Abstract
This study investigated whether chronic stress-induced spatial memory deficits were caused by changes in the hypothalamic-pituitary-adrenal axis, such as corticosterone (CORT) elevations on the day of memory assessment, rather than the consequence of structural changes in the hippocampus. Male Sprague–Dawley rats were restrained for 6 h/day/21 days, and spatial memory was assessed on the Y-maze on day 22. Ninety minutes before training, rats received a subcutaneous injection of vehicle or metyrapone, a CORT synthesis inhibitor, and then spatial memory was determined 4-h later. The highest dose of metyrapone (75 mg/kg, s.c.) was most effective at preventing stress-induced spatial memory deficits. Chronic stress increased total CORT levels following Y-maze exposure, while acute metyrapone treatment dose-dependently attenuated total and free (unbound) CORT levels in both stress and control conditions. Blood samples taken from a separate subset of chronically stressed rats showed that baseline CORT levels were similar across the restraint period. Finally, chronic stress down-regulated glucocorticoid, but not mineralocorticoid, receptor mRNA expression within the hippocampus (dentate gyrus, CA1, CA2, CA3). These findings suggest that chronic stress-induced spatial memory deficits may be mediated by hypothalamic-pituitary-adrenal axis dysregulation. Specifically, CORT elevations and reductions in hippocampal glucocorticoid receptor expression, at the time of behavioural assessment may be involved, as opposed to a direct effect that is solely dependent upon hippocampal structural changes. These results have significance for treating cognitive decline in conditions associated with elevated glucocorticoids that include subpopulations in ageing, depression, Cushing's disease and Alzheimer's disease.
Keywords: glucocorticoid receptor, hippocampus, hypothalamic-pituitary-adrenal axis, mineralocorticoid receptor, Sprague–Dawley rats
Introduction
The hippocampus contributes to cognitive functions, such as spatial memory. Rodents with damage to the hippocampus or its afferents show impaired performance in the Morris water maze (Morris et al., 1982), radial arm maze (Olton et al., 1978), and Y-maze (Conrad et al., 1996). A recent review, however, suggests that the role of the hippocampus on memory processing may be ancillary to physiological regulation, which includes regulating the hypothalamus-pituitary-adrenal (HPA) axis (Lathe, 2001). Supporting this hypothesis is the finding that spatial memory deficits following CA3 hippocampal lesion are prevented with a single pretesting injection of metyrapone, a corticosterone (CORT) synthesis blocker (Roozendaal et al., 2001). These findings indicate that spatial memory deficits may arise from HPA axis dysregulation following hippocampal damage, rather than as a direct effect of hippocampal injury.
The hypothesis that the hippocampus mediates spatial memory deficits via HPA axis dysregulation may change the interpretation of many models investigating hippocampal function. Our findings and others have shown that chronic stress reliably produces hippocampal CA3 dendritic retraction (Watanabe et al., 1992b; Magariños & McEwen, 1995a, b; Galea et al., 1997; Conrad et al., 1999; McKittrick et al., 2000; Vyas et al., 2002), and spatial memory deficits (Luine et al., 1994; Conrad et al., 1996; Luine et al., 1996). These data have been interpreted as supporting the idea that chronic stress compromises the hippocampus, which then impairs spatial memory. In light of the recent findings by Roozendaal et al. (2001), however, chronic stress may produce spatial memory deficits via HPA axis dysregulation, as opposed to its direct actions on hippocampal morphology. In his review, Roozendaal (2002) suggests that chronic stress gradually elevates baseline levels of CORT, which are responsible for chronic stress-induced spatial memory impairments. Yet, restraint produces varied findings, with some studies showing increases in baseline CORT levels (Watanabe et al., 1992b), and others showing no change (Magariños & McEwen, 1995b; Galea et al., 1997). Therefore, additional studies are necessary to help resolve the mechanism by which chronic stress produces spatial memory impairments.
We tested the hypothesis that chronic restraint stress produces spatial memory impairment by altering the hypothalamic-pituitary-adrenal axis, such as elevating CORT on the day of memory assessment. Rats were restrained for 6 h a day for 21 days, a procedure that reliably produces hippocampal dendritic retraction. Control and chronically stressed rats were given vehicle or metyrapone once to reduce CORT elevations on the day of Y-maze behavioural assessment. If chronic stress impairs spatial memory via CORT elevations, then chronically stressed rats given acute metyrapone treatment were expected to demonstrate functional spatial memory. However, if chronic stress impairs spatial memory via stress-induced dendritic retraction directly, then chronically stressed rats given acute metyrapone treatment were expected to demonstrate poor spatial memory.
Materials and methods
Subjects
Arizona State University's Animal Care and Use Committee approved all procedures, in accordance with applicable portions of the Animal Welfare Act and ‘Guide for the Care and Use of Laboratory Animals’ by DHHS. All efforts were made to minimize the number of animals used. Male Sprague–Dawley rats (225–250 g, n = 96) were pair-housed in temperature-controlled chambers, maintained on a 12-h light : 12-dark cycle (lights on at 18:00 h), and given food and tap water ad libitum. Rats were allowed to acclimatise to the facilities for one week prior to any manipulation. All procedures were performed during the dark phase of the light cycle.
Experiment 1 – Influence of 35 mg/kg metyrapone and chronic stress on Y-maze performance and total serum CORT levels
Restraint and blood sampling
Rats were restrained 6 h a day for 21 days in wire mesh restrainers (16.5 cm circumference × 24 cm long, or 19 cm circumference × 27 cm long as the rats grew) in their home cages. Control rats remained undisturbed during the restraint period. Body weights were obtained on days 1, 7, 14 and 21 to validate whether restraint was effective, as chronically stressed male rats gain less weight compared to controls across the period of restraint (Conrad et al., 2003). One subset of stressed rats (n = 8) was used solely for blood sampling during restraint and was not used for behavioural testing. Blood was repeatedly taken on days 1, 7, 14, and 21 of restraint, with sampling occurring outside of the animal colony. On each day of blood sampling, blood (∼300 μL) was taken at 0, 30, 120, 240 min from the lateral saphenous vein by quick puncture using a 20 gauge needle and drawn into heparinized capillary tubes (model # 0266810, Fischer Scientific). The initial blood sampling (time = 0) on each day represents baseline, when blood was taken within 2–4 min of removal from the animal colony room. A third set of rats (n = 48) was used to obtain blood samples 30 min following Y-maze training, when CORT levels peak, and immediately following testing. For these rats, trunk blood was collected via rapid decapitation.
Y-maze apparatus
The symmetrical Y-maze, as developed by Dellu et al. (1992), was used to assess hippocampal-dependent spatial recognition memory and requires extra maze cues to navigate (Conrad et al., 1996). The Y-maze consisted of three identical black Plexiglas © arms (50 cm L, 16 cm W, 32 cm H) with multiple extra-maze cues located around the perimeter of the maze. To prevent rats from utilizing cues located inside the maze, the floor of the Y-maze was covered with corncob bedding and mixed between trials, and the maze was rotated between training and testing. Hence, the arms, termed Novel, Start and Other, refer to the location of the arm in the room and not the actual arm. An overhead video camera recorded movement of the rats for later quantification, and the investigator was not visible to the rat during training or testing. Rats were not tested on the Y-maze within 1-h of the light cycle change to avoid changes in activity levels related to these transitional periods. Rats were tested on the Y-maze with a 4-h delay between training and testing on the day after restraint ended. Y-maze navigation relies upon a rats innate tendency to explore novel environments (Ennaceur & Delacour, 1988), which is not reduced by chronic stress (Wright & Conrad, 2005; Bellani et al., 2006; Kleen et al. 2006). In the present experiments rats that can recognize and choose the Novel arm more than the Other arm will be defined as having intact spatial memory, whereas rats that enter the Novel and Other arms similarly will be considered to have impaired spatial memory.
