Abstract
Adenovirus vectors induce acute inflammation of infected tissues due to activation of the innate immune system and expression of numerous chemokines and cytokines in transduced target cells. In contrast, adeno-associated virus (AAV) vectors are not associated with significant inflammation experimentally or clinically. We tested the ability of AAV vectors to induce the expression of chemokines in vitro and to activate the innate immune system in vivo. In human HeLa cells and murine renal epithelium-derived cells (REC cells) the adenovirus vector AdlacZ induced the expression of multiple inflammatory chemokines including RANTES, interferon-inducible protein 10 (IP-10), interleukin-8 (IL-8), MIP-1β, and MIP-2 in a dose-dependent manner. The use of AAVlacZ did not induce the expression of these chemokines above baseline levels despite 40-fold-greater titers than AdlacZ and greater amounts of intracellular AAVlacZ genomes according to Southern and slot blot analysis. This finding confirmed that the lack of AAVlacZ induction of chemokine was not due to reduced transduction. In DBA/2 mice, the intravenous administration of 2.5 × 1011 particles of AAVlacZ resulted in the rapid induction of liver tumor necrosis factor alpha (TNF-α), RANTES, IP-10, MIP-1β, MCP-1, and MIP-2 mRNAs. However, 6 h following injection, chemokine mRNA levels returned to baseline. As expected, administration of 10-fold less AdlacZ caused an induction of liver TNF-α and chemokine mRNAs that persisted for more than 24 h posttransduction. Whereas intravenous administration of 2.5 × 1011 particles of AAVlacZ triggered a transient infiltration of neutrophils and CD11b+ cells into liver, this response stood in contrast to widespread inflammation and toxicity induced by AdlacZ. Kupffer cell depletion abolished AAVlacZ but not AdlacZ-induced chemokine expression and neutrophil infiltration. In summary, these results show that AAV vectors activate the innate immune system to a lesser extent than do adenovirus vectors and offer a possible explanation for the reduced inflammatory properties of AAV compared to adenovirus vectors.
Gene therapy holds promise for the treatment of many inherited and acquired human diseases. Despite numerous early clinical trials, the effectiveness of human gene transfer and expression remains disappointingly low due to limitations in the current generation of vectors (36). The ideal vector for human application should permit targeted, highly efficient, long-term gene delivery and be devoid of significant toxicity (26, 36). Adenovirus vectors have several advantages. Recombinant adenoviruses are easy to manipulate and produce in high titer, can package large quantities of DNA, and have a broad cell tropism. Potent host inflammatory and immune responses, however, limit adenovirus vectors, resulting in transient gene expression and significant morbidity in vivo (17). Adeno-associated virus (AAV) vectors are increasingly being developed for human gene therapy. AAV vectors demonstrate long-term gene expression in vivo, a broad host range, and the ability to infect growth-arrested cells. In contrast to adenovirus vectors, AAV vectors have not been associated with acute inflammation (7, 8, 17). Since the use of AAV in human gene therapy protocols is on the rise, it is important to understand the biology of the host response to these vectors.
The acute inflammation induced by adenovirus vectors is due to the activation of target cells and the innate immune system (5, 32). It was previously demonstrated that adenovirus vectors induce the expression of chemokines following transduction in epithelial cells in vitro (5, 27). The induction of chemokines in these target cells is direct and occurs independently of virus transcription. In vivo, adenovirus vectors also activate the innate immune system (32, 40). The innate immune system is comprised of numerous cellular components including neutrophils, natural killer cells, and macrophages that recognize pathogens according to the pattern of their surface components rather than their specific antigenic sequences (14). An invading pathogen induces a cell to respond rapidly (within minutes to hours) and release an array of inflammatory mediators including cytokines and chemokines (31). The activation of innate immune responses in vivo also occurs in the absence of virus gene transcription (24, 32). The activation of target cells and the innate immune system is an important component of host recognition and response to adenovirus vectors (14).
The direct activation of target cells and the innate immune system by psoralen-inactivated adenovirus vectors suggests a role for the viral capsid in these responses. Thus, the differential induction of acute inflammation in vivo arising from adenovirus and AAV infection is not clear given the similar features of both vectors. Adenoviridae contain a linear double-stranded DNA genome, which is encapsulated in an icosahedral protein shell that is approximately 70 nm in diameter (17). Adenovirus particles bind to the 46-kDa coxsackievirus adenovirus receptor and are internalized by receptor-mediated endocytosis in clathrin-coated vesicles. αv integrins (αvβ1, αvβ3, αvβ5) are essential for the efficient internalization of adenovirus vectors (21, 28, 38). The AAV genome is encapsulated as a single-stranded DNA molecule. The nonenveloped virion is also icosahedral in shape but measures only 20 to 25 nm in diameter (7). Initial attachment of AAV to cells is mediated by interaction with heparan sulfate proteoglycan (34). Fibroblast growth factor receptor 1 has also been implicated in virus attachment (29). Using αvβ5 integrins as a coreceptor, AAV vectors gain entry into cells via receptor-mediated endocytosis in a manner similar to that of adenoviruses (2, 33).
