Abstract
Cyclophilin A (CypA) is necessary for effective human immunodeficiency virus type 1 (HIV-1) replication. However, the functions of CypA and the precise steps at which CypA acts in the HIV-1 life cycle remain to be determined. By using a methodology that bypasses the need for attachment factors—spinoculation—we present evidence that CypA participates in both entry and postentry events.
Human immunodeficiency virus type 1 (HIV-1) requires the incorporation of a host protein, cyclophilin A (CypA), to efficiently replicate in target cells (2, 4,7, 25). CypA is specifically incorporated into nascent viruses by binding to the capsid (CA) region of the Gag precursor (5, 7). It has been shown that the hydrophobic pocket of CypA interacts with an exposed loop of CA (3, 6, 10). The immunosuppressive drug cyclosporin (CsA) also binds to the hydrophobic pocket of CypA and competitively inhibits CypA-CA/Gag interactions (1, 8,12, 13, 14, 17). Mutations in the CypA packaging signal of CA or the presence of CsA hampers CypA incorporation into nascent particles (2, 5,7, 29). Importantly, these viruses, which lack CypA, exhibit low levels of infectivity compared to wild-type viruses (1, 2,7, 8, 25).
Several functions in the HIV-1 life cycle have been attributed to CypA. Initially, CypA was proposed to act as an uncoating factor (15). This notion arose from the observation that CypA is a cis-trans isomerase (11, 12) and that CypA binds CA (1, 10), the main component of the shell that surrounds the viral genome. In this model, the enzymatic activity of CypA induces subtle changes in CA-CA interactions that trigger the disassembly of the HIV-1 core, allowing the delivery of the viral genome into the host cytosol. More recently, we and others presented evidence that suggests that CypA participates in the initial uptake of HIV-1 by target cells (21, 24). Specifically, we showed that CypA-deficient viruses attach to target cells less efficiently than wild-type viruses (21). Most recently, it has been shown that HIV-1 entry depends on interactions between virus-associated CypA and cell surface CD147 (20). Indeed, antibodies directed against CD147 inhibit the entry of HIV-1 into target cells, but not that of viruses that do not require CypA for replication, such as simian immunodeficiency virus (20).
The two latter observations imply that CypA participates in HIV-1 entry, whereas the original function attributed to CypA, as an uncoating factor, suggests that CypA participates in postentry events. We recently obtained evidence that seems to argue against the possibility that CypA acts as an uncoating factor. Specifically, we demonstrated that the isomerase activity of CypA is not required for HIV-1 infection. By using an in trans incorporation system (28), we found that a Vpr-CypA chimera rescues the infectivity of viruses lacking CypA. Furthermore, we observed that a Vpr-CypA mutant that has no isomerase activity and no capacity to bind to CA also rescues HIV-1 infectivity (23). Thus, these data argue against the participation of the isomerase activity of CypA in HIV-1 replication and thus seem to plead against the possibility that CypA acts as an uncoating factor. However, this does not rule out the possibility that CypA plays a postentry role in HIV-1 infection. In the present study, we specifically asked whether CypA is only required for an efficient HIV-1 entry or is also necessary for subsequent postentry events.