Procedure
Ninety minutes prior to training on the Y-maze, control (C) and stressed (S) rats received either a 35 mg/kg injection (s.c.) of metyrapone (M35) dissolved in 40% polyethylene glycol and 60% saline, Aldrich (Roozendaal et al., 2001) or vehicle (V), giving rise to the following four groups: control + vehicle (CV), control + metyrapone (CM35), stress + vehicle (SV), stress + metyrapone (SM35). During training, one arm (Novel) of the Y-maze was blocked with a piece of black Plexiglas ©, allowing the rats to explore the Start and Other arms for 15 min. The black Plexiglas © that blocked the novel arm was the height of the arms (32 cm), preventing rats from rearing and seeing into the novel arm or viewing the spatial cues visible only from the novel arm. Following training, rats were returned to their home chambers during the 4-h delay. After the delay, the black Plexiglas © blocking the Novel arm was removed, and rats were placed in the same Start arm, and allowed to explore the Y-maze for 5 min (for an extensive review of the Y-maze see Conrad, 2006).
An investigator unaware of the treatment groups determined the number of entries made into, and time spent (dwell) by each rat in, the Novel, Start, and Other arm across all five minutes. An entry was counted when the forearms of the rat entered the arm.
CORT assay
Blood was centrifuged for 30 min at 3217 g. using a Heraeus centrifuge (model Megafuge 1.0R, VWR). Serum was removed and stored at −70 °C. Samples were diluted 1 : 10 and processed in duplicate. Final values for each subject were averaged and represented as μg/100 mL. CORT levels were determined using an Enzyme Immunoassay kit (# 026-AC-14F1, American Laboratory Products, Windham, NH). Antibody cross-reactivity to other steroids did not exceed 0.05%. Optical density values were measured at 450 nm using a microplate reader (model = LabSystems Multiskan RC, Fisher Scientific).
Experiment 2 – Influence of 35 and 75 mg/kg metyrapone and chronic stress on Y-maze performance: determination of free CORT and hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) mRNA
Experiment 2 was conducted to expand upon the findings of Experiment 1 by adding a 75 mg/kg dose of metyrapone (SM75), measuring CORT binding globulin (CBG) to determine free (unbound) CORT and determining hippocampal MR/GR mRNA. The procedures were the same as Experiment 1, but after decapitation, brains were dissected, frozen on dry ice and stored at −70 °C until further use.
CORT binding globulin (CBG) assay
The CBG assay was conducted as described by (Deviche et al., 2001). Blood was collected as described above and was kept cold on wet ice prior to being centrifuged and stored. Individual plasma samples were diluted 1 : 4 in a solution containing 1% charcoal and 1% dextran in order to eliminate steroids from plasma. Samples were incubated for 15 min and then centrifuged for 10 min at 4500 r.p.m. Supernatant was collected and further diluted 1 : 66.67 with Tris buffer (pH 7.4). A radioligand binding assay for CBG was conducted in triplicate by incubating 50 μL diluted plasma with 50 μL 3H-CORT and 50 μL Tris buffer (final dilution 1 : 800) for 1 h. Free and bound 3H-CORT were separated by rapid filtration over Whatman GF/B glass fibre filters that were soaked for 1 h in cold buffer containing 0.3% polyethylenimine (Sigma). After filtration filters were rinsed three times with 3 mL ice-cold Tris buffer and placed into glass scintillation vials. Radioactivity was quantified by standard liquid scintillation spectroscopy using a gamma-counter (Beckman, LS 6500) and the median value for each rat was analysed further. Free CORT was derived from CBG values as described in (Barsano & Baumann, 1989). Specifically, free CORT was calculated as follows with Kd as the dissociation constant for CORT:
Insitu hybridization for GR and MR mRNA
Five to six rats from four conditions CV, CM75, SV, SM75 were selected a-priori based upon their behavioural profile on the Y-maze representing the group's mean performance. Only these brains were cut and processed further. Brain sections (20 μm) at the level of the dorsal hippocampus were cut on a cryostat (Reichert-Jung Frigocut 2800), and processed to measure the expression of MR and GR mRNA using riboprobes. Sections were fixed with paraformaldehyde and prehybridized as described (e.g. Meijer & de Kloet, 1994). For GR, we used a 500-bp fragment (exon 2, coding for the N-terminus of the receptor, courtesy of Dr M. Bohn) of the original full-length GR clone (courtesy of Dr K. Yamamoto). The MR probe was generated from a 500-bp fragment coding for rat MR exon two in pGEM4 (courtesy of Dr J. Arriza). Linearized plasmids were transcribed with the appropriate RNA polymerase in the presence of 35S-labelled UTP to get antisense and (negative control) sense probes. Per slide, 100 μL of hybridization mix, containing 50% formamide, 10% dextran sulphate, 10 mm DTT, 1 × Denhardt's, 3 × SSC, tRNA and ssDNA, and 2*106 c.p.m. of the labelled probe were used to hybridize overnight at 55 °C. After hybridization, sections were washed in 2 × SSC at room temperature and incubated with RNAse A in 0.1 m Tris, pH = 8.0 for 15 min at 37 °C. Subsequently, different wash protocols were used for each probe, each with increasing stringency up to 0.1 × SSC at 65 °C for 30 min. After dehydration in an ethanol series, slides were put in a cassette and a Biomax-MR film (Kodak) was exposed for 4 (MR) to 10 (GR) days. Autoradiograms were quantified from film using NIH Image software, after film and tissue background correction.
Statistics
Parametric data were analysed by anova and significant effects were further investigated with Newman–Keuls posthoc tests or planned comparisons. Nonparametric data were analysed by Wilcoxon matched pairs tests.
Results
Experiment 1 – Influence of 35 mg/kg metyrapone and chronic stress on Y-maze performance and total CORT levels
A mixed factor anova was performed on total entries made into each arm during each minute (history × drug × minute) to determine the minutes with the most entries for further analysis. Otherwise, potential effects may be masked because rats habituate quickly while exploring the Y-maze. The repeated effect of minute was highly significant, F4,132 = 20.59, P < 0.0001, but no other effects reached significance. A posthoc test indicated that rats made more entries in minute one compared to all other minutes (two, three, four, five). Therefore, minute one was used for subsequent analyses.
Metyrapone, administered prior to training, prevented chronic stress-induced spatial memory deficits on the Y-maze after a 4-h delay.
