Abstract
In a Hungarian family with triosephosphate isomerase (TPI; d-glyceraldehyde-3-phosphate keto-isomerase, EC 5.3.1.1) deficiency, two germ-line identical, but phenotypically differing compound heterozygote brothers (one of them with neurological disorder) have been identified with the same very low (<5%) TPI activity and 20- or 40-fold higher erythrocyte dihydroxyacetone phosphate levels as compared with normal controls. Our present studies with purified TPI and hemolysates revealed the binding of TPI, and the binding of human wild-type and mutant TPIs in hemolysate, to the red cell membrane, and the interference of binding with other hemolysate proteins. The binding of the mutant TPI is enhanced as compared with the wild-type enzyme. The increased binding is influenced by both the altered structure of the mutant and the changes in the red cell membrane. Compared with binding of glyceraldehyde-3-phosphate dehydrogenase, the isomerase binding is much less sensitive to ionic strength or blocking of the N-terminal tail of the band-3 transmembrane protein. The binding of TPIs to the membrane decreases the isomerase activity, resulting in extremely high dihydroxyacetone phosphate levels in deficient cells. In cell-free brain extract, tubulin copolymerizes with TPI and with other cytosolic proteins forming highly decorated microtubules as shown by immunoblot analysis with anti-TPI antibody and by electron microscopic images. The efficacy order of TPI binding to microtubules is propositus > brother without neurological disorder > normal control. This distinct microcompartmentation of mutant proteins may be relevant in the development of the neurodegenerative process in TPI deficiency and in other, more common neurological diseases.
The last 10 years witnessed an explosion of knowledge on different factors involved in the process of neurodegeneration. Different mutations have been described in various proteins initiating neurodegenerative diseases (β42 and PrPsc in Alzheimer's disease, expansion of preexisting polyglutamine sequences in huntingtin, ataxin, frataxin, and other mutant proteins in peroxisomal diseases, and in synuclein in familial Parkinson's disease) (1). The ultimate stage of the neurodegenerative process, apoptosis of focal groups of neurons, has also been studied in detail (reviewed in ref. 2). However, the cellular biochemical changes induced by the initiating effect of the mutant proteins and the crucial mechanisms leading to progressive apoptosis remain an enigma. In the past few years, we have studied different cellular factors involved in the process of neurodegeneration in a unique human model, in two Hungarian compound heterozygote brothers having a triosephosphate isomerase (TPI; d-glyceraldehyde-3-phosphate keto-isomerase, EC 5.3.1.1) deficiency caused by germ-line identical mutant proteins but completely different clinical phenotype (3–5).
TPI deficiency is a rare autosomal recessive disease associated with chronic nonspherocytic hemolytic anemia, progressive neurological disorders, and early death of the homozygotes and compound heterozygotes. The TPI of both Hungarian brothers harbor a 240 (TTC[Phe] → CTC[Leu]) missense mutation and a 145 (GAG[Glu] → TAG[stop codon]) mutation (6, 7). Both have the same level of hemolytic anemia, equally low (<3%) red cell TPI activity, and an extremely high level of dihydroxyacetone phosphate (DHAP), but only the propositus has a severe extrapyramidal disorder; the other compound heterozygote brother is neurologically intact. To date, we have found manifest differences between the two brothers in lymphocyte TPI activity (3), in red blood cell membrane fluidity and membrane enzyme activities, in the molecular species composition of phospholipid subclasses (4, 5), in the extent of the decrease of ethanolamine plasmalogens in lymphocytes, and in the level of antioxidants (8). Lack of plasmalogens was also found in other neurodegenerative diseases (reviewed in ref. 9). Plasmalogens are known to be indispensable for adequate enzyme functions, signal transduction, fusion of neurotransmitter membranes, and prevention of oxidative stress [reviewed by de Kruijff (10)].
In our earlier publications (11, 12), attention was drawn, in addition to the differences in the cellular environment of the mutant TPI, to the modulating effect of the binding of enzymes to subcellular structural elements, e.g., on the compartmentation of enzyme functions. The hypothesis was raised that the phenotypic differences between the two compound heterozygote brothers might originate from differences in the binding of TPI to subcellular structural elements (12), to band 3 protein in erythrocytes, and to tubulin in nerve cells (4, 5). In fact, in red blood cells, band 3 protein is considered a major target of the binding of glycolytic enzymes (13, 14).
The present study was aimed to investigate the in vitro binding of wild-type and mutant TPI from hemolysates (i) to the red cell membranes from the two brothers with germ-line identical TPI deficiency and (ii) to microtubules (MTs) purified from bovine brain extract. Our results revealed significantly higher level of binding of the mutant than the wild-type TPI to a yet unidentified component of the red cell membrane and in even higher level to MTs. The possible role of TPI binding to subcellular components in the pathomechanism of TPI deficiency is being discussed.
Materials and Methods
Patients.
The main clinical and biochemical characteristics of the TPI-deficient Hungarian family were published earlier (3). Informed consents were obtained from all members of the family and the control.
Materials.
TPI, glycerol-3-phosphate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (all from rabbit muscle), glyceraldehyde-3-phosphate, NADH, taxol, 2-[N-morpholino]ethanesulfonic acid, ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid, GTP, and monoclonal anti-band 3 protein antibody (Sigma B-9277, LOT 17H4856) were purchased from Sigma. All other chemicals were reagent-grade commercial preparations.
