Abstract
The plasmatic strong ion difference (SID) is the difference between positively and negatively charged strong ions. At pH 7.4, temperature 37°C and partial carbon dioxide tension 40 mmHg, the ideal value of SID is 42 mEq/l. The buffer base is the sum of negatively charged weak acids ([HCO3-], [A-], [H2PO4-]) and its normal value is 42 mEq/l. According to the law of electroneutrality, the amount of positive and negative charges must be equal, and therefore the SID value is equal to the buffer base value. The easiest assessment of metabolic acidosis/alkalosis relies on the base excess calculation: buffer baseactual - buffer baseideal = SIDactual - SIDideal. The SID approach allows one to appreciate the relationship between acid–base and electrolyte equilibrium from a unique perspective, and here we describe a comprehensive model of this equilibrium. The extracellular volume is characterized by a given SID, which is a function of baseline conditions, endogenous and exogenous input (endogenous production and infusion), and urinary output. Of note, volume modifications vary the concentration of charges in the solution. An expansion of extracellular volume leads to acidosis (SID decreases), whereas a contraction of extracellular volume leads to alkalosis (SID increases). A thorough understanding of acid–base equilibrium mandates recognition of the importance of urinary SID.
Traditionally, the assessment of metabolic acidosis and alkalosis relies on measurement of the base excess, which is the difference between the 'ideal' buffer base [1] (i.e. the sum of the negatively charged forms of weak acids, [A-] + [HCO3-] + [H2PO4-], at standard conditions (pH 7.4, temperature 37°C, partial carbon dioxide tension 40 mmHg) and the 'actual' buffer base [2]):
Base excess = buffer baseactual - buffer baseideal (1)
During the past few years a novel approach based on assessment of the strong ion difference (SID) has been introduced to evaluate metabolic acidosis and alkalosis. For simplicity, we limit our discussion to these two disturbances.
Please note that in the following discussion we will refer to the amount of strong ion difference as SID (mEq), while we will refer to the strong ion difference concentration as [SID] (mEq/l).
By definition, strong ions are always dissociated in a solution. In plasma, as well as in interstitial fluids, the sum of positively charged ions (primarily Na+, K+, Ca2+ and Mg2+) exceeds the sum of the negatively charged strong ions (primarily Cl- and lactate-) of about 42 mEq/l. This difference is called the SID, and according to the Stewart model [3] its variation is one of the determinants of acid–base status. Looking at Figure 1, the connection between base excess and SID is apparent. The buffer base and SID are equivalent. In fact, because the ideal SID is equal to 42 mEq/l (as is the normal buffer base), it follows that
Figure 1.
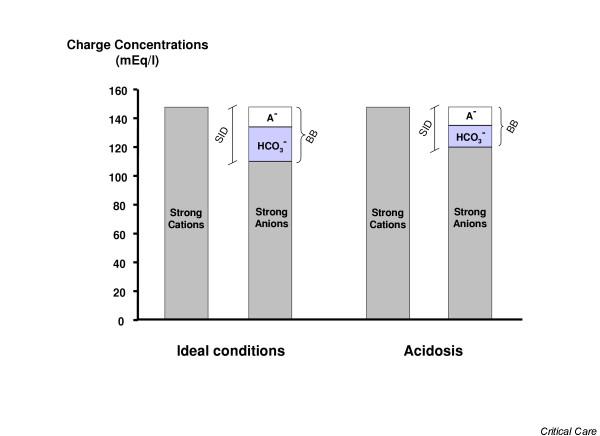
Gamblegram. The figure shows gamblegrams during ideal conditions and during acidosis. In ideal conditions the difference between positively and negatively charged strong ions is equal to 42 mEq/l (the strong ion difference [SID]) and, according to the law of electro-neutrality, is equivalent to the buffer base (BB; i.e. the sum of [HCO3-], [A-] and [H2PO4-], where A- are the weak acids in dissociated form, mainly albumin). During acidosis, SID decreases but the law of electro-neutrality is still satisfied. It follows that base excess = BBactual - BBideal = SIDacidosis - SIDideal.
Base excess = SIDactual - SIDideal = buffer baseactual - buffer baseideal (2)
Because computation of the actual SID is rather complicated, requiring the determination of all of the strong ion concentrations, we believe that the base excess approach may be easier, more rapid and adequate for clinical purposes. Indeed, the frequent debate involving the comparison of the 'SID approach' with the 'base excess approach' to assessment of metabolic acidosis [4,5] appears futile because their physiological meanings, as well as their variations, are identical. In other words, the two approaches look at the same thing from different points of view.
The picture is different when one considers the 'understanding' of acid–base and electrolyte equilibria, which everyone has studied in separate chapters of the textbooks. The great merit of the Stewart approach is that it considers electrolytes and acid–base status in a common framework. Here, we would like to propose a comprehensive model that may explain, at least qualitatively, many of the findings observed in clinical practice and in the literature.
