Abstract
Introduction
β2 agonists have several properties that could be beneficial in acute lung injury (ALI). We therefore chose to study the effect of inhaled β2 agonist use (salbutamol) on duration and severity of ALI.
Methods
We undertook a retrospective chart review of 86 consecutive mechanically ventilated patients with ALI, who had varying exposure to inhaled salbutamol. The cohort was divided into two groups according to the average daily dose of inhaled salbutamol they received ('high dose' ≥ 2.2 mg/day and 'low dose' <2.2 mg/day). Severity of ALI and non-pulmonary organ dysfunction was compared between the groups by calculating the days alive and free of ALI and other organ dysfunctions.
Results
The high dose and low dose groups received a mean of 3.72 mg and 0.64 mg salbutamol per day, respectively. The high dose salbutamol group had significantly more days alive and free of ALI than the low dose group (12.2 ± 4.4 days versus 7.6 ± 1.9 days, p = 0.02). There were no associations between dose of β agonist and non-pulmonary organ dysfunctions. High dose salbutamol (p = 0.04), APACHE II score (p = 0.02), and cause of ALI (p = 0.02) were independent variables associated with number of days alive and free of ALI in a multivariate linear regression model.
Conclusion
Our retrospective study suggests that salbutamol, an inhaled β2 agonist, is associated with a shorter duration and lower severity of ALI. A dose greater than 2.2 mg/day of inhaled salbutamol could be a minimal effective dose to evaluate in a randomized controlled trial.
Introduction
Acute lung injury (ALI) is defined by impaired oxygenation (arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) <300 mmHg) and bilateral infiltrates on a chest radiograph without clinical evidence of left atrial hypertension [1]. Pulmonary edema in ALI is caused by damage to the alveolar-capillary interface and increased permeability that leads to accumulation of protein rich edema fluid in the interstitial and alveolar spaces. Reabsorptive mechanisms to clear alveolar edema fluid are impaired in acute lung injury [2-4]. Furthermore, there is a dose effect indicated by the association of the greater degree of impaired edema clearance with longer duration of mechanical ventilation and decreased survival [5,6].
β2 agonists have several properties that could be beneficial in ALI. First, inhaled β2 agonists improve respiratory mechanics in patients with ALI as shown by decreased airflow resistance and peak airway pressures and increased dynamic compliance [7-9]. Second, β2 agonists have anti-inflammatory properties. β2-agonists attenuate the release of tumor necrosis factor-α and increase the production of IL-10 in response to endotoxin in animal models [10,11].
Intravenous dobutamine (which has β1 and β2 agonist action) attenuates pro-inflammatory cytokine expression in the lungs of a rat model of septic acute lung injury [12]. Third, β agonists increase alveolar edema fluid clearance in animal models of ALI [13-22], in the ex vivo human lung [19] and in patients with ALI [23]. Studies on the selective β blockers show that it is the β2 agonist activities that cause the enhanced edema fluid clearance [24].
To date, there have been no studies on the dose association of inhaled β agonists with duration or severity of human ALI. Our hypothesis was that a higher dose of inhaled β2 agonist use, compared to a lower dose, is associated with more days alive and free of ALI (a measure of duration of severity of ALI) in critically ill patients with acute lung injury.
Materials and methods
This study was approved by the Research Ethics Board of Providence Health Care and the University of British Columbia, which waived the requirement of informed consent because of the retrospective nature of this study.
Cohort of patients who had acute lung injury
Between September 2001 and August 2003, consecutive patients admitted to a tertiary care medical-surgical intensive care unit (ICU) at St Paul's Hospital, Vancouver, Canada, were screened and 86 of these met the American-European consensus conference definition of ALI who were on mechanical ventilation [1].
Quantification of inhaled β2 agonist
Salbutamol was the only inhaled β2 agonist used clinically in the ICU. Salbutamol was administered through the ventilator circuit by metered dose inhaler (8 to 10 puffs at 100 μg/puff) or by nebulization of 2.5 to 5 mg of salbutamol solution (2.5 to 5 ml). The total daily dose of salbutamol administered and the route of delivery (metered dose inhaler or nebulizer) was recorded for each patient by retrospective chart review. We recorded salbutamol dose for each day in the ICU for 28 days or until discharge from the ICU (if less than 28 days). We calculated the average daily dose of salbutamol (mg/day) while in the ICU as the sum of total metered dose inhaler and nebulization dose (in mg) divided by the number of days in the ICU.