Protein Determination.
Protein concentration was measured by the Bradford method (15). Hemoglobin concentration was determined spectrophotometrically with an absorption coefficient (414 nm, 0.1%) of 8.77.
Gel Electrophoresis and Immunoblotting.
Proteins of brain cell-free extract were separated by SDS/PAGE according to Laemmli (16) and electrotransferred to nitrocellulose membranes. The filters were subjected to immunoblotting with antisera directed against rabbit muscle TPI. Antibody binding was revealed by using anti-rabbit IgG coupled to alkaline phosphatase (Sigma).
Cell-Free Extracts.
Hemolysate.
Packed human red blood cells (from normal control and from the two TPI-deficient compound heterozygote brothers) were prepared from washed isotonic red blood cell preparations by centrifugation at 5,000 × g at 4°C for 20 min, then the separated cells were lysed by diluting 3-fold into 10 mM Tris⋅HCl buffer (pH 8.0), containing 0.1 mM ethylenediaminetetraacetic acid and 5 mM mercaptoethanol (buffer A), followed by three cycles of freezing in liquid N2 and thawing (12). These lysed cells were used as hemolysate after centrifugation at 24,000 × g at 4°C for 25 min.
Brain extract.
Cell-free cytosol fractions of bovine brain were prepared as described by Liliom et al. (17).
Inside-Out Vesicles (IOV) Preparation.
Preparation of hemoglobin-free erythrocyte ghosts—i.e., IOVs—were carried out as described by Sarkadi et al. (18). The average ratio of the IOV and ROV (rightside-out vesicle) in the preparations was 65 ± 5% determined on the basis of acethylcholinesterase activity measurements (5). The IOV preparations were practically free of bound TPI.
MT Preparation.
MAP-free tubulin was purified from bovine brain as described by Na and Timasheff (19). Tubulin was dialyzed in 50 mM 2-[N-morpholino]ethanesulfonic acid buffer (pH 6.8) at 4°C for at least 3 h, then centrifuged at 4°C at 100,000 × g for 20 min. The supernatant was polymerized into MTs adding 20 μM taxol to 10 mg/ml tubulin and by incubation at 37°C for 30 min.
Enzyme Activity Assays.
The activities of TPI and GAPDH were measured with glyceraldehyde-3-phosphate as substrate according to Beutler (20).
Binding Assays.
Binding to IOV.
A total of 50–200 μg of IOV were washed twice by suspension in buffer A and centrifugation was performed at 24,000 × g at 4°C for 25 min, then resuspended with 300–700 μl of the corresponding diluted hemolysates or with diluted commercial rabbit muscle TPI. The dilutions were carried out with buffer A or with buffer A containing 100 mM KCl. The suspensions were incubated for 30 min at 25°C, then the samples were centrifuged again as above. The pellets were resuspended in 150 μl buffer A with and without 100 mM KCl, and aliquots were taken for TPI and GAPDH activity assays (at least four determinations). The enzyme activities of the supernatants were also measured. The standard error of the determinations was ± 15–20% (n = 3–4).
Binding to MT.
Taxol-stabilized MT at a final concentration of about 2 mg/ml was incubated with TPI or with hemolysates of normal and deficient red cells in 100 mM 2-[N-morpholino]ethanesulfonic acid (pH 7.0) containing 5 mM MgCl2 (buffer B) at 37°C for 15 min. The samples were centrifuged (50,000 × g, 30 min, 37°C), and the pellet fractions were resuspended in buffer A containing 100 mM KCl. Then, the fractions were analyzed by SDS/PAGE and activity measurements. The standard error of the determinations was ± 15% (n = 3–6).
Kinetic Experiments in the Presence of IOVs.
The mixtures of 50 μl of hemolysate and washed IOV were incubated for 30 min at 25°C, in the presence or absence of antibody raised against the N-terminal tail of band 3 protein and assayed for TPI activity. Reference samples without IOV were handled similarly. The standard error of the determinations was ± 20% (n = 3).
Preparation of Anti-TPI Antibody.
Rat anti-TPI antibody was prepared by immunizing three rats with commercial rabbit muscle enzyme; 25 μg protein was dissolved in 250 μl sterile PBS, and the solution was homogenized and emulsified with 250 μl Freund's complete adjuvant. Two s.c. injections, 250 μl each, were given in the back of the animals. After 1, 2, and 3 wk, the same injections were repeated, but with incomplete adjuvant. The animals were bled on the 4th week. The serum raised against TPI was purified for IgG as described earlier (21).
Transmission Electron Microscopy.
MT samples containing brain extract were pelleted, and the pellets were prefixed and stained as described in ref. 17. The specimens were examined and photographed in a JEOL CX 100 electron microscope operated on accelerating voltage of 80 kV. Magnification was calibrated with a diffraction grating replica (2160 line/mm; Balzers).
Results
Binding of TPI to Plasma Membrane.
In vitro binding experiments were carried out with IOV and hemolysate prepared of erythrocytes from normal human controls. For comparison, purified rabbit TPI was also used because it was shown to have a 98.8% homology with the human isoform (22) because of the highly conserved structure of this housekeeping glycolytic enzyme (23).
