mean difference; SD, standard deviation of the difference; SEL, standard error of limit; SEM, standard error of the mean difference; SpO2trachea, mixed venous oxygen saturation measured through the left main bronchus; SvO2blood, oxygen saturation from pulmonary artery samples.
Figure 5.
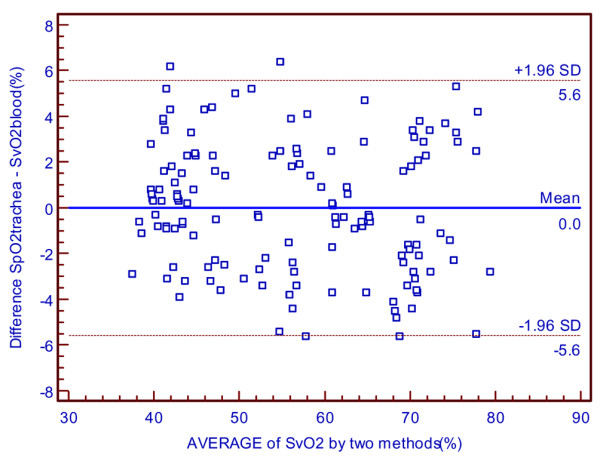
The accuracy of the new method in hemorrhagic shock status. Shown is a Bland–Altman graph comparing the difference between mixed venous oxygen saturation through left main bronchus (SpO2trachea) and oxygen saturation from pulmonary artery samples (SvO2blood) versus the mean oxygen saturation by the 'gold standard' and the new method in hemorrhagic shock status.
Discussion
SvO2 reflects the balance between oxygen delivery and demand. It decreases when oxygen delivery has been compromised or systemic oxygen demands have exceeded supply. Its ability to give a real-time indication of tissue oxygenation makes it a preferred parameter for monitoring the adequacy of hemodynamics. In comparison with traditional parameters such as arterial oxygen saturation and cardiac output, SvO2 allows a more precise understanding of the adequacy of cardiac and pulmonary function. Declines in SvO2 precede the onset of inadequate myocardial function, shock, or the development of arrhythmias, even though vital signs may be normal. Its use as an end point for determining the adequacy of hemodynamics (blood pressure, cardiac output/cardiac index), measurement of right to left shunt, and prediction of potential hemodynamic instability makes this parameter invaluable for the knowledgeable clinician. There is now evidence that the timing of diagnostic and therapeutic intervention using this technology may be a critical determinant of outcome [7].
The PAC, otherwise known as the Swan–Ganz catheter, was developed by cardiologists HJC Swan and William Ganz in 1970. It is a flexible balloon-tipped flow-directed catheter that, when inserted via central venous access, can be guided into a branch of the pulmonary artery. Its ability to provide continuous measurements of SvO2 in critically ill patients makes its use invaluable in the provision of quality medical care. However, controversy surrounding the efficacy and safety of the PAC has been going on for many years. The complications can be categorized as those of the initial venous cannulation (subclavian or carotid artery laceration, pneumothorax, thoracic duct laceration, phrenic nerve injury, and air embolism) and those due to the catheter itself (ie, arrhythmias, infection, valvular damage, thrombosis, pulmonary infarction, and rupture of the pulmonary artery). At the same time, the device requires a trained operator and is time-consuming. Moreover, it is expensive, bringing high healthcare costs.
There is therefore a powerful need for a method to measure SvO2 more safely. Other researchers have developed the technique of deriving oximetry readings of arterial blood through the trachea, or right and left ventricular oximetry through the esophagus [5,8]. The pulmonary artery is known to lie just proximal to the left bronchus. This evaluation of the anatomy made it practical to measure oximetry readings from the mixed venous circulation through the left main bronchus. However, so far no such studies have been reported. The present study establishes the first investigation to assess SvO2 microinvasively according to the above anatomic and technological bases.