The objective of this study is to determine the effects of AAV vector transduction on the host chemokine induction in vitro and on the activation of innate immune responses in vivo. We demonstrate that adenovirus but not AAV vectors induce chemokine expression in target cells at equivalent titers and transduction efficiencies in vitro. Furthermore, we show for the first time that AAV vectors transiently activate innate immune responses in vivo. These results increase our understanding of viral vector biology and provide a basis for the reduced inflammatory properties exhibited by AAV vectors.
MATERIALS AND METHODS
Virus vectors.
The type 5, E1-deleted, E3-defective (pJM17-based) adenovirus expressing Escherichia coli β-galactosidase (AdlacZ) under the control of the cytomegalovirus promoter (3, 16) was propagated in 293 cells and purified as previously described (4). After purification, virus was dialyzed against 4 liters of dialysis buffer containing 3% sucrose, 150 mM NaCl, 10 mM Tris (pH 7.4), and 1 mM MgCl2 for 4 h at 4°C and stored at −70°C. The control vehicle was made by dialyzing 2-ml aliquots of CsCl (1.34 mg/ml) without virus against the same dialysis buffer as that used for the viral preparations. The adenovirus vector concentration was determined by measuring the optical density at 260 nm as described by Mittereder et al. (25) and was expressed as particles per cell (part/cell). Vectors were screened for replication-competent adenovirus by plaque assay on HeLa cells and PCR and their concentration remained consistently <1 per 1010 particles.
AAVlacZ vectors were prepared from producer cell lines using wild-type Ad5 as described by Clark et al. (9) and purified by high-pressure liquid chromatography with a POROS HE1/M heparin column. Final vector preparations were dialyzed into buffer as above and stored at −70°C. The absence of replication-competent adenovirus in the AAVlacZ preparations was assayed by passing 1% of the purified vector stock onto 293 cells and scoring for adenovirus cytopathic effect after 7 days. Adenovirus contamination was consistently less than 1 infectious Ad particle per 1012 AAV particles, when evidenced at all. DNase-resistant particle values were determined using a Perkin-Elmer Applied Biosystems (Foster City, Calif.) Prism 7700 sequence detector as described previously (9).
Endotoxin testing.
Low-endotoxin H2O, buffers, and tissue culture reagents were used for vector production and experiments. Adenovirus and AAV vectors were routinely tested for the presence of endotoxin using the Limulus Amebocyte Lysate kit (Bio-Whittaker, Walkersville, Md.). All vectors contained <0.1 endotoxin units/ml of endotoxin.
Animal studies.
Male DBA/2 (H-2d) mice were obtained from Charles River Laboratories (Wilmington, Mass.) and housed under standard conditions. All animals were used at 10 to 12 weeks of age (25 to 30 g). Under methoxyfluorane general anesthesia, 2.5 × 109 to 2.5 × 1011 particles of AdlacZ or AAVlacZ was injected via the femoral vein in a total volume of 100 μl (vector plus sucrose vehicle). Control animals were treated with 100 μl of sucrose vehicle alone (3% sucrose, 150 mM NaCl, 10 mM Tris [pH 7.4], 1 mM MgCl2). Animals were allowed to recover and then were sacrificed at predetermined time points, and the livers and sera were harvested for analysis. For Kupffer cell depletion experiments, gadolinium chloride (GdCl3) (10 mg/kg of body weight in a total volume of 100 μl) was injected via the tail vein 48 and 24 h prior to viral vector administration. All animal studies were performed in accordance with the Animal Care Committee guidelines at the University of Calgary.
Cell culture.
The immortalized, nontransformed, epithelium-derived DBA/2 mouse kidney cell line (renal epithelium-derived cells [REC cells]) (5, 39) and human HeLa cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% penicillin-streptomycin (GIBCO-BRL, Rockville, Md.). Viral transductions were performed in six-well plates with 106 cells/well. Cells were incubated with 1 ml of medium per well containing viral vectors followed by incubation at 37°C in 5% CO2 for 1 or 6 h for RNase protection assays. Depending on the experiment, cells were harvested with trypsin or by direct lysis.
RNase protection assays.