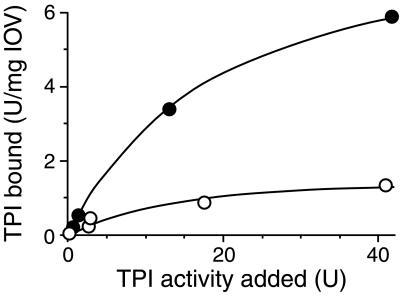
Fig. 1 shows representative titration curves; purified TPI as well as normal hemolysate were added in varying concentrations to 100 μg IOVs. The samples were centrifuged to separate the IOV-bound proteins in the pellets from the unbound ones in the supernatant. TPI activity in the pellets was assayed after resuspension and extensive dilution. Under this condition, the activity of TPI is proportional with the enzyme amount because the sum of the two phases equals the total TPI activity added to IOV (data not shown). The data presented in Fig. 1 show that both the commercial TPI purified from rabbit muscle and the human red cell TPI from hemolysate do bind to the red cell membrane; however, the extent of the binding is more intensive in the case of the purified enzyme, indicating that the binding of TPI interferes with that of other proteins.
Figure 1.
Binding of normal TPI to IOV. Binding experiments were carried out as described in Materials and Methods. IOV purified from a healthy donor's blood (129 μg) was washed twice and then suspended with the same person's hemolysate (○) or with purified, commercial rabbit muscle TPI (●) solved in buffer A, at 25°C for 30 min. Then, the samples were centrifuged, pellets were resuspended in buffer A, and aliquots were assayed for TPI activity.
Differences Between the Binding of TPI (in Hemolysates) from the TPI-Deficient Brothers and from Normal Controls to Their Own and to Each Other's IOVs.
The bindings of mutant TPIs of the hemolysates from the two brothers and that of the wild-type TPI of the hemolysates from normal controls to their own IOV and their crossbindings to each other's IOV have been compared. The added hemolysates had the same protein content and hemoglobin concentrations but differed in TPI activities between the TPI-deficient and control samples.
The partitions of the TPI in the pellet and supernatant fractions are given in percentages, which show the ratio of the bound and free TPI based on TPI activity measurements. In addition to these relative values, the absolute amounts of bound TPIs per mg IOVs were estimated on the basis of the following information: (i) the activity ratio of normal and mutant TPIs in the hemolysates at identical hemoglobin concentrations (cf. Table 1); and (ii) the specific activity of the wild-type human TPI (9800 units/mg; ref. 22). The bound TPI amounts presented according to this rationale in Table 1 show that significantly higher percentages of mutant TPI is bound to their own IOVs than wild-type TPI from normal controls to their IOVs. In addition, the binding of the TPI from the neurologically intact brother to IOV is higher than that of the propositus.
Table 1.
Binding of TPI to IOV
| Sample | Hemolysate, gHb/liter | TPI added, milliunits | TPI bound
|
||
|---|---|---|---|---|---|
| Percent | milliunits/mg IOV | ng/mg IOV | |||
| Propositus | 62.7 | 189 | 3.27 | 30.93 | 306.3 |
| 441 | 2.54 | 55.85 | 558.5 | ||
| Brother | 60.4 | 346 | 4.24 | 72.94 | 486.0 |
| 806 | 4.26 | 172.6 | 1016 | ||
| Control | 62.8 | 17,400 | 0.66 | 576.6 | 57.7 |
| 40,600 | 0.50 | 1,015.2 | 101.5 | ||
Binding experiments were carried out as described in Materials and Methods. A total of 200 μg of IOV from the normal and deficient cells were incubated with 300 or 700 μl of hemolysates from the same sources at 25°C for 30 min. The total protein contents of the hemolysates were similar. The estimation of the amount of the bound enzyme was based on the measured activities by using specific activities of 300 units/mg, 450 units/mg, and 9,800 units/mg for propositus, brother, and normal control TPIs, respectively. “Percent” represents the bound fraction of added TPI. The standard error of the determinations was ± 15% (n = 4). Data are from a typical set of experiments.
Table 2 summarizes the results of the binding of normal and mutant TPIs to IOVs in different combinations. Two different concentrations of hemolysates were used for these studies at identical IOV amounts, which were adjusted for protein content. It can be seen in Table 2 that when the same hemolysate was added to IOVs prepared from normal and TPI-deficient red cells, there was a modest difference in the amount of bound TPI; whereas adding normal and mutant cell hemolysates to the same IOV caused the bound TPI amount to vary significantly. The same qualitative result can be seen at lower (Table 2, Expt. A) and higher (Table 2, Expt. B) hemolysate concentrations. Higher amounts of bound TPI were obtained when the hemolysate and the IOV originated from the same donor; among these, the greatest binding was measured in the case of the neurologically symptom-free brother. Therefore, one can conclude that the difference in the association of TPI to the plasma membrane observed in the normal and the two types of deficient red cells is due to the combined effects of the mutation of the isomerase and the difference in the integrity of IOV as well as to additional factor(s) that makes the two deficient systems dissimilar.
Table 2.