Entry
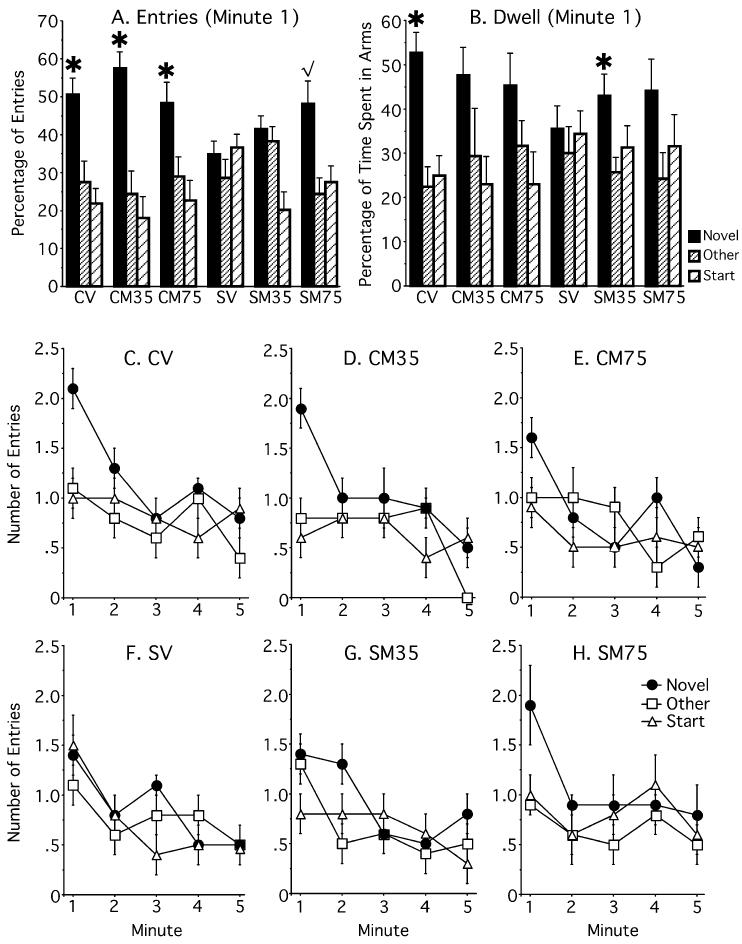
Wilcoxon nonparametric tests showed that chronically stressed rats injected with vehicle (SV, n = 9) entered the Novel and Other arm similarly (P = 0.12), whereas chronically stressed rats injected with metyrapone (SM35, n = 8) entered the Novel arm significantly more than the Other arm (P = 0.02, Fig. 1). Moreover, controls entered the Novel arm more than the Other arm (CM35, n = 10, P = 0.02; CV, n = 10, P = 0.06).
Fig 1.
Experiment 1 – Percentage of entries and time spent (Dwell) in all arms of the Y-maze. (A) Control and stressed rats given metyrapone (CM and SM, 35 mg/kg) prior to training displayed intact spatial memory by entering the novel arm more than the other arm. Control rats given vehicle showed a tendency to enter the novel arm more than the other arm. In contrast, stressed rats given vehicle entered the novel and other arms similarly (P = 0.12). (B) For time spent in the novel arm, the controls given vehicle (CV) and stressed rats treated with metyrapone spent more time in the novel arm compared to the other arm. Note that the stress rats given vehicle (SV) consistently performed poorly in both the entry measure and dwell measure. (C–F) Raw data for the number of entries made into each arm across the full five minutes of Y-maze exploration for each group. All groups showed high number of entries in the first minute, which declined in subsequent minutes, a common phenomenon when using the Y-maze. In the first minute, the CV, CM35, and SM35 groups clearly prefer the novel arm by entering it more often than the other arm, while the SV group enters all arms similarly. *P < 0.05, √P = 0.06. Data represent means ± SEM.
Total arm entries were used to determine whether motivation and/or motor skills differed among groups. Stressed rats injected with vehicle and control rats injected with metyrapone made more entries compared to stressed rats injected with metyrapone and control rats injected with vehicle (Table 1, interaction between treatment and drug, F1,33 = 6.617, P < 0.01). The main effects were not significant (stress, P = 0.58; drug, P = 0.80).
Table 1.
Total arm entries
| Experiment and group | Total arm entries |
|---|---|
| Experiment 1 | |
| CV | 4.0 ± 0.4 |
| CM | 5.3 ± 0.3* |
| SV | 5.4 ± 0.5* |
| SM | 4.4 ± 0.2 |
| Experiment 2 | |
| CV | 4.3 ± 0.4 |
| CM35 | 3.3 ± 0.3 |
| CM75 | 3.5 ± 0.3 |
| SV | 4.0 ± 0.5 |
| S35 | 3.4 ± 0.4 |
| SM75 | 3.5 ± 0.3 |
Data presented as means ± SEM.
P < 0.05 compared to CV or SM.
Dwell
CV rats spent more time in the novel arm compared to the other arm (P < 0.05) and SM35 rats showed a tendency to spend more time in the novel arm than the other arm (P = 0.06) while CM35 and SV groups showed no significant preference (P > 0.1, Fig. 1). Overall, the SV condition was the only group that consistently failed to enter and spend more time (dwell) in the novel arm compared to the other arm. The other groups (CV, CM35, SM35) entered the novel arm more than the other arm and/or spent more time in the novel arm compared to the other arm.
Physiological measures
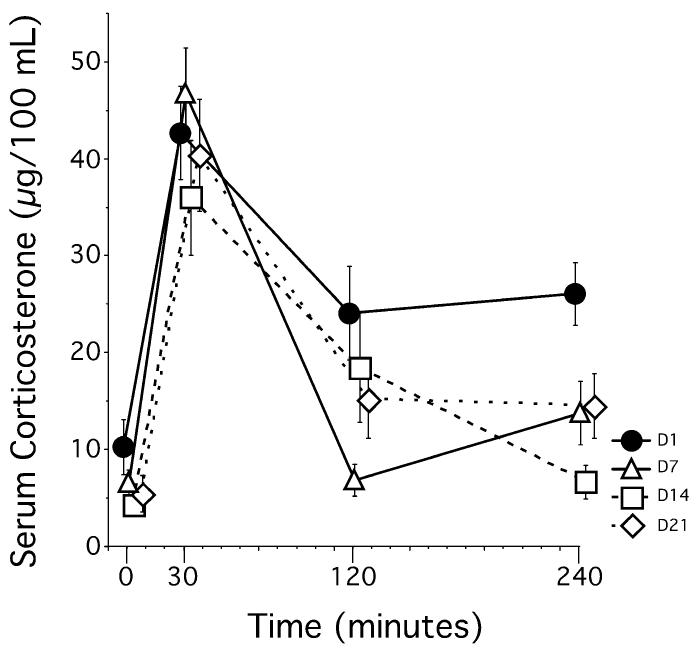
Serum total CORT levels (bound and unbound) measured from a subset of rats (n = 8) revealed that baseline levels of total CORT failed to increase during 21 days of restraint. The repeated measures anova (day × time) showed significant effects of day, F3,21 = 4.132, P = 0.02, time, F3,21 = 50.47, P < 0.01, and a day by time interaction, F9,63 = 2.617, P = 0.01. While baseline total CORT levels (T = 0 min) were similar across days (D1, D7, D14, D21), total CORT levels within 30 min after the start of restraint (T = 30 min) were significantly elevated on all days, and these levels did not statistically differ from each other (Fig. 2). Finally, levels of total CORT after recovery (T = 240 min) were reduced by repeated restraint, with total CORT levels on day 1 being significantly elevated compared to total CORT levels on days 7, 14, and 21.
Fig 2.
Experiment 1 – Serum CORT levels across 21 days of restraint. CORT levels at baseline (within 2–4 min of disturbance) did not differ across the 21 days of restraint (time = 0). CORT levels on day 1 (D1) stayed elevated above baseline 240 min after restraint onset, but did return to baseline on subsequent days (D7, D14, D21). Peak CORT levels (time= 30) were similar across all days of restraint. Data represent means ± SEM. Note that data are intentionally shifted on the x-axis to help visualize the means and SEM.
