Abstract
CD1d-deficient mice have normal numbers of T lymphocytes and natural killer cells but lack Vα14+ natural killer T cells. Respiratory syncytial virus (RSV) immunopathogenesis was evaluated in 129×C57BL/6, C57BL/6, and BALB/c CD1d−/− mice. CD8+ T lymphocytes were reduced in CD1d−/− mice of all strains, as shown by cell surface staining and major histocompatibility complex class I tetramer analysis, and resulted in strain-specific alterations in illness, viral clearance, and gamma interferon (IFN-γ) production. Transient activation of NK T cells in CD1d+/+ mice by α-GalCer resulted in reduced illness and delayed viral clearance. These data suggest that early IFN-γ production and efficient induction of CD8+-T-cell responses during primary RSV infection require CD1d-dependent events. We also tested the ability of α-GalCer as an adjuvant to modulate the type 2 immune responses induced by RSV glycoprotein G or formalin-inactivated RSV immunization. However, immunized CD1-deficient or α-GalCer-treated wild-type mice did not exhibit diminished disease following RSV challenge. Rather, some disease parameters, including cytokine production, eosinophilia, and viral clearance, were increased. These findings indicate that CD1d-dependent NK T cells play a role in expansion of CD8+ T cells and amplification of antiviral responses to RSV.
The CD1 proteins represent a distinct lineage of antigen-presenting molecules that are evolutionarily related to the classical major histocompatibility complex (MHC) class I and class II glycoproteins (64). These molecules have evolved to bind lipids and glycolipids rather than peptides. The murine CD1d molecule can bind glycosphingolipids and cellular phospholipids (33, 43, 67). CD1d is required for the development of a group of T cells, termed natural killer (NK) T cells, that express receptor structures of both conventional T cells and NK cells (9, 56). NK T cells express intermediate levels of a semi-invariant T-cell receptor (Vα14-Jα281 paired with Vβ8.2, -7, or -2), as well as NK cell receptors (members of the NKR-P1 and Ly49 families). These cells are found in the thymus, spleen, liver, and bone marrow but are rare in lymph nodes. When stimulated through their T-cell receptors, NK T cells quickly produce a variety of cytokines, including the typical T helper type 1 (Th1) cytokine gamma interferon (IFN-γ) and the typical Th2 cytokine interleukin-4 (IL-4) (51, 86). It was therefore proposed that NK T cells can modulate adaptive immune responses by establishing the early cytokine environment and thereby affect disease pathogenesis (51, 86). The synthetic glycolipid α-galactosylceramide (α-GalCer) has been shown to activate NK T cells to rapidly produce both IL-4 and IFN-γ (16, 46). NK T cells have been implicated in immune responses against the lethal parasite Toxoplasma gondii (19), Listeria monocytogenes (23, 24), malaria parasites (67), mycobacteria (5), Borrelia burgdorferi (49), encephalomyocarditis virus (25), and murine cytomegalovirus (12). Additionally, activated NK T cells have recently been shown to protect against autoimmune diabetes in nonobese diabetic mice (35).
Respiratory syncytial virus (RSV) is a negative-sense, single-stranded RNA virus that causes seasonal epidemics of respiratory infection (36). RSV is an important pathogen of early childhood, resulting in >130,000 hospitalizations in the United States each year (70). RSV infection normally results in upper respiratory tract infection and mild to moderate illness. However, in children with underlying conditions such as prematurity, congenital heart disease, and atopy, progression of infection to the lower respiratory tract often leads to severe disease requiring hospitalization and mechanical ventilation. Severe RSV disease is also associated with the occurrence of childhood asthma (54, 55). Recent studies have shown that RSV is also an important pathogen in the elderly (21, 26) and in bone marrow transplant patients, for whom infection is associated with high mortality (83, 84). Thus, development of RSV vaccines and therapies is of high priority. However, vaccine development has been hampered by a failed vaccine trial in which children immunized with formalin-inactivated alum-precipitated RSV (FI-RSV) were not protected against subsequent natural RSV infection and, in fact, experienced more severe disease than nonimmunized children (45, 47).
Animal models have shown that primary RSV infection induces a response characterized by NK cells, CD8+ cytotoxic T lymphocyte (CTL) activity, and IFN-γ production, with very low levels of IL-4, IL-5, and IL-13 (3, 8, 13, 27, 30, 31, 34, 38-40, 42, 58, 59, 62, 72, 73, 75, 77-79). In contrast, in mice immunized with FI-RSV or with recombinant vaccinia virus expressing RSV attachment glycoprotein G, disease following RSV challenge is more severe and is associated with production of type 2 cytokines and pulmonary eosinophilia (30, 42, 58, 74). In this study we have examined the role of CD1d expression and antigen presentation in RSV pathogenesis using CD1d-deficient mice and the NK T-cell ligand α-GalCer.
MATERIALS AND METHODS
Mice.
CD1d−/− mice were generated on a 129/Sv×C57BL/6 (129×B6) background by targeted gene disruption (56) and subsequently backcrossed onto C57BL/6 (B6) and BALB/c backgrounds (six and nine backcrossings, respectively). 129×B6 CD1d+/+ mice were similarly maintained as controls. B6 and BALB/c CD1d+/+ controls were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, Ind.). All mice were housed in pathogen-free barrier cages and cared for in accordance with the Guide for the Care and Use of Laboratory Animals as previously described (32).
Virus and cell stocks.
A stock of RSV (A2 strain) was generated in HEp2 cells and stored at −70°C as described previously (32). A stock of FI-RSV was prepared as previously described (30). Recombinant vaccinia virus expressing the secreted form of RSV G (vvGs), a gift of Gail Wertz (University of Alabama, Birmingham), was grown and purified as previously described (42). Vaccinia virus expressing β-galactosidase (vac-lac; a gift of Bernard Moss, National Institutes of Health) was used as a control. HEp-2 cells were maintained in Eagle's minimum essential medium (MEM) supplemented with 10% fetal calf serum and antibiotics. All cell lines and viral stocks were determined to be free of mycoplasma by PCR (American Type Culture Collection, Rockville, Md.).
RSV infection of mice and analysis of subsequent immune responses.
CD1d−/− and CD1d+/+ mice (8 to 10 weeks old) were anesthetized and intranasally infected with 0.1 ml of supernatant containing 107 PFU of RSV as described previously (32). Infected mice were weighed and graded for illness following challenge as described previously (42). Six weeks after primary infection, mice were reinfected with 107 PFU of RSV. In immunization experiments, BALB/c CD1d+/+ and CD1d−/− mice were immunized intradermally at the base of the tail with 5 × 105 PFU (in 0.5 ml) of vaccinia virus. BALB/c CD1d+/+ and CD1d−/− mice were immunized with 107 PFU of FI-RSV (in 0.1 ml) intramuscularly. Six weeks after immunization mice were challenged intranasally with 0.1 ml containing 107 PFU of live RSV. The mice were weighed and scored for illness for 7 days postinfection (p.i.).
(i) Viral titers.
At the indicated times p.i., mice were sacrificed and lungs were removed as previously described (42). Viral titers were measured by plaque assay on HEp2 monolayers. Data are represented as the geometric mean log10(PFU/gram) of lung tissue at the dilution producing more than five plaques per well. The limit of detection is 1.5 log10(PFU/g).
(ii) Cytokine production.
Levels of IL-4, IL-5, IL-10, IL-13, IFN-γ, and eotaxin in lung supernatants were quantitated by enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, Minn.).
(iii) BAL and eosinophil counting.
Seven days after RSV challenge, mice were euthanized and a tracheotomy was performed. Bronchoalveolar lavage (BAL) was performed by injecting 0.5 ml of 1% bovine serum albumin in phosphate-buffered saline (PBS). The BAL cells were pelleted and resuspended in 0.1 ml of PBS, and cytosmears were made. After air drying, the cells were differentially stained with Diff-Quik (Fisher Scientific, Fair Lawn, N.J.). Percentages of eosinophils were determined by counting at least 300 differentially stained BAL cells. Total BAL cell counts were determined by trypan blue exclusion.
