Abstract
The accessory protein Nef plays a crucial role in primate lentivirus pathogenesis. Nef enhances human immunodeficiency virus type 1 (HIV-1) infectivity in culture and stimulates viral replication in primary T cells. In this study, we investigated the relationship between HIV-1 replication efficiency in CD4+ T cells purified from human blood and two various known activities of Nef, CD4 downregulation and single-cycle infectivity enhancement. Using a battery of reporter viruses containing point mutations in nef, we observed a strong genetic correlation between CD4 downregulation by Nef during acute HIV-1 infection of activated T cells and HIV-1 replication efficiency in T cells. In contrast, HIV-1 replication ability was not significantly correlated with the ability of Nef to enhance single-cycle virion infectivity, as determined by using viruses produced in cells lacking CD4. These results demonstrate that CD4 downregulation by Nef plays a crucial role in HIV-1 replication in activated T cells and underscore the potential for the development of therapies targeting this conserved activity of Nef.
The accessory protein Nef is encoded by the primate immunodeficiency viruses human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus. The available evidence indicates that Nef is a virulence factor. Adult rhesus macaques infected with simian immunodeficiency virus with nef deleted are dramatically delayed in their progression to AIDS (29). In humans, a small but significant subset of HIV-infected long-term nonprogressors harbor viruses carrying deletions in nef (30, 40, 49). In vitro, Nef is not required for efficient HIV-1 replication in immortalized T-cell lines (41, 57) but enhances HIV-1 replication in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC), as well as purified macrophages and activated primary T cells (21, 41, 57, 61).
Several major distinct activities of Nef have been demonstrated in culture, including downregulation of cell surface CD4 molecules, downregulation of cell surface major histocompatibility complex (MHC) class I molecules, enhancement of viral infectivity, and modulation of T-cell signaling pathways. Of these, CD4 downregulation is perhaps the best understood (48). Nef triggers the rapid endocytosis of cell surface CD4 molecules, resulting in their degradation in lysosomes (2, 50). CD4 downregulation by Nef prevents superinfection (8, 36) and facilitates incorporation of Env protein into virions by preventing Env-CD4 interactions at the time of virus budding. Recent reports have also shown that Nef-mediated CD4 downregulation stimulates HIV-1 production (51) and infectivity (17, 31). However, these conclusions were based on studies of viruses produced in 293T cells or immortalized Jurkat T lymphocytes overexpressing CD4 molecules. The importance of Nef-induced CD4 downregulation for HIV-1 replication in primary T cells has not been determined.
Nef also enhances HIV-1 infectivity. Wild-type HIV-1 particles produced in CD4-negative COS, HeLa, or 293T cells are 4- to 40-fold more infectious than those with nef deleted in single-cycle infection assays (1, 4, 13, 42, 45). This activity of Nef is independent of CD4 expression in either the producer or target cells, as HIV-1 particles pseudotyped with amphotropic murine leukemia virus envelope, which does not use cellular CD4 as a receptor, also require Nef protein for efficient infectivity (4, 42). Recent studies suggest that Nef may function to enhance infectivity at the level of virus-cell fusion (54), potentially by modifying the viral lipid envelope during HIV-1 assembly (68, 69).
To evaluate the relative contributions of CD4 downregulation and CD4-independent infectivity enhancement to Nef-enhanced HIV-1 replication, we first established a purified CD4+-T-cell culture system in which a strong and reproducible effect of Nef on HIV-1 replication is exhibited. CD4+ primary cells were prepared through positive selection by using anti-CD4 antibody-conjugated Dynabeads as previously described (62). This purification protocol typically resulted in >99% purity of CD4+ T lymphocytes, as determined by fluorescence-activated cell sorter (FACS) analysis following staining with CD3- and CD4-specific monoclonal antibodies (data not shown). Immediately following purification, purified T cells were stimulated with staphylococcal enterotoxin B (100 ng/ml; Sigma), a bacterial superantigen, and mitomycin C-killed dendritic cells (1:10 dendritic-cell/T-cell ratio) and cultured for 6 to 8 days prior to infection in medium containing interleukin-2 (250 U/ml). This method of activation, compared to PHA stimulation, more closely mimics antigen-specific stimulation of T cells.
For replication assays, cultures of CD4+ primary cells (5 × 104) in 200 μl of medium were inoculated with HIV-1 stocks (1 ng of p24) in medium containing interleukin-2. Every 2 days following inoculation, one-half of the culture medium was removed for quantification of virus production by p24 enzyme-linked immunosorbent assay (64) and an equivalent volume of fresh medium was added. In this culture system, we observed strong and highly reproducible attenuation of the nef-null mutant virus relative to wild-type HIV-1, which replicated efficiently, yielding p24 concentrations of up to 1,000 ng/ml (Fig. 1A).
