Abstract
Bovine respiratory syncytial virus (BRSV) escapes from cellular responses to alpha/beta interferon (IFN-α/β) by a concerted action of the two viral nonstructural proteins, NS1 and NS2. Here we show that the NS proteins of human RSV (HRSV) are also able to counteract IFN responses and that they have the capacity to protect replication of an unrelated rhabdovirus. Even combinations of BRSV and HRSV NS proteins showed a protective activity, suggesting common mechanisms and cellular targets of HRSV and BRSV NS proteins. Although able to cooperate, NS proteins from BRSV and HRSV showed differential protection capacity in cells from different hosts. A chimeric BRSV with HRSV NS genes (BRSV h1/2) was severely attenuated in bovine IFN competent MDBK and Klu cells, whereas it replicated like BRSV in IFN-incompetent Vero cells or in IFN-competent human HEp-2 cells. After challenge with exogenous IFN-α, BRSV h1/2 was better protected than wild-type BRSV in human HEp-2 cells. In contrast, in cells of bovine origin, BRSV h1/2 was much less resistant to exogenous IFN than wild-type BRSV. These data demonstrate that RSV NS1 and NS2 proteins are major determinants of host range. The differential IFN escape capacity of RSV NS proteins in cells from different hosts provides a basis for rational development of attenuated live RSV vaccines.
Interferons (IFNs) are involved in mounting both innate and adaptive host immune responses. Genes encoding IFN-α and IFN-β are induced by virus infection or double-stranded RNA in many cell types, whereas IFN-γ expression is restricted to activated T cells and natural killer cells. IFNs bind to independent cell surface receptors and activate distinct but related signal transduction pathways, culminating in the activation of an overlapping set of IFN-stimulated genes (ISGs) (23). A variety of ISGs code for enzymes with antiviral function, such as PKR, 2′-5′ oligoadenylate synthetase, and Mx proteins. In addition, IFNs can inhibit cell growth and promote apoptosis, thereby restricting virus spread (19).
To counteract IFN, viruses have evolved mechanisms that interfere with IFN induction or signaling or directly with the function of antiviral IFN-induced proteins. The speed and efficiency by which a virus circumvents IFN induction or IFN response are critical determinants for its ability to establish an infection, for its pathogenicity and host range as well as for its resistance to IFN treatment.
Most members of the Paramyxoviridae family are able to affect IFN signaling using different mechanisms. Human parainfluenza virus 2 (hPIV2; genus Respirovirus) inhibits IFN signaling by degradation of STAT2 (40) whereas Sendai virus and simian virus 5 (SV5) (genera Respirovirus and Rubulavirus, respectively) do so by blocking STAT1 activation (16, 24) or by promoting STAT1 degradation (8, 9, 15). A rather distinctive feature seems to apply to respiratory syncytial virus (RSV) of the Pneumovirus genus, as this virus, without affecting IFN signaling, was found to be highly resistant to the cellular response induced by exogenously added IFN (40).
Human RSV (HRSV), the prototype of the Pneumovirus genus within the Pneumovirinae subfamily, is among the most important respiratory pathogens (5). Worldwide, HRSV is the most common cause of bronchiolitis-associated hospitalizations in infants (18, 25). HRSV is also a significant cause of excess morbidity and mortality in adult patients with compromised immune status and chronic inflammatory lung disease and in the elderly (10, 12, 13, 37). Since an effective vaccine for HRSV is not yet available, various alternative treatments such as the application of IFN-α/β are being actively pursued.
Sung et al. (32) reported that treatment of RSV-infected children with IFN did improve their clinical course. However, in clinical trials involving adult volunteers, recombinant IFN-2α did not prove to be effective as a therapeutic agent against RSV infection (21). The latter is supported by in vitro studies with both laboratory strains (1, 40) and clinical isolates of HRSV (unpublished results), which revealed a high intrinsic resistance of HRSV towards exogenously added recombinant IFN-α/β in cell cultures. This applies also to the bovine counterpart of HRSV, BRSV, which causes severe losses in stock breeding (31, 35).
We have recently identified the BRSV nonstructural proteins NS1 and NS2 as the factors mediating the resistance of BRSV to IFN-induced cell response (29). The presence of two NS genes is a characteristic of all members of the Pneumovirus genus. Both are located at the 3′ end of the negative-strand RNA genome (3′-NS1-NS2-N-P-M-SH-G-F-M2.1/M2.2-L-5′) and do not have counterparts in other Paramyxoviridae genera (6, 28). The abundantly expressed HRSV NS proteins (11, 36) are nonessential for virus replication in vitro (3, 33). However, the deletion of either NS gene severely attenuates HRSV in vivo (22, 33, 34, 38), indicating important defects in virus-host interplay. The close relationship of HRSV and BRSV NS proteins, with 69 and 84% identity for NS1 and NS2, respectively, further suggests that HRSV proteins may also function in IFN escape.