Flow cytometric analysis of infiltrating cell populations.
The kinetics of lymphocyte recruitment were examined by flow cytometry. Mice were sacrificed at various times after primary RSV infection or RSV challenge. Lung tissue was disrupted, and the cell suspension was overlaid on a 3-ml cushion of Fico-Lite (Atlantic Biologics, Norcross, Ga.), and the mononuclear cell band was isolated after centrifugation at 800 × g for 20 min. The cells were counted and standardized to 107 cells/ml in 10% RPMI. For surface staining, 106 cells were stained with 100 ng of fluorochrome-labeled antibodies to CD3, CD4, CD8, NK1.1, or DX5 for 30 min at 4°C, fixed in 4% paraformaldehyde, and analyzed on a FACSCaliber flow cytometer (Becton Dickinson, Mountain View, Calif.). CD3−NK1.1+ cells in 129×B6 and B6 mice and CD3-DX5+ cells were defined as NK cells. All antibodies were purchased from PharMingen, Inc. (San Diego, Calif.).
Quantitation of RSV-specific CD8+ T lymphocytes.
RSV-specific CD8+ T cells were quantitated by flow cytometric analysis of MHC tetramer-positive cells as follows. BALB/c mice were euthanized after RSV infection, and mononuclear cells were isolated as described above. H-2Kd MHC class I tetramers loaded with the RSV M2 peptide, a defined CTL epitope (48), were prepared by John Altman (Emory University, Atlanta, Ga.) as described previously (57). Cells (106) were incubated with phycoerythrin-conjugated M2-loaded tetramers, fluorescein isothiocyanate-conjugated anti-CD4, and Cy-chrome-conjugated anti-CD8, fixed, and analyzed. To verify specificity, the H-2Kd tetramer was loaded with a CTL epitope of influenza virus (50).
α-GalCer treatment.
α-GalCer was synthesized by Kirin Brewery Company (Takasaki-shi, Gunma, Japan) as previously described (46). Mice were injected intraperitoneally with 2 μg (in 0.2 ml) of α-GalCer diluted in PBS (stock solution: 220 μg/ml in 0.5% polysorbate-0.9% NaCl) on days −1, 2, and 5 around primary infection. Control mice were injected with 0.2 ml of similarly diluted polysorbate vehicle. To test the adjuvant activity of α-GalCer, BALB/c CD1d+/+ mice were injected with 2 μg (in 0.2 ml) of α-GalCer or 0.2 ml of polysorbate vehicle on days −1, 2, 5, and 10 around vvGs and FI-RSV immunization.
RESULTS
Induction of RSV-specific CD8+-T-cell responses in CD1d−/− mice.
NK T cells have been implicated in the disease process of several pathogens (5, 19, 23-25, 49, 67). To determine if NK T cells contribute to RSV pathogenesis, CD1d+/+ and CD1d−/− mice were infected with live RSV. CD1d-deficient mice were initially generated on the 129×B6 genetic background. However, genetic drift may occur in this mixed background since wild-type and transgenic colonies are maintained separately. Therefore, the CD1d-deficient mice were backcrossed to B6 and BALB/c backgrounds while initial studies were performed in the 129×B6 mice.
Induction of an early NK cell response and subsequent generation of a virus-specific CD8+ CTL response are required for efficient clearance of RSV (2, 17, 29, 34). However, an exuberant CD8+ CTL response (producing IFN-γ) has been shown to result in severe RSV disease (17, 29, 77, 79), and interference with CTL induction or function diminishes illness while delaying viral clearance (17, 27, 29). Therefore, the kinetics of lymphocyte recruitment following RSV infection of CD1d+/+ and CD1d-deficient mice were examined by cell surface staining and fluorescence-activated cell sorter (FACS) analysis. In all strains, lymphocyte subsets infiltrated the lung with similar kinetics, with infiltration of CD3+ and CD4+ cells paralleling that of CD8+ T cells (Fig. 1). As previously described (39), very few NK T cells were found in RSV-infected CD1d+/+ lung tissue (data not shown), but a detectable NK cell response occurred in all strains. In 129×B6 and BALB/c mice no significant differences were observed between CD1d+/+ and CD1d−/− mice (Fig. 1B). However, CD1d−/− B6 mice had significantly more NK cells at day 4 than did CD1d+/+ B6 animals (Fig. 1B; P = 0.0002). These data suggest that genetic factors in addition to CD1d contribute to NK cell activation in response to RSV infection and that the impact of these factors varies among mouse strains.
FIG. 1.
CD8+ T and NK cells in CD1d−/− mice following RSV infection. CD1d+/+ and CD1d−/− mice on 129×B6, B6, and BALB/c genetic backgrounds were infected intranasally with 107 PFU of live RSV. Recruitment of CD8+ T cells (A) and NK cells (B) to the lung was examined at the indicated time points after RSV infection by cell surface staining and FACS analysis. The data are the means ± standard errors of the means (SEMs); n = 8 for 129×B6 mice (three experiments combined), n = 9 for B6 mice (two experiments combined), and n = 18 for BALB/c mice (four experiments combined) for each time point shown.
Most striking were the alterations in CD8+-T-cell recruitment following RSV infection (Fig. 1A). Maximal CD8+-T-cell infiltration occurred at days 7 to 8 p.i. However, in all three strains of mice, fewer CD8+ T cells were present in CD1d−/− mice than in CD1d+/+ controls, although this difference was statistically significant only at day 7 p.i. in B6 mice (P < 0.04 and P = 0.006 for 129×B6 and BALB/c mice, respectively, at days 7 and 10 p.i. and P = 0.01 at day 7 for B6 mice). These data demonstrate that CD1d-dependent cells contribute to the activation, recruitment, and/or expansion of efficient CD8+-T-cell responses following RSV infection. Furthermore, the data suggest that in some genetic backgrounds (such as B6 mice), alternative immune responses such as NK cells play a more significant role in the generation of RSV-specific immune responses to compensate for the immune deficiency resulting from the constitutive loss of CD1d.
A single CD8+ CTL epitope from the M2 matrix protein has been defined in the mouse model of RSV infection and is H-2Kd restricted (48). Using MHC class I tetramer staining we demonstrated that reduced numbers of M2-specific T cells were recruited to RSV-infected lungs in BALB/c CD1d−/− mice (Fig. 2A; P < 0.001 at days 7 and 10 p.i.). However, the percentage of CD8+ T cells that are M2 specific is similar between CD1d+/+ and CD1d−/− mice (Fig. 2B).
FIG. 2.
Induction of RSV-specific CD8+-T-cell responses. The specificity of CD8+ T cells was examined by MHC tetramer staining using an H-2Kd-restricted CTL epitope from RSV M2 at the indicated time points after infection of BALB/c CD1d+/+ and CD1d−/− mice. The numbers of CD8+ T cells (A) and the percentage of M2-specific CD8+ T cells (B) were examined. The data represent the means ± SEMs for 18 mice from four experiments for each time point.
No significant changes were seen in CD4+-T-cell numbers in any strain of mice. Reflecting the decreases in CD8+-T-cell activation or recruitment, significantly lower numbers of CD3+ cells were found in the lungs of 129×B6, B6, and BALB/c mice (data not shown).
Viral clearance is delayed in CD1d-deficient mice.