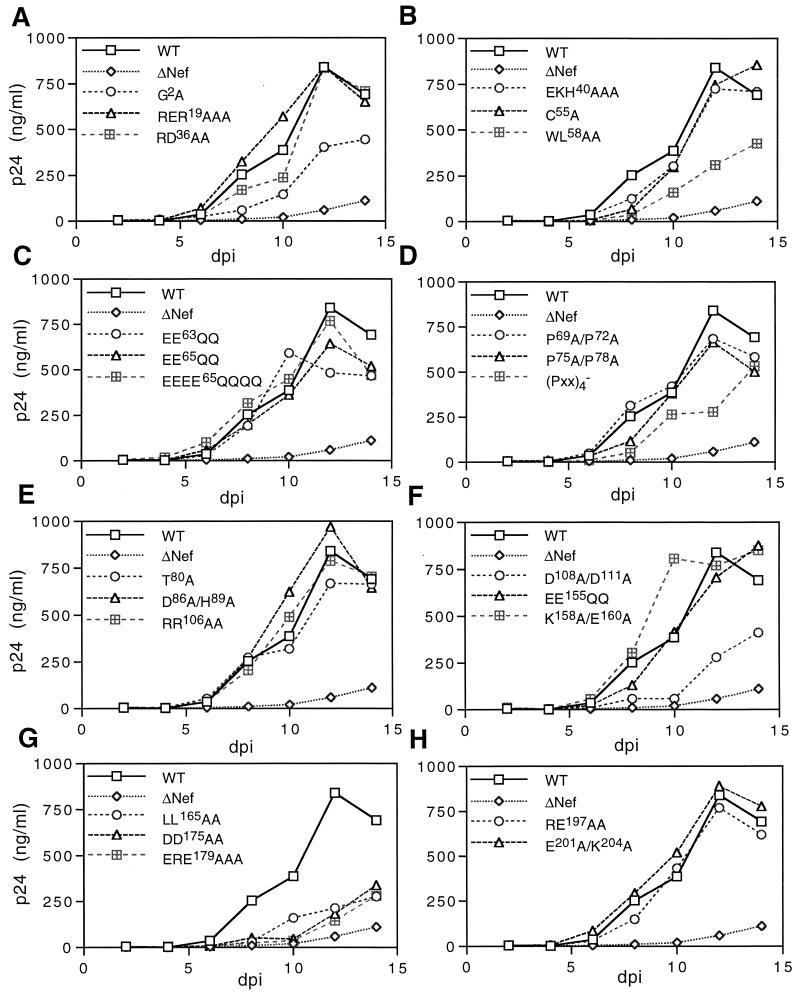
FIG. 1.
Replication of nef point mutant viruses in primary human T cells. Activated CD4+ T cells were inoculated with wild-type HIV-1 and nef mutant HIV-1 (1 ng of p24 antigen), and supernatants from cell cultures were sampled every 2 days following inoculation and assayed for virus production by p24 enzyme-linked immunosorbent assay. The data shown are averages of duplicate parallel cultures and are representative of three independent experiments. Wild-type (WT) and nef-defective (ΔNef) virus replication kinetics are shown in each panel for comparison. dpi, days postinfection.
Replication of nef point mutant viruses in CD4+ T cells.
To determine the relative contributions of CD4 downregulation and single-cycle infectivity enhancement by Nef to HIV-1 replication efficiency, we quantified the abilities of a large collection of nef point mutant viruses to replicate in activated primary T cells. The wild-type (R8) and nef-defective (R8ΔN) proviral constructs used were described previously (23). R8-derived point mutant viruses were constructed by transferring BamHI-XbaI fragments from expression vectors containing the nef point mutations (3) into the corresponding region of R8. A total of 23 mutant viruses were analyzed, together mutating 42 of the 206 Nef residues. The mutations targeted polar or charged residues that would likely be involved in interactions with other cellular or viral proteins, as well as residues previously shown to selectively impair each of Nef's known activities. Virus stocks were produced by transfection of 293T cells and assayed for p24 as previously described (1).
In addition to the nef-null mutant virus (ΔNef), seven additional mutant viruses [G2A, WL58AA, (Pxx)4−, D108A/D111A, LL165AA, DD175AA, and ERE179AAA] were significantly delayed in replication relative to wild-type HIV-1 (Fig. 1A, B, D, F, and G). Of these seven mutants, the (Pxx)4− virus was found to be only mildly delayed in replication while the G2A and WL58AA viruses were more severely impaired. The most severely impaired viruses were the D108A/D111A, LL165AA, DD175AA, and ERE179AAA mutants. Interestingly, none of the point mutant viruses was as markedly delayed for replication as the control Nef-defective virus.
CD4 downregulation by Nef correlates with viral replication in CD4+ primary T cells.
Previous mutational studies of Nef-induced CD4 downregulation employed Nef expression vectors in the absence of other HIV-1 proteins (3, 9, 18, 22, 25, 37, 38). To probe the relationship between Nef-induced CD4 downregulation and Nef-accelerated HIV-1 replication, we quantified the abilities of nef point mutant viruses to downregulate cell surface CD4 expression during acute HIV-1 infection of primary T cells. Because of the difficulty of synchronously infecting a high percentage of cells with HIV-1, we employed recombinant viruses encoding murine heat-stable antigen (HSA; CD24) in place of the vpr open reading frame (28), thereby allowing identification of infected cells by staining for surface expression of CD24 and analysis by flow cytometry. For analysis of CD4 downregulation, approximately 2 × 105 cells were infected with HIV-1 at a relatively high multiplicity of infection. Samples (105 cells) were removed at 3, 6, and 9 days following infection. Staining was performed with FACS staining buffer (2% fetal bovine serum, 0.1% sodium azide in phosphate-buffered saline) for 30 min by using fluorescein isothiocyanate-conjugated anti-HSA and phycoerythrin (PE)-conjugated anti-CD4 antibodies (Pharmingen). Cells were washed once with staining buffer (1 ml) and fixed in 2% paraformaldehyde prior to data acquisition and analysis with a Becton Dickinson FACScalibur flow cytometer and Cellquest software.