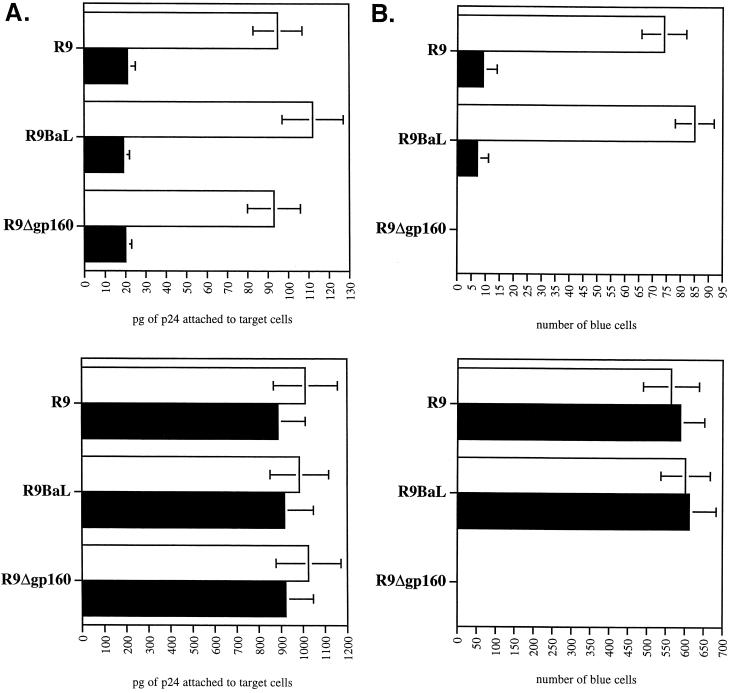
In order to examine postentry events exclusively, we took advantage of the recent work of Malim and colleagues demonstrating that spinoculation forces the deposition of viruses onto the surface of target cells (19). Importantly, this technique, by bypassing attachment requirements, allows the examination of postattachment steps of HIV-1 infection alone. First, we verified that this technique can substitute for HIV-1 attachment preconditions. We and others showed that cell surface heparan sulfates mediate HIV-1 attachment to adherent cells that express low CD4 levels, such as CD4+ HeLa cells or macrophages (18, 21, 22). Specifically, the removal of cell surface heparan sulfate by heparitinase diminishes both HIV-1 attachment and infectivity (18, 21, 22). We speculated that spinoculation would rescue the attachment of HIV-1 to cells devoid of cell surface heparan sulfate after heparitinase treatment. To test this hypothesis, wild-type R9 (NL4.3 derivative) (X4 virus) (9), R9 BaL (R5 virus) (27), or gp120-deleted (R9Δgp160) (21) viruses were produced from 293T cells as described previously (21). Twenty-four hours preinfection, CD4+ CXCR4+ CCR5+ HeLa cells were plated at 80,000 cells/well/ml in a 24-well plate. Adherent cells were pretreated or not with heparitinase for 6 h as described previously (22). Cells were then washed twice, and viruses were added in a final volume of 1 ml of complete Dulbecco's modified Eagle's medium. Plates were centrifuged at 2,000 × g (spinoculation) or not (sedimentation) for 3 h at room temperature. Cells were washed twice to remove unbound virus, and amounts of attached virus were quantified by p24 enzyme-linked immunosorbent assay (ELISA) as described previously (21). Without centrifugation, removal of heparan sulfate by heparitinase decreases the attachment of R9, R9 BaL, and R9Δgp120 viruses (fivefold) (Fig. 1A), further confirming that heparan sulfates promote HIV-1 adsorption to adherent cells (18, 21, 22). As expected, spinoculation increased the levels of attachment of R9, R9 BaL, and R9Δgp160 viruses (ninefold). Importantly, the amounts of viruses attached to the surface of untreated or heparitinase-treated cells were similar. Note that we obtained comparable results with CD4− HeLa cells (data not shown). This further suggests that HIV-1 attaches to adherent cells (HeLa or macrophages) in a gp120- and CD4-independent manner (21, 22). The infectivity of these attached viruses was then examined. Specifically, target cells were washed twice after spinoculation or sedimentation to remove unattached virus and carefully transferred to 37°C for 48 h. Infectivity was scored by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining as described previously (26). Without centrifugation, we observed that the removal of cell surface heparan sulfate by heparitinase reduced the levels of infectivity of both R9 and R9 BaL (8- and 12-fold, respectively) (Fig. 1B). This confirms previous observations that cell surface heparan sulfates, by facilitating HIV-1 attachment to target cells, promote HIV-1 infectivity (18, 21, 22). Correlating our attachment data (Fig. 1A), spinoculation increases the levels of infectivity of both R9 and R9 BaL (eight- and sevenfold, respectively). Most importantly, the levels of infectivity of R9 and R9 BaL of untreated and heparitinase-treated target cells were similar. As expected, gp120-deleted viruses (spinoculated or not) failed to infect CD4+ HeLa cells, further confirming the notion that gp120 is absolutely necessary for fusion. Together these results suggest that spinoculation, by forcing HIV-1 attachment, rescues HIV-1 infectivity in cells devoid of cell surface heparan sulfates. This also suggests that heparan sulfates do not play a major role in HIV-1 infection, beyond facilitating HIV-1 attachment to target cells. Altogether, these results demonstrate that spinoculation obviates the need for attachment factors, such as heparan sulfates.
