Abstract
Introduction
The lack of a standard definition for acute kidney injury has resulted in a large variation in the reported incidence and associated mortality. RIFLE, a newly developed international consensus classification for acute kidney injury, defines three grades of severity – risk (class R), injury (class I) and failure (class F) – but has not yet been evaluated in a clinical series.
Methods
We performed a retrospective cohort study, in seven intensive care units in a single tertiary care academic center, on 5,383 patients admitted during a one year period (1 July 2000–30 June 2001).
Results
Acute kidney injury occurred in 67% of intensive care unit admissions, with maximum RIFLE class R, class I and class F in 12%, 27% and 28%, respectively. Of the 1,510 patients (28%) that reached a level of risk, 840 (56%) progressed. Patients with maximum RIFLE class R, class I and class F had hospital mortality rates of 8.8%, 11.4% and 26.3%, respectively, compared with 5.5% for patients without acute kidney injury. Additionally, acute kidney injury (hazard ratio, 1.7; 95% confidence interval, 1.28–2.13; P < 0.001) and maximum RIFLE class I (hazard ratio, 1.4; 95% confidence interval, 1.02–1.88; P = 0.037) and class F (hazard ratio, 2.7; 95% confidence interval, 2.03–3.55; P < 0.001) were associated with hospital mortality after adjusting for multiple covariates.
Conclusion
In this general intensive care unit population, acute kidney 'risk, injury, failure', as defined by the newly developed RIFLE classification, is associated with increased hospital mortality and resource use. Patients with RIFLE class R are indeed at high risk of progression to class I or class F. Patients with RIFLE class I or class F incur a significantly increased length of stay and an increased risk of inhospital mortality compared with those who do not progress past class R or those who never develop acute kidney injury, even after adjusting for baseline severity of illness, case mix, race, gender and age.
Introduction
Acute kidney injury is well recognized for its impact on the outcome of patients admitted to the intensive care unit (ICU). Illness severity scores such as the Acute Physiology and Chronic Health Evaluation version III (APACHE III) scoring system [1] and the Sequential Organ Failure Assessment score (SOFA) [2] both weight kidney dysfunction heavily (20% and 16.6% of the total scores for acute physiology). Yet there is no consensus on the amount of dysfunction that defines acute kidney injury, with more than 30 definitions in use in the literature today [3]. The variety of definitions used in clinical studies may be partly responsible for the large variations in the reported incidence (1–31%) [4-6] and the associated mortality (19–83%) [3,6-9] of acute kidney injury. Indeed, the lack of a uniform definition for acute kidney injury is believed to be a major impediment to research in the field [10]. Acute kidney injury is generally defined as 'an abrupt and sustained decrease in kidney function'. Until recently there has not been a consensus on how best to assess kidney function; namely, what markers best reflect kidney function, and what values of those markers discriminate normal from abnormal kidney function.
To establish a uniform definition for acute kidney injury, the Acute Dialysis Quality Initiative formulated the Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) classification [11]. RIFLE defines three grades of increasing severity of acute kidney injury – risk (class R), injury (class I) and failure (class F) – and two outcome classes (loss and end-stage kidney disease) (see Table 1). A unique feature of the RIFLE classification is that it provides three grades of severity for acute kidney injury based on changes in either serum creatinine or urine output from the baseline condition. This allows classification of patients with acute kidney injury into one of the three RIFLE severity classes (Table 1).
Table 1.
Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) classification
| Class | Glomerular filtration rate criteria | Urine output criteria |
| Risk | Serum creatinine × 1.5 | < 0.5 ml/kg/hour × 6 hours |
| Injury | Serum creatinine × 2 | < 0.5 ml/kg/hour × 12 hours |
| Failure | Serum creatinine × 3, or serum creatinine ≥ 4 mg/dl with an acute rise > 0.5 mg/dl | < 0.3 ml/kg/hour × 24 hours, or anuria × 12 hours |
| Loss | Persistent acute renal failure = complete loss of kidney function > 4 weeks | |
| End-stage kidney disease | End-stage kidney disease > 3 months | |
For conversion of creatinine expressed in conventional units to SI units, multiply by 88.4. RIFLE class is determined based on the worst of either glomerular filtration criteria or urine output criteria. Glomerular filtration criteria are calculated as an increase of serum creatinine above the baseline serum creatinine level. Acute kidney injury should be both abrupt (within 1–7 days) and sustained (more than 24 hours). When the baseline serum creatinine is not known and patients are without a history of chronic kidney insufficiency, it is recommend to calculate a baseline serum creatinine using the Modification of Diet in Renal Disease equation for assessment of kidney function, assuming a glomerular filtration rate of 75 ml/min/1.73 m2. When the baseline serum creatinine is elevated, an abrupt rise of at least 0.5 mg/dl to more than 4 mg/dl is all that is required to achieve class Failure.
RIFLE represents a new classification system issued from a process of formal evidence appraisal and expert opinion [11,12]. Three studies were recently published that used the RIFLE classification to evaluate the occurrence rate and/or outcome of acute kidney injury in two relatively small cohorts (207 ICU patients treated with renal replacement therapy and 183 ICU patients with acute kidney injury) and one larger cohort (813 patients after cardiac surgery) [13-15]. The clinical characteristics and predictive ability of this classification have not, however, been clinically validated in a large general ICU population. The aims of this study were therefore to characterize acute kidney injury defined by the maximum RIFLE classification, to examine the progression between stages of the classification, and to relate this classification to the length of stay and mortality in a large cohort of critically ill patients.