Crossbinding between TPIs and IOVs from different sources
| Hemolysate | Control | IOVs from brother | Propositus |
|---|---|---|---|
| Expt. A | |||
| Control | 58 | 85 | 55 |
| Brother | 314 | 486 | — |
| Propositus | 212 | — | 307 |
| Expt. B | |||
| Control | 102 | 190 | 189 |
| Brother | 640 | 1,016 | — |
| Propositus | 296 | — | 559 |
Binding experiments were carried out as described in Table 1, except that IOVs were incubated with hemolysates of different sources. The numbers show the amount of the bound TPI in ng/mg IOV. Expt. A, 300 μl of hemolysate; Expt. B, 700 μl of hemolysate. The standard error of the determinations was ± 15% (n = 3).
Modulation of the Binding of TPI to IOVs.
We investigated the effect of salt concentration on the binding of TPI to the red cell membrane for two reasons. On one hand, to see whether the TPI binding takes place at physiological ionic strength; on the other hand, to test whether the N-terminal tail of band 3 is a potential receptor site for TPI binding. Because the salt-sensitive association of GAPDH to the plasma membrane of erythrocytes—especially to the band 3 protein—is well-documented (24, 25), in some sets of experiments the binding of GAPDH to IOV was also measured for comparative purposes.
Table 3 shows the results of pelleting experiments at low and high salt concentrations with the mixture of IOV and hemolysates, erythrocytes from normal control and from the propositus. Both the TPI and GAPDH activities were assayed in the pellet and the supernatant fractions, and the relative binding data are presented. These data show that a significant part (about 70%) of the total amount of GAPDH is bound to the membrane at low ionic strength; whereas, at high ionic strength, almost all GAPDH was released from the IOV. This result is in agreement with those of earlier reports (24, 25). In contrast to GAPDH, TPI association with the membrane appeared to be much less sensitive to ionic strength, and no difference in salt-sensitivity could be detected between the binding of normal and mutant TPI.
Table 3.
Modulation of bindings of TPI and GAPDH to IOVs
| Samples | Normal hemolysate, % | Propositus hemolysate, % |
|---|---|---|
| TPI | 100 | 100 |
| + 100 mM KCl | 80 ± 10 | 80 ± 10 |
| + Antibody | >80 | >80 |
| GAPDH | 100 | 100 |
| + 100 mM KCl | <5 | <5 |
| + Antibody | <5 | <20 |
IOVs were washed twice then resuspended with 700 μl of the corresponding diluted hemolysates (hemoglobin contents: 6.8 g/liter and 3.0 g/liter for propositus and control, respectively). The hemolysates were diluted with buffer A or with buffer A containing 100 mM KCl. The suspensions were incubated for 30 min at 25°C in the presence or absence of 15 μl of antibody raised against the N-terminal sequence of band 3. Then, the samples were analyzed as described in Materials and Methods. The standard error of the determinations was ± 20% (n = 3).
These results were further supported by the data of the next set of experiments. The N-terminal tail of the band 3 transmembrane protein was blocked by specific antibody raised against this protein, and the binding of TPI to this modified IOV preparation was investigated in comparison with that of GAPDH (Table 3). While the presence of the antibody significantly decreased the binding of GAPDH to IOV, the effect of the specific antibody on the binding of TPI to IOV was very small, if any. These data seem to exclude the possibility that TPI binds to the N-terminal, cytosolic fragment of the transmembrane band 3 protein.
Effect of IOV on the TPI Activity.
Extensive evidence accumulated that indicates that the binding of some glycolytic enzymes to subcellular particles, including red cell membrane, decreases their activities (e.g., phosphofructokinase, aldolase, GAPDH) (24–26), and the glycolytic flux is also significantly affected (14). However, no data for TPI association to IOV were available.
To clarify whether the binding of TPI affects its activity and whether the very low TPI activity of the deficient cells are further reduced by the binding of the enzyme, kinetic experiments were carried out. Since the relative amount of the bound TPI to the free form is low at the conditions used for the binding assay, we enforced the IOV/TPI ratio in the activity assay to enforce the TPI binding to IOV.
Fig. 2 illustrates the effect of IOV (TPI–IOV complex formation) on the activities of normal and mutant TPIs. The addition of 50 μg IOV prepared from both cell types significantly reduced the isomerase activity; the extent of reduction was similar in both cases. The increase of the concentration of IOV resulted in more inhibition. The binding causes unfavorable alteration in TPI activity, and because the deficient cell hemolysate has very low activity, any further decrease could significantly affect the energy production of the red cells as well as the DHAP level. These effects could be even more pronounced at physiological conditions where the protein concentrations are higher that favor enzyme associations.
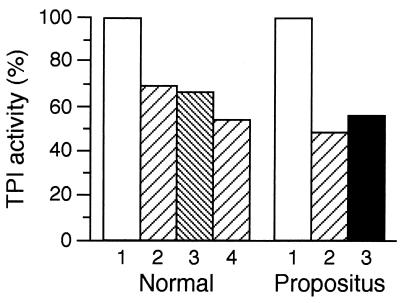
Figure 2.