Several different doses of inhaled β2 agonists have been reported in mechanically ventilated patients [7,25,26]. Atabai and colleagues [27] measured levels of albuterol in plasma and broncho-alveolar lavage fluid from patients with ALI and found that doses of 2.5 mg or more of nebulized albuterol resulted in physiologically efficacious levels. In the only dose-response study published for mechanically ventilated patients, Dhand and colleagues [28] reported that a dose of 0.36 mg was as effective as 1.08 mg and 2.52 mg. This dose given every 4 hours would result in a total daily dose of 2.2 mg. Based on this, we classified patients receiving equal to or greater that 2.2 mg/day as 'high dose' and those patients receiving less than 2.2 mg/day as 'low dose'.
Primary and secondary outcomes
The primary outcome was days alive and free of ALI over 28 days. Secondary outcomes were days alive and free of PaO2/FiO2 <300, days alive and free of cardiovascular, renal, hepatic, neurological, and hematological dysfunction, and 28-day mortality.
Organ dysfunction for each organ system was defined as being present during each 24 hour period if there was evidence of moderate, severe, or extreme organ dysfunction according to the Brussels criteria [29]. To assess duration of organ dysfunction and to correct organ dysfunction scoring for deaths in the 28-day observation period, we calculated days alive and free of organ dysfunction (DAF) as previously reported. Briefly, during each 24 hour period for each variable, DAF was scored as 1 if the patient was alive and free of organ dysfunction (normal or mild dysfunction). DAF was scored as 0 if the patient had organ dysfunction (moderate, severe, or extreme) or was not alive. Each of the 28 days after meeting the inclusion criteria was scored. A low score is indicative of more organ dysfunction because a low score indicates fewer days alive and free of organ dysfunction. Because data were not always available during the 24 hour period for each organ dysfunction variable, we used the carry forward assumption as defined previously [29]. For any 24 hour period in which there was no measurement of a variable, we carried forward the present or absent criteria from the previous 24 hour period. If any variable was never measured, it was assumed to be normal throughout the 28-day period.
Baseline demographics were age, gender, surgical versus medical diagnosis on admission to the ICU (based on the Acute Physiology and Chronic Health Evaluation (APACHE) III [30] diagnostic codes), admission APACHE II score [31], baseline PaO2/FiO2 ratio, history of chronic obstructive pulmonary disease (COPD), asthma, and/or smoking, cause of ALI (pulmonary versus extra-pulmonary), and proportion of patients that had sepsis or septic shock as defined by the ACCP/SCCM consensus conference [32].
Statistical analysis
A comparison between the high and low dose salbutamol groups was made using the t test for continuous baseline demographic variables and outcomes. A chi-squared test was used for categorical variables. A forward selection multivariate linear regression model was constructed to evaluate the independence of salbutamol (high or low dose) against days alive and free of ALI. In the forward selection model, the following covariates were included: salbutamol (high or low dose), age (as a continuous variable), gender (female versus male), surgical versus medical diagnosis, history of COPD, asthma, and/or smoking, APACHE II score on admission (as a continuous variable), cause of ALI (pulmonary versus extrapulmonary), presence or absence of septic shock, and severity of ALI as defined by presence or absence of PaO2/FiO2 ratio ≤ 200. Variables were entered sequentially from the smallest to largest univariate p values and removed if they no longer met the inclusion cut-off after adjustment for the other variables. A two-tailed p value of <0.05 was used for statistical significance. The data were analyzed using SPSS 11.5 for Windows (SPSS Inc., Chicago, IL, USA, 2003). Continuous variables are presented as mean ± standard deviation unless otherwise stated.
Results
The daily dose of salbutamol ranged from 0 to 6.4 mg/day. The cohort was divided into two groups using the cut-off point of 2.2 mg/day to compare the primary and secondary outcomes in those who received high dose salbutamol to those who received low dose. The mean salbutamol doses in the high and low dose groups were 3.72 mg/day and 0.64 mg/day respectively.