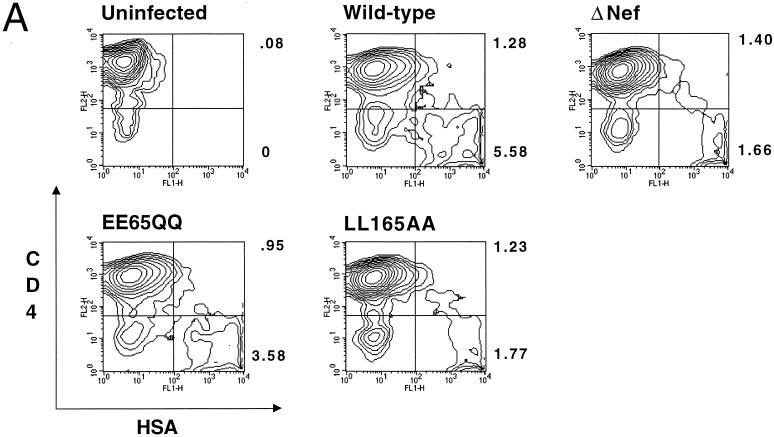
As expected, there were approximately 4.5-fold more CD4− than CD4+ cells (5.58 versus 1.28%, respectively) within the HSA+ population following infection with HIV-1 encoding wild-type Nef (Fig. 2A). In contrast, roughly equal numbers of CD4− and CD4+ cells were observed in the HSA+ population (1.66 and 1.40%, respectively) infected with Nef-defective HIV-1, demonstrating active downregulation of CD4 by Nef during acute HIV-1 infection, as previously reported (2, 12). Nef-independent CD4 downregulation was presumably mediated by Env and Vpu, which are expressed at later times than Nef following HIV-1 integration and prevent newly synthesized CD4 from reaching the plasma membrane (2, 12, 20, 31, 58, 65). Also shown are results for two representative point mutants, EE65QQ and LL165AA, which exhibited FACS profiles and CD4−/CD4+ ratios similar to those of wild-type and Nef-defective HSA-expressing viruses, respectively. The results demonstrate the utility of the assay system for quantification of CD4 downregulation by Nef during acute HIV-1 infection of primary CD4+ T cells.
FIG. 2.
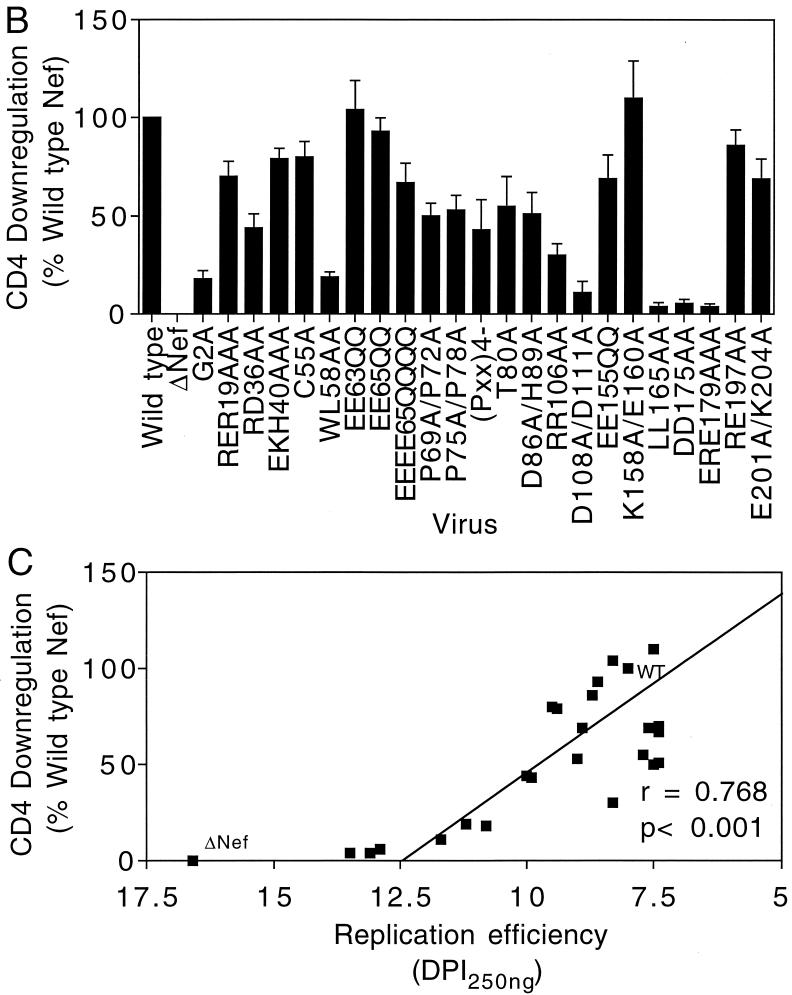
Nef-dependent CD4 downregulation correlates with HIV-1 replication efficiency. (A) FACS analysis of CD4 downregulation during HIV-1 infection of primary CD4+ T cells. Three days following infection with the indicated HSA-encoding viruses, cell surface CD4 levels were quantified by two-color flow cytometry following staining with HSA- and CD4-specific monoclonal antibodies. Percentages of HSA+ CD4+ and HSA+ CD4− populations are shown to the right of each FACS plot. (B) CD4 downregulation by mutant Nef proteins was calculated as a percentage of the difference in CD4 levels on cells infected with wild-type HIV-1 and those infected with nef-defective HIV-1. Shown are mean values and standard deviations of the six values obtained (two concentrations of input virus and three time points each) from a representative experiment. (C) Graphic representation of relative CD4 downregulation activity versus HIV-1 replication efficiency. Replication efficiency was determined by interpolation of the day following virus inoculation when p24 production exceeded 250 ng/ml; therefore, higher values represent slower replication kinetics. WT, wild type; DPI, days postinfection.