Effect of TPI binding to IOV on the isomerase activity in the absence and presence of salt or anti-band 3 antibody. IOVs were washed twice and then resuspended with 50 μl of the corresponding diluted hemolysates (hemoglobin contents: 6.8 g/liter and 3.0 g/liter for propositus and control, respectively). The hemolysates were diluted with buffer A or with buffer A containing 100 mM KCl. The suspensions were incubated for 30 min at 25°C in the presence or absence of 10 μl antibody raised against the N-terminal sequence of band 3. Then, the whole sample was used for TPI assay as described in Materials and Methods. The standard error of the determinations was ± 20% (n = 3). Samples: Normal: 1, control (no IOV); 2, 50 μg IOV; 3, 50 μg IOV + 10 μl anti-band 3 antibody; 4, 100 μg IOV; Propositus: 1, control (no IOV); 2, 100 μg IOV; 3, 100 μg IOV+ 100 mM KCl.
Fig. 2 also shows, in agreement with the binding measurements, that the salt concentration does not significantly modify the IOV-reduced TPI activity, and blocking by antibody the N-terminal tail of band 3 protein also does not interfere with IOV binding.
MTs Are Also Target for TPI Binding.
Neurological disorder is a crucial symptom of severe TPI deficiency. TPI is an enzyme involved in the energy production that is mediated exclusively by glycolysis in red cells and to a very high degree in brain cells. Because TPI activity decreases as a result of binding to the red cell membrane, based on some similarities between the association of glycolytic enzymes to the red cell membrane and MTs (27), we analyzed the possibility that MTs may function as potential receptors for TPI. For these reasons, two sets of experiments were designed: (i) the copolymerization of TPI with endogenous tubulin was investigated in bovine brain cell-free extract, and (ii) the binding of TPI to purified bovine brain MT was determined by using hemolysates from normal and TPI-deficient cells.
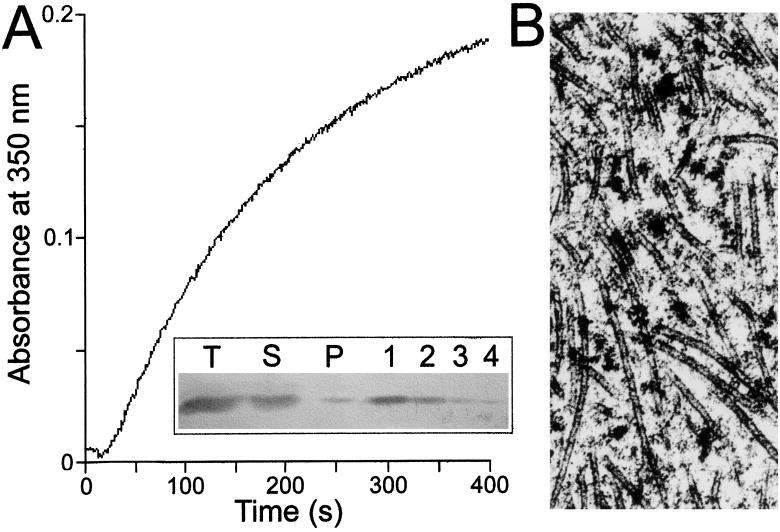
In the first set of experiments, endogenous tubulin was polymerized in bovine brain cell-free extract, and the polymerization was induced by addition of taxol at 37°C. The polymerization was followed by turbidimetry as shown in Fig. 3A. The formed MTs were pelleted then visualized by electron microscopy (Fig. 3B), which indicated that intact tubules were produced under our experimental conditions and the tubules were highly decorated by protein-size macromolecules, including MAPs. To identify whether TPI is among these attached proteins, immunoblot assay was carried out. Anti-TPI antibody raised against rabbit muscle enzyme was applied to identify TPI in the MT fractions. As shown in Fig. 3A Inset, TPI does exist in the pellet, indicating that the isomerase is able to associate to MT from brain extract. According to the densitomeric analysis, this fraction is about 4% compared with the total TPI present in the brain extract. However, under in vivo conditions where the total protein and especially the MT concentration is higher, more extensive TPI association might occur. The major objective for these investigations was to look for a difference in the binding of normal and mutant TPI to MTs, as well as for an eventual difference between the binding of TPI from the two affected brothers. These hypotheses were checked by using isolated MAP-free MT plus TPI in the hemolysates.
Figure 3.
MT assembly in bovine brain extract (A) and decoration of MTs by endogenous proteins (B). The cell-free extract concentration was 20 mg/ml with roughly 15% tubulin content. The polymerization was initiated at 37°C by addition of 20 μM taxol in buffer B. Inset shows the immunoblot data using anti-TPI antibody raised against rabbit muscle TPI. T, S, and P are samples from total, supernatant, and pellet phases, respectively; 1, 2, 3, and 4 are controls using 600, 300, 150, and 75 ng of isolated TPI, respectively. Electron microscopic image was prepared as described in Materials and Methods.