Patients who received high dose salbutamol had significantly more days alive and free of ALI (12.2 ± 4.4 days versus 7.6 ± 1.9 days, p = 0.02; Figure 1). Similarly, there was an association between the higher average daily dose of salbutamol and more days alive and free of PaO2/FiO2 ratio <300 (p = 0.05; Figure 2). There was no association between salbutamol dose and days alive and free of any of the non-pulmonary organ dysfunctions (Table 1). Mortality was not significantly different between the low and high dose groups (46.9% versus 50.0%, respectively).
Figure 1.
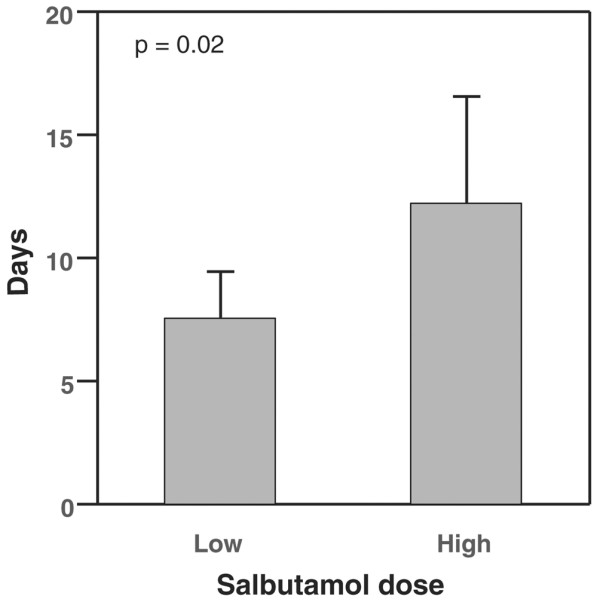
Days alive and free of acute lung injury in low dose (<2.2 mg/day) and high dose (≥ 2.2 mg/day) salbutamol groups (mean and 95% confidence interval).
Figure 2.
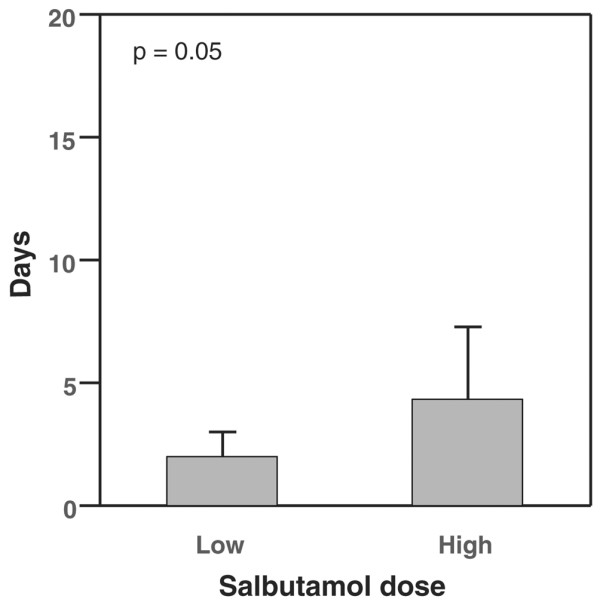
Days alive and free of PaO2/FiO2 <300 in low dose (<2.2 mg/day) and high dose (≥ 2.2 mg/day) salbutamol groups (mean and 95% confidence interval).
Table 1.
Comparison between the low versus high dose salbutamol groups and non-pulmonary organ dysfunction and mortality
| Days alive and free | Low dose (n = 64)a | High dose (n = 22)a | p value |
| Cardiovascular | 11.5 ± 2.6 | 13.2 ± 4.2 | 0.50 |
| Renal | 14.3 ± 2.9 | 16.0 ± 4.5 | 0.55 |
| Hepatic | 17.4 ± 2.8 | 19.6 ± 4.4 | 0.42 |
| Hematological | 15.9 ± 2.9 | 19.6 ± 4.5 | 0.10 |
| Neurological | 16.6 ± 2.6 | 19.0 ± 3.9 | 0.35 |
| Mortality (%) | 46.9% | 50.0% | 0.80 |
aValues are mean ± standard error of the mean.