Liver tissues and cells were processed for total RNA using RNeasy (Qiagen, Chatsworth, Calif.) according to the manufacturer's protocol. RNase protection assays were performed using the RiboQuant Multi-Probe RNase protection assay system (PharMingen, San Diego, Calif.). Briefly, by using the multiprobe template set mCK5-mCK3 for mouse cells or hCK5 for human cells, a [32P]UTP-labeled RNA probe was transcribed using T7 polymerase followed by phenol-chloroform extraction and ethanol precipitation. The concentration of the probe was adjusted to 3 × 105 cpm/μl (mCK3 and mCK5) or 3.1 × 105 (hCK5) cpm/μl. Seven micrograms of RNA per sample was hybridized to 6 × 105 cpm of total probe overnight at 56°C. Samples were then digested with RNase, followed by proteinase K treatment and phenol-chloroform extraction. After ethanol precipitation with 4 M ammonium acetate, protected samples were resuspended in 1× loading buffer and separated on 5.7% acrylamide-bisacrylamide urea gels. After drying, the gels were visualized by autoradiography.
Slot and Southern blot analysis.
To obtain total cell DNA, transduced cells were trypsinized for 5 min and washed several times with phosphate-buffered saline (PBS) to remove bound virus from the cell surface. The pellet was lysed in 300 μl of lysis buffer (100 mM NaCl, 10 mM Tris [pH 8.0], 25 mM EDTA [pH 8.0], 0.5% sodium dodecyl sulfate [SDS], 0.1 mg of proteinase K per ml) and incubated at 50°C for 2 h. Liver samples were lysed in 500 μl of the same buffer and incubated at 50°C overnight. Nucleic acids were thoroughly extracted with phenol-chloroform-isoamyl alcohol (25:24:1), and the aqueous layer was ethanol precipitated. The DNA was resuspended in double-distilled water. For Southern analysis, 3 μg of total DNA was digested with HindIII and separated on a 1% agarose gel. The gel was washed in 0.25 M HCl followed by another wash in a 0.4 M NaOH solution, and the DNA alkaline was transferred onto a Hybond XL nylon membrane (Amersham Pharmacia). A 3,121-bp fragment spanning the entire lacZ gene was labeled with [32P]dCTP with the Redprime random prime labeling system (Amersham Pharmacia) and served as probe. Hybridizations were performed by using 2 ng of labeled probe per ml in 5 ml of ExpressHyb solution (Clontech, Palo Alto, Calif.) at 60°C for 1 h. The membrane was then washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS for 30 min and twice in 0.2× SSC-0.1% SDS for 30 min at 50°C and exposed to radiographic film. For slot blot analysis 1 μg of total DNA from tissue culture cells or 2 μg of total DNA from liver samples was diluted with NaOH and EDTA to a final concentration of 0.4 M NaOH and 10 mM EDTA, heat denatured, and fixed onto a positively charged Hybond XL nylon membrane (Amersham Pharmacia) using a slot blot manifold. The membrane was subsequently rinsed with 0.4 M NaOH and hybridized as described above.
Histology and liver function tests.
Mice were sacrificed, and livers were harvested, rinsed in PBS, and fixed in 10% formalin. A portion of the liver was also snap-frozen in O.C.T. compound (Tissue Tek) and stored at −70°. Formalin-fixed tissues were embedded in paraffin. Five-micrometer-thick sections were stained with eosin and hematoxylin (for neutrophils, with chloroacetate esterase staining [Leder stain]) and analyzed by light microscopy in a blinded fashion.
Immunohistochemistry was performed by fixing 5-μm-thick frozen tissue sections for 10 min in acetone at 4°C followed by three washes in cold PBS for 10 min each. Sections for F4/80 staining to detect Kupffer cells were quenched in 0.3% H2O2 in PBS for 10 min. Sections were then blocked with rabbit serum for 30 min and avidin-biotin for 10 min (Vector Laboratories, Burlingame, Calif.) and were then incubated with a 1:500 dilution of rat anti-mouse CD11b (Mac-1) antibody (BD Pharmingen) or 1:100 dilution of the Kupffer cell-specific F4/80 (Serotec) for 1 h at room temperature. After being washed with PBS, sections were incubated with rabbit anti-rat immunoglobulin G (IgG) antibody for 30 min at room temperature and then stained using the ABC protocol and DAB substrate (Vector Laboratories). Leukocyte numbers were determined by counting the number of positive-stained cells over 40 fields at a magnification of ×40. The mean number of positive cells per high power field (hpf) was then calculated.
Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) determinations were performed on mouse serum using the Sigma Diagnostics transaminase kit as per the manufacturer protocol. Results are expressed as Sigma-Frankel (SF) units per milliliter.
Statistical analysis.
All experiments were performed at least in triplicate. Values are expressed as the mean ± standard deviation.
RESULTS
Adenovirus but not AAV vectors induce the expression of chemokines in vitro.