FIG. 1.
Spinoculation bypasses the need for heparan sulfates in HIV-1 attachment and infectivity. (A) Effect of spinoculation on HIV-1 attachment to target cells devoid of heparan sulfates. CD4+ CXCR4+ CCR5+ HeLa cells were (▪) or were not (□) pretreated with heparitinase for 6 h and exposed to R9 (X4 virus), R9 BaL (R5 virus), and R9Δgp160 viruses (3 ng of p24). Plates containing adherent target cells were centrifuged (spinoculation) (bottom panel) or not (sedimentation) (top panel) for 3 h at room temperature. Cells were washed twice to remove unbound virus, and the amounts of attached virus were quantified by p24 ELISA. Results are expressed in picograms of p24 (total amount attached) and represent the average of four independent experiments. (B) Effect of spinoculation on HIV-1 infectivity in target cells devoid of heparan sulfates. As above, target cells were (▪) or were not (□) pretreated with heparitinase and exposed to R9, R9 BaL, or R9Δgp160 viruses (3 ng of p24). Cells were centrifuged (bottom panel) or not (top panel), washed twice, and carefully transferred to 37°C for 48 h. Infectivity was scored by X-Gal staining. Results are expressed in number of blue cells (total number of infected cells) and represent the average of four independent experiments.
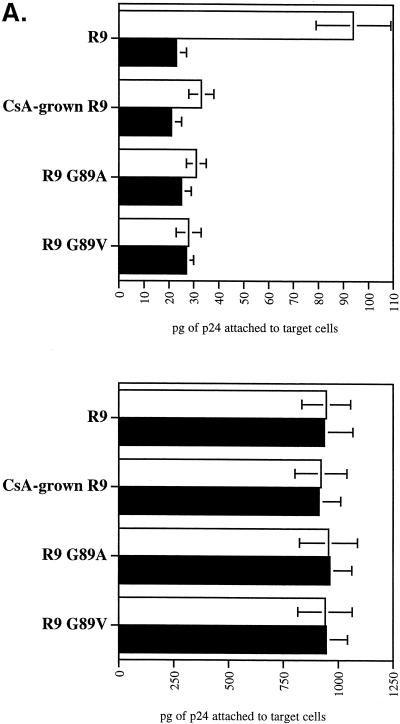
We took advantage of this technique to examine if CypA is needed for postattachment events in HIV-1 infection. First, we asked whether the infectivity of CypA-deficient viruses can be fully rescued by spinoculation. Wild-type (R9) and CypA-deficient (R9 G89V, R9 G89A, and CsA-grown R9) viruses were produced from 293T cells as described previously (21). Centrifuged or sedimentated viruses were then tested for attachment and infectivity as described above. We found that spinoculation greatly enhances the adsorption of both wild-type and CypA-deficient viruses to target cells (10- and 30-fold, respectively) (Fig. 2A). Importantly, similar amounts of wild-type and CypA-deficient viruses were attached to target cells. This indicates that spinoculation overcomes the previously reported defect for CypA-deficient viruses (21).
FIG. 2.