To facilitate comparison of CD4 downregulation by the mutant Nef proteins, we expressed the CD4 downregulation data as percentages of the difference between wild-type HIV-1 and Nef-defective HIV-1 (Fig. 2B). Interestingly, the six Nef point mutants that exhibited the strongest impairments in CD4 downregulation, i.e., G2A (18% of wild-type Nef activity), WL58AA (19% of wild-type Nef activity), D108A/D111A (11% of wild-type Nef activity), LL165AA (4% of wild-type Nef activity), DD175AA (6% of wild-type Nef activity), and ERE179AAA (4% of wild-type Nef activity), were also the most delayed in replication. In contrast, the (Pxx)4− mutant was only mildly impaired for both CD4 downregulation (43% of wild-type Nef activity) and replication (Fig. 1D).
To analyze the relationship between CD4 downregulation and replication efficiency, we plotted CD4 downregulation efficiency as a function of viral replication, which was defined arbitrarily as the time required for p24 production in the culture to reach 250 ng (Fig. 2C). Statistical analysis using Student's t test revealed a strong correlation between CD4 downregulation and HIV-1 replication efficiency (P < 0.001). Mutants that replicated with wild-type kinetics retained a minimum of 30% of wild-type Nef CD4 downregulation activity, suggesting that a threshold level of CD4 downregulation is required for efficient HIV-1 replication in primary T cells. Interestingly, none of the point mutants was as delayed in replication as the Nef-defective control, even though several of these (LL165AA, DD175AA, and ERE179AAA) failed to downregulate CD4.
Infectivity enhancement by Nef does not correlate with HIV replication.
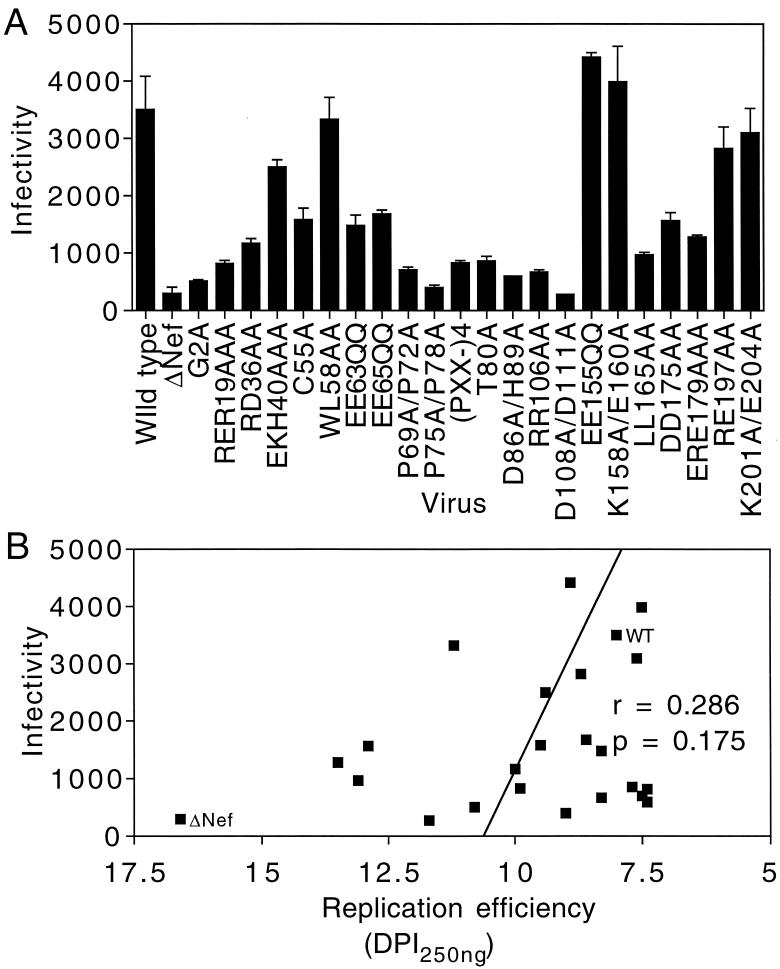
We next assessed the abilities of mutant Nef proteins to enhance HIV-1 infectivity in a single round of infection. Viruses containing nef point mutations were produced in CD4-negative 293T cells, and their titers were determined on HeLa CD4 long terminal repeat LacZ reporter cells (P4 cells) as previously described (4, 11). As reported previously, Nef enhanced the infectivity of HIV-1 by 10-fold (Fig. 3A). Many of the nef mutant viruses, including EE155QQ, K158E/E160A, and K201A/E204A, were minimally impaired in infectivity, while mutants T80A, D86A/H89A, and D108A/D111A were severely impaired (Fig. 3A). Although several of the mutants, including G2A, D108A/D111A, and (Pxx)4−, were impaired for both single-cycle infectivity and replication in primary T cells, the WL58AA mutant replicated with delayed kinetics but was as infectious as wild-type HIV-1 in the single-cycle infection assay. In addition, several mutants (P69A/P72A, P75A/P78A, T80A, and D86A/H89A) exhibited marked reductions in single-cycle infectivity yet replicated in T cells with kinetics similar to those of wild-type HIV-1 (Fig. 1). To test for a correlation between single-cycle infectivity enhancement and replication efficiency in T cells, we plotted the infectivity of each of these mutants as a function of the time of virus emergence (Fig. 3B). We observed no significant correlation (P = 0.175) between single-cycle infectivity enhancement by Nef and the efficiency of HIV-1 replication in CD4+ primary T cells.