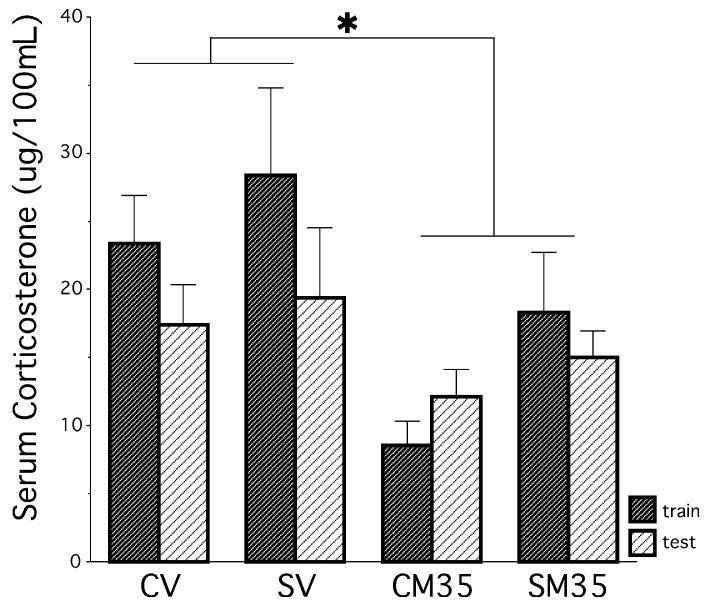
In another group of rats, serum total CORT levels in response to the procedure of training and testing on the Y-maze were analysed to determine whether chronic stress altered the total CORT response to the Y-maze procedure and whether metyrapone treatment successfully reduced total CORT levels. Metyrapone reduced total CORT levels in all control and stressed rats, as shown by a significant main effect of drug, F1,40 = 9.800, P = 0.003, following a 2 × 2 × 2 anova (stress history × drug × train/test sampling). A main effect of stress history approached significance, F1,40 = 3.338, P = 0.08 (Fig. 3), which contributed to running additional experiments in study 2 to show that chronically stressed rats released more total CORT following training and testing compared to control rats, regardless of whether metyrapone or vehicle was injected. No other main effects or interactions approached significance (P > 0.15).
Fig 3.
Experiment 1 – Serum CORT levels in response to Y-maze training and testing. Chronically stressed rats showed a tendency to release more CORT than controls in response to the Y-maze (P = 0.08). Metyrapone attenuated CORT levels in both stressed and control groups. *P < 0.05. Data represent means ± SEM.
Chronically stressed rats gained weight more slowly than controls. A 3 × 4 mixed factors anova for treatment (control, stress and rats in the stress-blood sampling group) and day (1, 7, 14 and 21) revealed a significant main effect of stress F1,45 = 30.19, P < 0.01, a significant main effect of day F3,135 = 450.66, P < 0.01 and an interaction between stress and day F3,135 = 111.43, P < 0.01. Chronically stressed rats that were tested on the Y-maze or used for blood sampling procedures gained less weight as measured on days 7, 14, 21 compared to controls (Table 2).
Table 2.
Body weights (g)
| Body weights (g) |
|||||
|---|---|---|---|---|---|
| Experiment | Group | Day 1 | Day 7* | Day 14* | Day 21* |
| Experiment 1 | |||||
| A | Control group used in Y-maze testing | 290.0 ± 2.2 | 337.9 ± 3.0 | 377.5 ± 4.2 | 398.6 ± 4.6 |
| B* | Stress group used in Y-maze testing | 303.4 ± 3.5 | 301.4 ± 3.8 | 323.5 ± 4.2 | 339.1 ± 5.4 |
| C* | Stressed group used for blood sampling | 306.2 ± 4.9 | 296.5 ± 6.1 | 313.5 ± 7.1 | 327.3 ± 8.5 |
| Experiment 2 | |||||
| A | Control group | 315.6 ± 2.4 | 345.0 ± 2.2 | 376.9 ± 2.8 | 395.8 ± 3.8 |
| B* | Stress group | 313.2 ± 2.4 | 318.0 ± 2.9 | 341.7 ± 3.5 | 360.3 ± 3.9 |
Data presented as means ± SEM.
p< 0.05 for stressed rats in conditions B and C compared to control rats A.
Experiment 2 – Influence of 35 and 75 mg/kg metyrapone and chronic stress on Y-maze performance: determination of free CORT and hippocampal MR/GR mRNA
Briefly, Experiment 2 expanded upon the findings from Experiment 1 by adding a 75 mg/kg dose of metyrapone (SM75), measuring CBG to determine free (unbound) CORT and determining hippocampal MR/GR mRNA.
A mixed factor anova on total entries made in the Y-maze for each minute was performed again in Experiment 2 to validate the choice of using minute one. The repeated effect of minute was highly signifi-cant, F4,168 = 41.72, P < 0.0001, following a 2 × 3 × 5 mixed factor anova (history × drug × minute). Posthoc tests indicated that rats made more entries in minute one compared to all other minutes (two, three, four, five), and validated the use of minute one in subsequent analyses. No other effects reached significance (P > 0.1).
The results show that metyrapone administered prior to training, attenuated chronic stress-induced spatial memory deficits on the Y-maze after a 4-h delay.
Entry
Wilcoxon nonparametric tests revealed that chronically stressed rats treated with vehicle (SV, n = 8) or 35 mg/kg of metyrapone (SM35, n = 8) entered the Novel and Other arms similarly (P > 0.42) whereas chronically stressed rats injected with 75 mg/kg of metyrapone (SM75 n = 8) entered the Novel arm more than the Other arm (P = 0.06). Moreover, all nonstressed controls (n = 8) entered the novel arm more than the other arm regardless of metyrapone treatment (P < 0.05; Fig. 4). As SV rats were treated the same in both experiments their data was combined and compared to the SM75 group with a one-tailed t-test. This analysis revealed that SM75 rats entered the novel arm more than SV rats (P < 0.05). Total entries made in the Y-maze were statistically similar among groups, 2 × 3 anova (stress history × drug, P > 0.1, Table 1), suggesting similar motivational/motor influences.
Fig 4.
Experiment 2 – Percentage of entries and time spent (Dwell) in all arms of the Y-maze. (A) Control rats and stressed rats given 75 mg/kg metyrapone prior to training displayed intact spatial memory by entering the novel arm more than the other arm. Stressed rats given vehicle or 35 mg/kg metyrapone showed no arm preference. (B) For time spent in the novel arm, the controls given vehicle (CV) and stressed rats treated with metyrapone (SM35) spent more time in the novel arm compared to the other arm. Note that the stress rats given vehicle (SV) consistently performed poorly in both the entry measure and dwell measure. (C–H) Raw data for the number of entries made into each arm across the full five minutes of Y-maze exploration for each group. In the first minute, the CV, CM35, CM75 and SM75 groups clearly prefer the novel arm by entering it more often than the other arm, while the SV and SM35 groups enter all arms similarly. CM35, control, 35 mg/kg metyrapone; CM75, control, 75 mg/kg metyrapone; SM35, stress, 35 mg/kg metyrapone; SM75, stress, 75 mg/kg metyrapone. *P < 0.05, √P = 0.06. Data represent means ± SEM.