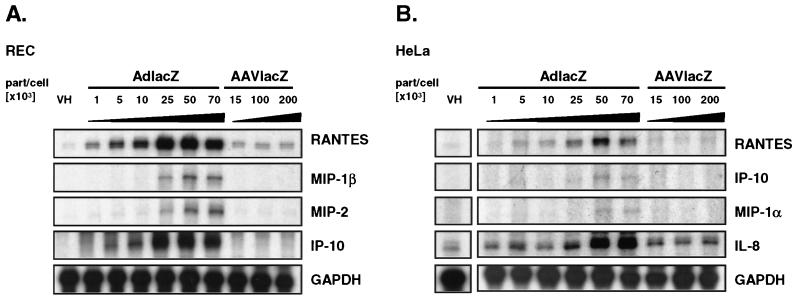
Chemokines are early mediators of the host inflammatory response to a variety of infectious agents (31). Adenovirus vectors have been shown to induce the expression of chemokines in nonmacrophage target cells independently of viral gene transcription (5, 41). To determine the ability of AAV vectors to induce chemokine expression in nonmacrophage cells, we transduced 106 REC cells (39) and human HeLa cells with increasing doses of AdlacZ and AAVlacZ. At 6 h following transduction, total RNA was analyzed for the induction of chemokine expression by RNase protection assay. Adenovirus vectors efficiently induced the expression of RANTES and IP-10 in REC cells at titers as low as 5 × 103 part/cell. AdlacZ also induced the expression of MIP-1β and MIP-2 but to a lower extent and at higher titers (25 × 103 to 70 × 103 part/cell). Transduction with AAVlacZ using titers as high as 200 × 103 part/cell failed to increase chemokine expression compared to vehicle-treated cells (Fig. 1A). The titer (200 × 103 part/cell) of AAV is 40-fold greater than that of the lowest concentration of adenovirus required to induce chemokine expression. In HeLa cells, AdlacZ efficiently induced the expression of RANTES and interleukin-8 (IL-8) in a dose-dependent manner. IP-10 and MIP-1α mRNAs were also increased following transduction with AdlacZ but to a much lower extent. As in REC cells, AAVlacZ failed to increase expression of these chemokines above baseline despite transduction with higher titers (Fig. 1B). Chemokine induction was not detected in REC or HeLa cells 1 h following transduction with either AAVlacZ or AdlacZ (data not shown).
FIG. 1.
Adenovirus and AAV vector-induced chemokine expression in vitro determined by an RNase protection assay of AdlacZ- or AAVlacZ-transduced cells. (A) REC cells were transduced with increasing titers of AdlacZ (1 × 103 to 70 × 103 part/cell) or AAVlacZ (15 × 103 to 200 × 103 part/cell). AdlacZ induced the expression of RANTES, MIP-1β, MIP-2, and IP-10 in a dose-dependent manner. In contrast, AAVlacZ failed to induce chemokine expression above baseline at titers 40-fold greater. (B) HeLa cells transduced with increasing titers of AdlacZ (1 × 103 to 70 × 103 part/cell) are induced to express RANTES, IP-10, MIP-1α, and IL-8 in a dose-dependent manner. AAVlacZ (15 × 103 to 200 × 103 part/cell) does not induce chemokine expression in HeLa cells. VH, sucrose vehicle.
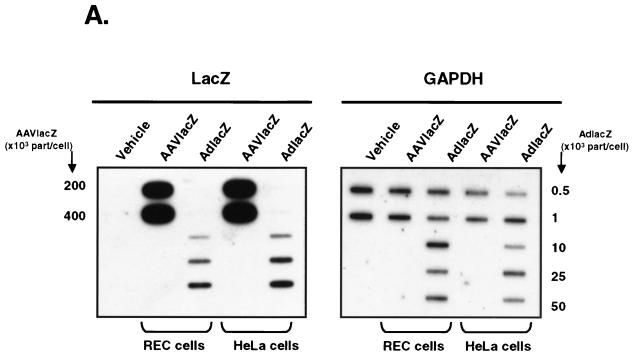
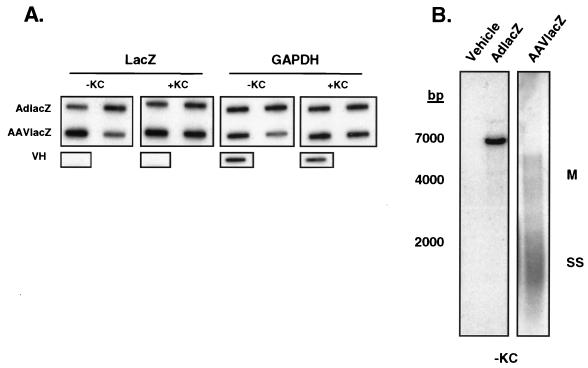
One potential explanation for the differential chemokine induction by AdlacZ and AAVlacZ may be from differences in cell transduction. To examine this possibility, we compared the transductions of HeLa cells and REC cells by AdlacZ and AAVlacZ from total DNA harvested 6 h following transduction. Vector genomes in total DNA were probed for the LacZ transgene in slot blot analyses. To ensure that equivalent amounts of total cell DNA were loaded in each slot, the blot was also hybridized with the housekeeping gene control glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Results (Fig. 2A) showed that the signal corresponding to AdlacZ vector DNA increased with increasing titers in both REC and HeLa cells. Total DNA from AAVlacZ-transduced REC and HeLa cells demonstrated a very strong lacZ signal corresponding with the high titers used (2 × 1011 to 4 × 1011 part/cell) (Fig. 2A). These findings confirmed that vector genomes in AAVlacZ- and AdlacZ-transduced REC and HeLa cells correlated with the infecting particle number.