FIG. 3.
Infectivity of nef point mutant viruses does not correlate with replication efficiency in primary T cells. (A) Viruses containing nef point mutations were produced by transfection of 293T cells and assayed for infection of P4 reporter target cells. Infected cells were quantified following 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining, and infectivity was calculated as the number of infected cells per nanogram of p24 in the inoculum. The values shown are averages of triplicate infections with three independently generated virus stocks. (B) Graphic representation of infectivity as a function of virus replication in activated CD4+ T cells. WT, wild type; DPI, days postinfection.
Implications of the genetic data.
The purpose of our study was to determine the relationship between several known activities of Nef and the ability of Nef to stimulate HIV-1 replication in activated primary T cells. By performing analyses on a large array of engineered nef mutant viruses, we observed a strong correlation between the CD4 downregulation capacity of Nef and HIV-1 replication efficiency. In contrast, there was no significant correlation between Nef-mediated infectivity enhancement and HIV-1 replication efficiency. These results are in agreement with those of a recent study (24) in which a limited number of patient-derived nef genes were analyzed for enhancement of HIV-1 replication in PBMC and CD4 downregulation in transfected Jurkat T cells. Our study extends the findings of Glushakova et al. by including a genetic analysis of CD4 downregulation during productive HIV-1 infection of primary T cells.
Seven of 23 nef mutant viruses exhibited significantly impaired replication in activated CD4+ primary T cells. Although several of these mutants were essentially devoid of CD4 downregulation activity, all replicated more efficiently than the nef-defective virus, suggesting that an additional function of Nef promotes HIV-1 replication. Six of these seven mutants were impaired for CD4 downregulation. The four viruses that were most severely defective in replication and CD4 downregulation encoded the D108A/D111A, LL165AA, DD175AA, and ERE179AAA mutant Nef proteins. All of these mutant proteins exhibit defects in interactions with known cellular intracellular trafficking components. Asp108 is necessary for the interaction of Nef with the cellular protein thioesterase II, and its alteration prevents CD4 downregulation (15). The segment of Nef containing residues 148 through 180 comprises a large exposed loop structure on the surface of Nef (5, 33), and the mutations in this region impair Nef's interactions with adaptor protein complexes (9, 26, 39, 46) and vacuolar H+ ATPase (38), both components of the cellular endocytic machinery. These nef mutations overlap U3 sequences and therefore also have the potential for alterations in the transcriptional efficiency of viral genes. However, we do not believe that these mutations affected replication efficiency through unintended transcriptional effects for the following reasons. First, all of the mutants replicated with wild-type kinetics (data not shown) in the T-cell line CEM, which exhibits only a minimal requirement for Nef in HIV-1 replication (41, 57), demonstrating the absence of severe transcriptional defects resulting from alterations in U3 sequences. Second, single-cycle infectivity experiments (in which infected cells were treated with zidovudine to prevent viral spread) revealed no obvious defects in HSA reporter expression in primary T cells infected with these mutants (data not shown). We conclude that the major defect in replication of the D108A/D111A, LL165AA, DD175AA, and ERE179AAA mutants likely arises from expression of mutant Nef proteins and not from effects on U3 promoter function.
With the exception of the (Pxx)4− mutant, mutants that were only moderately impaired for CD4 downregulation, such as RD36AA and RR106AA, replicated with wild-type kinetics. This suggests that a threshold level of CD4 downregulation activity is necessary for efficient HIV-1 replication. This hypothesis would also account for the phenotypes of the G2A and WL58AA mutants, which downregulated CD4 inefficiently (∼20% of wild-type Nef activity) but were only moderately defective for replication.
Mutations of the diglutamate motif (residues 154 and 155) were previously reported to interfere with Nef's interaction with the beta subunit of the coatomer complex (β-COP) hypothesized to target endocytosed CD4 molecules for lysosomal degradation (47). The ability of the EE155QQ Nef mutant to downregulate CD4 efficiently and replicate with wild-type kinetics argues against a role for this function in stimulating HIV-1 replication in activated T cells. This stands to reason, however, because the normal fate of CD4 molecules following endocytosis in T cells is degradation in lysosomes (52). Nevertheless, our results do not exclude a possible role of lysosomal targeting of CD4 by Nef during HIV-1 infection of resting T cells (67) or in other cell types.