Dwell
Rats in the CV and SM35 groups spent more time in the novel arm compared to the other arm (P < 0.01) while no other groups showed a significant preference (P > 0.16; Fig. 4). As observed in Experiment 1, the SV condition was the only group that consistently failed to enter and spend more time (dwell) in the novel arm compared to the other arm. The remaining groups (CV, CM35, CM75, SM35, SM75) showed a significant preference for the novel arm as determined by one or both indices of entry and dwell measures.
Physiological measures
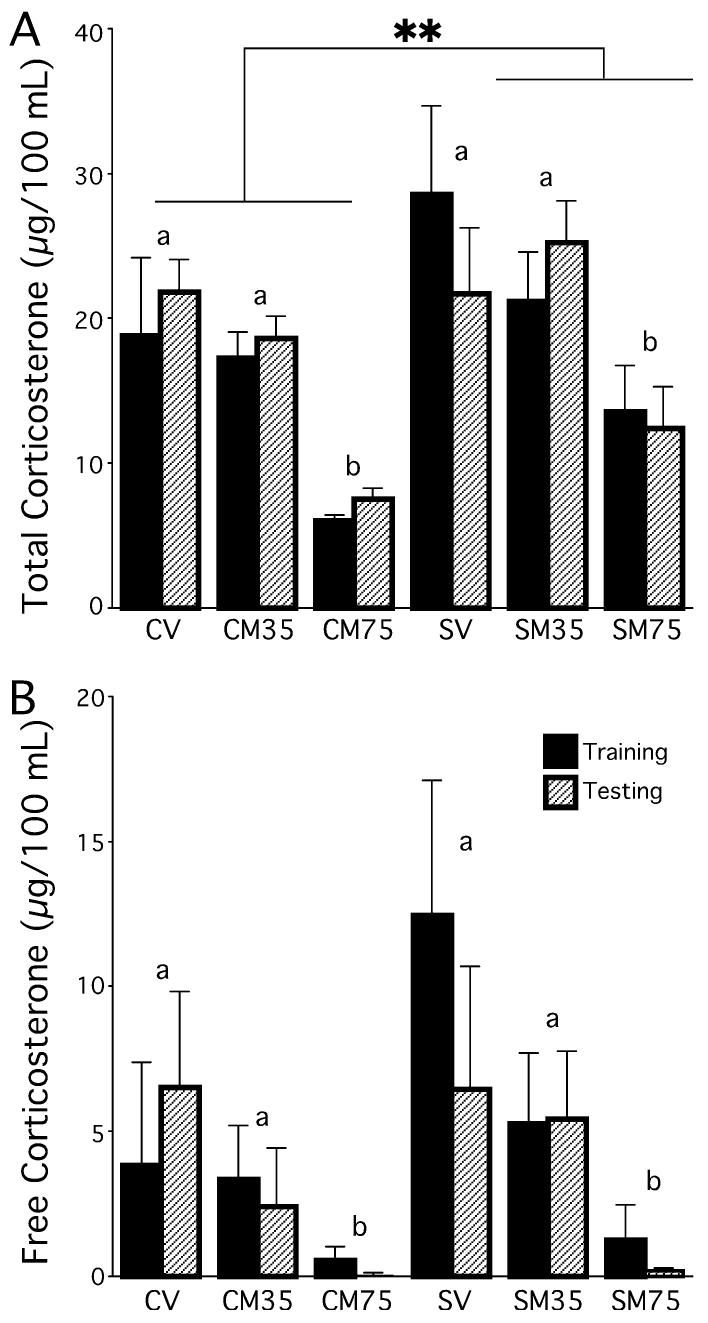
Chronically stressed rats released more total serum CORT than did controls following training and testing (significant main effect of group, F1,72 = 8.33, P < 0.01, following a 2 × 3 × 2 anova). Moreover, metyrapone reduced total CORT levels for both stress and control conditions (significant main effect of drug, F1,72 = 17.89, P < 0.001, Fig. 5A). No other main effects or interactions approached significance (P > 0.4).
Fig 5.
Experiment 2 – Total and free (unbound) serum CORT levels in response to Y-maze training and testing. (A) Chronically stressed rats released more total CORT than did controls. Metyrapone dose dependently reduced total CORT levels after training and testing in both stressed and control groups. (B) Rats given 75 mg/kg metyrapone had significantly lower CORT levels than rats given no metyrapone or 35 mg/kg. CV = control vehicle; CM35 = control, 35 mg/kg metyrapone; CM75 = control, 75 mg/kg metyrapone; SV = stress vehicle, SM35 = stress, 35 mg/kg metyrapone; SM75 = stress, 75 mg/kg metyrapone. Groups with the same letter are statistically similar, whereas groups with different letters are statistically significantly different. **P < 0.01. Data represent means ± SEM.
Metyrapone reduced free CORT levels in both stress and control conditions (significant main effect of drug, F1,72 = 6.520, P < 0.01, following a 2 × 2 × 2 anova, Fig. 5B). No other main effects or interactions approached significance (P > 0.12).
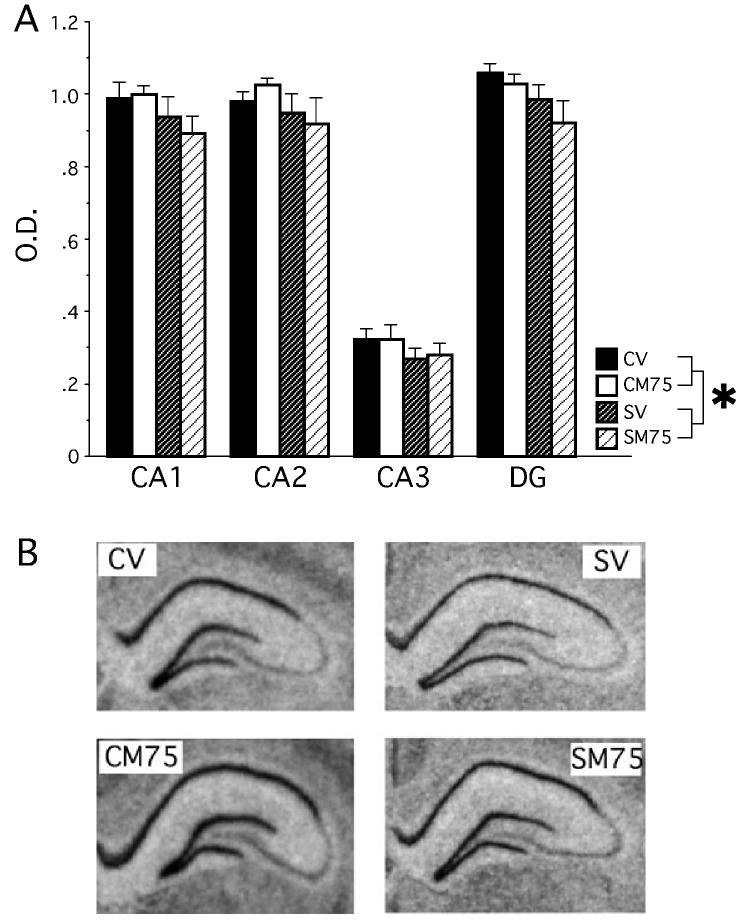
Chronic stress down-regulated the expression of GR mRNA within the dentate gyrus of hippocampus. The condition with the highest dose of metyrapone (M75) was used in these analyses because stressed rats given metyrapone (M75) showed improved Y-maze behaviour while stressed rats given metyrapone (M35) had inconsistent Y-maze behaviour. A 2 × 2 × 4 mixed factors anova for stress history, drug, and hippocampal region (dentate gyrus, CA1, CA2, CA3) revealed a significant main effect of stress history, F1,19 = 4.688, P < 0.05 with chronic stress down-regulating GR mRNA throughout the hippocampus (Fig. 6). A significant effect of region, F3,57 = 448, P < 0.0001, supports the finding that the CA3 region expresses less GR mRNA than the CA1, CA2 and dentate gyrus. All other main effects and interactions failed to reach significance (P > 0.55).