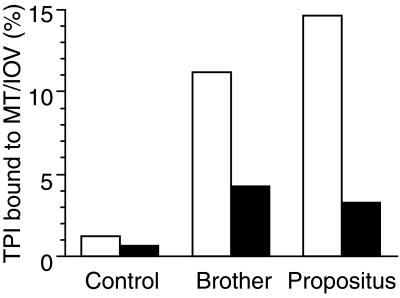
The binding experiments with MAP-free MT preparations were carried out as described with the binding to IOVs. MTs were pelleted, separating the MT-bound and free TPI. The TPI was assayed in both fractions by activity measurements after extensive dilution as described earlier. Fig. 4 shows the partition of TPI of the hemolysates from the normal controls and from the TPI-deficient brothers in the presence of 2 mg/ml MT. As shown in the figure, the amount of the bound TPI to MTs from the control hemolysate is relatively low, as compared with that from the TPI-deficient brothers. These data are qualitatively similar to that obtained with IOVs (cf. Table 2). The quantitative comparison of these data is difficult because the concentration of the receptor sites, at least in the case of IOVs, is unknown. However, the binding of the mutant TPI from the hemolysates of the two affected brothers to either normal IOV or to brain MT can be compared. One can expect identical binding of the TPIs carrying the same mutations to any of these receptors. Interestingly, the comparison clearly indicates that the extent of the binding is different, which strongly suggests the presence of an as yet unidentified factor(s).
Figure 4.
Comparison of normal and mutant TPIs from normal and deficient hemolysates to normal IOV (filled columns) and MT (open columns). A total of 150 μl hemolysates were added to 100 μg normal IOV or to 2 mg/ml MT in buffer A or buffer B, respectively. After incubation for 30 min at 25°C, the samples with the bound proteins were pelleted and assayed for TPI activity. For other details see Materials and Methods.
Discussion
The binding of glycolytic enzymes to subcellular structures has been well documented (13, 28), and a regulatory role in the red cell metabolism has been attributed to their reversible binding (14). No data were available for either an eventual binding of TPI to the cell membrane and about an ensuing effect of this binding. We have raised earlier the hypothesis (4) that the binding of mutant TPI to band 3 protein in erythrocytes and to tubulin in nerve cells might be involved in the process of neurodegeneration in TPI defects.
A well-characterized receptor site on the normal red blood cell membrane is the band 3 transmembrane protein. The binding of various glycolytic enzymes, e.g., phosphofructokinase, aldolase, GAPDH, to the cytoplasmic negatively charged N-terminal tail of this anion transporter protein has been reported (24–26). In the present study, we first titrated the binding of wild-type human TPI in hemolysates from normal human donors vs. the binding of commercial rabbit muscle TPI (Fig. 1.). The results showed that the human TPI binds definitely to the membrane of IOVs, although to a much lesser degree than the purified rabbit TPI. Because the sequence of TPI is highly conservative (22), a plausible explanation for the distinct binding curves is that other proteins that are present in the hemolysate interfere with the binding of TPI. The mutant TPI from the two compound heterozygote brothers were shown to bind to their own IOV in a significantly higher amount than the wild-type from normal donors to their own IOV. The binding of both wild-type and mutant TPIs to IOVs was only slightly sensitive to changes in salt concentration, in contrast to GAPDH, which was almost entirely released from the membrane at high ionic strength. No difference was found between the salt-sensitivity of wild-type and mutant TPIs. These results indicate distinct binding sites on the plasma membrane for the two glycolytic enzymes and disfavor the hypothesis of the binding of TPI to the negatively charged N-terminal tail of the band 3 protein. These findings are easily explained on the basis of the large difference in the isoelectric points (29) of GAPDH (pI = 8.5) and TPI (pI = 6.5).
This hypothesis was validated in the next set of experiments, which revealed that the blocking of the N-terminal tail of band 3 by specific antibody decreased the binding of TPI only modestly in contrast to the drastic decrease of GAPDH binding.
The most striking metabolic abnormality in TPI-deficient erythrocytes is the 20- to 60-fold increase in the concentration of DHAP, the substrate for TPI, suggestive of an almost complete metabolic block at this step (30). In a previous work, we emphasized that there is a discrepancy between DHAP levels and TPI activities determined in cell-free hemolysates of deficient cells (12).
In the present work, we demonstrated that TPI activity is decreased by increasing the amount of IOVs in the reaction mixture of red cell membrane and TPI, which revealed that aside from the structural consequences, the association of TPI to the plasma membrane caused functional alteration as well. The relative decrease in activity was found to be even more pronounced when mutant TPI was bound to the cell membrane from the TPI-deficient brothers. This effect may lead to extreme reduction of enzyme function in TPI deficiency and may explain the very high DHAP levels in homozygote and compound heterozygote carriers of TPI defects. In the earlier TPI deficiency literature, neurotoxic effect had been attributed to the high level of DHAP (31). The toxic effect could, however, never been substantiated.
DHAP is the crucial precursor of ethanolamine plasmalogens. In spite of this fact, plasmalogen level was found to be decreased in the lymphocytes from TPI-deficient individuals (5), just like in all investigated neurodegenerative processes (9, 32). Decreased plasmalogen levels can, however, originate not only from defects in synthesis but also from increased degradation by plasmalogen sensitive phospholipase A2 that was already reported in some neurodegenerative diseases (33).