Patients and methods
Study population
We constructed a retrospective cohort of all adult hospitalizations during a 12 month period (1 July 2000–30 June 2001) at the University of Pittsburgh Medical Center that were admitted to one of its seven ICUs during their hospital stay. We excluded patients receiving chronic hemodialysis (n = 146) from the study cohort, and we only considered the first admission for patients who were readmitted to the ICU during the study period (n = 327). The University of Pittsburgh Medical Center is a tertiary care academic medical center with seven ICUs and more than 120 ICU beds serving medical, surgical, neurological, trauma and solid organ transplant patients.
Data collection
The study was approved by the Institutional Review Board of the University of Pittsburgh Medical Center. Data from different sources were merged by a non-investigator data manager (such as, an honest broker) and were stripped of all identifying information to preserve patient anonymity and to comply with local and federal regulations. Demographic data were retrieved from the electronic hospital database, laboratory data were retrieved from the laboratory database, and patient data were retrieved from the electronic hospital records. After merging data from the different sources, we performed automated and manual data verification. The patient data included demographic, administrative, physiologic, laboratory and hospital outcome information. Ethnicity, reported as white, black or other, was reported by the admitting nurse, and was used to calculate the glomerular filtration rate assessed by the Modification of Diet in Renal Disease (MDRD) equation [16]. High-density (every two hours) physiologic data were only available while patients were in the ICU, while other data sources covered the entire hospitalization. Urine output was recorded at least once every two hours, and serum creatinine was measured at least once daily.
RIFLE criteria
We classified patients according to the maximum RIFLE class (class R, class I or class F) reached during their hospital stay. The RIFLE class was determined based on the worst of either glomerular filtration rate criteria or urine output criteria. We used the change in serum creatinine level and urine output to classify patients according to the RIFLE criteria.
Patients who met any of the criteria of the RIFLE classification were classified as acute kidney injury patients. For patients without chronic kidney insufficiency as reported in the medical history, we calculated a serum creatinine level using the MDRD equation [16] (CrMDRD) as recommended by the Acute Dialysis Quality Initiative, by solving the MDRD equation for serum creatinine assuming a glomerular filtration rate of 75 ml/minute/1.73 m2. We then used the lowest creatinine value among the hospital admission creatinine, the ICU admission creatinine or the CrMDRD creatinine as the baseline value. Approximately one-half of patients were classified using the CrMDRD as a baseline. None of these values differed by very much, however (mean difference between creatinine on admission and CrMDRD = 0; interquartile range -0.3 to 0.3), and our results are not qualitatively different regardless of which baseline is used. For patients with a history of kidney insufficiency (but not on chronic dialysis) we used their hospital admission creatinine as their baseline. We did not evaluate the outcome classes of RIFLE (loss and end-stage kidney disease criteria) in this study.
Severity of illness
The APACHE III [1] and the SOFA [2] scores were calculated based on the worst variables recorded during the first 24 hours of ICU admission. The nonrenal total SOFA score was calculated from the total SOFA score minus the points for kidney insufficiency. In addition, we calculated the SOFA score and the nonrenal SOFA score on basis of the worst variables recorded during the 24 hours preceding the maximum RIFLE class.
Statistical analysis
The central tendency for continuous data is expressed as the mean ± standard deviation or the median (interquartile range). We tested continuous variables for normality by distribution plots. We compared means using the Student's t test when normally distributed, and the Mann-Whitney U test when not. Comparisons across multiple groups were performed using the F test, with Bonferroni correction for multiple comparisons [17]. When data were not normally distributed, we used the Kruskal-Wallis H analysis of variance test; significant changes over the observation period were tested with the Mann-Whitney U test.
We performed univariate and multivariable logistic regression to assess the impact of different baseline characteristics found to be significantly different over the four groups, on the occurrence of acute kidney injury and on maximum RIFLE class F. In the multivariable model, all covariates were entered simultaneously (enter method). We analyzed for collinearity by assessing correlation between covariates. For continuous variables we analyzed the relationship between the outcome and the variable with locally weighted scatterplot smoothing in order to assess whether categorization was necessary. Finally, the goodness of fit of the model was tested by means of the Hosmer-Lemeshow statistic.
We analyzed hospital survival across groups using the chi-square and the Kaplan-Meier methods, and we tested differences between groups using the log-rank test. Patients alive at hospital discharge were censored. We performed a Cox proportional hazards regression analysis to examine whether the maximum RIFLE class and the incidence of acute kidney injury (defined as patients who fulfilled one of the RIFLE classes) were associated with mortality.
To correct for differences in patient characteristics, we included simultaneously age, gender, race, the main reason for ICU admission, the medical or surgical admission category, and the nonrenal SOFA score on ICU admission or at the maximum RIFLE class in the model (enter method). The nonrenal SOFA score was chosen as a covariate to control for multicollinearity between the RIFLE classification and scoring systems that include points for kidney insufficiency such as the APACHE III and SOFA scores. Interactions between the 'main reason for admission' and the maximum RIFLE class were explored, and were found not to be significant.