The SID reflects the difference in electrical charges of the strong ions in the volume of the extracellular compartment (V). At time 0, it will be equal to V(0) × [SID(0)]. For example, if at time 0 the SID is normal (i.e. 42 mEq/l) then the net amount of electrical charge in the extracellular fluid (15 l) will be 630 mEq. During a given period of time there may be an addition of volume to the system (e.g. infusion of a solution) with its own SID (SIDinfusion). Consequently, a net amount of charge equal to Vinfusion × [SIDinfusion] will be added to the system. Similarly, the urinary system will excrete a volume of urine (Vurine) with its own SID (SIDurine). The last variable that must be taken into account is endogenous production of SID (sulphates, phosphates, lactate and ketoacids, among other components). It follows that the SID at a given time 't' may be derived from a series of equations, which may appear to be complicated in their expression but simple in their meaning. Eqn 3 (below) indicates that, in a system, the net amount of electrical charges due to the strong ions is equal to the net electrical charge of the system at time zero plus the net electrical charge added as a result of metabolism plus the net electrical charge added with volume infusion minus the net electrical charge extracted via urine.
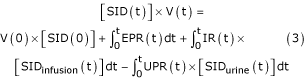
where EPR(t) is the 'endogenous production rate' of SID (mEq/min), IR(t) is the volume infusion rate and UPR(t) is the urine production rate. At a given time 't', the net fluid volume of the extracellular compartment is equal to the initial volume of the system plus the volume added with infusion minus the volume extracted in the form of urine.
![]()
Because what matters in terms of acid–base status is the concentration, rather than the net amount of electrical charge, the SID at a given time 't' may be expressed from the above equations as shown in equation 5 at the foot of the page:
![]()
It is important to remember that an increase in SID will lead the system to become more basic whereas a decrease in SID will lead the system to become more acidic. In general, Eqn 5 indicates that metabolic acidosis or alkalosis may occur either by changing the net electrical charge at constant extracellular volume or by changing the extracellular volume at constant electrical charge.
Looking at Eqn 5, we may make several comments. To maintain the metabolic acid–base status of a system (i.e. the baseline SID), two conditions must be satisfied: the input quantity of SID should equal the output quantity of SID; and the distribution volume of SID should remain constant. To the best of our knowledge, the only studies in which the strong ion balance (input and output) was investigated were conducted in cows [6-8]; different amounts of SID in the diet caused corresponding changes in urinary SID. Unfortunately, no such investigation has been conducted in critically ill patients. As discussed above, SID has been studied in comparison with base excess but without any physiological rationale [9]. The SID approach has been also proposed to explain metabolic acidosis during saline infusion (SID input) [10], but only a few papers have tackled and discussed the problem of urinary SID (SID output) [11-13]. What we lack is the entire picture of the system; unfortunately, this requires frequent assessment of urine electrolytes.
Some clinical findings may be viewed from the perspective of the general framework of Eqn 5. It is well known that rapid infusion of saline induces metabolic acidosis. This has been attributed to changes in SID due to hyperchloraemia [10]. By looking at Eqn 3 we derive a different point of view. Because the SID of saline is equal to 0, it follows that, if the urinary output of electrical charge and metabolic production remain constant, the net difference of electrical charges in the system (i.e. the numerator in Eqn 5) does not change. What causes the acidosis is the expansion of the extracellular volume (volume input greater than volume output), which leads to decreased concentration of the net amount of electrical charge (i.e. the SID).
Unfortunately, it is not easy to consider the urinary SID. In fact, although 40–42 mEq/l of plasmatic negative charge may be derived from the dissociated weak acids ([A-], [HCO3-] and [H2PO4-]), the amount of weak acids is far less in urine and, overall, the range of urinary pH is an order of magnitude greater than that in plasma. Once again, the problem is simpler when one considers the entire picture. In fact, as far as the plasmatic acid–base equilibrium is concerned, we must consider only the components of urinary [SID] that may affect the plasmatic [SID] (i.e. [K+], [Na+] and [Cl-]). In fact, in urine
[Na+] + [K+] + [Un+] = [Cl-] + [Un-] (6)
where Un+ and Un- are the positive and negative unmeasured ions. It follows that
[Na+] + [K+] - [Cl-] = [Un-] - [Un+] (7)
Quantitatively, the most important anion in urine is SO42-, which is derived from the metabolism of sulphur amino acids, whereas the most important cation is NH4+. In normal conditions, the sum of urinary [Na+] + [K+] - [Cl-] amounts to 42 mEq/l [14]. It follows that the concentration of unmeasured anions exceeds the concentration of unmeasured cations of 42 mEq/l. When a strong ion such as lactate is added to the plasma, the plasmatic SID will decrease. Consequently, the urinary system will react by increasing its excretion of chloride, thereby decreasing the plasma chloride concentration (while [Na+] and [K+] must be maintained within normal ranges). The increased excretion of chloride will decrease the urinary SID. Therefore, the difference between [Un-] and [Un+] should decrease (Eqn 7). This is accomplished by increasing the excretion rate of NH4+, which is a way to augment elimination of Cl- without Na+ [11,15].