In the mouse model peak RSV replication occurs between days 3 and 5 after infection, and most of the virus is cleared by day 8 (32), correlating with recruitment of RSV-specific CD8+ CTL into the infected lung. At 4 days p.i., there were no significant differences in peak viral titers between CD1d+/+ and CD1d−/− mice of any strain (Table 1). However, there was a significant delay in RSV clearance from lungs of infected 129×B6 and B6 CD1d−/− mice, for which viral titers were significantly greater than in CD1d+/+ mice of the same strain (P = 0.03 for 129×B6 and P = 0.02 for B6 mice). Yet in BALB/c mice virus clearance occurred at similar rates in CD1d+/+ and CD1d−/− mice, with no significant difference seen at any time p.i. Thus, the absence of CD1d had strain-specific effects on the induction of antiviral immune responses resulting in RSV clearance, and the clearance of RSV was associated with the heightened NK cell activity in the B6 strain of mice relative to the BALB/c genetic background.
TABLE 1.
RSV titers following primary infection of CD1d−/− mice
| Strain | Day p.i. | RSV titer ina:
|
|
|---|---|---|---|
| CD1d+/+ mice | CD1d−/− mice | ||
| 129×B6 | 4 | 4.68 ± 0.97 | 4.72 ± 1.71 |
| 7 | 2.70 ± 1.33 | 4.72 ± 1.19b | |
| B6 | 4 | 4.79 ± 0.65 | 5.61 ± 0.38 |
| 7 | 3.92 ± 0.98 | 4.86 ± 1.40b | |
| BALB/c | 4 | 6.68 ± 0.53 | 6.72 ± 0.48 |
| 7 | 2.79 ± 0.62 | 2.32 ± 0.71 | |
Data are presented as means ± SEMs of the log10(PFU/gram of lung); n = 15 for 129×B6 mice (three experiments combined), n = 8 for B6 mice (two experiments combined), and n = 18 for BALB/c mice (four experiments combined).
Statistically significant difference (P < 0.05 relative to CD1d+/+ mice of the same strain).
IFN-γ and IL-4 production following primary RSV infection.
CD8+ T lymphocytes and NK cells have dual roles in antiviral immune responses, a direct role of cytolytic killing of virally infected cells and an indirect role through production of immunoregulatory cytokines such as IFN-γ. Since the lack of CD1d expression resulted in decreased activation or recruitment of CD8+ T cells during primary RSV infection (Fig. 1), we measured IL-4 and IFN-γ concentrations to determine whether cytokine production was also altered in CD1d-deficient mice. Consistent with previous work (29, 30), primary RSV infection of CD1d+/+ mice induced significant IFN-γ production but only very low levels of IL-4 in all strains (Table 2). IFN-γ production parallels infiltration of CD8+ T lymphocytes and peaks at day 7 to 8 p.i. In CD1d-deficient B6 mice IFN-γ production at day 7 p.i. was not significantly different from levels in wild-type mice. However, in CD1d-deficient 129×B6 and BALB/c mice IFN-γ production was significantly decreased relative to CD1d+/+ mice of the same strain (P < 0.05). These data demonstrate that either NK T cells or other CD1-dependent events are required to induce IFN-γ production after RSV infection. This lack of IFN-γ may also contribute to the reduced efficiency of CD8+-T-cell activation in the 129×B6 and BALB/c CD1d−/− mice. However, there are additional genetic factors, potentially the increased NK cell population, which may compensate for the lack of CD1d expression and NK T cells, resulting in similar levels of IFN-γ production in B6 CD1d+/+ and CD1d−/− mice.
TABLE 2.
IFN-γ and IL-4 levels in the lung following primary infection of CD1d−/− mice
| Strain or cytokine | Day p.i. | Cytokine level (pg/ml) ina:
|
P valueb | |
|---|---|---|---|---|
| CD1d+/+ mice | CD1d−/− mice | |||
| 129 × B6 | ||||
| IFN-γ | 4 | 400.1 ± 156.8 | 477.1 ± 140.2 | 0.71 |
| 7 | 2,746.7 ± 467.6 | 1,970.4 ± 733.1 | 0.038 | |
| 10 | 10.2 ± 10.2 | 1.6 ± 1.6 | 0.049 | |
| IL-4 | 4 | 0.58 ± 0.07 | 2.69 ± 0.98 | 0.063 |
| 7 | 5.45 ± 1.20 | 14.63 ± 4.75 | 0.09 | |
| 10 | 3.40 ± 1.62 | 1.75 ± 0.31 | 0.42 | |
| B6 | ||||
| IFN-γ | 4 | 16.0 ± 9.9 | 30.0 ± 16.0 | 0.47 |
| 7 | 235.9 ± 57.4 | 415.1 ± 117.0 | 0.21 | |
| 10 | 7.4 ± 2.8 | 5.8 ± 2.3 | 0.65 | |
| IL-4 | 4 | 0.49 ± 0.14 | 8.36 ± 1.08 | 0.005 |
| 7 | 5.03 ± 1.87 | 9.50 ± 3.24 | 0.27 | |
| 10 | 6.33 ± 2.91 | 7.09 ± 2.90 | 0.86 | |
| BALB/c | ||||
| IFN-γ | 4 | 507.3 ± 90.2 | 778.7 ± 235.5 | 0.30 |
| 7 | 3,131.7 ± 776.7 | 1,177.6 ± 218.9 | 0.023 | |
| 10 | 92.4 ± 28.7 | 68.9 ± 18.3 | 0.049 | |
| IL-4 | 4 | 5.77 ± 2.13 | 8.24 ± 3.15 | 0.52 |
| 7 | 7.31 ± 2.84 | 12.90 ± 3.54 | 0.23 | |
| 10 | 13.17 ± 6.98 | 9.77 ± 5.15 | 0.70 | |
Data are presented as means ± SEMs of cytokine; n = 13 for 129×B6 mice (three experiments combined), n = 11 for B6 mice (two experiments combined), and n = 21 for BALB/c mice (four experiments combined). The limit of detection is 25 pg/ml for IFN-γ and 2 pg/ml for IL-4.
P values compare CD1d+/+ and CD1d−/− mice. Values in bold indicate statistically significant differences.
Illness following primary RSV infection of CD1d+/+ and CD1d−/− mice.
Illness in RSV-infected mice results from immune responses to the virus rather than from viral cytopathogenicity (17, 29, 77). Severe illness in RSV-infected mice may be produced by either type 1 or type 2 T-cell responses. Excessive CD8+ T cells (29, 79) or exogenous IL-12 (38, 79, 80) have been shown to exacerbate illness or attenuate type 2 CD4+-T-cell responses without alleviating illness in RSV-infected mice. In contrast, induction of aberrant Th2 CD4+ T cells also increases disease severity (1, 29, 42, 75, 79, 82). CD1d−/− and CD1d+/+ control mice were infected intranasally with RSV and weighed daily (Fig. 3). Weight loss was significantly reduced in the 129×B6 and BALB/c CD1d-deficient mice (Fig. 3; P < 0.05 at days 5 to 11). These significant differences may reflect the diminished CD8+-T-cell responses that, for BALB/c mice, were shown to be RSV specific (Fig. 1 and 2). While weight loss was greater in CD1d-deficient B6 mice than in CD1+/+ B6 mice, these differences were not significantly different (Fig. 3). Thus, the pathogenesis of primary RSV infection may be altered in CD1d−/− mice, but it is additionally influenced by the genetic background of the mice.
FIG. 3.
Illness during primary RSV infection of CD1d−/− mice. Mice were infected as for Fig. 1. The mice were then weighed and scored for illness each day. Data are represented as the percentages of initial body weight at day 0; n = 13 for 129×B6 mice (three experiments combined), n = 9 for B6 mice (two experiments combined), and n = 21 for BALB/c mice (four experiments combined).
α-GalCer treatment of CD1d+/+ mice during RSV infection.