Fig 6.
Experiment 2 – GR mRNA expression within the hippocampus. (A) Chronic stress down-regulated the expression of GR mRNA throughout the hippocampus and this effect was predominately carried by the dentate gyrus and the CA1 regions. Optical density (OD) values represent the mean value of each region after background was subtracted. *P < 0.05 compared to control (CV and CM75). Data represent means ± SEM. (B) Coronal sections of the hippocampus after exposure to film for 16 days. Note the reduced staining in the chronic stress conditions (SV, SM75).
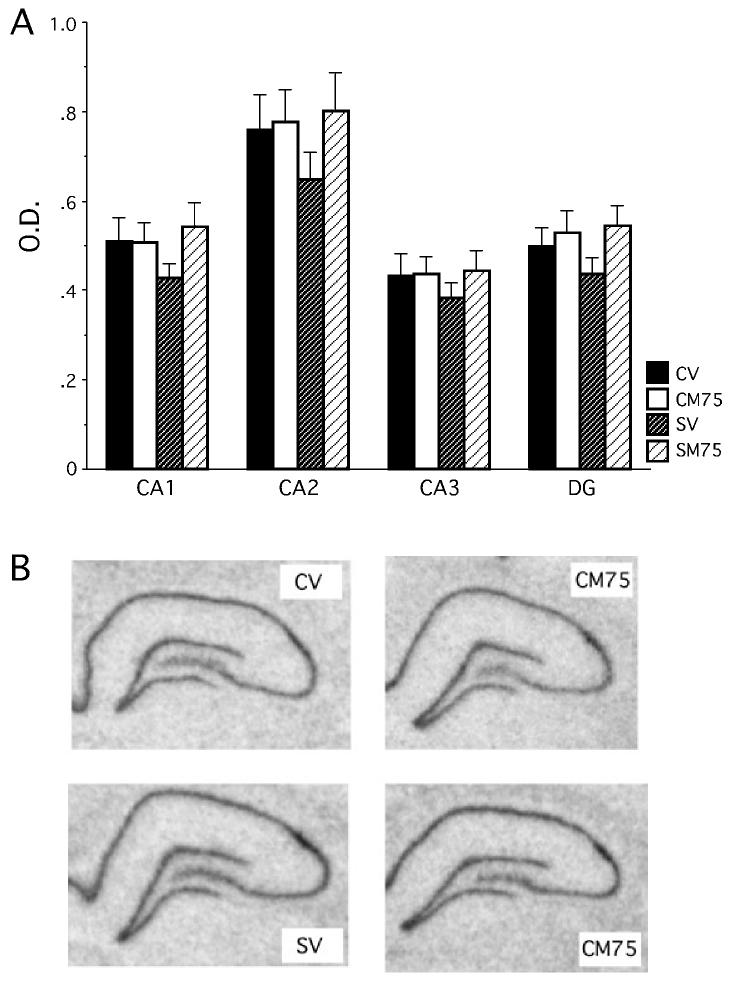
Unlike GR mRNA expression within the hippocampus, chronic stress and metyrapone treatment did not influence MR mRNA expression in any region of the hippocampus investigated (dentate gyrus, CA1, CA2, CA3) (Fig. 7). This was determined by a 2 × 2 × 4 a mixed-factor anova across all four regions (P > 0.2). The expression of MR mRNA did, however, vary depending upon the region with the CA2 region expressing the higher levels of MR mRNA than CA1, CA3, and the dentate gyrus, and with the dentate gyrus expressing more MR mRNA than the CA3 region.
Fig 7.
Experiment 2 – MR mRNA expression within the hippocampus. (A) Chronic stress and metyrapone failed to alter MR mRNA expression within the hippocampus. Optical density (OD) values represent the mean value of each region after background was subtracted. Data represent means ± SEM. (B) Coronal sections of the hippocampus after exposure to film for 8 days.
Chronically stressed rats gained weight more slowly than controls. A 2 × 4 mixed factors anova for treatment (control and stress) and day (1, 7, 14 and 21) revealed a significant main effect of stress, F1,82 = 47.74, P < 0.01, a significant main effect of day, F3,246 = 440.47, P < 0.01, and an interaction between stress and day, F3,246 = 33.73, P < 0.01 (Table 2).
Discussion
The current findings support the hypothesis that CORT elevations on the day of memory assessment play an important role in whether chronic stress impairs spatial memory. Both experiments showed that administering the CORT synthesis inhibitor, metyrapone prior to training on the Y-maze restored spatial memory in rats that were previously chronically stressed. Moreover, chronically stressed rats released more total CORT in response to the Y-maze procedure than did controls, and metyrapone dose-dependently attenuated CORT release in both groups. These data indicate an important mechanism whereby chronic stress may contribute to spatial memory impairments through alterations in CORT production and/or detection on the day of spatial memory determination. It is important to keep in mind, however, that metyrapone was given before training and could have been present throughout the procedure. Therefore, metyrapone could have influenced one or more stages of memory processing (acquisition, consolidation, memory). For the purposes of this paradigm, spatial memory refers to all memory processing stages.
While the general finding of these two experiments was that metyrapone prevents spatial memory deficits in chronically stressed rats, the results appeared to differ between the two studies. In Experiment 1, the 35 mg/kg dose of metyrapone was sufficient to restore spatial memory, but it failed to do so in Experiment 2. Total serum CORT levels from the 35 mg/kg group in Experiment 1 (15–18 μg/100 mL) more closely approximated the total serum CORT levels from the 75 mg/kg group in Experiment 2 (12–15 μg/100 mL) than the 35 mg/kg dose in Experiment 2 (21–25 μg/100 mL). Thus, 35 mg/kg in Experiment 1 and 75 mg/kg in Experiment 2 were more similar in their effect on total serum CORT levels and performance on the Y-maze than the 35 mg/kg dose in Experiment 2. These data also suggest that serum CORT levels may be varied following treatment with a 35 mg/kg dose; elevated CORT levels following a robust stressor may be more difficult to prevent with a 35 mg/kg dose than to a mild stressor. Overall, the general finding that reducing corticosterone prevents spatial memory deficits was found in both experiments.
Total entries on the Y-maze were measured to determine whether metyrapone or stress influenced motivation or motor abilities. Metyrapone may affect a rat's willingness to explore in the Y-maze because it reduces risk assessment in the elevated plus maze (Mikics et al., 2005), decreases immobility in a forced swim task (Rogoz et al., 2003), and prevents hyperactivity in the open field after hippocampal lesion (Ryan et al., 1985). Moreover, chronic stress may produce neophobia, which may hinder chronically stressed rats from seeking the novel arm. But, providing cues within the Y-maze allows chronically stressed rats to locate the novel arm, indicating that novelty-seeking remains intact in these animals (Wright & Conrad, 2005) Additionally, when the delay between training and testing is reduced to one minute to decrease task difficulty, chronically stressed rats will explore the novel arm (Bellani et al., 2006; Kleen et al., 2006). In these Y-maze paradigms, including the current study, the total number of entries (locomotion) did not predict spatial memory performance, indicating that altered motor or motivational factors did not confound the interpretation.