Here, we addressed the question of whether the HRSV NS proteins also possess IFN antagonist activity and whether this activity would be comparable to that of BRSV NS proteins. By exploiting a heterologous expression system based on recombinant rabies viruses (RVs), we could confirm that HRSV NS proteins are able to mediate IFN resistance. In a recombinant BRSV in which the NS genes were replaced with HRSV NS genes, the exchanged genes could fully substitute for BRSV NS in IFN-negative cell systems. However, in IFN-competent cells, host-specific differences became apparent. In particular, HRSV NS genes were much less protective in bovine cells, whereas in human cells they were more efficient than BRSV NS proteins. This indicates adaptation of RSVs to counteract host-specific IFN responses and provides a rationale for the design of attenuated live vaccines.
MATERIALS AND METHODS
Cells and virus.
Recombinant BRSV (rBRSV) was derived from BRSV strain A51908 (American Type Culture Collection) (27) variant Atue51908 (GenBank accession no. AF092942) and grown in Vero cells as described previously (3). For preparation of virus stocks, 80% confluent Vero cell monolayers were infected at a multiplicity of infection (MOI) of 0.1 in serum-free Dulbecco's modified Eagle's medium (DMEM). After 1 h of adsorption, the inoculum was removed, and the cells were incubated at 37°C in DMEM supplemented with 2.5% fetal calf serum (FCS) in a 5% CO2 atmosphere until extensive cytopathic effect (CPE) was observed. Virus was released by freezing and thawing.
Virus titers were determined on Vero cells by limiting dilution in microwell plates and counting of infected cell foci after indirect staining with an antibody recognizing the fusion protein. Stocks of BRSV h1/2 were prepared accordingly. For preparation of RV stocks, BSR cells were infected at an MOI of 1, and supernatants were harvested 3 days postinfection. RV titers were determined by limiting dilution and counting of infected cell foci after immunostaining with a fluorescein isothiocyanate conjugate (Centocor) recognizing RV N protein.
Construction of BRSV h1/2 and rRVs.
For the construction of BRSV h1/2, cDNAs of both HRSV NS genes were obtained by reverse transcription (RT)-PCR using total RNA isolated from HRSV (Long)-infected Vero cells as the template with the following primers for first-strand synthesis: hNS1NcoI (5′-ATTGACCATGGGCAGCAATTCATT-3′) for HRSV NS1 and hNS2NcoI (5′-ATTGACCATGGACACAACCCACA-3′) for HRSV NS2.
To replace the BRSV NS genes with their HRSV counterparts, a plasmid carrying nucleotides (nt) 1 to 957 of full-length BRSV cDNA was used. The NS1 gene was removed by restriction digestion with NotI and HpaI and a PCR fragment generated with primers hNS1NotI (5′-TATGAAGCGGCCGCCCCCTCTCTTCTTTCTACAGAAAATGGGCAGCAATTCATTGAG-3′) and hNS1EcoRI (5′-ATTGAGAATTCTTATGGATTAAGATCAAA-3′), representing the entire coding region of HRSV NS1, was inserted. In a subsequent step, the NS2 gene was removed in a restriction digestion using AseI and Acc65I, followed by the insertion of a PCR fragment generated with primers hNS2AseI (5′-ATACTTATTAATTGGGGCAAATAAATCAGTTCCCCAACCAGCCATGGACACAACCCACAATG-3′) and hNS2KpnI (5′-ATAAATGGTACCAAAAGATAACACTGTGTGAATTAAATTTTGAAAAGTGCTTATGGATTGAGATCATACTTG-3′), harboring the entire coding region of HRSV NS2. After digestion of the plasmid with NotI and Acc65I, the resulting fragment containing both HRSV NS genes was inserted into full-length BRSV cDNA, resulting in rBRSV h1/2 (see Fig. 3).
FIG. 3.
Construction of recombinant BRSVs. The locations of the protein-encoding frames (shaded bars) are shown relative to the viral genome (vRNA, black bar). In the enlargement, the organization of wild-type BRSV and the recombinant BRSV carrying the HRSV nonstructural proteins, rBRSV h1/2, is depicted. Leader RNA is marked by diagonal hatching, and the relative positions of the corresponding nucleotides and restriction sites used for cloning are indicated.