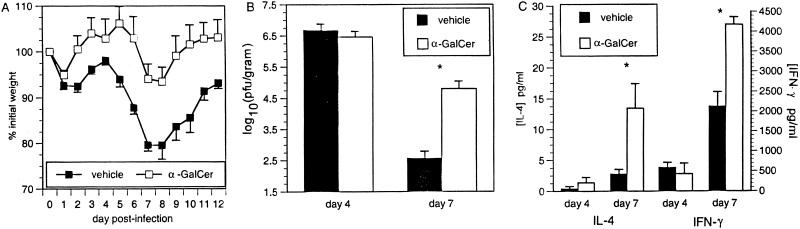
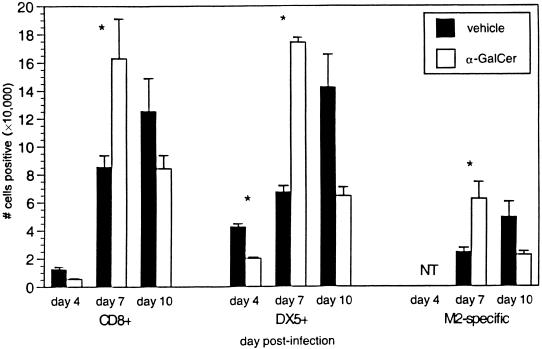
The synthetic glycolipid α-GalCer binds CD1d and activates Vα14 NK T cells to secrete IL-4 and IFN-γ before resulting in a transient depletion of NK T cells (15, 22, 46, 71). To determine if the altered disease profile in RSV-infected CD1d−/− mice was a result of the constitutive lack of CD1d antigen presentation or due to the absence of NK T cells, BALB/c CD1d+/+ mice were treated with α-GalCer during RSV infection. α-GalCer treatment of CD1d+/+ mice resulted in significantly less illness (Fig. 4A), significant delays in viral clearance (Fig. 4B), and increased production of both IL-4 and IFN-γ (Fig. 4C). FACS analysis of lung lymphocytes showed that α-GalCer-treated CD1d+/+ mice had significantly greater numbers of CD8+ T cells and NK cells at day 7 p.i. than vehicle-treated mice (Fig. 5). The increased cytokine production indicates activation of NK T cells by α-GalCer treatment, with the higher levels of IFN-γ resulting in greater recruitment or expansion of NK and CD8+ T cells in the RSV-infected lung. The increased numbers of CD8+ T cells in α-GalCer-treated mice may seem inconsistent with the increased viral titers at day 7. This apparent contradiction may be explained by decreased CTL function due to the increased production of IL-4 following α-GalCer treatment. Previous work has demonstrated that increased IL-4 levels delay viral clearance (4, 6, 27, 69), which—under some conditions—is due to an altered and less efficient mechanism of killing rather than to reduced numbers of CD8+ T cells (7). Consistent with decreased CD8+-T-cell function, illness is decreased in α-GalCer-treated CD1d+/+ mice. These data demonstrate a significant role for NK T-cell-produced cytokines in the induction of antiviral immune responses and suggest that it is the lack of NK T cells, and not CD1d, which is responsible for altered RSV pathogenesis in CD1d−/− mice.
FIG. 4.
α-GalCer treatment of CD1d+/+ mice during primary RSV infection. BALB/c mice were treated with α-GalCer during primary RSV infection. α-GalCer treatment (and depletion of NK T cells) decreases illness (A), delays viral clearance (B), and increases cytokine production (C) during RSV infection of BALB/c mice. Data are represented as the means ± SEMs for 14 mice at each time point from three combined experiments.
FIG. 5.
Lymphocyte recruitment in α-GalCer-treated BALB/c CD1d+/+ mice following primary RSV infection. Lung lymphocytes were isolated at the indicated times p.i. and stained with anti-CD8, anti-DX5 (NK cell marker), and M2-MHC class I tetramers. The data represent the means ± SEMs at each time point for 13 to 14 mice from three separate experiments.
The contribution of NK T cells to disease in vvGs- and FI-RSV-immunized mice.
RSV challenge of mice immunized with vvGs or FI-RSV results in the production of type 2 cytokines and pulmonary eosinophilia (30, 41, 42, 78). We therefore hypothesized that the lack of NK T cells in CD1d-deficient mice immunized with vvGs or FI-RSV would result in the attenuation of these type 2 T-cell responses. BALB/c CD1d+/+ and CD1d−/− mice were immunized with vvGs or FI-RSV and 6 weeks later were challenged with live RSV. Illness was similar between CD1d+/+ and CD1d−/− mice (data not shown). In contrast, in CD1d−/− mice peak RSV titers at day 4 p.i. were significantly greater in both vvGs- and FI-RSV-immunized mice (Table 3). This alteration in protective antiviral immune responses is further underscored by the significant reduction in vac-lac-primed CD1d−/− mice at day 4. Despite significant increases in peak viral titers in vvGs- and FI-RSV-immunized CD1d−/− mice, viral clearance was not altered, as evidenced by similar viral titers at day 7 postchallenge. Concentrations of type 1 and type 2 cytokines in the lung were measured by enzyme-linked immunosorbent assay. While some modest increases were observed in cytokine production (Table 4), particularly in FI-RSV-immunized mice, these changes were generally not statistically significant. These data correlate with eosinophil recruitment where CD1d-deficient mice have increased numbers of eosinophils in the BAL compartment following RSV challenge, although the differences were not statistically significant in either vvGs- or FI-RSV-immunized mice relative to vehicle-treated CD1d+/+ mice (Table 5).
TABLE 3.
RSV titers following challenge in α-GalCer-treated CD1d+/+ mice and in CD1d−/− mice immunized with vvGs or Fl-RSV
| Priming group | Day p.i. | RSV titer ina:
|
||
|---|---|---|---|---|
| CD1d+/+ mice
|
CD1d−/− mice | |||
| Vehicle treated | α-GalCer treated | Vehicle treated | ||
| vac-lac | 4 | 7.39 ± 0.06 | Not tested | 7.29 ± 0.03 |
| 7 | 2.70 ± 0.25 | Not tested | 2.01 ± 0.22b | |
| vvGs | 4 | 4.99 ± 0.39 | 6.16 ± 0.19b | 6.45 ± 0.24b |
| 7 | 2.02 ± 0.22 | 1.67 ± 0.09 | 1.88 ± 0.22 | |
| Fl-RSV | 4 | 1.98 ± 0.20 | 4.69 ± 0.15b | 2.84 ± 0.47b |
| 7 | 1.50 ± 0.00 | 1.50 ± 0.00 | 1.50 ± 0.00 | |
Data are presented as means ± SEMs of the log10(PFU/gram of lung); n = 5. The limit of detection is 1.50 log10(PFU/gram of lung).
Statistically significant (P < 0.05 relative to vehicle-treated CD1d+/+ mice of the same priming group).
TABLE 4.
Cytokine levels in the lungs of α-GalCer-treated vvGs- or Fl-RSV-immunized mice following RSV challenge
| Priming group | Cytokine | Cytokine level (pg/ml) ina:
|
||
|---|---|---|---|---|
| CD1d+/+ mice
|
CD1d−/− mice | |||
| Vehicle | α-GalCer | Vehicle | ||
| vvGs | IL-4 | 29.6 ± 2.7 | 19.6 ± 2.2 (0.02) | 16.8 ± 2.5 (0.009) |
| IL-5 | 70.9 ± 23.3 | 86.8 ± 15.9 | 105.9 ± 17.3 | |
| IL-10 | 12.6 ± 2.6 | 56.6 ± 20.3 | 48.5 ± 26.7 | |
| IL-13 | 493.0 ± 104.2 | 445.7 ± 61.6 | 372.5 ± 40.7 | |
| IFN-γ | 761.6 ± 332.8 | 1,687.7 ± 512.4 | 1,608.9 ± 187.3 | |
| Eotaxin | 753.9 ± 92.9 | 741.9 ± 58.21 | 860.6 ± 96.2 | |
| Fl-RSV | IL-4 | 18.3 ± 0.9 | 29.3 ± 4.2 | 20.4 ± 1.3 |
| IL-5 | 76.3 ± 18.7 | 138.8 ± 33.5 | 278.6 ± 89.2 | |
| IL-10 | 75.1 ± 36.8 | 128.6 ± 30.3 | 26.9 ± 14.2 | |
| IL-13 | 361.2 ± 32.9 | 672.2 ± 119.9 | 483.0 ± 32.1 (0.03) | |
| IFN-γ | 1,041.5 ± 161.5 | 284.8 ± 112.1 (0.006) | 690.2 ± 129.7 | |
| Eotaxin | 869.4 ± 81.4 | 1,165.9 ± 196.4 | 1,387.3 ± 183.5 (0.04) | |
Cytokines were measured in day 4 lung supernatants. Data are presented as means ± SEMs of cytokine for five mice. The limit of detection is 2 pg/ml for IL-4, IL-5, and IL-10 assays and 25 pg/ml for IL-13, IFN-γ, and eotaxin assays. Numbers in parentheses indicate statistical significance (P values relative to vehicle-treated CD1d+/+ mice).