FIG. 2.
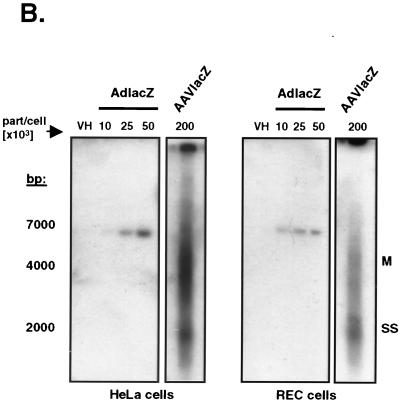
Transduction efficiency of adenovirus and AAV vectors in vitro. (A) Slot blot analysis of total DNA from AdlacZ- and AAVlacZ-transduced REC and HeLa cells. Probing for the lacZ reporter gene confirms that cellular viral genome content correlates directly with vector titer. (B) Southern blot of transduced cells probing for the lacZ reporter gene. Total DNA from AdlacZ-transduced REC and HeLa cells reveals a 7,500-bp fragment of adenovirus DNA containing the lacZ gene. AAVlacZ-transduced cells demonstrate multiple genome conformations characteristic of early AAV infection. SS, single-stranded vector genome; M, monomer.
Following cell entry, single-stranded AAV vector genomes slowly convert to double-stranded forms and concatemers. This process results in various intermediate forms during early infection (11, 23). Thus, to confirm that AAVlacZ genomes were intracellular as opposed to due to background contamination from cell surface-bound virus, we analyzed the DNA samples using Southern blot analyses. Probing for the lacZ transgene in HindIII-digested total DNA, a single 7,500-bp fragment was detected in AdlacZ-transduced REC and HeLa cells. The abundance of this band correlated with increasing titers of adenovirus vector used to transduce the cells (Fig. 2B). In contrast to the low-molecular-weight appearance of uninternalized AAV genomes (19), large-molecular-weight AAV vector genomes of various sizes were detected in AAVlacZ-transduced cells, characteristic of the early stages of AAV infection (11, 12, 23). Together these results demonstrate that in contrast to AdlacZ, AAVlacZ effectively transduces epithelium-derived target cells without inducing the expression of chemokines.
AAV vectors induce the expression of chemokines and cytokines in vivo.
The preceding studies were performed in cell culture and provided the basis for studies in whole animals. To determine the host response to AAV vectors in vivo, hepatic chemokine and cytokine mRNA levels were examined in DBA/2 mice following administration of adenovirus or AAV vectors. DBA/2 mice were injected intravenously with 2.5 × 109, 2.5 × 1010, or 2.5 × 1011 particles of AdlacZ or AAVlacZ. Animals were sacrificed at 1, 6, and 24 h following injection of virus. Both livers and sera were collected for analysis.
