Abstract
Acute hyperglycaemia has been associated with complications, prolonged intensive care unit and hospital stay, and increased mortality. We made an inventory of the prevalence and prognostic value of hyperglycaemia, and of the effects of glucose control in different groups of critically ill patients. The prevalence of hyperglycaemia in critically ill patients, using stringent criteria, approaches 100%. An unambiguous negative correlation between hyperglycaemia and mortality has been described in various groups of critically ill patients. Although the available evidence remains inconsistent, there appears to be a favourable effect of glucose regulation. This effect on morbidity and mortality depends on patient characteristics. To be able to compare results of future studies involving glucose regulation, better definitions of hyperglycaemia (and consequently of normoglycaemia) and patient populations are needed.
Introduction
Acute hyperglycaemia is frequently present in situations of stress, both in diabetic and in nondiabetic patients [1-3]. Because it is so common, it could be viewed as a physiologic adaptation during the 'fight or flight' response. On the other hand, it has been associated with complications, prolonged intensive care unit (ICU) and hospital stay, and increased mortality. The important issue is whether hyperglycaemia is just related to disease severity or is an independent risk factor that contributes to morbidity and mortality [4]. If hyperglycaemia is an independent risk factor, then tight glucose control (TGC) may have beneficial effects on morbidity and mortality. Conversely, if hyperglycaemia is not a risk factor per se, then the risks associated with glucose control may outweigh the benefits. We made an inventory of the prevalence and prognostic value of hyperglycaemia, and of the effects of glucose control in different groups of critically ill patients, in order to evaluate the available evidence.
Prevalence and prognostic value of hyperglycaemia
Table 1 provides an overview of various situations in which a correlation between hyperglycaemia and mortality has been demonstrated. Different authors use different threshold values to define hyperglycaemia.
Table 1.
Prognostic value of hyperglycaemia.
| Stress situation | Patients (n) | Hyperglycaemia (definition [mmol/l]) | Mortality (high versus lower BG) |
| Acute hospital admission [5] | 1886 | fasting > 7, twice > 11.1 | 16% versus 1.7% |
| Surgical intensive care patients [10] | 97 | ≥ 6.1 | 32% versus 8% |
| Trauma [2] | 738 | > 11.1 | 34.1% versus 3.7% |
| Trauma [20] | 1003 | > 11.1 | RR 2.2-fold higher |
| Severe burn injury [38] | 58 | > 7.8 | 27% versus 4% |
| Acute myocardial infarction [15] | 1856 | ≥ 6.1 | RR 4-fold higher |
| Myocardial infarction in diabetic patients [15] | 688 | ≥ 10 | RR 1.7-fold higher |
| Acute myocardial infarction [13] | 336 | > 11.1 | 40% versus about 10% |
| Cerebrovascular accident [19] | > 2000 | ≥ 6.1–7 | RR 3-fold higher |
| Cerebrovascular accident [18] | 656 | > 7.2 | 18% versus 11% |
| Severe brain damage [39] | 59 | > 11.1 | Higher mortality |
BG, blood glucose; RR, relative risk.
General hospital patients
Among patients admitted to a general hospital, 38% exhibited increased blood glucose (BG) values, defined as either fasting BG values above 7 mmol/l or two random values above 11.1 mmol/l [5]. In that retrospective study 16% of 223 patients admitted with new onset hyperglycaemia (without a history of diabetes mellitus) died during their stay in hospital, as compared with only 1.7% of 1168 patients without hyperglycaemia (P < 0.001). The cause of death in the hyperglycaemia group was more often related to infection (33% versus 20% without hyperglycaemia) or acute neurological complications (19% versus 10%). Patients with new onset hyperglycaemia had a longer hospital stay and were more often admitted to the ICU (29% versus 9%). In this study, diabetic patients had a better prognosis than newly hyperglycaemic patients.