Indeed, the effects of any volume infusion or other interventions cannot be understood if the urinary SID and volume are not taken into account. A merit of the report by Moviat and colleagues [13] is that, for the first time in critical care, attention is focused on the urinary system, which is the main regulator of SID. The authors found that the increase in urinary SID (indirectly induced by blocking carbonic anhydrase) was the key driver for correction of metabolic alkalosis. The message is important – urinary SID should be a key component of global acid–base assessment. We believe that urinary electrolyte monitoring may open a new perspective of research in critical care. Acid–base equilibrium, one of the oldest research areas in medicine, is still an open field for new discoveries and approaches.
Abbreviations
SID = strong ion difference.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Luciano Gattinoni, Email: gattinon@policlinico.mi.it.
Eleonora Carlesso, Email: ecarlesso@policlinico.mi.it.
Pietro Caironi, Email: pietro.caironi@policlinico.mi.it.
References
- Singer RB, Hastings AB. An improved clinical method for the estimation of disturbances of the acid-base balance of human blood. Medicine. 1948;27:223–242. doi: 10.1097/00005792-194805000-00003. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen O. The Acid-base Status of the Blood. Copenhagen: Munksgaard; 1974. [Google Scholar]
- Stewart PA. How to Understand Acid-base A Quantitative Acid-base Primer for Biology and Medicine. New York: Elsevier; 1981. [Google Scholar]
- Siggaard-Andersen O, Fogh-Andersen N. Base excess or buffer base (strong ion difference) as measure of a non-respiratory acid-base disturbance. Acta Anaesthesiol Scand Suppl. 1995;107:123–128. doi: 10.1111/j.1399-6576.1995.tb04346.x. [DOI] [PubMed] [Google Scholar]
- Fencl V, Leith DE. Stewart's quantitative acid-base chemistry: applications in biology and medicine. Respir Physiol. 1993;91:1–16. doi: 10.1016/0034-5687(93)90085-O. [DOI] [PubMed] [Google Scholar]
- Roche JR, Dalley D, Moate P, Grainger C, Rath M, O'Mara F. Dietary cation-anion difference and the health and production of pasture-fed dairy cows 2. Nonlactating periparturient cows. J Dairy Sci. 2003;86:979–987. doi: 10.3168/jds.S0022-0302(03)73681-9. [DOI] [PubMed] [Google Scholar]
- Roche JR, Dalley D, Moate P, Grainger C, Rath M, O'Mara F. Dietary cation-anion difference and the health and production of pasture-fed dairy cows. 1. Dairy cows in early lactation. J Dairy Sci. 2003;86:970–978. doi: 10.3168/jds.S0022-0302(03)73680-7. [DOI] [PubMed] [Google Scholar]
- Vagnoni DB, Oetzel GR. Effects of dietary cation-anion difference on the acid-base status of dry cows. J Dairy Sci. 1998;81:1643–1652. doi: 10.3168/jds.S0022-0302(98)75732-7. [DOI] [PubMed] [Google Scholar]
- Cusack RJ, Rhodes A, Lochhead P, Jordan B, Perry S, Ball JA, Grounds RM, Bennett ED. The strong ion gap does not have prognostic value in critically ill patients in a mixed medical/surgical adult ICU. Intensive Care Med. 2002;28:864–869. doi: 10.1007/s00134-002-1318-2. [DOI] [PubMed] [Google Scholar]
- Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Ring T, Frische S, Nielsen S. Clinical review: Renal tubular acidosis: a physicochemical approach. Crit Care. 2005;9:573–580. doi: 10.1186/cc3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey HE, Vallo A, Rodriguez-Soriano J. An analysis of renal tubular acidosis by the Stewart method. Pediatr Nephrol. 2006;21:206–211. doi: 10.1007/s00467-005-2081-8. [DOI] [PubMed] [Google Scholar]
- Moviat M, Pickkers P, van der Voort PH, van der Hoeven JG. Acetazolamide-mediated decrease in strong ion difference accounts for the correction of metabolic alkalosis in critically ill patients. Crit Care. 2006;10:R14. doi: 10.1186/cc3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle DC, Hizon M, Cohen E, Gutterman C, Gupta R. The use of the urinary anion gap in the diagnosis of hyperchloremic metabolic acidosis. N Engl J Med. 1988;318:594–599. doi: 10.1056/NEJM198803103181002. [DOI] [PubMed] [Google Scholar]
- Kellum JA. Determinants of blood pH in health and disease. Crit Care. 2000;4:6–14. doi: 10.1186/cc644. [DOI] [PMC free article] [PubMed] [Google Scholar]