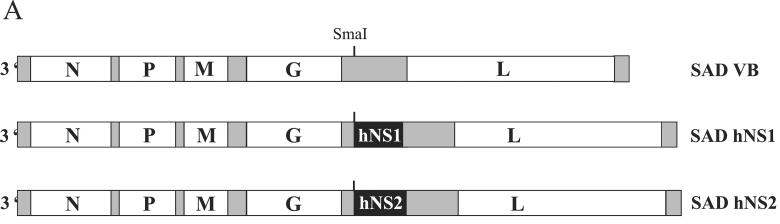
rRVs carrying the HRSV NS1 or NS2 gene (Fig. 1A) were constructed on the basis of a full-length RV cDNA (SAD L16) (30) containing an extra transcriptional stop-restart sequence in the 3′ noncoding sequence of the G gene (SAD VB) (26). The PCR fragments obtained by RT-PCR were digested with NotI and EcoRI in the case of NS1 or digested with AseI and Acc65I in the case of NS2, followed by filling in with Klenow polymerase. The resulting fragments were inserted into a unique SmaI site in pSAD VB immediately downstream of the additional transcription start signal, leading to SAD hNS1 and SAD hNS2, respectively.
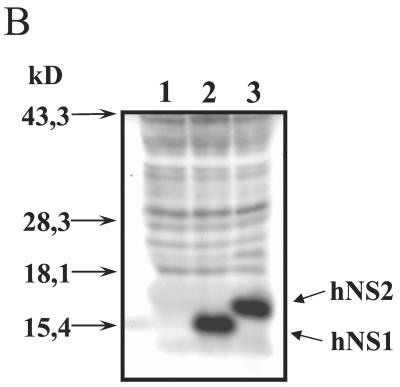
FIG. 1.
(A) Organization of rRV containing HRSV (Long) NS1 or NS2 open reading frames between the RV G and L genes. (B) Western blot analysis of HRSV NS1 and HRSV NS2 expression in infected BSR cells. NS1 and NS2 proteins were detected using a serum raised against a C-terminal peptide of NS1 that cross-reacts with NS2 (kindly provided by J. A. Melero, Madrid, Spain). The two bands representing the HRSV nonstructural proteins are indicated. Lane 1, mock infection; lane 2, SAD hNS1; lane 3, SAD hNS2.
Recovery of BRSVs and rRVs.
rBRSV h1/2 was rescued as described previously (3) using T7 promoter-controlled plasmids. For BRSV, plasmids encoding BRSV proteins N and P (pTITB-N and pTITB-P, respectively; 4 μg of each) and L and M2 (pTITB-L and pTITB-M2, respectively; 2 μg of each) were cotransfected with the respective virus cDNA (10 μg) into approximately 106 BSR T7/5 cells stably expressing phage T7 RNA polymerase (14) (Mammalian Transfection Kit; Stratagene). For RV, plasmids encoding RV proteins N (pTIT-N, 5 μg) and P and L (pTIT-P and pTIT-L, respectively; 2.5 μg) were cotransfected with 10 μg of the respective virus cDNA. The transfection medium was removed after 4 h, and the cells were further incubated in BHK-21 medium (Gibco) containing 5% FCS for BRSV and 10% calf serum for RV. Cells transfected with BRSV cDNA were split every 4 days at a ratio of 1:3 until CPE was detectable. For RV recovery (30), cell culture supernatants were harvested 5 days posttransfection and transferred onto fresh BSR cells. Infectious RV was detected by immunostaining with Centocor.
Western blot analysis.
To monitor the expression of HRSV NS1 and NS2, BSR cells were mock infected or infected with SAD hNS1 or SAD hNS2 at an MOI of 1. Three days postinfection, cells were lysed with lysis buffer (6.25 mM Tris [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 6 M urea, 5% methanol, 0.01% bromophenol blue, 0.01% phenol red), and equivalent amounts of cell extracts were loaded onto a 12% gel for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell) using a semidry transfer apparatus (OWL Scientific). After incubation with blocking solution (5% dry milk and 0.05% Tween 20 in phosphate-buffered saline [PBS-T]) at room temperature for 1 h, membranes were incubated overnight with peptide rabbit antisera raised against a C-terminal peptide of NS1 that cross-reacts with NS2 (α-IC/C; 1:5,000 in PBS-T; kindly provided by J. A. Melero, Madrid, Spain). The blot was then incubated for 2 h with peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG; 1:10,000 in PBS-T; Dianova), and proteins were visualized by chemifluorescence (Renaissance; NEN).
Infection experiments and treatment with IFN.
To determine the growth characteristics of BRSV h1/2, Vero, MDBK, HEp-2, and Klu cells were infected in suspension with either wild-type rBRSV or rBRSV h1/2 at an MOI of 0.1 and incubated in 12-well dishes in DMEM containing 2.5% FCS. Virus was harvested at 1, 2, 3, and 4 days postinfection by two cycles of freezing and thawing, and virus titers were determined on Vero cells by limiting dilution and counting of infected cell foci as described above. To measure the effect of IFN-α/β on viral replication, cells were infected at an MOI of 0.1 as described above, and recombinant IFN-α A/D (PBL Biomedical Laboratories) was added to concentrations of up to 10,000 IU/ml directly after seeding. Virus titers were determined 3 days postinfection as described above.