Intensive care unit patients
In one study conducted in medical ICU patients [6], admission BG was above 11.1 mmol/l in 23%. In another study [7], conducted in thoracosurgical ICU patients, admission glucose was above 6.1 mmol/l in 86% and almost all patients (96%) became hyperglycaemic during their ICU stay. Freire and coworkers [8] reported a mean admission glucose of 7.8 mmol in 1185 mixed ICU patients. In a study conducted in nearly 5000 ICU patients, Egi and colleagues [9] recently found a mean glucose of 8.2 mmol/l. In mixed ICU patients with a mortality of 15%, BG during admission was above 11.1 mmol/l in 54%; all patients had BG levels above 6.1 mmol/l during their ICU stay [10]. Hyperglycaemia was a risk factor for increased morbidity and mortality in critically ill surgical patients (n = 97) but not in medical patients (n = 38). However, the number of medical patients was relatively small, and so no firm conclusions can be drawn. In various ICU populations, the association between hyperglycaemia and in-hospital mortality was not uniform; hyperglycaemia was an independent risk factor only in patients without a history of diabetes in the cardiac, cardiothoracic and neurosurgical ICUs [11]. A retrospective study conducted in a mixed ICU population of 1826 patients [12] showed that even a modest degree of hyperglycaemia was associated with an increase in hospital mortality; admission BG as well as mean BG were higher in nonsurvivors than in survivors. However, in another retrospective study [4], conducted in 1085 consecutive mixed ICU patients (ICU mortality 20%), hyperglycaemia was not an independent risk factor for mortality in a multivariate model (Fig. 2).
Figure 2.
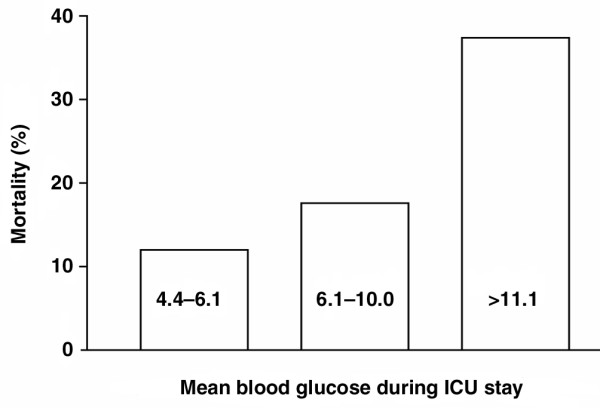
Relationship between mean blood glucose during ICU stay and ICU mortality. Blood glucose levels are given in mmol/l. Data are from 1085 consecutive mixed ICU patients [4]. ICU, intensive care unit.
Patients with acute myocardial infarction
In a study of 336 patients with acute myocardial infarction (AMI) [13], the admission BG value in 15% was 11.1 mmol or greater; more than 40% of these patients with a BG value of 11.1 mmol/l or more on admission died within 1 year, as compared with approximately 10% of patients with normal or slightly elevated BG values. In a prospective study of 305 patients with AMI [14] one out of four patients appeared to have diabetes mellitus, with an admission BG of 17.1 mmol/l. In a meta-analysis of 1856 AMI patients [15] patients without known diabetes mellitus but with BG values above 6.1 mmol/l had a fourfold increased chance of dying compared with patients with lower BG values. In diabetic patients with elevated BG values (> 8.0 mmol/l), mortality risk was nearly doubled. In another study [16], conducted in 846 AMI patients, admission BG level appeared to be an independent predictor of long-term mortality in patients with and in those without known diabetes. In that study, patients were stratified according to their level of hyperglycaemia, and a clear correlation between level of hyperglycaemia and increased risk for mortality was identified (Figure 1). In the DIGAMI (Diabetes Insulin-Glucose in Acute Myocardial Infarction) 2 study [17] glucose level was a strong and independent predictor of long-term mortality in diabetic patients with AMI.
Figure 1.