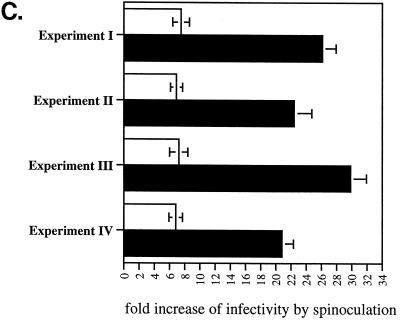
Spinoculation by bypassing attachment preconditions reveals a postattachment role for CypA in HIV-1 infection. (A) Spinoculation rescues the attachment of CypA-deficient viruses. CD4+ CXCR4+ CCR5+ HeLa cells were exposed to wild-type (R9) or CypA-deficient (CsA-grown R9, R9 G89V, or R9 G89A) viruses (3 ng of p24). CsA was used at a concentration of 10 μM. Adherent cells and viruses were centrifuged (spinoculation) (bottom panel) or not (sedimentation) (top panel) for 3 h at room temperature. Cells were washed twice to remove unbound virus, and amounts of attached virus were quantified by p24 ELISA. Results are expressed in picograms of p24 and represent the average of two independent experiments. □, no treatment; ▪, heparitinase treatment. (B) Spinoculation only partially rescues the infectivity of CypA-deficient viruses. As above, target cells were exposed to wild-type (R9) or CypA-deficient (CsA-grown R9, R9 G89V, or R9 G89A) viruses (3 ng of p24). Cells were (bottom panel) or were not (top panel) centrifuged, washed twice, and carefully transferred to 37°C for 48 h. Infectivity was scored by X-Gal staining. Results are expressed in number of blue cells and represent the average of three independent experiments. □, no treatment; ▪, heparitinase treatment. (C) Disproportionate increase of infectivity between wild-type and CypA-deficient viruses by spinoculation. The number of infected cells with sedimentated or spinoculated viruses (as above) was compared in four independent experiments. Results are expressed as a ratio of the number of infected cells by spinoculation divided by the number of infected cells by sedimentation. □, R9; ▪, R9G89V.
After demonstrating that spinoculation deposits similar amounts of both wild-type and CypA-deficient viruses onto the cell surface, levels of infectivity of these attached viruses were examined. Without centrifugation (sedimentation), CypA-deficient viruses exhibited decreased levels of infectivity compared to wild-type virus (about eightfold) (Fig. 2B) as reported previously (2, 7,21, 25). This confirms the requirement for CypA in HIV-1 infection. Levels of infectivity of centrifuged wild-type viruses were enhanced (about sevenfold) compared to sedimentated viruses. Importantly, levels of infectivity of CypA-deficient viruses were dramatically enhanced by spinoculation (20-fold). The disparate enhancement of infectivity by spinoculation between wild-type (about 7-fold) and CypA-deficient (about 20-fold) viruses further suggests that CypA is required for effective HIV-1 attachment and infectivity. This disparate enhancement of infectivity by spinoculation between wild-type and CypA-deficient viruses was observed in several independent experiments (Fig. 2C). The increase of infectivity of wild-type virus by spinoculation is remarkably constant among independent experiments. It is important to note that the use of higher doses of virus masks the attachment defect for CypA-deficient viruses. Indeed, the disproportionate rescue of infectivity observed was more pronounced at the lowest inoculum (3 ng of p24). This is consistent with our observation that detectable attachment of HLA-DR- or GFP-labeled viruses to target cells requires a high inoculum (100 ng to 1 μg of p24), which obscures the contribution of CypA-mediated attachment (data not shown). Nevertheless, it is important to emphasize that the postentry defect of CypA-deficient viruses is manifested at either a low or high virus inoculum, demonstrating that spinoculation does not mask this defect.
Most importantly, although spinoculation greatly rescues the infectivity of CypA-deficient viruses, the absolute levels of infectivity of these viruses were still decreased compared to those of wild-type viruses (2.5-fold) (Fig. 2B). Given that identical amounts of centrifuged CypA-deficient and wild-type viruses were deposited onto the surface of target cells (Fig. 2B), this suggests that CypA not only participates in HIV-1 attachment, but also participates in postattachment events. Note that this defect in infectivity is observed in a single round of infection and would be amplified in multiple rounds of infection. Thus, we expect that the observed postattachment defect of CypA-deficient viruses (2.5-fold defect) will result in a profound diminishment of virus propagation. Supporting this hypothesis, several studies showed that replication of CypA-deficient viruses is severely attenuated in human T-cell lines (1, 2,7, 8,17, 25).