Infections of MDBK cells with rRVs SAD VB, SAD hNS1, and SAD hNS2 were done in suspension at an MOI of 5. For coinfections with SAD hNS1 and SAD hNS2, an MOI of 2.5 was used for each recombinant. Recombinant IFN-α A/D was added to concentrations of up to 500 U/ml immediately after seeding. Cell supernatants were harvested 2 days postinfection, and virus titers were determined by limiting dilution and immunostaining with a fluorescein isothiocyanate conjugate against RV N protein (Centocor).
RESULTS
HRSV NS proteins protect RV from IFN-induced responses.
To assess the IFN-antagonistic activity of HRSV NS proteins, we first used an assay based on expression of HRSV NS cDNA from rRVs to monitor replication of the rRVs in the absence or presence of IFN (29). RVs expressing either HRSV NS1 (SAD hNS1) or NS2 cDNA (SAD hNS2 ) were generated by insertion of the additional gene between the G and the L genes of the infectious SAD L16 full-length antigenomic cDNA clone (7, 26) (Fig. 1A). Efficient expression of either HRSV NS protein was confirmed by Western blot analysis using NS-specific peptide antisera (Fig. 1B). No adverse influence on viral replication, growth kinetics, or infectious titers of the recombinant RVs was observed in BSR cells (data not shown).
For demonstration of IFN-antagonistic activity of the expressed HRSV proteins, infection experiments were performed with MDBK cells, as replication of RV in these cells is highly sensitive to exogenous IFN. Cells were infected with parental RV (SAD VB) or with each recombinant at an MOI of 5 or coinfected with both recombinants SAD hNS1 and SAD hNS2 at an MOI of 2.5 each. The infected cultures were then treated with increasing amounts of recombinant IFN-α, and the production of infectious RV was analyzed 2 days postinfection. Upon addition of 50 IU of IFN-α, infectious titers of wild-type RV and of NS-expressing viruses from single infections dropped by 3 log units, confirming a highly effective IFN-stimulated antiviral response in MDBK cells (Fig. 2A). However, in cells coexpressing hNS1 and hNS2, RV replication was protected in the presence of up to 150 IU of IFN-α. A comparable degree of protection was observed in MDBK cells which were coinfected with the previously described rRVs expressing BRSV NS proteins (SAD bNS1 and SAD bNS2) as a positive control. Thus, HRSV NS proteins are also able to antagonize IFN-mediated antiviral responses and do so only when expressed together.
FIG. 2.
IFN resistance of RV in cells coinfected with RVs expressing HRSV NS1 or NS2. (A) Infections of MDBK cells with wild-type SAD VB (VB wild type), SAD hNS1 (H1), or SAD hNS2 (H2) or coinfections with SAD bNS1 (B1) and SAD bNS2 (B2) or SAD hNS1 and SAD hNS2. Indicated concentrations of recombinant IFN-α A/D were added immediately after seeding. Virus titers were determined 2 days postinfection. Results represent the mean values of three independent experiment, with error bars indicating standard deviation. (B) Infection of MDBK cells using various combinations of RVs expressing BRSV or HRSV nonstructural proteins.
HRSV and BRSV NS proteins are able to cooperate.
The similar behavior of the BRSV and HRSV NS proteins prompted us to investigate further whether combinations of BRSV and HRSV NS proteins are functional in mediating IFN resistance. MDBK cells were infected with two pairs of recombinants, SAD bNS1 and SAD hNS2 or SAD hNS1 and SAD bNS2, and treated with IFN as described above. Indeed, both pairs were able to increase the IFN resistance of RV (Fig. 2B). While coexpression of BRSV NS1 and HRSV NS2 was as effective as the two homologous combinations, the inverse combination of HRSV NS1 and BRSV NS2 did not rescue viral growth in the presence of more than 50 U of IFN-α, as observed in several independent experiments. Nevertheless, the cooperativity of HRSV and BRSV NS proteins in IFN escape strongly suggests that they have identical functions and similar target proteins.
Chimeric BRSV expressing HRSV NS genes.
To investigate HRSV NS protein function in the context of a pneumovirus infection, we generated a recombinant BRSV possessing both NS genes from HRSV instead of its own NS genes (rBRSV h1/2; Fig. 3). cDNAs of the HRSV NS genes were obtained by RT-PCR from Vero cells infected with HRSV strain Long and were used to replace BRSV NS genes of an infectious full-length antigenomic cDNA clone, prBRSV, as described in Materials and Methods. Recombinant virus was recovered by cotransfection of BRSV full-length cDNA with support plasmids encoding BRSV N, P, L, and M2 proteins into BSR T7/5 cells as described previously (3). Virus stocks were produced in Vero cells, as these lack an intact IFN-α/β system.