The baseline demographics (Table 2) were similar between the groups except for a lower age in the low dose versus the high dose group (54.7 ± 16.6 years versus 65.7 ± 15.1 years, p< 0.05) and a lower proportion of patients with a history of COPD, asthma, and/or smoking in the low dose group versus the high dose group (15.6% versus 45.5%, p< 0.05).
Table 2.
Baseline characteristics of patients who had acute lung injury
| Characteristic | Low dose (n = 64) | High dose (n = 22) | p value |
| Mean salbutamol dose (mg/day, range) | 0.64 (0–2.19) | 3.72 (2.2–6.4) | <0.001 |
| Age (years) | 54.7 ± 16.6 | 65.7 ± 15.1 | 0.007 |
| Gender (% female) | 41% | 45% | 0.70 |
| Surgical diagnosis | 31.3% | 31.8% | 0.96 |
| APACHE II | 27.2 ± 8.1 | 25.2 ± 7.1 | 0.27 |
| Cause of ALI | |||
| Pulmonary | 51.6% | 50% | |
| Extra-pulmonary | 48.4% | 50% | 0.90 |
| History of COPD, asthma, and/or smoking | 15.6% | 45.5% | 0.007 |
| PaO2/FiO2 ≤ 200 | 81.3% | 68.2% | 0.20 |
| Sepsis | 95% | 100% | 0.41 |
| Septic shock | 81.3% | 72.7% | 0.29 |
ALI, acute lung injury; COPD, chronic obstructive pulmonary disease; FiO2, fraction of inspired oxygen; PaO2, arterial oxygen partial pressure.
Because of these differences at baseline between the two groups in age and in COPD/asthma/smoking status, a multivariate linear regression model was used to determine whether high dose salbutamol was independently associated with days alive and free of ALI when adjusting for other factors. High dose salbutamol remained a predictor of days alive and free of ALI in this model (p = 0.04). APACHE II score (p = 0.02) and cause of ALI (p = 0.02) were also independently associated with days alive and free of ALI (Table 3).
Table 3.
Multivariate linear regression model for prediction of days alive and free of acute lung injury
| Covariates | β (slope) | 95% CI of β | p value | |
| Salbutamol (high dose) | 4.08 | 0.17 | 8.00 | 0.04 |
| APACHE II | -0.25 | -0.47 | -0.03 | 0.02 |
| Cause of ALI | -3.96 | -7.37 | -0.56 | 0.02 |
Covariates: salbutamol (high referenced to low dose), age (as a continuous variable), gender (female referenced to male), surgical versus medical diagnosis, history of chronic obstructive pulmonary disease, asthma, and/or smoking, APACHE II score on admission (as a continuous variable), cause of acute lung injury (ALI; pulmonary referenced to extrapulmonary), presence or absence of septic shock, and severity of ALI as defined by presence or absence of arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio ≤ 200. CI, confidence interval.
Discussion
We found that high dose salbutamol, an inhaled β2 agonist, was associated with more days alive and free of ALI in critically ill patients who had ALI. This finding was supported by a similar significant association between dose of salbutamol and days alive and free of PaO2/FiO2 <300, a marker of severity of lung injury. Even after adjusting for differences in baseline characteristics between the high dose and low dose groups using a multivariate analysis, salbutamol was an independent predictor of more days alive and free of ALI.
Supporting the theory that β agonists have a direct effect on the pathophysiology of ALI, salbutamol dose was not significantly associated with days alive and free of any non-pulmonary organ dysfunction. To the best of our knowledge, this is the first study to show an association of the dose of an inhaled β-adrenergic agonist with a measure of duration of severity of ALI. Furthermore, this study suggests that a dose greater than 2.2 mg/day would be a reasonable dose to evaluate in a future prospective randomized controlled trial.
Our findings could be explained by one or more potentially beneficial actions of β2 agonists. β2 agonists such as salbutamol can improve pulmonary dysfunction in ALI by at least three mechanisms: increased alveolar fluid clearance, anti-inflammatory effects, and bronchodilation. The actions of β2 agonists in acute lung injury have recently been reviewed [33,34].