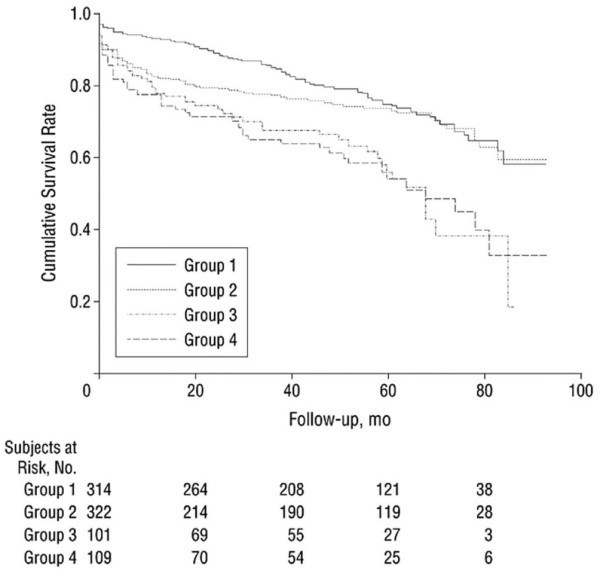
Survival by blood glucose level. Shown are Kaplan-Meier survival curves for patients without known diabetes mellitus and admission blood glucose levels less than 141 mg/dl (7.8 mmol/l; group 1), 141–199 mg/dl (7.8–11.0 mmol/l; group 2) and 200.0 mg/dl (11.1 mmol/l) or higher (group 3), and patients with previously diagnosed with diabetes (group 4). Adapted from Stranders and coworkers [16].
Patients with cerebrovascular accident
In a study of 656 patients with an established cerebrovascular accident (CVA) [18], 25% had a BG above 10.0 mmol/l. In that retrospective study acute hyperglycaemia predicted increased mortality after 1 year; 18% of 258 patients with a BG of 7.2 mmol/l or more had died compared with 11% of 385 patients with a BG below 7.2 mmol/l. In another study conducted in CVA patients without known diabetes mellitus but with elevated BG values (> 6.1 mmol/l) [19], the risk for dying within 30 days was threefold.
Trauma patients
In a prospective study conducted in 738 trauma patients [2], 'moderate' hyperglycaemia (BG > 11.1 mmol/l) but also 'mild' hyperglycaemia (BG > 7.5 mmol/l) were independent predictors of mortality, infection, and hospital and ICU length of stay. Sung and coworkers [20] stratified 1003 consecutive trauma patients by admission glucose level (<11.1 mmol/l versus ≥ 11.1 mmol/l) and found a 2.2 times greater mortality risk in the hyperglycaemic group. In a retrospective study conducted in 865 trauma and 5234 nontrauma patients (mortality in both groups 12%) [21], the relation between hyperglycaemia and mortality was stronger in trauma patients than in other surgical ICU patients.
Summary
The prevalence of hyperglycaemia in critically ill patients approaches 100%. For the majority of studies, a negative correlation between hyperglycaemia and survival was demonstrated.
Can tight glucose control affect outcome in critically ill patients?
To date, only a few studies that reached their goal for TGC have been reported [7,22,23]; the results of ongoing multicentre studies (Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation [NICE-SUGAR] and Comparing the Effects of Two Glucose Control Regimens by Insulin in Intensive Care Unit Patients [GLUCONTROL]) will be available in due course. Reviews comparing the results of glucose regulation studies in critically ill patients expose important drawbacks; the (various) targets appear difficult to achieve, blood glucose determinations are not standardized and the number of patients is often limited [3,24]. Furthermore, perioperative studies aiming to achieve better glucose control and studies conducted in patients with AMI generally have a very limited period of observation [24]. In most studies conducted before 2001 normoglycaemia was not a goal; this changed following the impressive results reported in thoracosurgical ICU patients by van den Berghe and coworkers in 2001 [7]. Glycaemic goals became tighter, with a target range between 4 and 8 mmol/l.
Intensive care unit patients
In a prospective randomized single centre study conducted in 765 cardiosurgical ICU patients, van den Berghe and coworkers [7] showed that TGC decreased mortality and morbidity substantially. Mean morning BG was 5.7 ± 1.1 mmol/l; 5.1% of patients had hypoglycaemic episodes (< 2.2 mmol/l). Krinsley [23] found a beneficial effect of glucose regulation (mean BG 7.2 mmol/l; <1% hypoglycaemic episodes) on mortality, using a historical control group. However, in a recent prospective study conducted in a medical ICU population, van den Berghe and coworkers [22] found that reduced BG levels did not significantly reduce in-hospital mortality (40%) for the group as a whole but just for the subgroup of patients with an ICU stay of 3 or more days. Furthermore, TGC appeared to be more difficult to achieve in medical ICU patients, among other patients, resulting in an increase in hypoglycaemic events. In that study, the potential benefit of glucose regulation may be small because of the high mortality caused by the underlying diseases (malignancy, chronic obstructive pulmonary disease, heart failure) and as a result of 'dilution' of the study with patients whose conditions were not relevant to the study goals. The number needed to treat to prevent an ICU death and the associated risk for hypoglycaemia (number needed to harm) with TGC may vary widely according to baseline mortality, case mix and case selection [9].