Growth of chimeric BRSV h1/2 is attenuated only in bovine cells.
The growth behavior of rBRSV h1/2 in Vero cells was indistinguishable from that of wild-type BRSV. Both viruses produced infectious titers of 5 × 105 PFU 2 days after infection at an MOI of 0.1 (Fig. 4A). The identical growth in Vero cells suggests that the HRSV NS proteins are able to fulfill the function(s) of their bovine counterparts which is important for efficient RNA synthesis. We then used a cell line of bovine origin, MDBK, which has an intact IFN system. In this cell line, growth of BRSV h1/2 was markedly attenuated. Whereas wild-type BRSV reached titers of 106 PFU after 3 days, BRSV h1/2 grew only up to 4 × 104 PFU (Fig. 4C). In another bovine cell line isolated from embryonic calf lung tissue, Klu, the attenuation of BRSV h1/2 was even more pronounced. Here, wild-type BRSV grew to highest titers of 1.5 × 106 PFU 2 days postinfection, whereas the chimeric BRSV h1/2 was barely able to grow, reaching titers of only 3 × 102 PFU (Fig. 4D).
FIG. 4.
Growth of BRSV h1/2 is attenuated in bovine cells. Vero (A), HEp-2 (B), MDBK (C), and Klu (D) cells were infected in suspension with either rBRSV wild type (▪) or rBRSV h1/2 (□) at an MOI of 0.1. Virus was harvested at the indicated time points, and virus titers were determined by limiting dilution. Bars show standard deviation for at least two independent experiments.
In contrast, in HEp-2 cells, a human cell line harboring an intact IFN system, the growth characteristics of wild-type BRSV and BRSV h1/2 were again comparable (Fig. 4B). Both viruses reached similar titers of 3 × 104 PFU after 3 days. This indicated that in the context of an IFN-competent human cell system, the HRSV NS proteins may be at least an adequate substitute for their counterparts in BRSV, whereas in bovine cell IFN systems, they are obviously less efficient.
Host cell-dependent IFN-antagonistic activity of RSV NS proteins.
Additional stimulation of infected cells with increasing doses of exogenous recombinant IFN-α was then used to directly challenge the protective capacity to cellular IFN-induced responses in cell lines of different origin. The African green monkey cell line Vero cannot produce IFN-α/β, yet it is able to mount an effective antiviral response when exogenous IFN is added (10, 29). In Vero cells infected with BRSV or BRSV h1/2, the replication of both viruses was largely resistant to high IFN doses (Fig. 5A). Only after application of more than 5,000 IU of IFN-α was an approximately 10-fold reduction in infectious titers observed at 3 days postinfection.
FIG. 5.
IFN resistance of BRSV h1/2 is cell type dependent. Vero (A), MDBK (B), or HEp-2 (C) cells were infected at an MOI of 0.1 with the indicated viruses. Recombinant IFN-α A/D was added to concentrations up to 10,000 U/ml directly after seeding. Virus titers were determined 3 days postinfection. Bars show standard deviation for at least two independent experiments.
Notably, the chimeric virus expressing the HRSV NS proteins appeared slightly more resistant than wild-type BRSV in this cell line (Fig. 5A), although its growth in the absence of IFN was at most equal to that of wild-type BRSV. In the bovine MDBK cells, the resistance of BRSV wild-type to IFN-α was even more pronounced than in Vero cells. After application of 10,000 IU of IFN-α, virus titers were only fivefold lower than in the untreated control. In striking contrast, BRSV h1/2 showed a marked and dose-dependent sensitivity towards the IFN-induced antiviral response of MDBK cells, with 5,000 U of IFN-α reducing viral titers about 10-fold and 10,000 U causing a 100-fold reduction (Fig. 5B). Thus, while the IFN resistance of BRSV h1/2 in Vero cells is comparable to that of wild-type BRSV, BRSV h1/2 is not able to thoroughly counteract the IFN-induced antiviral response in cells of bovine origin.
On the contrary, in the human HEp-2 cell line, which is derived from a human nasopharyngeal carcinoma, BRSV h1/2 was more effective in counteracting the IFN-induced antiviral state than wild-type BRSV. Although in untreated HEp-2 cells growth of the viruses was similar, BRSV h1/2 was superior to wild-type BRSV in coping with the effects of exogenous IFN (Fig. 5C). The addition of 10,000 U of IFN-α led to an approximately 10-fold reduction in BRSV h1/2 titers, whereas the same dose reduced the yield of wild-type BRSV 100-fold. These results indicate that the NS proteins of BRSV and HRSV are adapted to optimally counteract the cellular IFN responses of their natural host.