Stimulation of alveolar epithelial β2 receptors activates amiloride-sensitive sodium channels and ouabain-sensitive Na+/K+-ATPase to increase transepithelial sodium transport and alveolar fluid clearance via cAMP second messenger systems [35-37], which increases alveolar fluid clearance and alveolar epithelial function [38]. Beta-adrenergic agonists increase alveolar fluid clearance in normal lung [13-19] and in several animal models of acute lung injury [20-22] as well as in ex vivo human lungs [19] and in patients with ALI [23]. Terbutaline increases sodium transport across intact alveolar epithelium in isolated perfused rat lung, an effect that was inhibited by propranolol, indicating the importance of β receptor agonist activity [13]. Terbutaline also increases alveolar fluid clearance in anesthetized ventilated sheep [14], in dog lung [15], and in several models of ALI, such as hyperoxic lung injury [20], high tidal volume-associated lung injury [21] and the in vivo hypoxic rat model [22]. Resolution of alveolar edema is accelerated by isoproterenol [16,17,21] and epinephrine. Salmeterol, a specific β2 agonist, increased fluid clearance in both ex vivo human and rat lung [19]. In a recent double-blinded placebo controlled trial, intravenous salbutamol was shown to reduce extra vascular lung water in patients with ALI [23]. We did not measure lung water in our study so we cannot comment on whether salbutamol changed edema clearance in our study.
Beta-adrenergic agonists also have anti-inflammatory properties as β agonists decrease polymorphonuclear cell chemotaxis and accumulation in the lung [39] and decrease IL-1 [40], tumor necrosis factor-α [41] and IL-6 [42] production from macrophages. In a murine model of endotoxin-induced lung injury, dobutamine and dopexamine (both β1 and β2 agonists) decreased lung IL-6 protein and mRNA expression, and attenuated neutrophil accumulation in the lung [12]. We did not measure markers of inflammation in our study.
The third potential benefit of salbutamol on lung function in ALI is bronchodilation. β2 agonists decrease the elevated respiratory system resistance and airway pressure of patients who have acute respiratory distress syndrome (ARDS) [7-9]. In particular, both nebulized salbutamol (1 mg through the endotracheal tube) [7] and continuous intravenous infusion of salbutamol (15 μg/minute for at least 30 minutes) [9] decrease respiratory system resistance and airway pressure in ARDS. Wright and colleagues [8] also showed that a β2 agonist, aerosolized metaproterenol (5 mg), not only decreases high airway resistance and improves oxygenation, but also increases static compliance in human ARDS. This improvement of static compliance may be related to decreased lung edema or reduction in intrinsic positive end-expiratory pressure [7]. Overall, there may be clinical benefit from a reduction in respiratory resistance by β2 agonists in ALI because of a potential to decrease the risk of barotrauma.
There are few studies on the effects of β2 agonists on respiratory function in human ALI. Ware and Matthay [6] demonstrated that alveolar fluid clearance is impaired in most patients with ALI/ARDS and that impaired clearance is associated with a poor outcome. Basran and colleagues [43] studied the effect of intravenous terbutaline on plasma protein extravasation in ten patients with ALI/ARDS. Systemic terbutaline significantly reduced plasma transferrin movement into the lungs, a marker of lung permeability, in survivors but not non-survivors of ALI/ARDS. Perkins and colleagues [23] have recently reported that patients with ALI randomized to receive intravenous salbutamol (15 μg/kg/hr) for 7 days had a significant reduction in extra-vascular lung water index at days 4 and 7 compared to patients receiving placebo. They did not report any outcome data.
Several limitations of our study should be considered. First, there are limitations of retrospective studies such as ours. For example, the indications for salbutamol and the dose given were not controlled because our study was retrospective. Indeed, previous studies suggest even the high dose we defined (average of 3.7 mg/day) may be inadequate to attempt to increase alveolar fluid clearance. An alveolar concentration of 10-6 M of salmeterol was associated with increased alveolar fluid clearance in an ex vivo human lung study [19]. An average dose of 3.5 ± 2.6 mg of albuterol in the previous 6 hours was associated with alveolar edema albuterol levels of 10-6 M in patients who had ALI [27]. The intravenous dose Perkins and colleagues [23] reported is approximately ten-fold greater than our inhaled high dose threshold. A second limitation of our study is that other medications that can affect alveolar fluid clearance (such as infused catecholamines, diuretics, and corticosteroids) were not measured. However, Ware and Matthay [6] did not find a significant association between these medications and rate of edema fluid clearance. Therefore, these three medications may not have had a significant influence on alveolar fluid clearance in our patients. A third limitation is that our study was an association study that did not address mechanisms of improvement.