Waterproofing is crucial for the proper function of oximeters in the humid environment of the trachea. Our experiment employed medical silica gel as a surface coat, because silica gel is waterproof and is nontoxic to humans. It can solidify fully at normal temperature, thus avoiding potential damage to the oximeter caused by thermal treatment. Moreover, it can endure a certain level of friction and tension after solidification. Because the pulmonary artery and the bronchus run nearly parallel, with sufficient overlapping area in the longitudinal direction, the light emitter and sensor of the oximeter are affixed along the same direction on the tracheal tube. As a result, the probe turned from a penetrating model (the light emitter and sensor being aligned opposite each other) into a reflecting model (the two terminals lying side by side). Experimental results indicate that the optimum distance between the emitter and sensor should be close to 1 cm. If the two terminals are too close, transmitting signals will be attenuated, which will affect the stability and accuracy of the data. Conversely, an increase in distance will negatively affect the reception efficiency of the infrared reflection signal.
Despite the above changes to the oximetry probe, high-quality signals were still available. We found that SpO2refit of the tongue was accurate at different inspiratory oxygen concentrations, in different head and neck positions, and over a prolonged period, suggesting good stability and sensitivity of the refitted probe.
The ability to localize the oximetry probe accurately is pivotal to the experiment. An experiential position 2 to 3 cm deep in the bronchus and an orientation of 15 to 20° left-leaning to the midline for the tracheal tube was found in our pilot study. To ensure that the tube was advanced to the optimal location, the animal should be fixed beforehand, and the position of the tube should be confirmed by electrocardiography.
Supported by the foregoing statement, our data showed that the reading of SpO2trachea was close to SvO2blood in stable physiological situations at 10 to 60 cmH2O cuff pressure. The readings obtained at zero cuff pressure were probably low because of a lack of contact between the probe and the trachea. The SpO2trachea was thought not to be derived primarily from the tracheal mucosa, because tracheal mucosal perfusion ceases when the intracuff pressure exceeds 50 cmH2O, and there was no decrease in the accuracy of SpO2trachea with increasing intracuff pressure. The blood flowing through the left pulmonary artery was speculated to be the mass of tissue sampled by the tracheal oximetry probe. At the same time, our study showed that SpO2trachea was consistent with SvO2blood in low cardiac output status during the hemorrhagic shock period. This measurement demonstrated that the precision of measuring SvO2 through the left main bronchus was not influenced in a pathological state, suggesting great reliability of this technique in operation and for patients in intensive care units. Although ventilation with a double-lumen tube is itself an invasive procedure, its advantage in causing much fewer lesions than PAC cannulation, and in avoiding the multiple complications that accompany the PAC device, makes this technique particularly appropriate for critically ill patients.
However, several limitations of the present investigation should be noted. First, our device was homemade, with the oximeter probe fixed to the endoscope by tape. Damage to the mucosa of the trachea is possible, and accidental inhalation would occur if the probe exfoliated. Furthermore, to reduce complications, a small tracheal tube and thin wire were required. However, it would be possible to incorporate the oximeter within the cuff and the wire within the tube and in so doing to reduce the complication of damage or accidental inhalation and allow a larger tube to be used to decrease the risk of trauma. Secondly, there were difficulties with locating the probe in the left bronchus. In addition to adjusting the tube repeatedly, ultrasound is required to confirm the position of the oximeter. The technique for location merits further investigation.
Conclusion
Measurement of SpO2 via the left main bronchus is feasible and provides similar readings to SvO2blood in both hemodynamically stable status and hemorrhagic shock status. Tracheal oximetry readings are not derived primarily from the tracheal mucosa. This technique is capable of providing continuous and microinvasive measurements of SvO2 despite the difficulty in achieving proper location of the probe. Further improvement is required for convenience of operation.
Key messages
• An appropriately located and directed bronchial oximetry probe is able to derive oximetry readings from the pulmonary artery, because the artery lies in close proximity to the bronchus with only some connective tissues in between, thus providing a microinvasive tool for the assessment of mixed venous oxygen saturation (SvO2).