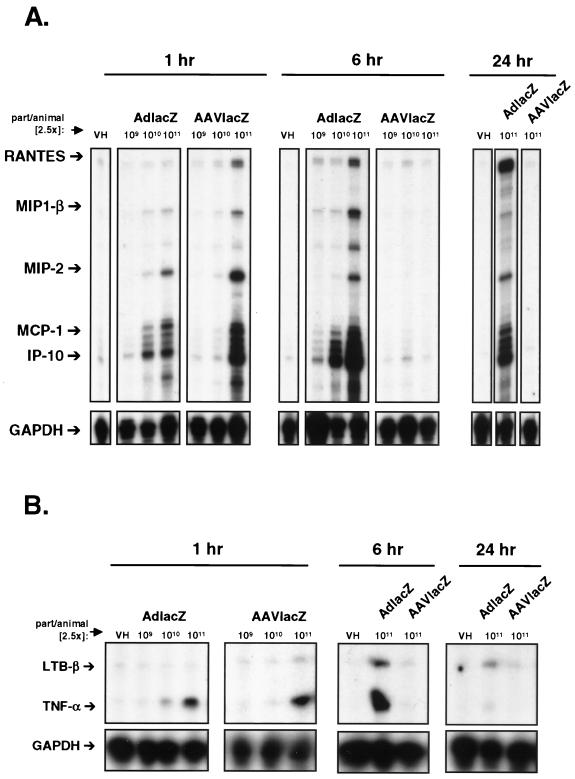
As previously reported, AdlacZ induced the expression of many inflammatory chemokines in the liver as determined by RNase protection assay (27). Results (Fig. 3A) showed that MIP-1β, MIP-2, MCP-1, and IP-10 mRNAs were induced in a dose-dependent manner as early as 1 h following the administration of AdlacZ. Furthermore, chemokine mRNA levels increased from 1 to 6 h and remained elevated for the duration of the experiment that was terminated at 24 h (Fig. 3A). Surprisingly, AAVlacZ also induced the expression of chemokine mRNAs in vivo. One hour following the intravenous administration of 2.5 × 1011 particles of AAVlacZ, RANTES, MIP-1β, MIP-2, MCP-1, and IP-10 mRNA levels were significantly upregulated. In contrast to what was observed with AdlacZ, however, chemokine mRNA expression was not induced following transduction with lower titers (2.5 × 109 and 2.5 × 1010 particles of AAVlacZ), suggesting a higher and more distinct threshold for AAVlacZ-induced chemokine expression in vivo. Interestingly, AAVlacZ-induced chemokine mRNA expression was transient and returned to undetectable levels within 6 h. Similar patterns of induction (Fig. 3B) were seen with the cytokines lymphotoxin β (LTB-β) and tumor necrosis factor alpha (TNF-α). At 6 h postinjection 2.5 × 1011 particles of AdlacZ induced the expression of TNF-α and LTB-β mRNAs with the highest level of induction. The same titer of AAVlacZ up-regulated TNF-α and LTB-β mRNAs at 1 h following vector administration but returned to baseline before 6 h (Fig. 3B). Transcripts for IL-6, IL-1β, and gamma interferon (data not shown) were not detectable at any time following the administration of AAVlacZ. These results show that AAV vectors induce the expression of chemokines and cytokines in vivo but that the response is transient and occurs at a higher threshold titer than that for adenovirus vectors.
FIG. 3.
Adenovirus and AAV vector-induced chemokine and cytokine expression in vivo. RNase protection assay of DBA/2 mouse liver RNA following the intravenous administration of 2.5 × 109, 2.5 × 1010, and 2.5 × 1011 particles of AdlacZ or AAVlacZ. (A) Chemokine mRNA expression. One, six, and twenty-four hours following injection, AdlacZ induced the expression of RANTES, MIP-1β, MIP-2, MCP-1, and IP-10 in a dose-dependent manner. AAVlacZ induced the expression of proinflammatory chemokines only 1 h following the administration of 2.5 × 1011 particles. Chemokine mRNA was not increased in mouse liver 6 and 24 h following the administration of 2.5 × 1011 particles of AAVlacZ. (B) Cytokine mRNA expression. LTB-β and TNF-α mRNAs are induced in a pattern similar to that of chemokines following AdlacZ and AAVlacZ administration. Data are representative samples of experiments performed with three animals per time point.
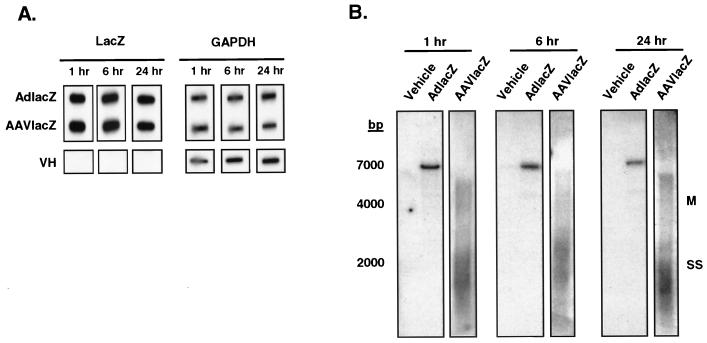
To confirm that both AAVlacZ and AdlacZ transductions of liver were equivalent, total liver DNA was analyzed using slot and Southern blot analysis for the LacZ transgene. Slot blot analysis revealed equivalent quantities of AdlacZ and AAVlacZ vector genomes in mouse livers at 1, 6, and 24 h following transduction (Fig. 4A). Furthermore, Southern blot analysis confirmed the variable sizes of AAV vector genomes typical of its intracellular location (Fig. 4B). These data confirm that differential inductions of chemokine and cytokine expression arising from adenovirus and AAV vectors are not due to differences in transduction efficiency.
FIG. 4.