We tested whether it was appropriate to treat continuous variables as continuous by a residuals plot. We tested the assumption of proportionality of hazards by plotting hazard rates against time for the four different categories, as well as by the numerical method proposed by Lin and colleagues [18] derived from cumulative sums of martingale residuals. We found no evidence of violating the proportional hazards assumption. Finally, we tested the qualitative goodness of fit of the model with residual plots. A double-sided P value less than 0.05 was considered significant. Analysis was performed with the statistical software package SPSS 11.0.1 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of patients with acute kidney injury
A total of 5,383 patients was evaluated. The baseline characteristics of the patient cohort are presented according to the maximum RIFLE class in Table 2. The four groups differed in age, race, pre-existing chronic kidney insufficiency, admission type, severity of illness on admission and at the time of maximum RIFLE class, APACHE III score, SOFA score and the nonrenal SOFA score, and the proportion of patients already admitted inhospital to another non-ICU ward.
Table 2.
Baseline characteristics of patients classified according to the maximum Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) class
| No acute kidney injury | Risk | Injury | Failure | |
| n | 1,766 (32.8%) | 670 (12.4%) | 1,436 (26.7%) | 1,511 (28.1%) |
| Sex (male) | 988 (55.9%) | 372 (55.5%) | 841 (58.6%) | 570 (57.0%) |
| Age (years)* | 56.6 (18.2) | 63.4 (17.0) | 62.6 (16.6) | 62.1 (16.4) |
| Race (n = 5,101)** | ||||
| White | 1,491 (89.5%) | 587 (91.3%) | 1,237 (90.7%) | 1,255 (87.9%) |
| Black | 146 (8.8%) | 45 (7.0%) | 111 (8.1%) | 156 (10.9%) |
| Other | 29 (1.7%) | 11 (1.7%) | 16 (1.2%) | 17 (1.2%) |
| Chronic kidney insufficiency* | 17 (1.0%) | 4 (0.6%) | 17 (1.2%) | 121 (8.0%) |
| Admission type* (n = 5,375) | ||||
| Medical | 778 (44.2%) | 277 (41.4%) | 566 (39.4%) | 545 (36.1%) |
| Surgical | 984 (55.8%) | 392 (58.6%) | 870 (60.6%) | 963 (63.9%) |
| Reason for admission according to organ system* (n = 5,375) | ||||
| Cardiovascular disease | 446 (25.3%) | 236 (35.3%) | 446 (31.1%) | 513 (34.0%) |
| Neurological disease | 311 (17.7%) | 109 (16.3%) | 314 (21.9%) | 327 (21.7%) |
| Pulmonary disease/infection | 226 (12.8%) | 101 (15.1%) | 232 (16.2%) | 291 (19.3%) |
| Trauma | 317 (18.0%) | 85 (12.7%) | 203 (14.1%) | 163 (10.8%) |
| Malignancy | 117 (6.6%) | 34 (5.1%) | 38 (2.6%) | 35 (2.3%) |
| Gastrointestinal disease | 67 (3.8%) | 22 (3.3%) | 32 (2.2%) | 26 (1.7%) |
| Other | 278 (15.8%) | 82 (12.3%) | 171 (11.9%) | 153 (10.1%) |
| APACHE III score* (n = 3,400) | 36 (26–47) | 46 (35–57) | 46 (36–58) | 56 (41–73) |
| SOFA score* | 5.1 (3.3) | 6.3 (4.0) | 6.8 (4.1) | 7.8 (4.5) |
| SOFAnonrenal score* | 4.5 (3.1) | 5.3 (3.5) | 5.6 (3.5) | 5.9 (3.9) |
| SOFA RIFLEmax score* (n = 4,994) | 3.7 (3.2) | 5.3 (3.8) | 5.9 (3.8) | 6.7 (4.3) |
| SOFAnonrenal RIFLEmax score* (n = 4,994) | 3.2 (2.8) | 4.5 (3.5) | 5.0 (3.4) | 5.0 (3.7) |
| Inhospital before ICU admission* | 527 (29.8%) | 243 (36.3%) | 476 (33.1%) | 592 (39.2%) |
| Pre-ICU LOS* (days) | 1 (1–4) | 2 (1–4) | 2 (1–5) | 2 (1–6) |
| Time to RIFLEmax* (days) | 2 (1–3) | 2 (1–4) | 2 (1–7) | |
| RIFLE class on glomerular filtration rate criteria* | 463 (69.1%) | 929 (64.7%) | 1,110 (73.5%) |
Continuous variables are presented as the mean (standard deviation) when normally distributed or as the median (interquartile interval) when not normally distributed. Categorical variables are presented as percentages. No acute kidney injury is those patients without any occurrence of RIFLE criteria; APACHE III, Acute Physiology and Chronic Health Evaluation, version III; SOFAnonrenal, Sequential Organ Failure Assessment score without points for renal insufficiency, SOFA RIFLEmax, Sequential Organ Failure Assessment score at the time of maximum RIFLE class; pre-ICU LOS, length of hospital stay before intensive care unit admission (only for patients who were in hospital before intensive care unit admission). *P < 0.001, **P = 0.035.