Finally, there were differences between the two dose groups in age and history of COPD, asthma and/or smoking, which could confound the association we found between the high dose of salbutamol and more days alive and free of ALI. To address this limitation, we did a multivariate analysis to adjust for differences in baseline characteristics. Importantly, the higher salbutamol dose remained independently associated with significantly more days alive and free of ALI even after multivariate analysis adjustment of baseline characteristics.
Conclusion
This preliminary retrospective study demonstrates for the first time that the aerosolized β2 agonist salbutamol at a dose greater than 2.2 mg/day (average dose of 3.72 mg/day) given to mechanically ventilated patients with ALI was associated with more days alive and free of ALI. This possible beneficial association requires prospective studies, such as a rigorous randomized controlled trial, to determine whether inhaled β2 agonists improve relevant outcomes of ALI.
Key messages
• β2 agonists have several properties that could be beneficial in ALI, including improving respiratory mechanics, reducing inflammation and increasing edema clearance.
• To date there have been no published studies examining the effect of β2 agonists on outcome from ALI.
• This retrospective study demonstrates an improved outcome from ALI with higher doses (average 3.72 mg/day) of inhaled salbutamol.
• A prospective randomized controlled trial examining the effect of β2 agonists on outcome from acute lung injury is required.
Abbreviations
ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; COPD = chronic obstructive pulmonary disease; DAF = days alive and free (of organ failure); FiO2 = fraction of inspired oxygen; ICU = intensive care unit; IL = interleukin; PaO2 = arterial oxygen partial pressure.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SM, ES and HG collected and analyzed the data. ACG analyzed the data. KRW and JAR conceived and coordinated the study. All the authors contributed to, read and approved the final manuscript.
Acknowledgments
Acknowledgements
Sanjay Manocha is a Post-doctoral Fellow of the Canadian Institutes of Health Research IMPACT program and a Post-doctoral Fellow of the Michael Smith Foundation for Health Research. Anthony C Gordon is a recipient of the UK Intensive Care Society Visiting Fellowship and a recipient of the Merck Frosst/Canadian Lung Association Fellowship. Keith R Walley is a Michael Smith Foundation for Health Research Distinguished Scholar
See related letter by Eisenhut, http://ccforum.com/content/10/3/412
Contributor Information
Anthony C Gordon, Email: agordon@mrl.ubc.ca.
Keith R Walley, Email: kwalley@mrl.ubc.ca.
James A Russell, Email: jrussel@mrl.ubc.ca.
References
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Saldias F, Comellas A, Ridge K, Guerrero C, Sznajder JI. Ventilator-associated lung injury decreases lung ability to clear edema in rats. Am J Respir Crit Care Med. 1999;159:603–609. doi: 10.1164/ajrccm.159.2.9805050. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Wiener-Kronish JP, Serikov V, Matthay MA. Resistance of the alveolar epithelium to injury from septic shock in sheep. Am J Respir Crit Care Med. 1995;151:1093–1100. doi: 10.1164/ajrccm.151.4.7697237. [DOI] [PubMed] [Google Scholar]
- Modelska K, Pittet JF, Folkesson HG, Courtney Broaddus V, Matthay MA. Acid-induced lung injury. Protective effect of anti-interleukin-8 pretreatment on alveolar epithelial barrier function in rabbits. Am J Respir Crit Care Med. 1999;160:1450–1456. doi: 10.1164/ajrccm.160.5.9901096. [DOI] [PubMed] [Google Scholar]
- Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol. 1999;87:1301–1312. doi: 10.1152/jappl.1999.87.4.1301. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- Morina P, Herrera M, Venegas J, Mora D, Rodriguez M, Pino E. Effects of nebulized salbutamol on respiratory mechanics in adult respiratory distress syndrome. Intensive Care Med. 1997;23:58–64. doi: 10.1007/s001340050291. [DOI] [PubMed] [Google Scholar]
- Wright PE, Carmichael LC, Bernard GR. Effect of bronchodilators on lung mechanics in the acute respiratory distress syndrome (ARDS) Chest. 1994;106:1517–1523. doi: 10.1378/chest.106.5.1517. [DOI] [PubMed] [Google Scholar]
- Pesenti A, Pelosi P, Rossi N, Aprigliano M, Brazzi L, Fumagalli R. Respiratory mechanics and bronchodilator responsiveness in patients with the adult respiratory distress syndrome. Crit Care Med. 1993;21:78–83. doi: 10.1097/00003246-199301000-00016. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kim YK, Govindarajan A, Baba A, Binnie M, Marco Ranieri V, Liu M, Slutsky AS. Effect of adrenoreceptors on endotoxin-induced cytokines and lipid peroxidation in lung explants. Am J Respir Crit Care Med. 1999;160:1703–1710. doi: 10.1164/ajrccm.160.5.9903068. [DOI] [PubMed] [Google Scholar]
- Dhingra VK, Uusaro A, Holmes CL, Walley KR. Attenuation of lung inflammation by adrenergic agonists in murine acute lung injury. Anesthesiology. 2001;95:947–953. doi: 10.1097/00000542-200110000-00025. [DOI] [PubMed] [Google Scholar]
- Crandall ED, Heming TA, Palombo RL, Goodman BE. Effects of terbutaline on sodium transport in isolated perfused rat lung. J Appl Physiol. 1986;60:289–294. doi: 10.1152/jappl.1986.60.1.289. [DOI] [PubMed] [Google Scholar]
- Berthiaume Y, Staub NC, Matthay MA. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest. 1987;79:335–343. [Google Scholar]
- Berthiaume Y, Broaddus VC, Gropper MA, Tanita T, Matthay MA. Alveolar liquid and protein clearance from normal dog lungs. J Appl Physiol. 1988;65:585–593. doi: 10.1152/jappl.1988.65.2.585. [DOI] [PubMed] [Google Scholar]
- Garat C, Carter EP, Matthay MA. New in situ mouse model to quantify alveolar epithelial fluid clearance. J Appl Physiol. 1998;84:1763–1767. doi: 10.1152/jappl.1998.84.5.1763. [DOI] [PubMed] [Google Scholar]
- Norlin A, Finley N, Abedinpour P, Folkesson HG. Alveolar liquid clearance in the anesthetized ventilated guinea pig. Am J Physiol. 1998;274:L235–243. doi: 10.1152/ajplung.1998.274.2.L235. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Folkesson HG, Matthay MA. Relationship of interstitial fluid volume to alveolar fluid clearance in mice: ventilated versus in situ studies. J Appl Physiol. 2000;89:672–679. doi: 10.1152/jappl.2000.89.2.672. [DOI] [PubMed] [Google Scholar]
- Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, Matthay MA. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med. 1997;155:506–512. doi: 10.1164/ajrccm.155.2.9032186. [DOI] [PubMed] [Google Scholar]
- Lasnier JM, Wangensteen OD, Schmitz LS, Gross CR, Ingbar DH. Terbutaline stimulates alveolar fluid resorption in hyperoxic lung injury. J Appl Physiol. 1996;81:1723–1729. doi: 10.1152/jappl.1996.81.4.1723. [DOI] [PubMed] [Google Scholar]
- Saldias FJ, Lecuona E, Comellas AP, Ridge KM, Rutschman DH, Sznajder JI. Beta-adrenergic stimulation restores rat lung ability to clear edema in ventilator-associated lung injury. Am J Respir Crit Care Med. 2000;162:282–287. doi: 10.1164/ajrccm.162.1.9809058. [DOI] [PubMed] [Google Scholar]
- Vivona ML, Matthay M, Chabaud MB, Friedlander G, Clerici C. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats: reversal by beta-adrenergic agonist treatment. Am J Respir Cell Mol Biol. 2001;25:554–561. doi: 10.1165/ajrcmb.25.5.4420. [DOI] [PubMed] [Google Scholar]