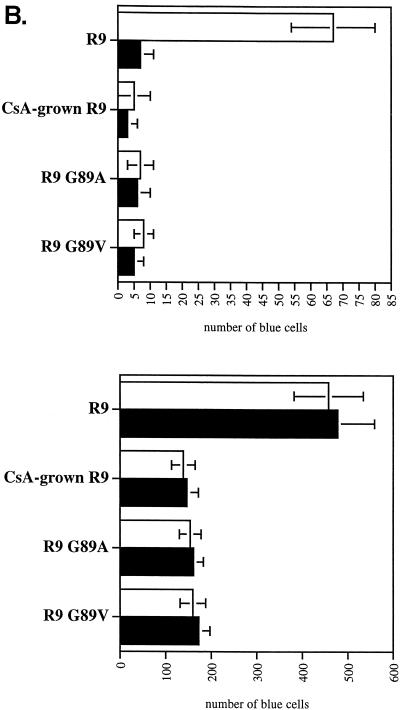
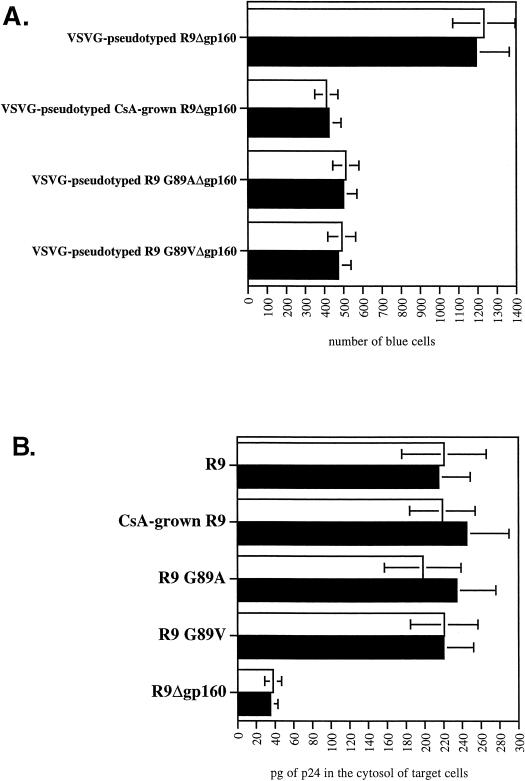
To distinguish between a postattachment and postentry role for CypA, we performed similar experiments with viruses pseudotyped with the vesicular stomatitis virus G envelope (VSVG), which bypasses the need for CD4 and chemokine receptors for fusion. Specifically, wild-type (R9Δgp160) and CypA-deficient (R9 G89VΔgp160, R9 G89AΔgp160, and CsA-grown R9Δgp160) viruses were pseudotyped with VSVG as described previously (21) and tested for infectivity after spinoculation. Like nonpseudotyped CypA-deficient viruses (Fig. 2B), we found that the absolute levels of infectivity of VSVG-pseudotyped CypA-deficient viruses were still decreased compared to pseudotyped wild-type viruses (2.5-fold) (Fig. 3A). That the use of the VSVG envelope bypasses the conventional CD4/CXCR4/CCR5 entry pathway suggests that CypA participates in postentry events. To further demonstrate that spinoculated CypA-deficient viruses enter efficiently into target cells, we measured the amount of virus internalized into the cytosol of target cells after spinoculation as described previously (16). Importantly, we found that the cytosolic amounts of CypA-deficient (CsA-grown R9, R9 G89V, and R9 G89A) and wild-type (R9) viruses were similar (Fig. 3B). The residual cytosolic amounts of R9Δgp160 likely correspond to background contamination, as reported previously (16). Thus, if spinoculated CypA-deficient viruses enter target cells efficiently, but fail to infect these cells, this further suggests that CypA is required for a postentry event in HIV-1 infection.
FIG. 3.
Spinoculation by bypassing attachment preconditions reveals a postentry role for CypA in HIV-1 infection. (A) VSVG pseudotyping does not rescue the infectivity of spinoculated CypA-deficient viruses. CD4+ CXCR4+ CCR5+ HeLa cells were exposed to VSVG-pseudotyped wild-type (R9Δgp160) or CypA-deficient (CsA-grown R9Δgp160, R9 G89AΔgp160, or R9 G89VΔgp160) viruses (3 ng of p24). Adherent cells and viruses were centrifuged (spinoculation) for 3 h at room temperature. Cells were washed twice to remove unbound virus and carefully transferred to 37°C for 48 h. Infectivity was scored by X-Gal staining. Results are expressed in number of blue cells and represent the average of two independent experiments. (B) Spinoculated CypA-deficient viruses efficiently enter target cells. CD4+ CXCR4+ CCR5+ HeLa cells were exposed to wild-type (R9), CypA-deficient (CsA-grown R9, R9 G89A, or R9 G89V) or gp120-deleted (R9Δgp160) viruses (30 ng of p24). Adherent cells and viruses were centrifuged (spinoculation) for 3 h at room temperature. Cells were washed twice to remove unbound virus, carefully transferred to 37°C for 2 h for internalization, and treated with pronase to remove uninternalized virus. Entry levels were quantified by the measure of p24 in the cytosolic fraction of target cells by p24 ELISA as described previously (16). □, no treatment; ▪, heparitinase treatment.