Patients with acute myocardial infarction
In an overview of nine studies of glucose–insulin–potassium (GIK) infusion conducted in patients with AMI (n = 932) [25], treatment was associated with a decrease in 30-day mortality from 21% to 16.1% (P = 0.004). In four 'high-dose' GIK studies (288 patients), differences in mortality were not statistically significant. In the DIGAMI study (n = 620) [26], an absolute reduction in mortality of 7.5% was achieved. The more recent DIGAMI 2 trial [17] did not support the evidence that an acutely introduced, long-term insulin regimen improves survival or lowers the number of reinfarctions in patients with type 2 diabetes following AMI. In that study, only one out of five patients was treated with coronary artery bypass grafting or primary percutaneous coronary intervention. Several other studies using GIK infusion failed to demonstrate a beneficial effect on mortality: the ECLA (Estudios Cardiologicos Latinoamerica) study (n = 490) [27], the Pol-GIK (Polish-Glucose-Insulin-Potassium) trial (n = 954) [28], the GIPS (Glucose-Insulin-Potassium Study) study [29] (n = 940), the REVIVAL (Reevaluation of Intensified Venous Metabolic Support for Acute Infarct Size Limitation) trial (n = 312) [30], and the CREATE-ECLA (Clinical Trial of Metabolic Modulation in Acute Myocardial Infarction-ECLA) trial (n = > 20,000) [31].
Most studies performed with GIK infusion protocols were originally not designed to achieve TGC; they do not result in adequate glucose regulation and may in some patients have unfavourable side effects. A recent report [32] suggests that GIK infusion, despite high insulin infusion rates, may cause refractory hyperglycaemia, which appeared to be an independent parameter for larger myocardial infarction. Optimal reperfusion therapy appears to be much more important for AMI patients.
Cardiac surgery patients
During cardiac surgery glucose regulation results in a reduction in complications, but an effect on mortality has not been demonstrated. It was shown that arrhythmias were less frequent, resulting in a shorter hospital stay [33,34]. In a recently published trial conducted in 1127 high-risk patients undergoing coronary artery bypass grafting, the addition of 10 IU/l insulin to the GIK infusion did not yield any benefit; even in this high-risk group the perioperative mortality was only 2.2% [35]. There are various reasons why a favourable effect of GIK on mortality during cardiac surgery has not been shown: the low mortality risk in these patients requires a large study population, the optimal dose to be administered is unknown, and different studies describe different patient populations.
Patients with cerebrovascular accident
In the GIST (Glucose Insulin in Stroke Trial) study [36], GIK infusion in 53 hyperglycaemic patients with CVA did not result in lower BG values and did not reduce short-term mortality (32% versus 28%; not significant).
Summary
Taken together, most recent trials aiming to achieve TGC, there appears to be a tendency toward a favourable effect of glucose regulation in ICU patients. In AMI and CVA patients no such effect has yet been demonstrated.
To be able to judge and compare future studies, strict definitions of hyperglycaemia (and consequently of normo-glycaemia and hypoglycaemia) and of patient populations are needed. It would be feasible to define hyperglycaemia as any blood glucose value above 6.1 mmol/l measured in whole blood (or > 7.0 mmol/l measured in plasma), which is similar to the World Health Association and American Diabetes Association criteria [37].
Conclusion
The prevalence of hyperglycaemia in critically ill patients is considerable; using stringent criteria it approaches 100%. An clear negative correlation of hyperglycaemia with survival has been shown. It is therefore likely that there is a pathophysiological link between acute hyperglycaemia and complications/mortality. The underlying mechanisms may differ considerably in various situations of stress and various clinical conditions. Whether hyperglycaemia is an independent risk factor in critically ill patients can only be demonstrated in outcome trials involving TGC. No evidence of a favourable effect of TGC has yet been reported for patients with AMI and CVA. In ICU patients the findings remain unclear, although there is a tendency toward a favourable effect. Multicentre trials are underway and their findings will hopefully shed more light on this issue.