Results of the regression analyses examining the impact of the different baseline characteristics on the appearance of acute kidney injury and maximum RIFLE class F are presented in Table 3. Increasing age, greater severity of illness (APACHE III, SOFA and nonrenal SOFA scores), pre-existing chronic kidney insufficiency and a preceding admission to a non-ICU ward in the hospital were associated with increased risk for occurrence of acute kidney injury and maximum RIFLE class F. In addition, black patients had increased risk for development of maximum RIFLE class F. Medical admissions were less likely to result in acute kidney injury or RIFLE class F compared with surgical admissions.
Table 3.
Impact of baseline characteristics on the occurrence of acute kidney injury (multivariate logistic regression analysis)
| Characteristic | Covariates associated with occurrence of acute kidney injury | Covariates associated with occurrence of maximum RIFLE class failure | ||
| Odds ratio (95% confidence interval) | P | Odds ratio (95% confidence interval) | P | |
| Age (per year older) | 1.02 (1.02–1.03) | < 0.001 | 1.01 (1.00–1.01) | 0.001 |
| Race (reference white) | 0.130 | 0.001 | ||
| Black | 1.20 (0.96–1.50) | 0.111 | 1.50 (1.21–1.86) | < 0.001 |
| Other | 0.73 (0.44–1.23) | 0.237 | 0.78 (0.41–1.38) | 0.397 |
| Chronic kidney insufficiency | 4.19 (2.48–7.10) | < 0.001 | 8.86 (6.01–13.05) | < 0.001 |
| Medical admission (reference surgical) | 0.79 (0.69–0.90) | < 0.001 | 0.76 (0.66–0.87) | < 0.001 |
| Reason for admission according to organ system (reference cardiovascular disease) | < 0.001 | < 0.001 | ||
| Trauma | 0.64 (0.53–0.79) | < 0.001 | 0.64 (0.52–0.80) | < 0.001 |
| Neurological disease | 0.93 (0.78–1.13) | 0.481 | 1.02 (0.85–1.2) | 0.830 |
| Pulmonary disease and infection | 1.08 (0.88–1.32) | 0.461 | 1.16 (0.96–1.40) | 0.120 |
| Gastrointestinal disease | 0.51 (0.35–0.73) | < 0.001 | 0.51 (0.32–0.66) | 0.004 |
| Malignancy | 0.36 (0.27–0.49) | < 0.001 | 0.45 (0.31–0.66) | < 0.001 |
| Other | 0.57 (0.47–0.70) | < 0.001 | 0.60 (0.48–0.74) | < 0.001 |
| SOFAnonrenal (per point greater) | 1.19 (1.16–1.21) | < 0.001 | 1.08 (1.06–1.10) | < 0.001 |
| In hospital before ICU admission | 1.18 (1.03–1.36) | 0.015 | 1.19 (1.04–1.36) | 0.012 |
SOFAnonrenal, Sequential Organ Failure Assessment score without points for kidney insufficiency; ICU, intensive care unit. The odds ratios were calculated with logistic regression analysis. The goodness of fit of the multivariable regression model was tested by the Hosmer-Lemeshow statistic: P = 0.080 for the model with acute kidney injury as the endpoint, and P = 0.019 for the model with maximum Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) class failure as the endpoint.
Progression of acute kidney injury to maximum RIFLE class
The progression of acute kidney injury during the ICU stay to the maximum RIFLE class is shown in Figure 1. On the first day of ICU admission, 1,182 patients (21.9%) already had acute kidney injury, defined by the RIFLE criteria. During the entire ICU stay, 3,617 patients (67.2%) had an episode of acute kidney injury defined by RIFLE criteria. One-half of the patients reached the maximum RIFLE class within 2 days after ICU admission (Table 2).
Figure 1.
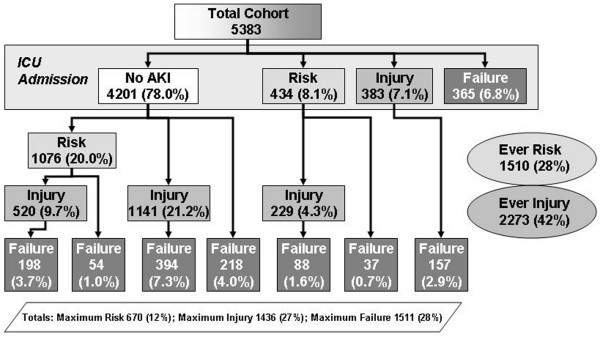
Flow chart of the clinical course of patients until the maximum RIFLE class. Data expressed as patient numbers who were identified at each level, and the percentage of the total number of patients. Patients who appear to skip a grade (class risk or class injury) do so because they did not remain at a transition state for at least 24 hours. 'Ever Risk' and 'Ever Failure' refers to the number of patients who could be identified at this stage. AKI, acute kidney injury; ICU, intensive care unit; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney Disease.
More than 50% of the patients with RIFLE class R progressed to RIFLE class I or class F, and more than one-third of the patients with RIFLE class I progressed to class F. The time to progress to class I was 1 (0.5–3.6) days and the time to progress to class F was 4 (1.4–9.5) days. Patients who progressed to a higher RIFLE class during the ICU stay were older compared to patients whose renal function did not deteriorate, (62.4 ± 16.6 years versus 58.5 ± 18.0 years, P < 0.001) and were already more severely ill on admission, as illustrated by the greater APACHE III score (47 (36–62) versus 41 (29–56), P < 0.001) and the greater nonrenal SOFA score (5.7 (3.6) versus 4.8 (3.4), P < 0.001).