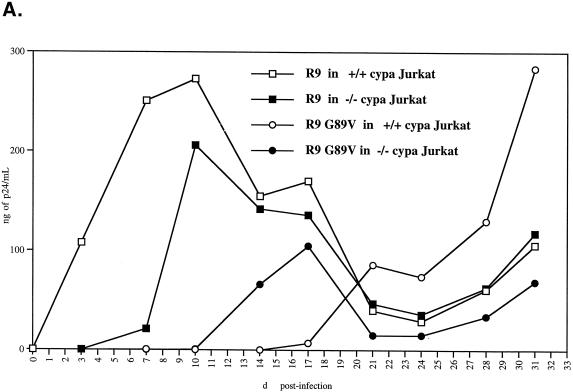
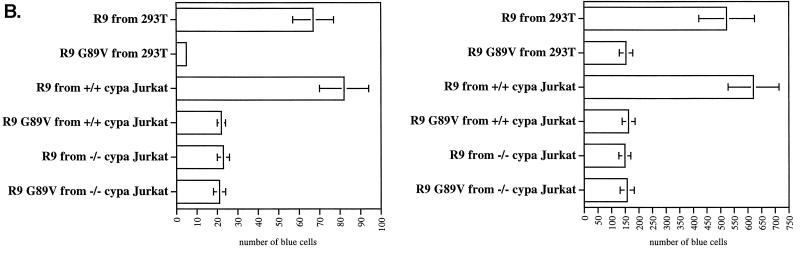
By the same methodology, we also examined CypA-deficient viruses produced from CypA-null Jurkat cells (4). Specifically, wild-type or CypA-null Jurkat cells were electroporated with wild-type (R9) or capsid mutant (R9 G89V) viruses. Viral replication was monitored by measuring the amount of p24 released into the medium. As previously reported (4), we found that wild-type R9 replication is delayed in CypA-null cells compared to that in wild-type Jurkat cells (delayed about 3 to 4 days) (Fig. 4A). This confirms that CypA regulates HIV-1 replication. Furthermore, we observed that the capsid mutant R9 G89V virus exhibits a profound delay in replication in wild-type Jurkat cells compared to wild-type R9 virus (more than 20 days) as described previously (4). Interestingly, the capsid mutant R9 G89V virus grew more efficiently in CypA-null cells than wild-type Jurkat cells (more than 6 days). It is crucial to note that these experiments have been repeated several times and that similar results have been obtained. At the peak of their growth, viruses were harvested and tested for attachment and infectivity with CD4+ HeLa cells. Interestingly, these CypA-deficient viruses derived from Jurkat cells did not exhibit the attachment defect observed above with CypA-deficient viruses produced from 293T cells (data not shown). Nevertheless, these viruses were still less infectious than wild-type viruses in CD4+ HeLa cells (Fig. 4B, top panel). Thus, we postulated that this decrease in infectivity arises exclusively from a postattachment defect. To address this issue, we asked whether spinoculation would rescue the infectivity of these CypA-deficient viruses. Importantly, all spinoculated CypA-deficient viruses, including R9 virus growing in CypA-null cells or R9 G89V viruses growing in wild-type or CypA-null cells, exhibited the postentry defect (threefold) (Fig. 4B, bottom panel). Remarkably, the relative decrease in infectivity of CypA-deficient viruses derived from Jurkat cells compared to wild-type virus observed by spinoculation (Fig. 4B, bottom panel) strikingly parallels that observed by sedimentation (Fig. 4B, top panel). Specifically, we found that spinoculation increases the levels of infectivity of R9 viruses produced from wild-type or CypA-null cells equally (both eightfold). This contrasts with the disproportionate rescue in infectivity of wild-type and CypA-deficient viruses produced from 293T cells (7- and 20-fold, respectively) (Fig. 2B and C). There are various explanations that may account for the different attachment phenotypes observed for CypA-deficient viruses derived from either Jurkat or 293T cells. Given that 293T cell membranes are rich in heparan sulfates, whereas Jurkat cell membranes express little heparan sulfates, this discrepancy as observed above may arise from differences in the electrostatic properties of the virus donor cell membrane. Furthermore, one can imagine that Jurkat cell-derived membranes of these viruses may be rich in other molecules that are known to facilitate interactions with HeLa cell membranes, such as adhesion molecules or lectins. We are currently investigating the influence of the donor cell membrane on HIV-1 attachment. Nevertheless, although these CypA-deficient viruses derived from Jurkat cells efficiently attach to CD4+ HeLa cells, they still fail to infect them. This further underscores the central message of this study that CypA is required for a postentry event in HIV-1 infection.