DISCUSSION
In this work we provide experimental evidence that the two nonstructural proteins of HRSV, NS1 and NS2, are able to mediate virus escape from IFN-α/β-induced cellular antiviral mechanisms. Furthermore, the presence of both NS proteins is required for this function, as previously found for the BRSV NS proteins. Using rRVs expressing individual HRSV NS proteins, we could demonstrate that only in cells coinfected with both recombinants was a significantly enhanced resistance of the RV vector to IFN stimulation observed.
How IFN resistance is mediated by the RSV NS proteins is not yet known. It was previously shown (40) that HRSV does not inhibit IFN signaling, yet it is able to replicate in human cells that produce and respond to IFN. Studies by Garofalo et al. (17) further showed that RSV-infected human respiratory epithelial cells increase expression of an ISG, the class I major histocompatibility complex (MHC) molecule, due to the autocrine action of IFN-β produced in response to RSV infection. Moreover, Atreya and Kulkarni (1) could demonstrate that HRSV is resistant to the antiviral effects of MxA. However, in IFN-stimulated cells coinfected with HRSV and PIV3, HRSV could not prevent the IFN-mediated inhibition of the MxA-sensitive PIV3 replication. The authors concluded that resistance of RSV replication to IFN may be due to insensitivity of RSV replication to certain ISGs in the presence of a viral factor rather than to active disruption of an IFN-stimulated antiviral state.
Our data clearly show that the NS protein-mediated IFN resistance can be transferred to an unrelated rhabdovirus which is MxA insensitive (unpublished results). Thus, we assume that the NS proteins block the action of specific IFN-induced antiviral gene products other than MxA to accomplish resistance to IFN. HRSV and BRSV NS proteins are highly similar in sequence, with 69 and 84% identity for the NS1 and NS2 proteins, respectively. This similarity and, moreover, the functional cooperativity of BRSV and HRSV NS1 and NS2 proteins suggest that BRSV and HRSV interfere with the same IFN-induced antiviral mechanism and that they target similar, if not the same, antiviral gene products.
Although closely related, bovine and human RSVs display a highly restricted host range in vivo. One reason might be restricted entry into host target cells due to differences in the viral surface glycoproteins and in their cellular receptor. In vitro, however, BRSV and HRSV are able to enter a wide variety of cells of different hosts, indicating that other virally encoded factors may also play a pivotal role in determining host range. In this study, we could demonstrate that the NS proteins of BRSV and HRSV represent important determinants of viral host range in that they display a differential ability to counteract innate responses in cells of different hosts. In cells unable to produce IFN, viral replication of wild-type BRSV and BRSV h1/2 was indistinguishable. This is notable, as the HRSV NS1 protein was shown to associate with the viral matrix protein M (11) and was reported to be a potent inhibitor of viral transcription and RNA replication in an HRSV minigenome system. A similar but far less pronounced inhibitory effect was also observed for the HRSV NS2 protein (2). The virtually identical growth in Vero cells suggests that these interactions and functions were not largely affected in the chimeric virus and that the HRSV NS proteins are able to accurately fulfill the function(s) of their bovine counterparts during viral replication. In cells harboring an intact IFN system, however, differences in the ability to counteract host-specific IFN responses became obvious and even more evident in IFN-treated cells. In cells of bovine origin, BRSV was perfectly protected even against high doses of exogenous IFN-α/β, whereas BRSV h1/2 was severely attenuated and highly sensitive towards IFN-α. In human cells, both viruses displayed similar growth characteristics, but BRSV h1/2 could cope better with antiviral responses triggered by exogenous IFN. Although IFN resistance of BRSV h1/2 in HEp-2 cells was greater, this did not result in higher titers in the absence of exogenous IFN. Most probably, IFN induction and the activation of antiviral mechanisms are not that efficient in these cells such that the weaker IFN resistance of BRSV was not a limiting factor. In contrast, infected MDBK and Klu cells are likely to be strong inducers of IFN and differences in IFN escape are immediately apparent.
The contribution of pneumovirus NS proteins to the permissivity of hosts to RSV infection is also supported by other studies. Hanada et al. (20) showed that the markedly restricted growth of HRSV in mouse embryo cells could be overcome by adding anti-mouse IFN serum to the medium. As a result, HRSV yields were enhanced and the infection spread in the entire monolayer. In addition, a recombinant BRSV that had its glycoproteins replaced with their HRSV counterparts displayed prolonged replication in chimpanzees. However, this virus failed to significantly protect against challenge with wild-type HRSV (4). Our results suggest that the BRSV NS proteins were not able to sufficiently counteract the primate IFN responses.