• The mixed venous oxygen saturation via the left main bronchus (SpO2trachea) was thought not to derive primarily from the tracheal mucosa, because it was lower than the oxygen saturation from pulmonary artery samples (SvO2blood) at zero cuff pressure.
• SpO2trachea was the same as SvO2blood in hemodynamically stable status.
• SpO2trachea also provides similar readings to SvO2blood in hemorrhagic shock status, suggesting great reliability of this technique in operation and for patients in intensive care units.
Abbreviations
PAC = pulmonary artery catheter; SaO2 = arterial oxygen saturation; SpO2origin = pulse oximetry obtained with the original oximetry probe; SpO2refit = pulse oximetry obtained with the refitted oximetry probe; SpO2trachea = SvO2 through the left main bronchus; SvO2 = mixed venous oxygen saturation; SvO2blood = oxygen saturation from pulmonary artery samples.
Competing interests
The study was funded by 'The Third Period of Hundred People Project, Shanghai City'.
Authors' contributions
XW conceived the study, participated in the design and execution of the study, and finalized and revised the manuscript. YZ participated in the animal experiments, performed the statistical analysis, and was involved in drafting the manuscript. JT participated in study design, interpretation of the results, and writing the manuscript. ZW and ZP participated in the animal experiments. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Lin-mei Zhi and Zu-ren Zhang, Institute of Animal Research Center at the Renji Hospital, for their invaluable help and assistance.
Contributor Information
Xiang-rui Wang, Email: xiangruiwang@vip.sina.com.
Yong-jun Zheng, Email: yjzheng1971@hotmail.com.
Jie Tian, Email: vaseline2001@hotmail.com.
Zheng-hong Wang, Email: hong_wzh@hotmail.com.
Zhi-ying Pan, Email: rshjsh@hotmail.com.
References
- Kearney TJ, Shabot MM. Pulmonary artery rupture associated with the Swan–Ganz catheter. Chest. 1995;108:1349–1352. doi: 10.1378/chest.108.5.1349. [DOI] [PubMed] [Google Scholar]
- Feng WC, Singh AK, Drew T, Donat W. Swan–Ganz catheter-induced massive hemoptysis and pulmonary artery false aneurysm. Ann Thorac Surg. 1990;50:644–646. doi: 10.1016/0003-4975(90)90206-l. [DOI] [PubMed] [Google Scholar]
- Vicenzi MN, Gombotz H, Krenn H, Dorn C, Rehak P. Transesophageal versus surface pulse oximetry in intensive care unit patients. Crit Care Med. 2000;28:2268–2270. doi: 10.1097/00003246-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Brimacombe J, Keller C. Successful pharyngeal pulse oximetry in low perfusion states. Can J Anaesth. 2000;47:907–909. doi: 10.1007/BF03019674. [DOI] [PubMed] [Google Scholar]
- Brimacombe J, Keller C, Margreiter J. A pilot study of left tracheal pulse oximetry. Anesth Analg. 2000;91:1003–1006. doi: 10.1097/00000539-200010000-00043. [DOI] [PubMed] [Google Scholar]
- Keller C, Brimacombe J, Agro F, Margreiter J. A pilot study of pharyngeal pulse oximetry with the laryngeal mask airway: a comparison with finger oximetry and arterial saturation measurements in healthy anesthetized patients. Anesth Analg. 2000;90:440–444. doi: 10.1097/00000539-200002000-00037. [DOI] [PubMed] [Google Scholar]
- Rivers EP, Ander DS, Powell D. Central venous oxygen saturation monitoring in the critically ill patient. Curr Opin Crit Care. 2001;7:204–211. doi: 10.1097/00075198-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Margreiter J, Keller C, Brimacombe J. The feasibility of transesophageal echocardiograph-guided right and left ventricular oximetry in hemodynamically stable patients undergoing coronary artery bypass grafting. Anesth Analg. 2002;94:794–798. doi: 10.1097/00000539-200204000-00005. [DOI] [PubMed] [Google Scholar]