Mortality, length of stay and renal replacement therapy
Less than 1% of patients with maximum RIFLE class I and 14.2% of patients with maximum RIFLE class F received renal replacement therapy (Table 4). Increasing severity of acute kidney injury was associated with an increasing length of ICU stay and hospital stay, and higher mortality (Table 5 and Figure 2). Patients with maximum RIFLE class R, class I and class F had hospital mortality rates of 8.8%, 11.4% and 26.3%, respectively, compared with 5.5% for patients without acute kidney injury. Patients with maximum RIFLE class F based on glomerular filtration rate criteria had a somewhat higher inhospital mortality compared with patients who had a maximum RIFLE class F on urine output criteria (27.9% versus 21.9%, P = 0.020). The unadjusted hazard ratios (95% confidence interval) for hospital mortality for acute kidney injury and RIFLE class R, class I and class F were, respectively, 2.1 (1.67–2.57, P < 0.001), 1.3 (0.91–1.93, P = 0.142), 1.9 (1.45–2.48, P < 0.001) and 3.4 (2.64–4.29, P < 0.001). After adjustment for covariates, acute kidney injury was still associated with an almost twofold increased hazard for hospital mortality (Table 5, panel A). Maximum RIFLE class I and class F were both associated with mortality in the covariate-adjusted Cox regression model (Table 5, panel B). These results were unchanged when the nonrenal SOFA at the time of maximum RIFLE class was substituted for the nonrenal SOFA at ICU admission.
Table 4.
Outcomes for all patients and for patients classified according to the maximum Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) class
| No acute kidney injury (n = 1,766) | Risk (n = 670) | Injury (n = 1,436) | Failure (n = 1,511) | All injury (n = 5,383) | |
| Renal replacement therapy* | 1 (0.1%) | 0 (0%) | 4 (0.3%) | 214 (14.2%) | 219 (4.1%) |
| Hospital LOS after reaching maximum RIFLE class (days)* | 5 (3–10) | 5 (3–10) | 7 (4–14) | 11 (5–23) | 7 (3–14) |
| ICU LOS (days)* | 3 (2–4) | 3 (2–6) | 5 (3–10) | 9 (4–21) | 4 (2–9) |
| Hospital LOS (days)* | 6 (4–10) | 8 (5–14) | 10 (6–19) | 16 (9–31) | 9 (5–19) |
| Hospital mortality* | 97 (5.5%) | 59 (8.8%) | 163 (11.4%) | 398 (26.3%) | 717 (13.3%) |
Continuous variables presented as the median (interquartile interval) and categorical variables presented as the percentage. LOS, length of stay; ICU, intensive care unit. *P = 0.001 between the four subgroups no acute kidney injury, RIFLE class risk, RIFLE class injury and RIFLE class failure.
Table 5.
Association of Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) criteria with mortality
| Hazard ratio (95% confidence interval) | P | |
| Panel A: association of AKI, defined by meeting any RIFLE criteria, with mortality | ||
| AKI (reference no AKI) | 1.7 (1.28–2.13) | < 0.001 |
| SOFAnonrenal score (per point) | 1.1 (1.10–1.15) | < 0.001 |
| Age (per 10 years) | 1.1 (1.07–1.18) | < 0.001 |
| Sex (female) | 0.9 (0.78–1.07) | 0.252 |
| Race (reference white) | 0.528 | |
| Black | 1.1 (0.88–1.45) | 0.324 |
| Other | 0.8 (0.41–1.68) | 0.608 |
| Medical admission (reference surgical admission) | 2.9 (2.44–3.40) | < 0.001 |
| Main reason for admission (reference cardiovascular disease) | < 0.001 | |
| Trauma | 0.9 (0.66–1.09) | 0.196 |
| Neurological disease | 1.3 (1.03–1.52) | 0.026 |
| Pulmonary disease, infection | 0.8 (0.65–1.03) | 0.087 |
| Gastrointestinal disease | 0.5 (0.21–1.25) | 0.140 |
| Malignancy | 0.2 (0.04–0.63) | 0.009 |
| Other | 0.4 (0.22–0.62) | < 0.001 |
| Panel B: association of maximum RIFLE class with mortality | ||
| RIFLEmax (reference no AKI) | < 0.001 | |
| Risk | 1.0 (0.68–1.56) | 0.896 |
| Injury | 1.4 (1.02–1.88) | 0.037 |
| Failure | 2.7 (2.03–3.55) | < 0.001 |
| SOFAnonrenal score (per point) | 1.1 (1.10–1.14) | < 0.001 |
| Age (per 10 years) | 1.1 (1.08–1.20) | < 0.001 |
| Sex (female) | 0.9 (0.76–1.06) | 0.190 |
| Race (reference white) | 0.673 | |
| Black | 1.1 (0.87–1.45) | 0.389 |
| Other | 0.9 (0.44–1.97) | 0.844 |
| Medical admission (reference surgical admission) | 2.7 (2.27–3.21) | < 0.001 |
| Main reason for admission (reference cardiovascular disease) | < 0.001 | |
| Trauma | 0.9 (0.69–1.18) | 0.463 |
| Neurological disease | 1.3 (1.06–1.60) | 0.011 |
| Pulmonary disease, infection | 0.8 (0.66–1.07) | 0.154 |
| Gastrointestinal disease | 0.6 (0.24–1.41) | 0.230 |
| Malignancy | 0.2 (0.04–0.69) | 0.013 |
| Other | 0.3 (0.20–0.60 | < 0.001 |
Covariate-adjusted Cox proportional hazard regression analysis. AKI, acute kidney injury, patients meeting at least one of the RIFLE criteria; RIFLEmax, maximum RIFLE class; SOFAnonrenal, Sequential Organ Failure Assessment score without points for kidney failure, determined on data from the first 24 hours of admission.