Analysis of adenovirus and AAV vector transduction in vivo. (A) Slot blot analysis of mouse liver total DNA 1, 6, and 24 h following transduction with 2.5 × 1011 particles of AdlacZ or AAVlacZ. Probing for the lacZ reporter gene reveals equivalent amounts of AAV and adenoviral genome DNA in transduced mouse livers. (B) Southern blot analysis of mouse liver total DNA 1, 6, or 24 h after injection of 2.5 × 1011 particles of AdlacZ or AAVlacZ. Probing for the lacZ gene reveals a 7,500-bp fragment of adenovirus DNA in AdlacZ-transduced mice. AAVlacZ-transduced livers demonstrate multiple genome conformations characteristic of early AAV infection. SS, single-stranded vector genome; M, monomer. Data are representative samples of experiments performed with three animals per time point.
AAV vectors induce transient hepatic leukocyte infiltration in vivo.
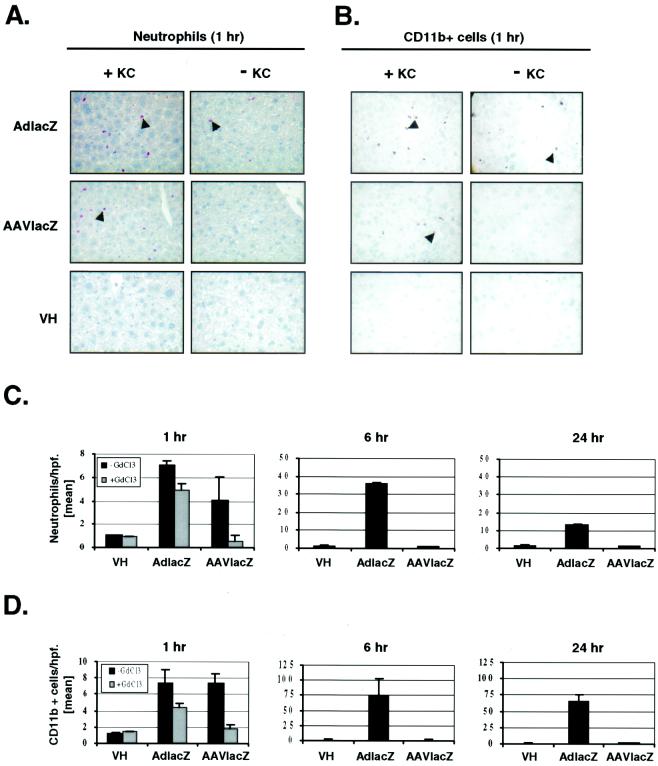
To determine whether AAV vector induction of chemokines and cytokines in the liver was associated with the recruitment of leukocytes and/or toxicity, livers of vector-injected animals were analyzed histologically. The intravenous administration of 2.5 × 1011 particles of AdlacZ increased both neutrophil (Fig. 5A) and CD11b+ (Fig. 5B) cell (neutrophils, natural killer cells, monocytes) recruitment to the liver over 1 h. Quantifying (Fig. 5C and D) the number of cells 1 h following viral administration showed that the livers transduced with AdlacZ contained 7.0 ± 0.5 (mean ± standard deviation [SD]) neutrophils per high-power field (hpf) (×40). This parameter increased to a maximum of 36.0 ± 1.0 cells/hpf followed by a decline to 13.0 ± 0.2 cells/hpf at 6 and 24 h, respectively (Fig. 5C). The recruitment of CD11b+ cells to the liver mirrored that of neutrophils. At 1 h following the administration of 2.5 × 1011 particles of AdLacZ, 7.1 ± 1.7 CD11b+ cells/hpf were detected in the liver. Recruitment of CD11b+ cells in the liver increased to 73.0 ± 30.0 cells/hpf followed by a reduction to 65.0 ± 11.0 cells/hpf at 6 and 24 h after AdlacZ administration, respectively (Fig. 5D).
FIG. 5.
Leukocyte infiltration following adenovirus or AAV vector transduction in vivo. (A) Leder (esterase) stain of liver sections showing infiltrating neutrophils in normal (+KC) or Kupffer cell-depleted (−KC) DBA/2 mice 1 h following the intravenous administration of 2.5 × 1011 particles of AdlacZ or AAVlacZ. (B) CD11b+ immunohistochemistry of liver sections showing infiltrating leukocytes in normal (+KC) or Kupffer cell-depleted (−KC) DBA/2 mice 1 h following the intravenous administration of 2.5 × 1011 particles of AdlacZ or AAVlacZ (magnification, ×40). Arrowheads indicate one representative stained cell. Quantitative analysis of neutrophil (C) and CD11b+ (D) cell infiltration in mouse liver 1, 6, or 24 h after intravenous administration of 2.5 × 1011 particles of AdlacZ or AAVlacZ. ▪, with Kupffer cells; ░⃞, depleted of Kupffer cells. Values represent mean cells per high power field ± SD (n = 3).