TABLE 5.
BAL eosinophilia in α-GalCer-treated vvGs- or FI-RSV-immunized mice following RSV challengea
| Priming group | No. of eosinophils (104) inb:
|
||
|---|---|---|---|
| CD1d+/+ mice
|
CD1d−/− mice | ||
| Vehicle | α-GalCer | Vehicle | |
| vac-lac | 1.49 ± 0.32 | Not tested | 0.75 ± 0.13 |
| vvGs | 12.63 ± 3.11 | 10.70 ± 2.76 | 17.41 ± 2.96 |
| FI-RSV | 11.86 ± 2.53 | 8.29 ± 1.43 (0.015) | 16.45 ± 2.13 |
Mice were immunized with vac-lac, vvGs, or FI-RSV and treated with α-GalCer or polysorbate vehicle. Six weeks later the mice were challenged with live RSV, and 7 days later BAL was performed. BAL cells were differentially stained and counted, and the numbers of eosinophils were calculated.
Data are presented as means ± SEMs. Numbers in parentheses are statistically significant P values (relative to vehicle-treated CD1d+/+ mice).
Data reported above (Fig. 4) demonstrate that α-GalCer treatment during primary RSV infection decreases illness and increases IFN-γ production, suggesting the potential use of α-GalCer as an adjuvant during RSV immunization. To further characterize the ability of cytokine production by activated NK T cells to influence the differentiation of RSV-specific immune responses, mice were treated with α-GalCer during vvGs or FI-RSV immunization and then challenged with RSV. While there was a 2-day delay in the onset of illness in vvGs- and FI-RSV-primed mice treated with α-GalCer relative to that in vehicle-treated mice, no significant difference was observed in peak illness (data not shown). As with CD1d-deficient mice, α-GalCer treatment of CD1d+/+ mice during vvGs or FI-RSV immunization resulted in higher peak virus titers after RSV challenge (Table 3), although viral titers at day 7 were similar. Lung cytokine levels were also minimally increased by α-GalCer treatment during immunization, although the differences were generally not significant (Table 4). The number of eosinophils recruited to the BAL following RSV challenge was reduced (Table 5), with statistically significant differences attained in FI-RSV-immunized mice (P = 0.015, comparing vehicle- and α-GalCer-treated FI-RSV-immunized mice). This may reflect the increased IFN-γ production by NK T cells since it has been demonstrated that IFN-γ reduces pulmonary eosinophilia in RSV G-primed mice (74).
DISCUSSION
Disease in RSV-infected mice results from a complex interaction of immune responses in which illness can be increased by an exaggerated type 2 CD4+-T-cell response, by excess numbers of CD8+ T cells, or by increased levels of type 1 CD4+ T cells. Much work has focused on the severe disease observed in mice immunized with FI-RSV or RSV G and challenged with live RSV. In these mice disease is clearly associated with the induction of aberrant type 2 CD4+ T cells that produce significant levels of IL-4, IL-5, and IL-13 and that result in pulmonary eosinophilia (1, 29, 30, 41, 42, 78, 79, 82). This disease profile is cytokine dependent in that inhibition of type 2 cytokine activity modulates the illness (41, 77). However, to say that Th2 CD4+ T cells are “bad” responses and IFN-γ-producing CD8+ T cells are “good” responses is an oversimplification. In fact, transfer experiments have shown that excessive RSV-specific CD8+ T cells in combination with RSV infection can enhance illness (17, 29, 79) and inhibition of CD8+ CTL is associated with a reduction of RSV-induced disease (75, 79). In addition, use of exogenous IL-12 can attenuate type 2 responses without altering illness (38, 79, 80). Thus, CD8+ T cells are required for efficient clearance of RSV, but the price to pay for clearing virus is some degree of illness. Therefore, identification of the additional immune mediators involved in the induction of CD8+ CTL and dissection of the roles of these mediators in T-cell maturation may allow the development of antiviral agents that promote efficient CD8+ CTL responses with minimal disease consequences. Therefore, the role of NK T cells in RSV pathogenesis was examined. In this paper we demonstrate that NK T cells contribute to the efficient induction of CD8+-T-cell immune responses against RSV and that in the absence of NK T-cell activation, early IFN-γ production may be reduced, resulting in diminished activation, recruitment, or expansion of RSV-specific CD8+ T cells and delayed virus clearance.
Our studies also demonstrate that, in the appropriate genetic background, redundant and compensatory antiviral mechanisms may develop in CD1d-deficient mice. This is not surprising because NK cell responses to tumors (85) and stressors (52, 68) vary between mouse strains. NK cells are an important element in the early immune response to viral infections, including RSV infection (2, 10, 14, 31, 34, 39, 81), and may account for the strain-dependent differences in viral clearance observed in RSV-infected mice of various strains (unpublished data). Primary functions of NK cells at the site of virus infection include both direct cytotoxic killing of infected cells (10, 39) and secretion of cytokines that subsequently serve to activate other components of the immune response (2, 28, 39, 53, 66).
In primary RSV infection NK cells have been shown to infiltrate the lung early in infection, with peak cytolytic activity detected at day 3 p.i. (2, 34). Depletion of asialo GM+ NK cells resulted in prolonged shedding of RSV from infected mice, demonstrating a crucial role for NK cells—directly or indirectly—in efficient RSV clearance (34). Subsequent work by Hussell and Openshaw showed that NK cells are the predominant population present at day 4 p.i. and that recruitment of these IFN-γ-secreting cells precedes activation and recruitment of CD8+ T cells (39). NK cell-produced type 1 and 2 interferons play critical roles in generation of antiviral immune responses (66) with IL-12 (18, 20) and IL-18 (63) differentially required for interferon production. IFN-α/β and IFN-γ may be differentially expressed by both NK cells and T cells during the course of viral infection (60). STAT-1 signaling through the IFN-α/β receptor is a critical immunoregulatory event in this process (60). Similarly, IFN-α/β and IFN-γ produced by NK T cells activated by a single injection of α-GalCer inhibit hepatitis B virus replication (44). Thus, cells of the innate response (such as NK and NK T cells) are vital to the development of adaptive immune responses during viral infection and clearly set the pattern (11) for adaptive immune responses. As the pathogenesis of multiple viruses is dissected in detail, it is becoming evident that elaborate and cooperative networks function to generate immune responses that may protect against or enhance disease. This cascade of events has been described for antiviral NK and NK T-cell responses in that it appears that NK T cells are activated early in viral infections (12, 25, 44) and the resulting cytokine production (particularly IFN-γ) then functions to activate NK cells that act either directly or indirectly to control viral infections. This amplification of NK and NK T cell responses subsequently serves to direct the development of T-cell responses (11, 76).