Figure 2.
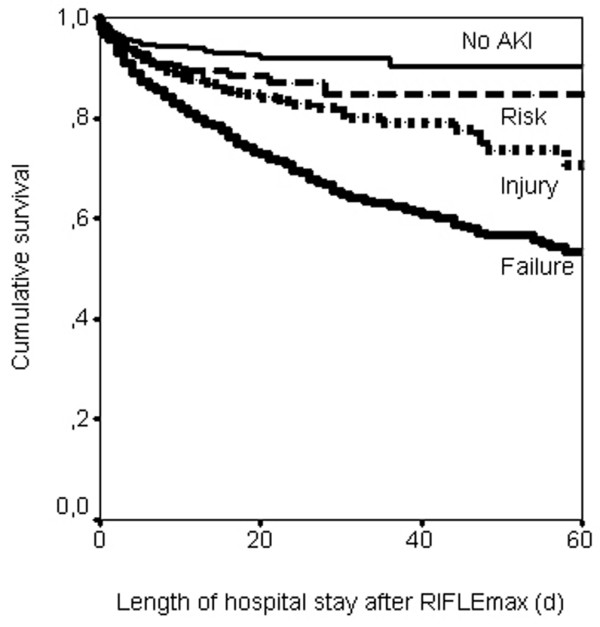
Kaplan-Meier curves for survival (inhospital) by maximum RIFLE class. Patients discharged alive were censored. Log-rank statistic, P < 0.001. AKI, acute kidney injury; RIFLEmax, maximum Risk, Injury, Failure, Loss, and End-stage Kidney Disease (RIFLE) class during the intensive care unit stay (days).
Discussion
We found that acute kidney injury, defined by the RIFLE classification, had a high incidence (67.2%) and was associated with an increased risk for hospital mortality compared with those who never developed acute kidney injury. The incidence of almost 70% may appear at odds with the existing literature [5]. Even when limiting cases to those with RIFLE class F (28%) we found a higher rate for ICU patients than typically reported. Fourteen percent of class F patients received renal replacement therapy, however, leading to a rate of 4–5% among ICU patients, consistent with previous reports [9,19]. Indeed, our study highlights the potential for under-reporting when renal replacement therapy is used to 'define' acute kidney injury. Importantly, even milder degrees of kidney dysfunction, RIFLE class R or class I, were still associated with excess mortality compared with patients who maintained normal function. RIFLE provided a well-balanced classification system for determination of patients with different severity of acute kidney injury, at least as far as risk of mortality or need for renal replacement therapy is concerned.
Not surprisingly, the occurrence of acute kidney injury and maximum RIFLE class F were associated with increased baseline severity of illness and older age (Tables 2 and 3). Patients developing acute kidney injury were slightly older and had higher APACHE III and SOFA scores, even when kidney dysfunction was not counted. However, the severity within acute kidney injury was not so affected by these factors. Patients progressing to RIFLE class I and class F were no older and their nonrenal SOFA scores no greater than patients remaining in RIFLE class R. Although, Herget-Rosenthal and colleagues have also described the progression of acute kidney injury in a selected cohort of 85 ICU patients [20], this is to our knowledge the first time that the progression of acute kidney injury has been examined in a large dataset of general ICU patients.
RIFLE class R would appear to be aptly named. More than one-half of the patients of class R progressed to more severe RIFLE classes, yet those that did not were not at increased risk of hospital mortality. Future studies could target this population for prevention. RIFLE class I may also have been fortuitously named, for this is the stage at which risk for hospital mortality increases even after controlling for covariates. It was commonly held until fairly recently that patients die 'with, and not of, acute renal failure'. Medication (for example, erythropoietin and diuretics) and renal replacement therapy were thought to 'replace' the loss of kidney function. It has already been demonstrated in critically ill patients that severe acute renal failure, defined as the need for renal replacement therapy or oliguria, is independently associated with mortality [4,5,19,21]. In addition, in a cardiothoracic surgery population and in a cohort of hospitalized patients, both with a lower baseline mortality compared with general ICU patients, small changes in serum creatinine were associated with a worse outcome [22,23]. In the present study we confirm the association of acute kidney injury with increased hospital mortality in a general ICU population. This is a remarkable finding considering how common this condition appears to be – 55% of all patients had RIFLE class I or class F. Furthermore, there was increasing mortality risk over RIFLE classes, despite the fact that these ICU patients had similar comorbidity, as reflected by the nonrenal SOFA score.