Several of the mutant Nef proteins (T80A, D108A/D111A, EE155QQ, K158A/E160A, and ERE179AAA) were previously shown to exhibit reduced stability when expressed in transfected 293 cells in the absence of other HIV-1 proteins (3). Because the goal of our study was to evaluate the relationships among CD4 downregulation, single-cycle infectivity enhancement, and replication efficiency in primary T cells, irrespective of the reason for the Nef activity impairments, we have included these mutants in our analyses. In general, the CD4 downregulation results obtained with HSA-expressing viruses during productive infection of primary T cells agree well with the results of our previous studies of CD4 downregulation using a 293 cell-based transient-transfection assay, with a few notable exceptions. Three of the unstable mutants, T80A, EE155QQ, and K158A/E160A, were less active for CD4 downregulation in the transient-transfection assay than during HIV-1 infection. It is possible that these mutant Nef proteins are more stable in infected T cells than in transfected 293 cells and that infected T cells therefore accumulate amounts of these mutant Nef proteins sufficient for downregulation of CD4. These discrepancies, although few, underscore the potential limitations of using nonnatural targets of HIV infection, such as epithelial tumor cell lines, for analysis of HIV-1 protein functions.
In contrast to Nef-induced CD4 downregulation, we found no significant correlation between single-cycle infectivity enhancement by Nef and the efficiency of HIV-1 replication in primary T cells. Several mutants replicated efficiently despite marked impairments in single-cycle infectivity. This result was surprising, as the 10-fold enhancement by Nef observed in single-cycle infection assays might also be expected to occur with each successive round of replication. Our results suggest that, in contrast to infection of P4 HeLa cells, Nef is not required for efficient cell-free infection of activated T cells. It is also possible that the dynamics of HIV-1 transmission in single-cycle and continuous replication assays differ significantly and that the infectivity enhancement by Nef observed in single-cycle assays is not required for efficient infection when HIV-1 is transmitted directly from an infected cell.
MHC class I downregulation by Nef does not enhance HIV-1 replication in primary T cells.
In addition to downregulation of CD4 molecules and enhancement of HIV-1 infectivity, Nef also downregulates cell surface expression of MHC class I by preventing its transport to the cell surface (59) and by stimulating its internalization and degradation (34, 35, 56). It has been suggested that selective MHC class I downregulation by Nef aids HIV-1 persistence and disease progression in vivo (10, 27, 43), presumably by protecting HIV-1-infected cells from killing by cytotoxic T lymphocytes (16) and natural killer cells (14). As expected, analysis of point mutant Nef proteins that were defective for MHC downregulation [EEEE65QQQQ, P69A/P72A, P75A/P78A, and (Pxx)4−] revealed no significant correlation between Nef-induced MHC class I downregulation and the efficiency of HIV-1 replication in primary T cells (Fig. 1 and data not shown), in agreement with previous studies (35, 60).
Although we did not specifically examine Nef's role in alteration of the T-cell signaling pathways involved in HIV-1 replication, two of the Nef point mutants in our collection were previously reported to be defective for binding of cellular proteins that play roles in T-cell activation (for a review, see reference 6). Mutation of the proline-rich motif of Nef, the binding site for SH3 domains of several Src family protein tyrosine kinases, in the (Pxx)4− mutant resulted in a moderate delay in HIV-1 replication. This result is consistent with those of previous studies using PHA-stimulated PBMC (19, 53, 66). Although the (Pxx)4− mutant was moderately impaired for CD4 downregulation (43% of wild-type Nef activity), this activity was greater than that of the RR106AA mutant (30% of wild-type Nef activity) and similar to that of the RD36AA mutant (44% of wild-type Nef activity), both of which replicated well in activated primary T cells. This exception suggests that the (Pxx)4− motif in Nef promotes HIV-1 replication through an additional mechanism, such as modulation of cellular signaling pathways (55, 63). Mutations in Nef residues 105 and 106 (RR106AA mutant), which abrogate binding and activation of cellular protein p21-activated kinase 1 (PAK) (44), did not affect HIV-1 replication efficiency. Although this result argues against a significant role of PAK in HIV-1 replication in our culture system, activation of PAK may be necessary for replication in T cells activated by different stimuli or in other natural targets of HIV-1.
Role of CD4 downregulation in HIV-1 replication.
Although our data indicate a functional link between CD4 downregulation by Nef and efficient HIV-1 replication, the molecular mechanism by which CD4 downregulation by Nef stimulates HIV-1 replication in activated CD4+ primary T cells remains undefined. Studies in our laboratory indicate that Nef-defective virions produced from infected primary T cells are at least 200-fold less infectious than wild-type HIV-1 when infectivity is assayed in CD4-expressing HeLa target cells (C.A.L. and C.A., unpublished data). This impairment is significantly greater than the 10-fold defect in infectivity of ΔNef virus produced in CD4-negative 293T producer cells (Fig. 3A). These observations are in agreement with previous reports that CD4 downregulation by Nef is essential for optimal infectivity of virus produced in CD4-expressing cells (31). Whether the defect in CD4 downregulation results in decreased progeny virus production from infected CD4+ primary T cells, as previous studies with CD4+ 293T producer cells have reported (51), remains to be determined.