In this report we examined the immune responses to RSV infection in CD1d-deficient mice in three different genetic backgrounds. Depending upon the strain of mouse, the absence of NK T cells also resulted in delayed viral clearance and decreased IFN-γ production. These data demonstrate a role for NK T cells in the induction of CD8+-T-cell responses in that activation of the local NK T cells (since RSV does not result in a systemic infection) contributes to the activation, expansion, or recruitment of CD8+ T cells. To examine the role of NK T-cell activation and cytokine production in the generation of these responses, CD1d+/+ mice were treated with α-GalCer around the time of RSV infection. While α-GalCer treatment did result in increased production of IFN-γ and increased numbers of CD8+ T cells infiltrating the lung, viral clearance was delayed. This may be due to the increased IL-4 levels, which have been shown to decrease CTL activity (4, 6, 27, 69) and can alter the mechanism of CTL killing to a less-efficient Fas-mediated pathway (7). The recent publication by Rutigliano et al. demonstrates that decreased viral clearance occurs in RSV-infected perforin-deficient mice despite increased numbers of CD8+ T cells and increased levels of IFN-γ (65). Thus, activation of NK T cells, and the concomitant production of IFN-γ, during RSV infection may result in amplification of CD8+-T-cell responses at the level of expansion and recruitment.
It has been reported that α-GalCer treatment activates NK T cells, resulting in increased IFN-γ and IL-4 production and increased CTL killing against tumor cell lines (61). Our data confirm increased cytokine production following α-GalCer activation of NK T cells in vivo and extend the earlier findings to demonstrate that numbers of antigen-specific CD8+ T cells are increased. While Nishimura et al. (61) observed enhanced CTL killing of tumors, we found reduced CTL killing activity, as evidenced by delayed viral clearance. One possible explanation for this apparent discrepancy is that in the studies of Nishimura et al. (61), α-GalCer-treated lymphocytes were cultured for 2 days in vitro in the presence of IL-2 and IL-12, resulting in enhanced CTL activity. This in vitro culture system may reverse the inhibitory effects of the in vivo IL-4 production we demonstrate here, which in our in vivo measure of cytotoxic function by viral clearance is delayed by α-GalCer treatment. Additionally, the differences in timing of α-GalCer administration may account for this discrepancy. While Nishimura et al. administered α-GalCer at a single time point, mice were given multiple injections of α-GalCer in our studies. During the performance of our studies, it was demonstrated that multiple injections of α-GalCer may predispose mice to the induction of IL-4-producing type 2 T cells (71). Thus, in our model system, activation of NK T cells contributes to the amplification of protective antiviral CD8+-T-cell responses during primary RSV infection.
The role of NK T cells in the generation of CD8+-T-cell responses is also evident in vvGs- and FI-RSV-immunized CD1d-deficient mice or α-GalCer-treated CD1d+/+ mice in which peak viral titers were increased. Thus, NK T cells also play a role in the amplification and generation of memory T cells that protect against RSV. It has been shown that IFN-γ production from CD8+ T cells or NK cells regulates pulmonary eosinophilia in RSV G-immunized mice (37, 39, 74, 75). Thus, activation of NK T cells (and concomitant IFN-γ production) by α-GalCer treatment during vvGs or FI-RSV immunization might be predicted to modulate differentiation of the RSV-specific type 2 T cells to a more type 1-like phenotype. However, this is not the case. The systemic NK T-cell activation following α-GalCer treatment of vvGs-primed mice did not reduce Th2 cytokine or eotaxin production nor did it inhibit pulmonary eosinophilia. Similarly, in FI-RSV-immunized mice significant induction of type 2 T-cell responses is evident, although greater increases in IL-5 and IL-13 responses were observed, potentially reflecting the IL-4 dependence of FI-RSV-induced responses, while RSV G-induced responses are IL-4 independent (41). Thus, while not directly shown, these results suggest that during induction of RSV-specific immune responses, NK T cells function as a means of amplifying the inherent immune responses to the RSV antigens rather than as a means influencing the differentiation to a type 1 or type 2 T-cell response.
In summary, our findings demonstrate that NK T cells contribute to the efficient induction of CD8+-T-cell responses and other antiviral immune responses to RSV and that, in the absence of NK T-cell activation, early IFN-γ production may be reduced, resulting in diminished RSV-specific CD8+-T-cell expansion and delayed viral clearance. These data are consistent with the established mouse model of RSV infection which demonstrates that CD8+ CTL are important for clearance of virus but are also responsible for immunopathology and disease. Our findings also demonstrate that, in the appropriate genetic background, compensatory mechanisms are present that diminish the importance of NK T cells in antiviral responses. In the setting of RSV immunization and challenge, we demonstrate that NK T cells amplify the intrinsic immune responses to RSV rather than serving a major role in selective T-cell differentiation. Furthermore, despite the ability of α-GalCer treatment to reduce illness in primary RSV infection, this intervention at immunization decreases protection without alleviating illness, arguing against the use of α-GalCer as an adjuvant in any RSV vaccine product. Nevertheless, as we learn more about the CD1d antigen presentation pathway, additional reagents may become available that will permit the selective activation of certain NK T-cell functions. Such reagents may be valuable as therapeutics and/or adjuvants.
REFERENCES
- 1.Alwan, W. H., W. J. Kozlowska, and P. J. M. Openshaw. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 179:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. J., J. Norden, D. Saunders, G. L. Toms, R. Scott, and P. L. Collins. 1990. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J. Gen. Virol. 71:1561-1570. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, L. J., C. Tsou, C. Potter, H. L. Keyserling, T. F. Smith, G. Ananaba, and C. R. M. Bangham. 1994. Cytokine response to respiratory syncytial virus stimulation of human peripheral blood mononuclear cells. J. Infect. Dis. 170:1201-1208. [DOI] [PubMed] [Google Scholar]
- 4.Andrew, M. E., and B. E. H. Coupar. 1992. Biological effects of recombinant vaccinia virus-expressed interleukin-4. Cytokine 4:281-286. [DOI] [PubMed] [Google Scholar]
- 5.Apostolou, I., Y. Takahama, C. Belmant, T. Kawano, M. Huerre, G. Marchal, J. Cui, M. Taniguchi, H. Nakauchi, J.-J. Fournie, P. Kourilsky, and G. Gachelin. 1999. Murine natural killer T (NKT) cells [correction of natural killer cells] contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc. Natl. Acad. Sci. USA 96:5141-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aung, S., Y.-W. Tang, and B. S. Graham. 1999. Interleukin-4 diminishes CD8+ T respiratory syncytial virus-specific cytotoxic T-lymphocyte activity in vivo. J. Virol. 73:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aung, S., and B. S. Graham. 2000. IL-4 diminishes perforin-mediated and increases Fas ligand-mediated cytotoxicity in vivo. J. Immunol. 164:3487-3493. [DOI] [PubMed] [Google Scholar]