Adaptation of viral proteins to the cellular environment of their natural host may in fact play a critical role in establishing an infection. The V protein of SV5, for example, is very effective in blocking the activation of IFN-responsive genes in primate cells but not in murine cells (8). A single amino acid substitution, however, renders the V protein fully functional to block IFN signaling in murine cells (39). Further studies with chimeric RSVs will reveal whether the species specificity in IFN escape can be attributed to one of the two NS proteins and may help to identify the involved sequences. In addition, mutations in the NS proteins might be identified that only partially knock out the IFN-antagonistic activity without compromising NS protein function in viral replication. This would lead to the generation of replication competent RSVs that display a reduced capability of antagonizing IFN in a given host.
The ability to adjust IFN resistance will also have important implications for the development of an efficacious live attenuated RSV vaccine. One method for attenuation of RSV is the deletion of nonessential genes. Indeed, deletion of either NS protein resulted in attenuated, highly IFN sensitive viruses (3, 33, 34, 38). However, replication was also affected in these mutants, resulting in reduced production of antigen and poor immunogenicity. The challenge in the development of a vaccine will be the elimination of residual virulence without compromising immunogenicity. BRSV and HRSV vaccines that possess intermediate ability to escape from the innate bovine and human response but are nevertheless able to keep their replicative capacity are a promising approach.
Acknowledgments
We thank J. A. Melero, Madrid, Spain, for providing the α-IC/C serum and R. Riebe from the Zellbank Riems, Germany, for providing Klu cells.
This work was supported in part by the Deutsche Forschungsgemeinschaft (SFB 455-A3, SPP 1089_Co260/1) and the European Commission (EC 5th FP-RSV Vac QLK2-CT-1999-00443).
REFERENCES
- 1.Atreya, P. L., and S. Kulkarni. 1999. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology 261:227-241. [DOI] [PubMed] [Google Scholar]
- 2.Atreya, P. L., M. E. Peeples, and P. L. Collins. 1998. The NS1 protein of human respiratory syncytial virus is a potent inhibitor of minigenome transcription and RNA replication. J. Virol. 72:1452-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchholz, U. J., H. Granzow, K. Schuldt, S. S. Whitehead, B. R. Murphy, and P. L. Collins. 2000. Chimeric bovine respiratory syncytial virus with glycoprotein gene substitutions from human respiratory syncytial virus (HRSV): effects on host range and evaluation as a live-attenuated HRSV vaccine. J. Virol. 74:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, P. L., K. McIntosh, and R. M. Chanock. 1996. Respiratory syncytial virus, p. 1313-1352. In D. M. Knipe et al. (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 6.Conzelmann, K. K. 1998. Nonsegmented negative-strand RNA viruses: genetics and manipulation of viral genomes. Annu. Rev. Genet. 32:123-162. [DOI] [PubMed] [Google Scholar]
- 7.Conzelmann, K.-K., and M. Schnell. 1994. Rescue of synthetic genomic RNA analogs of rabies virus by plasmid-encoded proteins. J. Virol. 68:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J. Virol. 73:3125-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowell, S. F., L. J. Anderson, H. E. Gary, Jr., D. D. Erdman, J. F. Plouffe, T. M. File, Jr., B. J. Marston, and R. F. Breiman. 1996. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J. Infect. Dis. 174:456-462. [DOI] [PubMed] [Google Scholar]
- 11.Evans, J. E., P. A. Cane, and C. R. Pringle. 1996. Expression and characterisation of the NS1 and NS2 proteins of respiratory syncytial virus. Virus Res. 43:155-161. [DOI] [PubMed] [Google Scholar]
- 12.Falsey, A. R., C. K. Cunningham, W. H. Barker, R. W. Kouides, J. B. Yuen, M. Menegus, L. B. Weiner, C. A. Bonville, and R. F. Betts. 1995. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J. Infect. Dis. 172:389-394. [DOI] [PubMed] [Google Scholar]
- 13.Falsey, A. R., J. J. Treanor, R. F. Betts, and E. E. Walsh. 1992. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J. Am. Geriatr. Soc. 40:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finke, S., and K.-K. Conzelmann. 1999. Virus promoters determine interference by defective RNAs: selective amplification of mini-RNA vectors and rescue from cDNA by a 3′ copy-back ambisense rabies virus. J. Virol. 73:3818-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcin, D., J. Curran, M. Itoh, and D. Kolakofsky. 2001. Longer and shorter forms of Sendai virus C proteins play different roles in modulating the cellular antiviral response. J. Virol. 75:6800-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcin, D., P. Latorre, and D. Kolakofsky. 1999. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J. Virol. 73:6559-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garofalo, R., F. Mei, R. Espejo, G. Ye, H. Haeberle, S. Baron, P. L. Ogra, and V. E. Reyes. 1996. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN beta and IL-1 alpha. J. Immunol. 157:2506-2513. [PubMed] [Google Scholar]