Abbreviations
AMI = acute myocardial infarction; BG = blood glucose; CVA = cerebrovascular accident; GIK = glucose–insulin–potassium; ICU = intensive care unit; TGC = tight glucose control.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
We thank Joline Lind, MD, for language editing.
Contributor Information
Anouk M Corstjens, Email: a.m.corstjens@anest.umcg.nl.
Iwan CC van der Horst, Email: i.c.c.vanderhorst@int.umcg.nl.
Jan G Zijlstra, Email: j.g.zijlstra@int.umcg.nl.
AB Johan Groeneveld, Email: johan.groeneveld@vumc.nl.
Felix Zijlstra, Email: f.zijlstra@thorax.umcg.nl.
Jaap E Tulleken, Email: j.e.tulleken@int.umcg.nl.
Jack JM Ligtenberg, Email: j.j.m.ligtenberg@int.umcg.nl.
References
- Lind L, Lithell H. Impaired glucose and lipid metabolism seen in intensive care patients is related to severity of illness and survival. Clin Intensive Care. 1994;5:100–105. [PubMed] [Google Scholar]
- Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–38. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004;164:2005–2011. doi: 10.1001/archinte.164.18.2005. [DOI] [PubMed] [Google Scholar]
- Ligtenberg JJ, Meijering S, Stienstra Y, Horst ICC, Vogelzang M, Nijsten MW, Tulleken JE, Zijlstra JG. Mean glucose level is not an independent risk factor for mortality in mixed ICU patients. Intensive Care Med. 2006;32:435–438. doi: 10.1007/s00134-005-0052-y. [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- Cely CM, Arora P, Quartin AA, Kett DH, Schein RM. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness. Chest. 2004;126:879–887. doi: 10.1378/chest.126.3.879. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Freire AX, Bridges L, Umpierrez GE, Kuhl D, Kitabchi AE. Admission hyperglycemia and other risk factors as predictors of hospital mortality in a medical ICU population. Chest. 2005;128:3109–3116. doi: 10.1378/chest.128.5.3109. [DOI] [PubMed] [Google Scholar]
- Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P, Li W, Bates S. Intensive insulin therapy in postoperative intensive care unit patients: a decision analysis. Am J Respir Crit Care Med. 2006;173:407–413. doi: 10.1164/rccm.200506-961OC. [DOI] [PubMed] [Google Scholar]
- Christiansen C, Toft P, Jorgensen HS, Andersen SK, Tonnesen E. Hyperglycaemia and mortality in critically ill patients. A prospective study. Intensive Care Med. 2004;30:1685–1688. doi: 10.1007/s00134-004-2325-2. [DOI] [PubMed] [Google Scholar]
- Whitcomb BW, Pradhan EK, Pittas AG, Roghmann MC, Perencevich EN. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit Care Med. 2005;33:2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- Bolk J, van der Ploeg T, Cornel JH, Arnold AE, Sepers J, Umans VA. Impaired glucose metabolism predicts mortality after a myocardial infarction. Int J Cardiol. 2001;79:207–214. doi: 10.1016/s0167-5273(01)00422-3. [DOI] [PubMed] [Google Scholar]
- Tenerz A, Lonnberg I, Berne C, Nilsson G, Leppert J. Myocardial infarction and prevalence of diabetes mellitus. Is increased casual blood glucose at admission a reliable criterion for the diagnosis of diabetes? Eur Heart J. 2001;22:1102–1110. doi: 10.1053/euhj.2000.2445. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- Stranders I, Diamant M, van Gelder RE, Spruijt HJ, Twisk JW, Heine RJ, Visser FC. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164:982–988. doi: 10.1001/archinte.164.9.982. [DOI] [PubMed] [Google Scholar]
- Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67–71. doi: 10.1212/wnl.59.1.67. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Sung J, Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;59:80–83. doi: 10.1097/01.ta.0000171452.96585.84. [DOI] [PubMed] [Google Scholar]