If CD4 downregulation is crucial for efficient replication, then why is Nef not required for HIV-1 replication in many immortalized T-cell lines (41, 57)? Assuming that CD4 downregulation by Nef is necessary for viral egress and/or optimal virion infectivity, a strong requirement for Nef would be expected for optimal HIV-1 replication in all CD4-expressing cell types. The requirement for Nef could be modulated by differences between cell-free HIV-1 infection and cell-to-cell transmission, which may vary between primary T cells and tumor cell lines. Furthermore, primary T cells may be more sensitive to superinfection, which could induce cell injury from overexpression of viral proteins, or to CD4-dependent signaling events leading to cell death by apoptosis (7, 32). Regardless of the reason for the discrepancy, primary T cells are likely more physiologically relevant than immortalized T-cell lines for studying the role of HIV-1 Nef in replication.
Although Nef is clearly a virulence factor, efforts to evaluate the potential of this protein as a therapeutic target have been confounded by the bewildering array of Nef activities observed in culture. The genetic correlation of HIV-1 replication efficiency with Nef-induced CD4 downregulation demonstrated herein should encourage efforts to target this highly conserved activity of Nef for antiviral drug discovery and development.
Acknowledgments
We thank Jerome Zack for the HSA reporter virus construct, NL-HSAS, which was obtained through the National Institutes of Health AIDS Research and Reference Reagent Program. We also thank members of the Aiken Laboratory and Paul Spearman for helpful suggestions.
This work was supported by grant AI40364 from the National Institutes of Health. C.A.L. was supported in part by the Vanderbilt University Medical Scientist Training Program. D.U. was supported by NIH grant AI42284.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71: 5871-5877. [DOI] [PMC free article] [PubMed]
- 2.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 3.Aiken, C., L. Krause, Y.-L. Chen, and D. Trono. 1996. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology 217:293-300. [DOI] [PubMed] [Google Scholar]
- 4.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arold, S., P. Franken, M. P. Strub, F. Hoh, S. Benichou, R. Benarous, and C. Dumas. 1997. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure 5:1361-1372. [DOI] [PubMed] [Google Scholar]
- 6.Arold, S. T., and A. S. Baur. 2001. Dynamic Nef and Nef dynamics: how structure could explain the complex activities of this small HIV protein. Trends Biochem. Sci. 26:356-363. [DOI] [PubMed] [Google Scholar]
- 7.Banda, N. K., J. Bernier, D. K. Kurahara, R. Kurrle, N. Haigwood, R.-P. Sekaly, and T. H. Finkel. 1992. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176:1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson, R. E., A. Sanfridson, J. S. Ottinger, C. Doyle, and B. R. Cullen. 1993. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef protein prevents viral super infection. J. Exp. Med. 177:1561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8:1235-1238. [DOI] [PubMed] [Google Scholar]
- 10.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charneau, P., G. Mirambeau, P. Roux, S. Paulus, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription: a termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 12.Chen, B. K., R. T. Gandhi, and D. Baltimore. 1996. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J. Virol. 70:6044-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. S. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 15.Cohen, G. B., V. S. Rangan, B. K. Chen, S. Smith, and D. Baltimore. 2000. The human thioesterase II protein binds to a site on HIV-1 Nef critical for CD4 down-regulation. J. Biol. Chem. 275:23097-23105. [DOI] [PubMed] [Google Scholar]
- 16.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 17.Cortes, M. J., F. Wong-Staal, and J. Lama. 2002. Cell surface CD4 interferes with the infectivity of HIV-1 particles released from T cells. J. Biol. Chem. 277:1770-1779. [DOI] [PubMed] [Google Scholar]
- 18.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig, H. M., M. W. Pandori, N. L. Riggs, D. D. Richman, and J. C. Guatelli. 1999. Analysis of the SH3-binding region of HIV-1 nef: partial functional defects introduced by mutations in the polyproline helix and the hydrophobic pocket. Virology 262:55-63. [DOI] [PubMed] [Google Scholar]
- 20.Crise, B., L. Buonocore, and J. K. Rose. 1990. CD4 is retained in the endoplasmic reticulum by the human immunodeficiency virus type 1 glycoprotein precursor. J. Virol. 64:5585-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Ronde, A., B. Klaver, W. Keulen, L. Smit, and J. Goudsmit. 1992. Natural HIV-1 Nef accelerates virus replication in primary human lymphocytes. Virology 188:391-395. [DOI] [PubMed] [Google Scholar]
- 22.Erdtmann, L., K. Janvier, G. Raposo, H. M. Craig, P. Benaroch, C. Berlioz-Torrent, J. C. Guatelli, R. Benarous, and S. Benichou. 2000. Two independent regions of HIV-1 Nef are required for connection with the endocytic pathway through binding to the μ1 chain of AP1 complex. Traffic 1:871-883. [DOI] [PubMed] [Google Scholar]
- 23.Gallay, P., S. Swingler, J. Song, F. Bushman, and D. Trono. 1995. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell 83:569-576. [DOI] [PubMed] [Google Scholar]
- 24.Glushakova, S., J. Münch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldsmith, M. A., M. T. Warmerdam, R. E. Atchison, M. D. Miller, and W. C. Greene. 1995. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 69:4112-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16:6964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iafrate, A. J., S. Carl, S. Bronson, C. Stahl-Hennig, T. Swigut, J. Skowronski, and F. Kirchhoff. 2000. Disrupting surfaces of Nef required for downregulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J. Virol. 74:9836-9844. [Erratum, 74:12002.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamieson, B., and J. Zack. 1998. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J. Virol. 72:6520-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 31.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622-631. [DOI] [PubMed] [Google Scholar]