- Perkins GD, McAuley DF, Thickett DR, Gao F. The Beta agonist lung injury trial [abstract] Thorax. 2004;59ii:A1. [Google Scholar]
- Tibayan FA, Chesnutt AN, Folkesson HG, Eandi J, Matthay MA. Dobutamine increases alveolar liquid clearance in ventilated rats by beta-2 receptor stimulation. Am J Respir Crit Care Med. 1997;156:438–444. doi: 10.1164/ajrccm.156.2.9609141. [DOI] [PubMed] [Google Scholar]
- Manthous CA, Chatila , Schmidt GA Hall JB. Treatment of bronchospasm by metered-dose inhaler albuterol in mechanically ventilated patients. Chest. 1995;107:210–213. doi: 10.1378/chest.107.1.210. [DOI] [PubMed] [Google Scholar]
- Benoit D, Vahdewoude K, Colardyn F. Effects of nebulised salbutamol in ARDS. Intensive Care Med. 1998;24:88–89. doi: 10.1007/PL00003758. [DOI] [PubMed] [Google Scholar]
- Atabai K, Ware LB, Snider ME, Koch P, Daniel B, Nuckton TJ, Matthay MA. Aerosolized beta(2)-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med. 2002;28:705–711. doi: 10.1007/s00134-002-1282-x. [DOI] [PubMed] [Google Scholar]
- Dhand R, Duarte AG, Jubran A, Jenne JW, Fink JB, Fahey PJ, Tobin MJ. Dose-response to bronchodilator delivered by metered-dose inhaler in ventilator-supported patients. Am J Respir Crit Care Med. 1996;154:388–393. doi: 10.1164/ajrccm.154.2.8756811. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Groshaus HE, Manocha S, Walley KR, Russell JA. Mechanisms of beta-receptor stimulation induced improvement of acute lung injury and pulmonary edema. Crit Care. 2004;8:234–242. doi: 10.1186/cc2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins GD, McAuley DF, Richter A, Thickett DR, Gao F. Bench-to-bedside review: β2-Agonists and the acute respiratory distress syndrome. Crit Care. 2004;8:25–32. doi: 10.1186/cc2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S, Benos DJ, Jackson RM. Biophysical and molecular properties of amiloride-inhibitable Na+ channels in alveolar epithelial cells. Am J Physiol. 1996;271:L1–22. doi: 10.1152/ajplung.1996.271.1.L1. [DOI] [PubMed] [Google Scholar]
- Goodman BE, Anderson JL, Clemens JW. Evidence for regulation of sodium transport from airspace to vascular space by cAMP. Am J Physiol. 1989;257:L86–93. doi: 10.1152/ajplung.1989.257.2.L86. [DOI] [PubMed] [Google Scholar]
- Saumon G, Basset G, Bouchonnet F, Crone C. cAMP and beta-adrenergic stimulation of rat alveolar epithelium. Effects on fluid absorption and paracellular permeability. Pflugers Arch. 1987;410:464–470. doi: 10.1007/BF00586526. [DOI] [PubMed] [Google Scholar]
- Minakata Y, Suzuki S, Grygorczyk C, Dagenais A, Berthiaume Y. Impact of beta-adrenergic agonist on Na+ channel and Na+-K+-ATPase expression in alveolar type II cells. Am J Physiol. 1998;275:L414–422. doi: 10.1152/ajplung.1998.275.2.L414. [DOI] [PubMed] [Google Scholar]
- Whelan CJ, Johnson M. Inhibition by salmeterol of increased vascular permeability and granulocyte accumulation in guinea-pig lung and skin. Br J Pharmacol. 1992;105:831–838. doi: 10.1111/j.1476-5381.1992.tb09065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff WC, Fann AV, Dunegan MA, Lachman LB. Catecholamine-induced suppression of interleukin-1 production. Lymphokine Res. 1986;5:239–247. [PubMed] [Google Scholar]
- Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992;148:3441–3445. [PubMed] [Google Scholar]
- van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62:2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basran GS, Hardy JG, Woo SP, Ramasubramanian R, Byrne AJ. Beta-2-adrenoceptor agonists as inhibitors of lung vascular permeability to radiolabelled transferrin in the adult respiratory distress syndrome in man. Eur J Nucl Med. 1986;12:381–384. doi: 10.1007/BF00252194. [DOI] [PubMed] [Google Scholar]