- 18.Gilchrist, S., T. J. Torok, H. E. Gary, Jr., J. P. Alexander, and L. J. Anderson. 1994. National surveillance for respiratory syncytial virus, United States, 1985-1990. J. Infect. Dis. 170:986-990. [DOI] [PubMed] [Google Scholar]
- 19.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 20.Hanada, N., T. Morishima, K. Nishikawa, S. Isomura, and Y. Nagai. 1986. Interferon-mediated self-limiting growth of respiratory syncytial virus in mouse embryo cells. J. Med. Virol. 20:363-370. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, P. G., G. I. Barrow, D. A. Tyrrell, D. Isaacs, and C. L. Gauci. 1990. The efficacy of intranasal interferon alpha-2a in respiratory syncytial virus infection in volunteers. Antiviral Res. 14:3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, H., H. Zhou, X. Cheng, R. Tang, M. Munoz, and N. Nguyen. 2000. Recombinant respiratory syncytial viruses with deletions in the NS1, NS2, SH, and M2-2 genes are attenuated in vitro and in vivo. Virology 273:210-218. [DOI] [PubMed] [Google Scholar]
- 23.Kalvakolanu, D. V. 1999. Virus interception of cytokine-regulated pathways. Trends Microbiol. 7:166-171. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu, T., K. Takeuchi, J. Yokoo, Y. Tanaka, and B. Gotoh. 2000. Sendai virus blocks alpha interferon signaling to signal transducers and activators of transcription. J. Virol. 74:2477-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Via, W. V., M. I. Marks, and H. R. Stutman. 1992. Respiratory syncytial virus puzzle: clinical features, pathophysiology, treatment, and prevention. J. Pediatr. 121:503-510. [DOI] [PubMed] [Google Scholar]
- 26.Mebatsion, T., M. J. Schnell, J. H. Cox, S. Finke, and K. K. Conzelmann. 1996. Highly stable expression of a foreign gene from rabies virus vectors. Proc. Natl. Acad. Sci. USA 93:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanty, S. B., A. L. Ingling, and M. G. Lillie. 1975. Experimentally induced respiratory syncytial viral infection in calves. Am. J. Vet. Res. 36:417-419. [PubMed] [Google Scholar]
- 28.Pringle, C. R. 1996. Virus taxonomy 1. Arch. Virol. 141:2251-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlender, J., B. Bossert, U. Buchholz, and K.-K. Conzelmann. 2000. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J. Virol. 74:8234-8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnell, M. J., T. Mebatsion, and K. K. Conzelmann. 1994. Infectious rabies viruses from cloned cDNA. EMBO J. 13:4195-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stott, E. J., L. H. Thomas, G. Taylor, A. P. Collins, J. Jebbett, and S. Crouch. 1984. A comparison of three vaccines against respiratory syncytial virus in calves. J. Hyg. (London) 93:251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung, R. Y., J. Yin, S. J. Oppenheimer, J. S. Tam, and J. Lau. 1993. Treatment of respiratory syncytial virus infection with recombinant interferon alfa-2a. Arch. Dis. Child. 69:440-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng, M. N., and P. L. Collins. 1999. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J. Virol. 73:466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng, M. N., S. S. Whitehead, A. Bermingham, M. St. Claire, W. R. Elkins, B. R. Murphy, and P. L. Collins. 2000. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J. Virol. 74:9317-9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Poel, W. H., A. Brand, J. A. Kramps, and J. T. Van Oirschot. 1994. Respiratory syncytial virus infections in human beings and in cattle. J. Infect. 29:215-228. [DOI] [PubMed] [Google Scholar]
- 36.Weber, E., B. Humbert, H. J. Streckert, and H. Werchau. 1995. Nonstructural protein 2 (NS2) of respiratory syncytial virus (RSV) detected by an antipeptide serum. Respiration 62:27-33. [DOI] [PubMed] [Google Scholar]
- 37.Wendt, C. H., and M. I. Hertz. 1995. Respiratory syncytial virus and parainfluenza virus infections in the immunocompromised host. Semin. Respir. Infect. 10:224-231. [PubMed] [Google Scholar]
- 38.Whitehead, S. S., A. Bukreyev, M. N. Teng, C. Y. Firestone, M. St. Claire, W. R. Elkins, P. L. Collins, and B. R. Murphy. 1999. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J. Virol. 73:3438-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young, D. F., N. Chatziandreou, B. He, S. Goodbourn, R. A. Lamb, and R. E. Randall. 2001. Single amino acid substitution in the V protein of simian virus 5 differentiates its ability to block interferon signaling in human and murine cells. J. Virol. 75:3363-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young, D. F., L. Didcock, S. Goodbourn, and R. E. Randall. 2000. Paramyxoviridae use distinct virus-specific mechanisms to circumvent the interferon response. Virology 269:383-390. [DOI] [PubMed] [Google Scholar]