- 8.Bembridge, G. P., J. A. Lopez, R. Cook, J. A. Melero, and G. Taylor. 1998. Recombinant vaccinia virus coexpressing the F protein of respiratory syncytial virus (RSV) and interleukin-4 (IL-4) does not inhibit the development of RSV-specific memory cytotoxic T lymphocytes, whereas priming is diminished in the presence of high levels of IL-2 or gamma interferon. J. Virol. 72:4080-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac, A., M. N. Rivera, S.-H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells. Development, specificity, and function. Annu. Rev. Immunol. 15:535-562. [DOI] [PubMed] [Google Scholar]
- 10.Bigger, J. E., I. Thomas, and S. S. Atherton. 1998. NK cell modulation of murine cytomegalovirus retinitis. J. Immunol. 160:5826-5831. [PubMed] [Google Scholar]
- 11.Biron, C. A. 1999. Initial and innate responses to viral infections--pattern setting in immunity or disease. Curr. Opin. Microbiol. 2:374-381. [DOI] [PubMed] [Google Scholar]
- 12.Biron, C. A., and L. Brossay. 2001. NK and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 13.Bright, H., T. Turnball, G. L. Toms, and R. Scott. 1995. Comparison of the T helper cell response induced by respiratory syncytial virus and its fusion protein in BALB/c mice. Vaccine 13:915-922. [DOI] [PubMed] [Google Scholar]
- 14.Brutkiewicz, R. R., and R. M. Welsh. 1995. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J. Virol. 69:3967-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 16.Burdin, N., L. Brossay, Y. Koezuka, S. T. Smiley, M. J. Grusby, M. Gui, M. Taniguchi, K. Hayakawa, and M. Kronenberg. 1998. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J. Immunol. 161:3271-3281. [PubMed] [Google Scholar]
- 17.Cannon, M. J., P. J. M. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cousens, L. P., R. Peterson, S. Hsu, A. Dorner, J. D. Altman, R. Ahmed, and C. A. Biron. 1999. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 189:1315-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denkers, E. Y., T. Scharton-Kersten, S. Barbieri, P. Caspar, and A. Sher. 1996. A role for CD4+ NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J. Exp. Med. 184:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doughty, L. A., K. B. Nguyen, J. E. Durbin, and C. A. Biron. 2001. A role for IFN-α/β in virus infection-induced sensitization to endotoxin. J. Immunol. 166:2658-2664. [DOI] [PubMed] [Google Scholar]
- 21.Dowell, S. F., L. J. Anderson, J. Gary, D. D. Erdman, J. F. Plouffe, J. File, B. J. Marston, and R. F. Breiman. 1996. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J. Infect. Dis. 174:456-462. [DOI] [PubMed] [Google Scholar]
- 22.Eberl, G., and H. R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985-992. [DOI] [PubMed] [Google Scholar]
- 23.Emoto, M., Y. Emoto, and S. H. E. Kaufmann. 1995. Interleukin-4-producing CD4+ NK1.1+ TCR alpha/beta intermediate liver lymphocytes are down-regulated by Listeria monocytogenes. Eur. J. Immunol. 25:3321-3325. [DOI] [PubMed] [Google Scholar]
- 24.Emoto, M., and S. H. E. Kaufmann. 1997. Transient control of interleukin-4-producing natural killer T cells in the livers of Listeria monocytogenes-infected mice by interleukin-12. Infect. Immun. 65:5003-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exley, M. A., N. J. Bigley, O. Cheng, S. M. A. Tahir, S. T. Smiley, Q. L. Carter, H. F. Stills, M. J. Grusby, Y. Koezuka, M. Taniguchi, and S. P. Balk. 2001. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 69:713-718. [PubMed] [Google Scholar]
- 26.Falsey, A. R., E. E. Walsh, and R. F. Betts. 1990. Serologic evidence of respiratory syncytial virus infection in nursing home patients. J. Infect. Dis. 162:568-569. [DOI] [PubMed] [Google Scholar]
- 27.Fischer, J. E., J. E. Johnson, R. K. Kuli-Zade, T. R. Johnson, S. Aung, R. A. Parker, and B. S. Graham. 1997. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J. Virol. 71:8672-8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovarelli, M., A. Santoni, C. Jemma, T. Musso, A. M. Giuffrida, G. Cavallo, S. Landolfo, and G. Forni. 1988. Obligatory role of IFN-γ in induction of lymphokine-activated and T lymphocyte killer activity, but not in boosting of natural cytotoxicity. J. Immunol. 141:2831-2836. [PubMed] [Google Scholar]
- 29.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham, B. S., G. S. Henderson, Y.-W. Tang, X. Lu, K. M. Neuzil, and D. G. Colley. 1993. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 151:2032-2040. [PubMed] [Google Scholar]
- 31.Graham, B. S. 1996. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 4:290-294. [DOI] [PubMed] [Google Scholar]
- 32.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 33.Gumperz, J. E., C. Roy, A. Makowska, D. Lum, M. Sugita, T. Podrebarac, Y. Koezuka, S. A. Porcelli, S. Cardell, M. B. Brenner, and S. M. Behar. 2000. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12:211-221. [DOI] [PubMed] [Google Scholar]
- 34.Harrop, J. A., J. J. Anderson, P. Hayes, N. Serin, and R. Scott. 1994. Characteristics of the pulmonary natural killer (NK) cell response to respiratory syncytial virus infection in BALB/c mice. Immunol. Infect. Dis. 4:179-185. [Google Scholar]
- 35.Hong, S., M. T. Wilson, I. Serizawa, L. Wu, N. Singh, O. V. Naidenko, T. Miura, T. Haba, D. C. Scherer, J. Wei, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 2001. The natural killer T-cell ligand α-galacatosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 7:1052-1056. [DOI] [PubMed] [Google Scholar]
- 36.Huang, Y. T., and G. W. Wertz. 1982. The genome of respiratory syncytial virus is a negative-stranded RNA that codes for at least seven mRNA species. J. Virol. 43:150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussell, T., C. J. Baldwin, A. O'Garra, and P. J. M. Openshaw. 1997. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 27:3341-3349. [DOI] [PubMed] [Google Scholar]
- 38.Hussell, T., U. Khan, and P. J. M. Openshaw. 1997. IL-12 treatment attenuates T helper cell type 2 and B cell responses but does not improve vaccine-enhanced lung illness. J. Immunol. 159:328-334. [PubMed] [Google Scholar]
- 39.Hussell, T., and P. J. M. Openshaw. 1998. Intracellular IFN-γ expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J. Gen. Virol. 79:2593-2601. [DOI] [PubMed] [Google Scholar]
- 40.Jackson, M., and R. Scott. 1996. Different patterns of cytokine induction in cultures of respiratory syncytial (RS) virus-specific human Th cell lines following stimulation with RS virus and RS virus proteins. J. Med. Virol. 49:161-169. [DOI] [PubMed] [Google Scholar]
- 41.Johnson, T. R., and B. S. Graham. 1999. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J. Virol. 73:8485-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and B. S. Graham. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 72:2871-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joyce, S., A. S. Woods, J. W. Yewdell, J. R. Bennink, A. D. De Silva, A. Boesteanu, S. P. Balk, R. J. Cotter, and R. R. Brutkiewicz. 1998. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 279:1541-1544. [DOI] [PubMed] [Google Scholar]
- 44.Kakimi, K., L. G. Guidotti, and Y. Koezuka. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapikian, A. Z., R. H. Mitchell, R. M. Chanock, R. A. Shvedoff, and C. E. Stewart. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89:405-421. [DOI] [PubMed] [Google Scholar]
- 46.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki, and M. Taniguchi. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278:1626-1629. [DOI] [PubMed] [Google Scholar]
- 47.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 48.Kulkarni, A. B., P. L. Collins, I. Bacik, J. W. Yewdell, J. R. Bennink, J. Crowe, and B. R. Murphy. 1995. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J. Virol. 69:1261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar, H., A. Belperron, S. W. Barthold, and L. K. Bockenstedt. 2000. CD1d deficiency impairs murine host defense against the spirochete Borrelia burgdorferi. J. Immunol. 165:4797-4801. [DOI] [PubMed] [Google Scholar]