- 32.Laurent-Crawford, A. G., B. Krust, Y. Riviere, C. Desgranges, S. Muller, M. P. Kieny, C. Dauguet, and A. G. Hovanessian. 1993. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res. Hum. Retrovir. 9:761-773. [DOI] [PubMed] [Google Scholar]
- 33.Lee, C.-H., K. Saksela, U. A. Mirza, B. T. Chait, and J. Kuriyan. 1996. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell 85:931-942. [DOI] [PubMed] [Google Scholar]
- 34.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 35.Le Gall, S., M.-C. Prevost, J.-M. Heard, and O. Schwartz. 1997. Human immunodeficiency virus type I Nef independently affects virion incorporation of major histocompatibility complex class I molecules and virus infectivity. Virology 229:295-301. [DOI] [PubMed] [Google Scholar]
- 36.Little, S. J., N. L. Riggs, M. Y. Chowers, N. J. Fitch, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1994. Cell surface CD4 downregulation and resistance to superinfection induced by a defective provirus of HIV-1. Virology 205:578-582. [DOI] [PubMed] [Google Scholar]
- 37.Liu, L. X., N. Heveker, O. T. Fackler, S. Arold, S. Le Gall, K. Janvier, B. M. Peterlin, C. Dumas, O. Schwartz, S. Benichou, and R. Benarous. 2000. Mutation of a conserved residue (D123) required for oligomerization of human immunodeficiency virus type 1 Nef protein abolishes interaction with human thioesterase and results in impairment of Nef biological functions. J. Virol. 74:5310-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu, X., H. Yu, S.-H. Liu, F. M. Brodsky, and B. M. Peterlin. 1998. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 8:647-656. [DOI] [PubMed] [Google Scholar]
- 39.Mangasarian, A., M. Foti, C. Aiken, D. Chin, J.-L. Carpentier, and D. Trono. 1997. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity 6:67-77. [DOI] [PubMed] [Google Scholar]
- 40.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69:570-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munch, J., N. Stolte, D. Fuchs, C. Stahl-Hennig, and F. Kirchhoff. 2001. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 75:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunn, M. F., and J. W. Marsh. 1996. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 70:6157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandori, M. W., N. J. S. Fitch, H. M. Craig, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1996. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J. Virol. 70:4283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piguet, V., Y. L. Chen, A. Mangasarian, M. Foti, J. L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 17:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piguet, V., F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J.-L. Carpentier, and D. Trono. 1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of b-COP in endosomes. Cell 97:63-73. [DOI] [PubMed] [Google Scholar]
- 48.Piguet, V., O. Schwartz, S. Le Gall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 49.Premkumar, D. R., X. Z. Ma, R. K. Maitra, B. K. Chakrabarti, J. Salkowitz, B. Yen-Lieberman, M. S. Hirsch, and H. W. Kestler. 1996. The nef gene from a long-term HIV type 1 nonprogressor. AIDS Res. Hum. Retrovir. 12:337-345. [DOI] [PubMed] [Google Scholar]
- 50.Rhee, S. S., and J. W. Marsh. 1994. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 68:5156-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross, T. M., A. E. Oran, and B. R. Cullen. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9:613-621. [DOI] [PubMed] [Google Scholar]
- 52.Ruegg, C. L., S. Gajasekar, B. S. Stein, and E. G. Englemen. 1992. Degradation of CD4 following phorbol-induced internalization in human T lymphocytes. J. Biol. Chem. 267:18837-18843. [PubMed] [Google Scholar]
- 53.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 96:8167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J.-M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 57.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevenson, M., C. Meier, A. M. Mann, N. Chapman, and A. Wasiak. 1988. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell 53:483-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swann, S. A., M. Williams, C. M. Story, K. R. Bobbitt, R. Fleis, and K. L. Collins. 2001. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI3-kinase-dependent pathway. Virology 282:267-277. [DOI] [PubMed] [Google Scholar]
- 60.Swigut, T., A. J. Iafrate, J. Muench, F. Kirchhoff, and J. Skowronski. 2000. Simian and human immunodeficiency virus Nef proteins use different surfaces to downregulate class I major histocompatibility complex antigen expression. J. Virol. 74:5691-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terwilliger, E. F., E. Langhoff, D. Gabuzda, E. Zazopoulos, and W. A. Haseltine. 1991. Allelic variation in the effects of the nef gene on replication of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 88:10971-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, J. K., E. Kiyokawa, E. Verdin, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA 97:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wehrly, K., and B. Chesebro. 1997. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods 12:288-293. [DOI] [PubMed] [Google Scholar]
- 65.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiskerchen, M., and C. Cheng-Mayer. 1996. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology 224:292-301. [DOI] [PubMed] [Google Scholar]
- 67.Wu, Y., and J. W. Marsh. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293:1503-1506. [DOI] [PubMed] [Google Scholar]
- 68.Zheng, Y. H., A. Plemenitas, T. Linnemann, O. T. Fackler, and B. M. Peterlin. 2001. Nef increases infectivity of HIV via lipid rafts. Curr. Biol. 11:875-879. [DOI] [PubMed] [Google Scholar]
- 69.Zhou, J., and C. Aiken. 2001. Nef enhances human immunodeficiency virus type 1 infectivity resulting from intervirion fusion: evidence supporting a role for Nef at the virion envelope. J. Virol. 75:5851-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]