Total serum CORT levels measured throughout the process of chronic restraint failed to explain why metyrapone treatment prevented stress-induced spatial memory deficits. In Experiment 1, chronic restraint did not elevate baseline total CORT levels. While total CORT levels increased within 30-min from the start of restraint across all days measured, they returned to baseline on all but the first day of restraint. Moreover, hippocampal MR mRNA levels were unaltered by stress or metyrapone treatment. Some have indicated that MR occupation mediates the basal HPA axis tone (Canny, 1990; de Kloet et al., 1993; Bradbury et al., 1994) and the lack of chronic stress to influence hippocampal MR mRNA expression supports the finding that chronic stress did not alter basal CORT levels to influence spatial memory in this study. Therefore, these findings support the interpretation that chronic stress impairs spatial memory through mechanisms that are unlikely to involve basal CORT levels.
Next, the CORT response to the Y-maze procedure was examined to determine whether CORT elevations on the day of memory assessment impair spatial memory in chronically stressed rats. Chronic stress increased total CORT levels in response to the Y-maze procedure and down-regulated hippocampal GR mRNA expression, which may have functional consequences at the level of receptor capacity. GR decrease or changes in other brain areas may have been responsible for the enhanced corticosterone response to the Y-maze procedure because GR are proposed to mediate CORT release during the stress response and diurnal rhythms (Canny, 1990; De Kloet et al., 1993; Bradbury et al., 1994) and alterations in extrahippocampal brain areas have been shown to be involved in an enhanced corticosterone response to novel stressors after chronic stress (Dallman, 1993; Bhatnagar & Dallman, 1998). The reduction in GR mRNA and an enhanced corticosterone response to the Y-maze procedure in chronically stressed rats is consistent with the hypothesis that stress-induced CORT elevations on the day of memory assessment (as opposed to elevated basal CORT levels) impair spatial memory in chronically stressed rats. However, two findings may appear to contradict this interpretation and these are discussed in the next paragraphs. First, biologically active CORT levels were unaltered in chronically stressed rats relative to controls. Second, CV, CM35, SV and SM35 rats in Experiment 2 had statistically similar total CORT levels, yet the controls (CV, CM35) showed intact spatial memory while the stressed rats (SV, SM35) were impaired.
These findings can be held against several interpretations in how memory is influenced by metyrapone following chronic stress. First, mRNA GR down-regulation in chronically stressed rats may alter the well-described inverted U-shaped function between glucocorticoid levels and memory, whereby very high and very low levels impair memory, while basal to moderate levels produce optimal memory (reviewed in Conrad, 2005). This inverted U-shaped function can also be described in terms of the ratio of MR to GR occupation, with high ratios facilitating and low ratios impairing memory (de Kloet et al., 1999). Fewer GR may indicate that lower CORT levels are needed to occupy the majority of GR and reach the negative slope of the inverted U-shaped function to impair spatial memory (see Lupien et al., 2005). Hence, similar CORT levels in control and chronically stressed rats may bind proportionately different numbers of GR. A second interpretation is that metyrapone decreased CORT levels, which allowed MR-mediated processes involved with novel situations to be favoured over GR-mediated processes (Oitzl & de Kloet, 1992; Oitzl et al., 1994) to enhance novelty-seeking in the Y-maze. Yet, novelty-seeking remains intact in chronically stressed rats (Wright & Conrad, 2005; Bellani et al., 2006; Kleen et al. 2006). Third, GR mRNA down-regulation may create glucocorticoid insensitivity, which is proposed to disrupt memory (Nichols et al., 2001). However, a decrease in CORT levels would not be expected to reverse memory impairment because reduced CORT levels would be harder to detect by an insensitive system. Further, novel stressors facilitate the CORT response in rats that have been previously chronically stressed (Dallman, 1993; Bhatnagar & Dallman, 1998), and this CORT elevation may lead to greater GR activation.
Finally, acute and chronic stress influence neurotransmitters, neurotrophins, and hormones within the HPA axis (corticotrophin-releasing hormone, adrenocorticotrophic hormone, see Diamond et al., 2005) and metyrapone may have restored spatial memory in chronically stressed rats through changes in these hormones. For instance, metyrapone reduces CORT secretion by inhibiting 11β-hydroxylase, which enhances progesterone and deoxycorticosterone accumulation. These hormones can alter GABA receptor function (Lambert et al., 1995), block voltage dependent Ca2+ channels (French-Mullen & Spence, 1991) and decrease hippocampal long-term potentiation (Dubrovsky et al., 1993). While metyrapone administration may affect learning and memory by increasing progesterone and deoxycorticosterone, it seems most likely that these hormones would impair memory, which contrasts to the memory facilitation shown in chronically stressed rats in the present research. In addition, metyrapone failed to enhance memory at either dose in control rats. Therefore, we favour the interpretation that spatial memory recovery may result from CORT attenuation at the time of training and/or testing through its effects in the context of changes brought about by the chronic stress procedure. Future experiments will investigate CORT replacement and spatial ability.
Substantial methodological differences existed between the experiment by Roozendaal et al. (2001) and the present research, but both showed similar outcomes. Roozendaal et al. (2001) performed selective CA3 lesions whereas we used chronic stress, which produces hippocampal dendritic retraction (Watanabe et al., 1992b; Magariños & McEwen, 1995a, b; Galea et al., 1997; Conrad et al., 1999). This difference is important because rats with hippocampal lesions show increased basal CORT levels (Fischette et al., 1980; Wilson et al., 1980), and the impairment reported by Roozendaal et al. (2001) may have been caused by increased basal CORT levels. In contrast, the present study did not observe elevated basal CORT levels. Additionally, Roozendaal et al. (2001) trained rats for 3 days and then administered metyrapone on day four when assessing memory retrieval on the water maze. In our paradigm, training and testing occurred within hours, so metyrapone administration occurred before the first Y-maze exposure to ensure that CORT levels were attenuated throughout training and testing. In spite of these significant differences, both studies support the hypothesis that attenuating CORT elevations on the day of spatial memory assessment may prevent spatial memory impairments by hippocampal lesion or chronic stress.
The present data shed light on the paradoxical findings between chronic stress and CORT treatment and their effects on spatial ability. Chronic stress produces hippocampal dendritic retraction and concurrent spatial memory deficits (Luine et al., 1994; Luine et al., 1996; Conrad et al., 1999). In contrast, chronic CORT treatment produces dendritic retraction (Watanabe et al., 1992a; Magariños et al., 1998), without altering spatial memory (Coburn-Litvak et al., 2003). The current hypothesis generates a plausible explanation for this difference. Chronic stress and the subsequent CORT hypersecretion increase adrenal weight (Watanabe et al., 1992b), which can release additional CORT in response to a stressor. However, chronic exogenous CORT shuts down the HPA axis through negative feedback and reduces adrenal size (Magariños et al., 1998). While both conditions produce hippocampal dendritic retraction, only chronic stress may permit an enhanced CORT response to a unique stressor or situation (compare CORT response following chronic stress, Bhatnagar & Dallman, 1998, with that following chronic CORT treatment, Johnson, 2006). Moreover, our data reveal that when biologically active CORT levels are similar between chronically stressed and control rats, spatial memory may still differ because chronic stress down-regulates hippocampal GR levels and produces CA3 dendritic retraction. Thus, the CORT response combined with a compromised hippocampus in chronically stressed rats renders them susceptible to spatial memory impairments (reviewed in Conrad, 2006).