FIG. 4.
CypA-deficient viruses produced from either 293T cells or CypA-null cells exhibit a postentry defect in CD4+ HeLa cells. (A) Production of CypA-deficient viruses from CypA-null Jurkat cells. Wild-type or CypA-null Jurkat cells (3 million cells/5 ml of complete RPMI medium) were electroporated with wild-type R9 or capsid mutant R9 G89V plasmids (20 μg). Replication was monitored by measuring the amount of capsid released in the medium by p24 ELISA. (B) CypA-deficient viruses produced from CypA-null cells fail to infect CD4+ HeLa cells due to a postentry defect. CD4+ CXCR4+ CCR5+ HeLa cells were exposed to R9 or R9 G89V viruses produced from either wild-type or CypA-null Jurkat cells or 293T cells (3 ng of p24). Adherent cells and viruses were centrifuged (spinoculation) (bottom panel) or not (sedimentation) (top panel) for 3 h at room temperature. Cells were washed twice to remove unbound virus and carefully transferred to 37°C for 48 h. Infectivity was scored by X-Gal staining. Results are expressed as the number of blue cells and represent the average of two independent experiments.
In conclusion, obtained by a methodology that bypasses the need for attachment factors—spinoculation—, these data suggest that CypA participates in both entry and postentry events. Furthermore, these results provide the first demonstration of a postentry role for CypA by an approach that permits the analysis of postentry events exclusively and experimentally eliminates the contribution of CypA to attachment.
Acknowledgments
We thank J. Kuhns for secretarial assistance. We also thank C. Aiken for the R9Δgp160 plasmid clone and J. Luban for wild-type and CypA-null Jurkat cells. P4 CCR5 cells were obtained through the AIDS Research and Reference Reagent Program.
This work was supported by U.S. Public Health Service grant no. AI46958 from the National Institute of Allergy and Infectious Diseases to P.A.G. A.C.S.S. was supported by a Scholar Award from the American Foundation for AIDS Research (amfAR).
Footnotes
This is publication no. 14317-IMM from the Department of Immunology, The Scripps Research Institute, La Jolla, Calif.