The finding that moderate degrees of kidney dysfunction pose a significant risk of death is particularly notable given that we know very little of why this should be. Acute kidney injury may simply be colinear with unmeasured elements of comorbidity, or it may be causally related to the increased mortality. Future studies should consider exploring whether alternative management of patients with mild degrees of kidney dysfunction could change the outcome. If the problem is actually the kidney, then possible mechanisms underlying the excess mortality associated with acute kidney injury are likely to be found in the pathophysiologic changes resulting from kidney insufficiency and adverse effects of renal replacement therapy [24,25]. Salt and water retention resulting in volume overload, hyperkalemia and acid-base derangements [26], perhaps leading to decreased blood pressure, cardiac output, hepatic and renal blood flow [27], to insulin resistance and protein breakdown, and even to alterations in innate immunity [28], all may contribute to the excess mortality in this group of patients. Furthermore, patients with acute kidney injury have a high incidence of infectious complications [29-31] and frequently develop anemia. Finally, acute kidney injury itself can lead to a non-infectious, proinflammatory response with activation of leukocytes, secretion of proinflammatory cytokines and recruitment of neutrophils and macrophages with resultant lung injury, as has been demonstrated in animal models of ischemia-reperfusion-induced acute renal failure [32,33]. All these changes may occur prior to, or even in, patients never receiving renal replacement therapy. These same mechanisms, however, may explain why patients who are treated with a lower dose of renal replacement therapy have a worse survival [34-36].
Our study has certain limitations. First, we did not attempt to compare RIFLE with other classification systems; nor did we compare urine output and creatinine criteria, but rather used the criteria as proposed by the Acute Dialysis Quality Initiative workgroup, as the worst classification by each criterion. It is possible that urine output and creatinine criteria provide complementary information, which is lost when these criteria are combined.
The Acute Dialysis Quality Initiative recommended the use of a baseline serum creatinine, yet a true baseline is often unknown for patients admitted to the ICU. Several possible baseline values existed for our patients (hospital admission, ICU admission, or a calculated baseline from the MDRD equation). Our use of the lowest of these values for any given patient may have lead to a higher estimate of change and therefore a higher estimate of the incidence of acute kidney injury. Although the MDRD equation was developed and validated on a large number of patients, conflicting results have been published regarding the validation of this equation in different patient populations. We acknowledge that this equation is only a substitute for the actual glomerular filtration rate, but validation of this equation or developing an alternative for the MDRD-derived baseline creatinine was beyond the scope of this study.
We also acknowledge that some members of our research group have contributed to the consensus process by which RIFLE was developed and by which MDRD recommendations were made. In addition, patient follow-up in our study was limited to hospital discharge information.
Some patients may have died shortly after hospital discharge. As shown in Figure 2, the curves continue to separate, particularly for those in the class F group. Longer follow-up would also be required to examine the RIFLE endpoints 'loss' and 'end-stage disease'. Early renal replacement therapy may theoretically influence the criteria, and patients that would have reached class F could be classified in our study as class R or class I. Only four class I patients were treated with renal replacement therapy, however, and reclassification of these patients to class F does not influence our results.
Although our study is relatively large and included seven ICUs, it was conducted at a single medical center whose case mix and referral patterns may not be representative of other centers. The case mix of this study cohort could have hindered the detection of specific conditions that influence the development of acute kidney injury. Finally, our retrospective study design, using existing medical records, limited our ability to look outside the ICU and to collect information on potential mechanisms of injury. Our design also prohibited the use of more sophisticated measures of kidney function. Indeed, our assessment of time to progression of acute kidney injury may have been artificially lengthened due to daily measurement of creatinine – some patients appeared to skip class R or class I because of this limitation.
Conclusion
In this general ICU population, acute kidney 'risk, injury, failure' as defined by the newly developed RIFLE classification is associated with increased hospital mortality and resource use. Patients with RIFLE class R are indeed at high risk of progression to class I or class F. Patients with RIFLE class I or class F incur a significantly increased length of stay and an increased risk of inhospital mortality compared with those who do not progress past class R or those who never develop acute kidney injury, even after adjusting for baseline severity of illness, case mix, race, gender and age.
Key messages
• The RIFLE classification is a very sensitive definition of acute kidney injury: acute kidney injury defined by the RIFLE classification occurred in two thirds of general ICU patients.
• RIFLE classes injury and failure are independently associated with increased risk for in-hospital dead.
• Patients who meet the very sensitive RIFLE "risk" criteria, are at significant risk for progression to injury or failure, and therefore in-hospital dead.
Abbreviations
APACHE III = Acute Physiology and Chronic Health Evaluation, version III; class F = failure, according to the RIFLE classification; class I = injury, according to the RIFLE classification; class R = risk, according to the RIFLE classification; CrMDRD = serum creatinine based upon the MDRD equation; ICU = intensive care unit; MDRD = Modification of Diet in Renal Disease; RIFLE = Risk, Injury, Failure, Loss, and End-stage Kidney; SOFA = Sequential Organ Failure Assessment score; SOFAnonrenal = SOFA score without points for renal insufficiency.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EAJH designed the study, analyzed the raw data set, performed the statistical analysis and contributed to writing of the paper. GC set up the raw data set, helped to design the study and contributed to writing of the paper. AK helped analyze the raw data set and helped in the design of the study. RV and DCA helped to design the study and contributed to writing the paper. DDB helped with the statistical analysis. JAK designed the study, analyzed the data and contributed to writing the paper. All authors read, edited and ultimately approved the final manuscript.