Although erythrocytes, just like all other cells, contain TPI and its substrates, DHAP and glyceraldehyde-3-phosphate, and thus can serve well for the diagnostics of TPI deficiency, they are not ideal for the research of the pathomechanism of the neurodegenerative disorder of the defect. The most important differences giving some clue to the development of neurodegeneration were found in lymphocytes (3–5), which share a number of characteristics with brain cells (34, 35). In addition, it turned out recently that the earliest stem cells of the hemopoietic and neural lines are identical (36, 37). The most convincing data would be obtained, however, investigating the structural and functional changes of TPI defects in brain cells. Since brain biopsy for research purposes would be unethical, we have chosen to investigate the binding of the wild-type and mutant TPI to tubulin purified from calf brain cells. The binding of some glycolytic enzymes to MT is well documented (27, 28). Here, we presented data on the association of TPI from different species to purified brain MTs. In fact, in brain extracts, TPI copolymerizes with tubulin forming intact tubules (cf. Fig. 3). It has to be noted that bovine tubulin used in our experiments does not significantly differ from the human (the homology with mammalian tubulins is at least 90–95%) (38). Therefore, TPI binding to MTs, which are a major component of neuronal cells, can be significant also in the human brain. In addition, the high protein concentration occurring in vivo could enormously force the enzyme binding.
The comparative data of our experiments (Fig. 4 A and B) showed that significantly higher amounts of TPI were bound to MTs from the hemolysate from the neurologically intact compound heterozygote brother than from the normal control (Fig. 4) and even higher levels of bound TPI could be detected with the hemolysate from the propositus. Since mutant TPIs from the two brothers are identical (6, 7), it may be surmised that either (i) an as a yet unidentified factor occurring/missing in one of the hemolysates, or (ii) differences in cellular membrane structure and function could be responsible for the distinct associative properties of TPI binding.
There are no data available on brain pathology of patients with severe TPI deficiency. Since the associative properties of mutant TPI are different from the wild-type enzyme, it is interesting to speculate that there might be analogy at the molecular level in the development of the neurodegenerative process in TPI deficiency and other neurodegenerative diseases. Recent research has documented abnormal protein deposition in chronic neurodegenerative diseases. The mutant proteins, just like the wild types are normally soluble. Their conversion to fibrillar or aggregated forms involves a change in the three-dimensional structure of these proteins. Lansbury (39) proposed a seeded polymerization for the development of the abnormal protein aggregation and deposition. Based on findings of protein deposits in peroxisomal diseases, Alzheimer's and Parkinson's diseases, it can be surmised that cerebral aggregates of mutant TPI might also occur in brain tissue, causing the neurological disorder of this defect. The mutant proteins like huntingtin, ataxin, and amyloid precursor protein are expressed just as TPI throughout the body. Disease pathology inducing aggregates, however, seems to be restricted to specific areas of the brain. Mutant misfolded proteins readily form aggregates by binding to integral proteins of subcellular membranes. The superstructure of the mutant TPI is difficult to predict because, in addition to the mutation with functional interference at the 240 (6) position, a TPI fragment due to the stop codon mutation at position 145 (7) is also present in the complex cytosolic compartment. The increased binding of the probably very unstable, sticky TPI chunk could produce small aggregates similar to those in other chronic neurodegenerative diseases. These ultramicro foci may be silent for a long time since the slowly progressive cell death can be compensated for many years. Intercurrent stimuli like viral diseases, immunological and inflammatory processes may induce the aggregation of the wild-type proteins around the initial seed.
Acknowledgments
We thank Emma Hlavanda and Mimi Nuridsány for their excellent technical contribution. This work was supported by grants from the Hungarian National Science Foundation OTKA [T-019638 (to S.H.), T-025291 (to J.O.), and T-029924 (to F.O.)], from the Hungarian Medical Research Council [ETT-408 (to S.H.)], and from the European Community [Grant ERBIC15CT960307 (to J.O.)]. L.K. has received a “Bolyai” fellowship of the Hungarian Academy of Sciences.
Abbreviations
- TPI
triosephosphate isomerase
- DHAP
dihydroxyacetone phosphate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IOV
inside-out vesicle
- MT
microtubule
- MAP
microtubule-associated protein
References