- 50.Lawson, C. M., J. R. Bennink, N. P. Restifo, J. W. Yewdell, and B. R. Murphy. 1994. Primary pulmonary cytotoxic T lymphocytes induced by immunization with a vaccinia virus recombinant expressing influenza A virus nucleoprotein peptide do not protect mice against challenge. J. Virol. 68:3505-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leite-De-Moraes, M. C., G. Moreau, A. Arnould, F. Machavoine, C. Garcia, M. Papiernik, and M. Dy. 1998. IL-4-producing NK T cells are biased towards IFN-gamma production by IL-12. Influence of the microenvironment on the functional capacities of NK T cells. Eur. J. Immunol. 28:1507-1515. [DOI] [PubMed] [Google Scholar]
- 52.Lu, Z. W., C. Song, A. V. Ravindran, Z. Merali, and H. Anisman. 1998. Influence of a psychogenic and a neurogenic stressor on several indices of immune functioning in different strains of mice. Brain Behavior Immun. 12:7-22. [DOI] [PubMed] [Google Scholar]
- 53.Mandelboim, O., S. Kent, D. M. Davis, S. B. Wilson, T. Okazaki, R. Jackson, D. Hafler, and J. L. Strominger. 1998. Natural killer activating receptors trigger interferon gamma secretion from T cells and natural killer cells. Proc. Natl. Acad. Sci. USA 95:3798-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez, F. D., A. L. Wright, L. M. Taissig, C. J. Holberg, M. Halonen, W. J. Morgan, and the Group Health Medical Associates. 1995. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 332:133-138. [DOI] [PubMed] [Google Scholar]
- 55.McIntosh, K. 1976. Bronchiolitis and asthma: possible common pathogenetic pathways. J. Allergy Clin. Immunol. 57:595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendiratta, S. K., D. W. Martin, S. Hong, A. Boesteanu, S. Joyce, and L. Van Kaer. 1997. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6:469-477. [DOI] [PubMed] [Google Scholar]
- 57.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. D. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 58.Neuzil, K. M., J. E. Johnson, Y.-W. Tang, J.-P. Prieels, M. Slaoui, N. Gar, and B. S. Graham. 1997. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine 15:525-532. [DOI] [PubMed] [Google Scholar]
- 59.Neuzil, K. M., and B. S. Graham. 1996. Cytokine release and innate immunity in respiratory viral infection. Semin. Virol. 7:255-264. [Google Scholar]
- 60.Nguyen, K. B., L. P. Cousens, L. A. Doughty, G. C. Pien, J. E. Durbin, and C. A. Biron. 2000. Interferon α/β-mediated inhibition and promotion of interferon γ: STAT1 resolves a paradox. Nat. Immunol. 1:70-76. [DOI] [PubMed] [Google Scholar]
- 61.Nishimura, T., H. Kitamura, K. Iwakabe, T. Yahata, A. Ohta, M. Sato, K. Takeda, K. Okumura, L. Van Kaer, T. Kawano, M. Taniguchi, M. Nakui, M. Sekimoto, and T. Koda. 2000. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 12:987-994. [DOI] [PubMed] [Google Scholar]
- 62.Okamoto, Y., K. Kudo, K. Ishikawa, E. Ito, K. Togawa, I. Saito, I. Moro, J. A. Patel, and P. L. Ogra. 1993. Presence of respiratory syncytial virus genomic sequences in middle ear fluid and its relationship to expression of cytokines and cell adhesion molecules. J. Infect. Dis. 168:1277-1281. [DOI] [PubMed] [Google Scholar]
- 63.Pien, G. C., A. R. Satoskar, K. Takeda, S. Akira, and C. A. Biron. 2000. Selective IL-18 requirements for induction of compartmental IFN-γ responses during viral infection. J. Immunol. 165:4787-4791. [DOI] [PubMed] [Google Scholar]
- 64.Porcelli, S. A., and R. L. Modlin. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297-329. [DOI] [PubMed] [Google Scholar]
- 65.Rutigliano, J. A., S. Aung, and B. S. Graham. 2001. Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease. J. Virol. 75:9918-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salazar-Mather, T. P., R. Ishikawa, and C. A. Biron. 1996. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J. Immunol. 157:3054-3064. [PubMed] [Google Scholar]
- 67.Schofield, L., M. J. McConville, D. Hansen, A. S. Campbell, B. Fraser-Reid, M. J. Grusby, and S. D. Tachado. 1999. CD1d-restricted immunoglobulin T formation to GPI-anchored antigens mediated by NKT cells. Science 283:225-229. [DOI] [PubMed] [Google Scholar]
- 68.Shanks, N., J. Griffiths, and H. Anisman. 1994. Norepinephrine and serotonin alterations following chronic stressor exposure: mouse strain differences. Pharmacol. Biochem. Behavior 49:57-65. [DOI] [PubMed] [Google Scholar]
- 69.Sharma, D. P., A. J. Ramsay, D. J. Maguire, M. S. Rolph, and I. A. Ramshaw. 1996. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J. Virol. 70:7103-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1980. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282:1440-1446. [DOI] [PubMed] [Google Scholar]
- 71.Singh, N., S. Hong, D. C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373-2377. [PubMed] [Google Scholar]
- 72.Spender, L. C., T. Hussell, and P. J. M. Openshaw. 1998. Abundant IFN-γ production by local T cells in respiratory syncytial virus-induced eosinophilic lung disease. J. Gen. Virol. 79:1751-1758. [DOI] [PubMed] [Google Scholar]
- 73.Srikiatkhachorn, A., W. Chang, and T. J. Braciale. 1999. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J. Virol. 73:6590-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific, CD8+ T lymphocytes down regulate Th2 type cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J. Virol. 71:678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su, H. C., K. B. Nguyen, T. P. Salazar-Mather, M. C. Ruzek, M. Y. Dalod, and C. A. Biron. 2001. NK cell functions restrain T cell responses during viral infections. Eur. J. Immunol. 31:3048-3055. [DOI] [PubMed] [Google Scholar]
- 77.Tang, Y.-W., and B. S. Graham. 1994. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J. Clin. Investig. 94:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang, Y.-W., K. M. Neuzil, J. E. Fischer, F. W. Robinson, R. A. Parker, and B. S. Graham. 1997. Determinants and kinetics of cytokine expression patterns in lungs of vaccinated mice challenged with respiratory syncytial virus. Vaccine 15:597-602. [DOI] [PubMed] [Google Scholar]
- 79.Tang, Y.-W., and B. S. Graham. 1997. T cell source of type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J. Clin. Investig. 99:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang, Y.-W., and B. S. Graham. 1995. Interleukin-12 treatment during immunization elicits a T helper cell type 1-like immune response in mice challenged with respiratory syncytial virus and improves vaccine immunogenicity. J. Infect. Dis. 172:734-738. [DOI] [PubMed] [Google Scholar]
- 81.Tay, C.-H., R. M. Welsh, and R. R. Brutkiewicz. 1995. NK cell response to viral infections in β2-microglobulin-deficient mice. J. Immunol. 154:780-789. [PubMed] [Google Scholar]
- 82.Tebbey, P. W., M. Hagen, and G. E. Hancock. 1998. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 188:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whimbey, E., R. E. Champlin, J. A. Englund, N. Q. Mirza, P. A. Piedra, J. M. Goodrich, D. Przepiorka, M. A. Luna, R. C. Morice, J. L. Neumann, L. S. Elting, and G. P. Bodey. 1995. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 16:393-399. [PubMed] [Google Scholar]
- 84.Whimbey, E., R. E. Champlin, R. B. Couch, J. A. Englund, J. M. Goodrich, I. Raad, D. Przepiorka, V. A. Lewis, N. Mirza, H. Yousuf, J. J. Tarrand, and G. P. Bodey. 1996. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin. Infect. Dis. 22:778-782. [DOI] [PubMed] [Google Scholar]
- 85.Whyte, A. L., and S. C. Miller. 1998. Strain differences in natural killer cell-mediated immunity among mice: a possible mechanism for the low natural killer cell activity of A/J mice. Immunobiology 199:23-38. [DOI] [PubMed] [Google Scholar]
- 86.Yoshimoto, T., A. Bendelac, C. Watson, J. Hu-Li, and W. E. Paul. 1995. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science 270:1845-1847. [DOI] [PubMed] [Google Scholar]