Acknowledgments
Acknowledgements
Part of this work has been presented in abstract form at the 24th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 2004. This study was conducted without external financial support.
Contributor Information
Eric AJ Hoste, Email: eric.hoste@ugent.be.
Gilles Clermont, Email: Clermontg@ccm.upmc.edu.
Alexander Kersten, Email: Spacemanspiff99de@mac.com.
Ramesh Venkataraman, Email: venkataramr@ccm.upmc.edu.
Derek C Angus, Email: angusdc@ccm.upmc.edu.
Dirk De Bacquer, Email: dirk.debacquer@ugent.be.
John A Kellum, Email: kellumja@ccm.upmc.edu.
References
- Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- Vincent J-L, Moreno R, Takala J, Willatts S, de Mendonça A, Bruining H, Reinhart CK, Suter PeterM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Levin N, Bouman C, Lameire N. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care. 2002;8:509–514. doi: 10.1097/00075198-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Levy EM, Hammermeister KM, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/S0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- Vivino G, Antonelli M, Moro M, Cottini F, Conti G, Bufi M, Cannata F, Gasparetto A. Risk factors for acute renal failure in trauma patients. Intensive Care Med. 1998;24:808–814. doi: 10.1007/s001340050670. [DOI] [PubMed] [Google Scholar]
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- Mora Mangano C, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT, for the Multicenter Study of Perioperative Ischemia Research Group Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- Guerin C, Girard R, Selli JM, Perdrix JP, Ayzac L. Initial versus delayed acute renal failure in the intensive care unit. A multicenter prospective epidemiological study. Rhone-Alpes Area Study Group on Acute Renal Failure. Am J Respir Crit Care Med. 2000;161:872–879. doi: 10.1164/ajrccm.161.3.9809066. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Kellum J, Ronco C. Acute renal failure: time for consensus. Intensive Care Med. 2001;27:1685–1688. doi: 10.1007/s00134-001-1120-6. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, the ADQI workgroup Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum JA. The Acute Dialysis Quality Initiative: methodology. Adv Ren Replace Ther. 2002;9:245–247. doi: 10.1053/jarr.2002.35564. [DOI] [PubMed] [Google Scholar]
- Bell M, Liljestam E, Granath F, Fryckstedt J, Ekbom A, Martling CR. Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant. 2005;20:354–360. doi: 10.1093/ndt/gfh581. [DOI] [PubMed] [Google Scholar]
- Abosaif NY, Tolba YA, Heap M, Russell J, El Nahas AM. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis. 2005;46:1038–1048. doi: 10.1053/j.ajkd.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettila V. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542–546. doi: 10.1016/j.athoracsur.2005.07.047. [DOI] [PubMed] [Google Scholar]
- K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. doi: 10.1053/ajkd.2002.32799. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80:557–572. doi: 10.2307/2337177. [DOI] [Google Scholar]
- Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–1030. doi: 10.1097/01.ASN.0000059863.48590.E9. [DOI] [PubMed] [Google Scholar]
- Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.ASN.0000130340.93930.DD. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- Klahr S, Miller SB. Acute oliguria. N Engl J Med. 1998;338:671–675. doi: 10.1056/NEJM199803053381007. [DOI] [PubMed] [Google Scholar]
- Hoste EA, Kellum JA. ARF in the critically ill: impact on morbidity and mortality. Contrib Nephrol. 2004;144:1–11. doi: 10.1159/000078872. [DOI] [PubMed] [Google Scholar]
- Rocktaeschel J, Morimatsu H, Uchino S, Goldsmith D, Poustie S, Story D, Gutteridge G, Bellomo R. Acid-base status of critically ill patients with acute renal failure: analysis based on Stewart-Figge methodology. Crit Care. 2003;7:R60–R66. doi: 10.1186/cc2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnerson KJ, Song M, Kellum JA. Influence of acid-base balance in patients with sepsis. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine 2004. Berlin: Springer-Verlag; 2004. pp. 58–67. [Google Scholar]
- Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286:R686–R692. doi: 10.1152/ajpregu.00564.2003. [DOI] [PubMed] [Google Scholar]
- Hoste EA, Blot SI, Lameire NH, Vanholder RC, De Bacquer D, Colardyn FA. Effect of nosocomial bloodstream infection on the outcome of critically ill patients with acute renal failure treated with renal replacement therapy. J Am Soc Nephrol. 2004;15:454–462. doi: 10.1097/01.ASN.0000110182.14608.0C. [DOI] [PubMed] [Google Scholar]
- Woodrow G, Turney JH. Cause of death in acute renal failure. Nephrol Dial Transplant. 1992;7:230–234. doi: 10.1093/oxfordjournals.ndt.a092111. [DOI] [PubMed] [Google Scholar]
- Thakar CV, Yared JP, Worley S, Cotman K, Paganini EP. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003;64:239–246. doi: 10.1046/j.1523-1755.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–2367. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G. Effect of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, Winearls C, Farrar J, White N, Day N. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895–902. doi: 10.1056/NEJMoa020074. [DOI] [PubMed] [Google Scholar]