REFERENCES
- 1.Billich, A., F. Hammerschmid, P. Peichl, R. Wenger, G. Zenke, V. Quesniaux, and B. Rosenwirth. 1995. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. J. Virol. 69:2451-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 70:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braaten, D., H. Ansari, and J. Luban. 1997. The hydrophobic pocket of cyclophilin is the binding site for the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 71:2107-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braaten, D., and J. Luban. 2001. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20:1300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colgan, J., H. E. H. Yuan, E. K. Franke, and J. Luban. 1996. Binding of the human immunodeficiency virus type 1 Gag polyprotein to cyclophilin A is mediated by the central region of capsid and requires Gag dimerization. J. Virol. 70:4299-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorfman, T., A. Weimann, A. Borsetti, C. T. Walsh, and H. G. Gottlinger. 1997. Active-site residues of cyclophilin A are crucial for its incorporation into human immunodeficiency virus type 1 virions. J. Virol. 71:7110-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke, E. K., H. E. H. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359-362. [DOI] [PubMed] [Google Scholar]
- 8.Franke, E. K., and J. Luban. 1996. Inhibition of HIV-1 replication by cyclosporine A or related compounds correlates with the ability to disrupt the Gag-cyclophilin A interaction. Virology 222:279-282. [DOI] [PubMed] [Google Scholar]
- 9.Gallay, P., T. Hope, D. Chin, and D. Trono. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. USA 94:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamble, T. R., F. F. Vajdos, S. Yoo, D. K. Worthylake, M. Houseweart, W. I. Sundquist, and C. P. Hill. 1996. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87:1285-1294. [DOI] [PubMed] [Google Scholar]
- 11.Gething, M.-J., and J. Sambrook. 1992. Protein folding in the cell. Nature 355:33-45. [DOI] [PubMed] [Google Scholar]
- 12.Handschumacher, R. E., M. W. Harding, J. Rice, and R. J. Drugge. 1984. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 226:544-547. [DOI] [PubMed] [Google Scholar]
- 13.Ke, H., D. Mayrose, P. J. Belshaw, D. G. Alberg, S. L. Schreiber, Z. Y. Chang, F. A. Etzkorn, S. Ho, and C. T. Walsh. 1994. Crystal structures of cyclophilin A complexed with cyclosporin A and N-methyl-4-[(E)-2-butenyl]-4,4-dimethylthreonine cyclosporin A. Structure 2:33-44. [DOI] [PubMed] [Google Scholar]
- 14.Luban, J., K. L. Bossolt, E. K. Franke, G. V. Kalpana, and S. P. Goff. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067-1078. [DOI] [PubMed] [Google Scholar]
- 15.Luban, J. 1996. Absconding with the chaperone: essential cyclophilin-Gag interaction in HIV-1 virions. Cell 87:1157-1159. [DOI] [PubMed] [Google Scholar]
- 16.Maréchal, V., M. C. Prevost, C. Petit, E. Perret, J.-M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mlynar, E., D. Bevec, A. Billich, B. Rosenwirth, and A. Steinkasserer. 1997. The non-immunosuppressive cyclosporin A analogue SDZ NIM 811 inhibits cyclophilin A incorporation into virions and virus replication in human immunodeficiency virus type 1-infected primary and growth-arrested T cells. J. Gen. Virol. 78:825-835. [DOI] [PubMed] [Google Scholar]
- 18.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pushkarsky, T., G. Zybarth, L. Dubrovsky, V. Yurchenko, H. Tang, H. Guo, B. Toole, B. Sherry, and M. Bukrinsky. 2001. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc. Natl. Acad. Sci. USA 98:6360-6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saphire, A. C., M. D. Bobardt, and P. A. Gallay. 1999. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. EMBO J. 18:6771-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saphire, A. C. S., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saphire, A. C. S., M. D. Bobardt, and P. A. Gallay. 2002. trans-complementation of cyclophilin A-deficient viruses reveals that the requirement for cyclophilin A in human immunodeficiency virus type 1 replication is independent of its isomerase activity. J. Virol. 76:2255-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherry, B., G. Zybarth, M. Alfano, L. Dubrovsky, R. Mitchell, D. Rich, P. Ulrich, R. Bucala, A. Cerami, and M. Bukrinsky. 1998. Role of cyclophilin A in the uptake of HIV-1 by macrophages and T lymphocytes. Proc. Natl. Acad. Sci. USA 95:1758-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thali, M., A. Bukovsky, E. Kondo, B. Rosenwirth, C. T. Walsh, J. Sodroski, and H. G. Göttlinger. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372:363-365. [DOI] [PubMed] [Google Scholar]
- 26.von Schwedler, U., J. Song, C. Aiken, and D. Trono. 1993. vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J. Virol. 67:4945-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Schwedler, U., R. S. Kornbluth, and D. Trono. 1994. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc. Natl. Acad. Sci. USA 91:6992-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu, X., H. Liu, H. Xiao, J. A. Conway, E. Hunter, and J. C. Kappes. 1997. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. EMBO J. 16:5113-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo, S., D. G. Myszka, C. Yeh, M. McMurray, C. P. Hill, and W. I. Sundquist. 1997. Molecular recognition in the HIV-1 capsid/cyclophilin A complex. J. Mol. Biol. 269:780-795. [DOI] [PubMed] [Google Scholar]