In summary, we present novel findings that chronic stress impairs spatial memory through changes in the HPA axis and that attenuating CORT levels can restore spatial memory. These findings are consistent with the recent hypothesis presented by Roozendaal et al. (2001); Roozendaal (2002) that elevated CORT levels at the time of memory assessment may mediate spatial memory impairment in rats with a compromised hippocampus. Our data, however, indicate that increased CORT secretion as a result of exposure to behavioural tasks may mediate this effect. These data have important ramifications for our understanding of the mechanism by which chronic stress induces spatial memory impairments and may lead to treatments to alleviate cognitive decline in conditions associated with elevated glucocorticoids such as depression, Cushing's disease, Alzheimer's disease, and a subpopulation of aged individuals.
Acknowledgements
This work was funded by MH64727 (Conrad) and the Howard Hughes Medical institute through the Undergraduate Biology Enrichment Program (Harman & Lightner). The contributions of the following individuals are greatly appreciated: Sarah Baran, Rudy Bellani, Cainan Foltz, Jonathan Kleen, Servane Lachize, Katie McLaughlin, Joe Nguyen, Serge Tsekhanov, and Lindsay Wieczorek. A special thank you to E. R. de Kloet.
Abbreviations
- CBG
CORT binding globulin
- CORT
corticosterone
- CV
control + vehicle
- CM
control + metyrapone
- GR
glucocorticoid receptor
- HPA
hypothalamus-pituitary-adrenal
- MR
mineralocorticoid receptor
- SV
stress + vehicle
- SM
stress + metyrapone
References
- Barsano CP, Baumann G. Simple algebraic and graphic methods for the apportionment of hormone (and receptor) into bound and free fractions in binding equilibria; or how to calculate bound and free hormone? Endocrinology. 1989;124:1101–1106. doi: 10.1210/endo-124-3-1101. [DOI] [PubMed] [Google Scholar]
- Bellani R, Luecken LJ, Conrad CD. Peripubertal anxiety profile can predict spatial memory impairments following chronic stress. Behav. Brain Res. 2006;166:263–270. doi: 10.1016/j.bbr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bradbury MJ, Akana SF, Dallman MF. Roles of type I and II corticosteroid receptors in regulation of basal activity in the hypothalamo-pituitary-adrenal axis during the diurnal trough and the peak: evidence for a nonadditive effect of combined receptor occupation. Endocrinology. 1994;134:1286–1296. doi: 10.1210/endo.134.3.8119168. [DOI] [PubMed] [Google Scholar]
- Canny BJ. Hippocampal glucocorticoid receptors and the regulation of ACTH secretion. Mol. Cell. Endocrinol. 1990;71:C35–C38. doi: 10.1016/0303-7207(90)90067-i. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol. Learn. Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Conrad CD. The relationship between acute glucocorticoid levels and hippocampal function depends upon task aversiveness and memory processing stage. Nonlinear. Biol., Toxicol. Med. 2005;3:57–78. doi: 10.2201/nonlin.003.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav. Cognit. Neurosci. Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y-Maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol. Learn. Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Dallman M. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol. Metab. 1993;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: Study in young and aged rats. Brain Res. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- Deviche P, Breuner C, Orchinik M. Testosterone, corticosterone, and photoperiod interact to regulate plasma levels of binding globulin and free steroid hormone in dark-eyed juncos. Junco hyemalis. Gen. Comp. Endocrinol. 2001;122:67–77. doi: 10.1006/gcen.2001.7613. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Campbell AM, Woodson JC. Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia. Hippocampus. 2005;15:1006–1025. doi: 10.1002/hipo.20107. [DOI] [PubMed] [Google Scholar]
- Dubrovsky B, Gijsbers K, Filipini D, Birmingham MK. Effects of adrenocortical steroids on long-term potentiation in the limbic system: basic mechanisms and behavioral consequences. Cell. Mol. Neurobiol. 1993;13:399–414. doi: 10.1007/BF00711580. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioural data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fischette CT, Komisaruk BR, Edinger HM, Feder HH, Siegel A. Differential fornix ablations and the circadian rhythmicity of adrenal corticosteroid secretion. Brain Res. 1980;195:373–387. doi: 10.1016/0006-8993(80)90073-6. [DOI] [PubMed] [Google Scholar]
- French-Mullen JM, Spence KT. Neurosteroids block Ca2+ channel current in freshly isolated hippocampal CA1 neurons. Eur. J. Pharmacol. 1991;202:269–272. doi: 10.1016/0014-2999(91)90303-8. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Johnson SA. Effect of different doses of corticosterone on depression-like behavior and HPA axis response to a novel stressor. Behav. Brain Res. 2006;168:280–288. doi: 10.1016/j.bbr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Kleen J, Sitomer M, Killeen P, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav. Neurosci. 2006 doi: 10.1037/0735-7044.120.4.842. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Functional implications of brain corticosteroid receptor diversity. Cell. Mol. Neurobiol. 1993;13:433–455. doi: 10.1007/BF00711582. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: Are corticosteroids good or bad guys? TINS. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol. Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lathe R. Hormones and hippocampus. J. Endocrinol. 2001;169:205–231. doi: 10.1677/joe.0.1690205. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol. Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Buss C, Schramek TE, Maheu F, Pruessner J. The good effects of a bad thing: Hormetic influence of glucocorticoids on human memory. Nonlinear Biol. Toxicol. Med. 2005;3:23–56. doi: 10.2201/nonlin.003.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison stressors. Neuroscience. 1995a;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995b;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Meijer OC, de Kloet ER. Corticosterone suppresses the expression of 5-HT1A receptor mRNA in rat dentate gyrus. Eur. J. Pharmacol. 1994;266:255–261. doi: 10.1016/0922-4106(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Mikics E, Barsy B, Barsvari B, Haller J. Behavioral specificity of non-genomic glucocorticoid effects in rats: effects on risk assessment in the elevated plus-maze and the open-field. Horm. Behav. 2005;48:152–162. doi: 10.1016/j.yhbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nichols NR, Zieba M, Bye N. Do glucocorticoids contribute to brain aging? Brain Res. Brain Res. Rev. 2001;37:273–286. doi: 10.1016/s0165-0173(01)00131-x. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav. Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, de Kloet ER. The effect of corticosterone on reactivity to spatial novelty is mediated by central mineralocorticosteroid receptors. Eur. J. Neurosci. 1994;6:1072–1079. doi: 10.1111/j.1460-9568.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Rogoz Z, Skuza G, Wojcikowski J, Daniel WA. Effects of combined treatment with imipramine and metyrapone in the forced swimming test in rats. Behavioral and pharmacokinetic studies. Pol. J. Pharmacol. 2003;55:993–999. [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Phillips RG, Power AE, Brooke SM, Sapolsky RM, McGaugh JL. Memory retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nature Neurosci. 2001;4:1169–1171. doi: 10.1038/nn766. [DOI] [PubMed] [Google Scholar]
- Ryan JP, Springer JE, Hannigan JH, Jr, Isaacson RL. Suppression of corticosterone synthesis alters the behavior of hippocampally lesioned rats. Behav. Neural. Biol. 1985;44:47–59. doi: 10.1016/s0163-1047(85)91166-5. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992b;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wilson M, Greer M, Roberts L. Hippocampal inhibition of pituitary-adrenocortical function in female rats. Brain Res. 1980;197:433–441. doi: 10.1016/0006-8993(80)91128-2. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]