- Vogelzang M, Nijboer JM, van der Horst IC, Zijlstra F, ten Duis HJ, Nijsten MW. Hyperglycemia has a stronger relation with outcome in trauma patients than in other critically ill patients. J Trauma. 2006;60:873–877. doi: 10.1097/01.ta.0000195715.63978.80. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe G, Wilmer A, Hermans G, Meersseman G, Wouters P, Milants I, Van Wijngaarden E, Bobbaers H, Bouillon R. Intensive Insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- Meijering S, Corstjens AM, Tulleken JE, Meertens JHJ, Zijlstra JG, Ligtenberg JJ. Towards a feasible algorithm for tight glycaemic control in critically ill patients: a systemic review of the literature. Crit Care. 2006;10:R19. doi: 10.1186/cc3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath-Ordoubadi F, Beatt KJ. Glucose-insulin-potassium therapy for treatment of acute myocardial infarction: an overview of randomized placebo-controlled trials. Circulation. 1997;96:1152–1156. doi: 10.1161/01.cir.96.4.1152. [DOI] [PubMed] [Google Scholar]
- Malmberg KA, Efendic S, Ryden LE. Feasibility of insulin-glucose infusion in diabetic patients with acute myocardial infarction. A report from the multicenter trial: DIGAMI. Diabetes Care. 1994;17:1007–1014. doi: 10.2337/diacare.17.9.1007. [DOI] [PubMed] [Google Scholar]
- Diaz R, Paolasso EA, Piegas LS, Tajer CD, Moreno MG, Corvalan R, Isea JE, Romero G. Metabolic modulation of acute myocardial infarction. The ECLA (Estudios Cardiologicos Latinoamerica) Collaborative Group. Circulation. 1998;98:2227–2234. doi: 10.1161/01.cir.98.21.2227. [DOI] [PubMed] [Google Scholar]
- Ceremuzynski L, Budaj A, Czepiel A, Burzykowski T, Achremczyk P, Smielak-Korombel W, Maciejewicz J, Dziubinska J, Nartowicz E, Kawka-Urbanek T, et al. Low-dose glucose-insulin-potassium is ineffective in acute myocardial infarction: results of a randomized multicenter Pol-GIK trial. Cardiovasc Drugs Ther. 1999;13:191–200. doi: 10.1023/a:1007787924085. [DOI] [PubMed] [Google Scholar]
- van der Horst IC, Zijlstra F, van't Hof AW, Doggen CJ, de Boer MJ, Suryapranata H, Hoorntje JC, Dambrink JH, Gans RO, Bilo HJ. Glucose-insulin-potassium infusion inpatients treated with primary angioplasty for acute myocardial infarction: the glucose-insulin-potassium study: a randomized trial. J Am Coll Cardiol. 2003;42:784–791. doi: 10.1016/s0735-1097(03)00830-1. [DOI] [PubMed] [Google Scholar]
- Pache J, Kastrati A, Mehilli J, Bollwein H, Ndrepepa G, Schuhlen H, Martinoff S, Seyfarth M, Nekolla S, Dirschinger J, et al. A randomized evaluation of the effects of glucose-insulin-potassium infusion on myocardial salvage in patients with acute myocardial infarction treated with reperfusion therapy. Am Heart J. 2004;148:e3. doi: 10.1016/j.ahj.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- Vogelzang M, Svilaas T, van der Horst IC, Nijsten MW, Zijlstra F. Refractory hyperglycaemia induced by glucose-insulin-potassium infusion in acute myocardial infarction. Neth Heart J. 2006;14:46–48. [PMC free article] [PubMed] [Google Scholar]
- Lazar HL, Chipkin S, Philippides G, Bao Y, Apstein C. Glucose-insulin-potassium solutions improve outcomes in diabetics who have coronary artery operations. Ann Thorac Surg. 2000;70:145–150. doi: 10.1016/s0003-4975(00)01317-5. [DOI] [PubMed] [Google Scholar]
- Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497–1502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- Rao V, Christakis GT, Weisel RD, Ivanov J, Borger MA, Cohen G. The insulin cardioplegia trial: myocardial protection for urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2002;123:928–935. doi: 10.1067/mtc.2002.121686. [DOI] [PubMed] [Google Scholar]
- Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyper-glycemia: the Glucose Insulin in Stroke Trial (GIST) Stroke. 1999;30:793–799. doi: 10.1161/01.str.30.4.793. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- Young B, Ott L, Dempsey R, Haack D, Tibbs P. Relationship between admission hyperglycemia and neurologic outcome of severely brain-injured patients. Ann Surg. 1989;210:466–472. doi: 10.1097/00000658-198910000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