- 1.Ross C A, Margolis R L, Becher M W, Wood J D, Engelender S, Cooper J K, Sharp A H. Prog Brain Res. 1998;117:397–419. doi: 10.1016/s0079-6123(08)64029-7. [DOI] [PubMed] [Google Scholar]
- 2.Pettmann B, Henderson C E. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- 3.Hollán S, Fujii H, Hirono A, Hirono K, Karro H, Miwa S, Harsányi V, Gyódi E, Inselt-Kovács M. Hum Genet. 1993;92:486–490. doi: 10.1007/BF00216456. [DOI] [PubMed] [Google Scholar]
- 4.Hollán S, Dey I, Szollár L, Horányi M, Magócsi M, Harsányi V, Farkas T. Proc Natl Acad Sci USA. 1995;92:268–271. doi: 10.1073/pnas.92.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollán S, Magócsi M, Fodor E, Horányi M, Harsányi V, Farkas T. Proc Natl Acad Sci USA. 1997;94:10362–10366. doi: 10.1073/pnas.94.19.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M-L, Artymiuk P J, Wu X, Hollán S, Lammi A, Maquat L E. Am J Hum Genet. 1993;52:1260–1269. [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider A, Cohen-Solal M. Blood Cells Mol Dis. 1996;22:82–84. doi: 10.1006/bcmd.1996.0011. [DOI] [PubMed] [Google Scholar]
- 8.Hollán S, Vécsei L, Karg E, Németh I, Horányi M, Inselt-Kovács M, Farkas T. C R Seances Soc Biol Fil. 1998;192:929–945. [PubMed] [Google Scholar]
- 9.Lohner K. Chem Phys Lipids. 1996;81:167–184. doi: 10.1016/0009-3084(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 10.de Kruijff B. Curr Opin Chem Biol. 1997;1:564–569. doi: 10.1016/s1367-5931(97)80053-1. [DOI] [PubMed] [Google Scholar]
- 11.Ovádi J. Cell Architecture and Metabolic Channeling. Austin; Springer, Heidelberg: R. G. Landes; 1995. [Google Scholar]
- 12.Orosz F, Vértessy B, Hollán S, Horányi M, Ovádi J. J Theor Biol. 1996;182:437–447. doi: 10.1006/jtbi.1996.0184. [DOI] [PubMed] [Google Scholar]
- 13.Wasseem A, Steck T L. Methods Enzymol. 1989;173:513–519. doi: 10.1016/s0076-6879(89)73035-4. [DOI] [PubMed] [Google Scholar]
- 14.Low P S, Rathinavelu P, Harrison M L. J Biol Chem. 1993;268:14627–14631. [PubMed] [Google Scholar]
- 15.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Nature (London) 1970;227:680–688. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Liliom K, Wágner G, Kovács J, Comin B, Cascante M, Orosz F, Ovadi J. Biochem Biophys Res Commun. 1999;264:605–610. doi: 10.1006/bbrc.1999.1547. [DOI] [PubMed] [Google Scholar]
- 18.Sarkadi B, Szász I, Gárdos G. Biochim Biophys Acta. 1980;598:326–338. doi: 10.1016/0005-2736(80)90010-3. [DOI] [PubMed] [Google Scholar]
- 19.Na C N, Timasheff S N. Biochemistry. 1986;25:6214–6222. doi: 10.1021/bi00368a057. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E, Blume K G, Kaplan J C, Löhr G W, Ramot B, Valentine W N. Br J Haematol. 1977;35:331–340. doi: 10.1111/j.1365-2141.1977.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 21.Tracey D E, Liu S H, Cebra J J. Biochemistry. 1976;15:624–629. doi: 10.1021/bi00648a027. [DOI] [PubMed] [Google Scholar]
- 22.Mande S C, Mainfroid V, Kalk K H, Goraj K, Martial J A, Hol W G J. Protein Sci. 1994;3:810–821. doi: 10.1002/pro.5560030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu H S, Yuan P M, Gracy R W. J Biol Chem. 1984;259:11958–11968. [PubMed] [Google Scholar]
- 24.Tsai I, Murthy S N P, Steck T L. J Biol Chem. 1982;257:1438–1442. [PubMed] [Google Scholar]
- 25.Rogalski A A, Steck T L, Waseem A. J Biol Chem. 1989;264:6438–6446. [PubMed] [Google Scholar]
- 26.Jenkins J D, Kezdy F J, Steck T L. J Biol Chem. 1985;260:10426–10433. [PubMed] [Google Scholar]
- 27.Knull H R, Walsh J L. Curr Top Cell Regul. 1992;33:15–30. doi: 10.1016/b978-0-12-152833-1.50007-1. [DOI] [PubMed] [Google Scholar]
- 28.Ovádi J, Orosz F. In: Channelling in Intermediary Metabolism. Agius L, Sherratt H S A, editors. London: Portland Press; 1996. pp. 237–268. [Google Scholar]
- 29.Malamud D, Drysdale J W. Anal Biochem. 1978;86:620–647. doi: 10.1016/0003-2697(78)90790-x. [DOI] [PubMed] [Google Scholar]
- 30.Valentine W N, Paglia D E. Blood. 1984;64:583–591. [PubMed] [Google Scholar]
- 31.Farooqui A A, Horrocks L A. Biochem Soc Trans. 1998;26:243–246. doi: 10.1042/bst0260243. [DOI] [PubMed] [Google Scholar]
- 32.Schneider A S, Dunn I, Ibsen K H, Weinstein I M. In: Hereditary Disorders of Erythrocyte Metabolism. Beutler E, editor. New York: Grune & Stratton; 1968. pp. 273–279. [Google Scholar]
- 33.Wells K, Farooqui A A, Liss L, Horrocks L A. Neurochem Res. 1995;20:1329–1333. doi: 10.1007/BF00992508. [DOI] [PubMed] [Google Scholar]
- 34.Singh V K, Fudenberg H. J Clin Psychiatry. 1986;47:592–595. [PubMed] [Google Scholar]
- 35.Szelényi J, Páldi-Haris P, Hollán S. Immunol Lett. 1987;16:49–54. doi: 10.1016/0165-2478(87)90060-5. [DOI] [PubMed] [Google Scholar]
- 36.Björklund A, Svendsen C. Nature (London) 1999;397:569–570. doi: 10.1038/17495. [DOI] [PubMed] [Google Scholar]
- 37.Bjornson C R, Rietze R L, Reynolds B A, Magli M C, Vescovi A I. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 38.Burns R G. Cell Motil Cytoskeleton. 1991;20:181–189. doi: 10.1002/cm.970200302. [DOI] [PubMed] [Google Scholar]
- 39.Lansbury P T. Neuron. 1997;19:1151–1154. doi: 10.1016/s0896-6273(00)80406-7. [DOI] [PubMed] [Google Scholar]