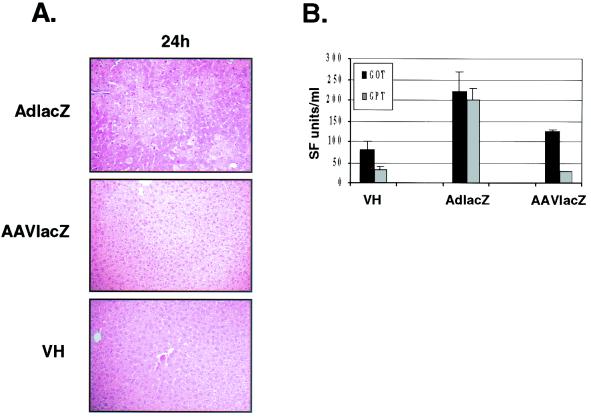
In contrast, the same titer of AAVlacZ only transiently induced leukocyte recruitment to the liver (Fig. 5A and B). At 1 h postinjection, AAVlacZ-transduced livers contained 7.5 ± 1.1 CD11b+ cells/hpf and 4.0 ± 2.0 neutrophils/hpf, similar to the leukocyte recruitment induced by AdlacZ. At 6 h following transduction, the number of infiltrating leukocytes had returned to baseline, similar to that of vehicle-treated animals (Fig. 5C and D). Morphologically, the administration of 2.5 × 1011 particles of AdlacZ induced extensive hepatic necrosis at 24 h as determined by hematoxylin and eosin staining. In contrast, the livers of animals that received vehicle or 2.5 × 1011 particles of AAVlacZ were histologically normal at 24 h (Fig. 6A). The lack of liver toxicity arising from the administration of AAVlacZ was consistent with reduced serum liver transaminase levels measured 24 h following vector administration (aspartate aminotransferase levels were 81 ± 28 SF units/ml for vehicle versus 221 ± 48 SF units/ml for AdlacZ versus 124 ± 4 SF units/ml for AAVlacZ [n = 3]; alanine aminotransferase levels were 31 ± 10 SF units/ml for vehicle versus 199 ± 27 SF units/ml for AdlacZ versus 26 ± 2 SF units/ml for AAVlacZ [n = 3]) (Fig. 6B). These results show that although AAV vectors induce leukocyte recruitment following liver transduction in vivo, it is transient and not associated with significant liver inflammation or toxicity. In contrast, adenovirus vectors are associated with a prominent and persistent host inflammatory response.
FIG. 6.
Liver morphology following adenovirus or AAV vector transduction in vivo. (A) Liver necrosis (seen as pale patchy areas) is detected at 24 h in mice administered 2.5 × 10 11 particles of AdlacZ, but not in AAV- or vehicle-treated animals (hematoxylin and eosin stain; magnification, ×20). Data are representative samples of experiments performed with three animals. (B) Serum aspartate aminotransferase (AST/GOT) (▪) and alanine aminotransferase (ALT/GPT) (░⃞) levels in mice 24 h following the administration of 2.5 × 1011 particles of AdlacZ or AAVlacZ. VH, vehicle-treated animals. Values represent mean SF units ± SD (n = 3).
AAV vector-induced chemokine expression and leukocyte recruitment are Kupffer cell dependent.
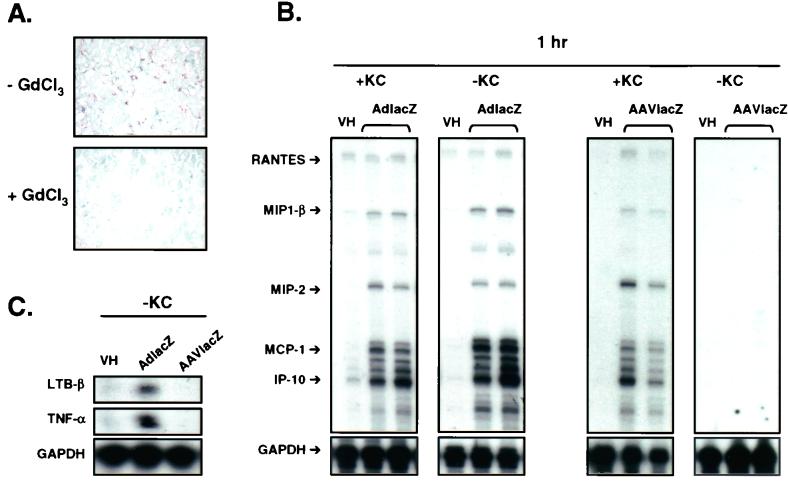
The source of chemokine and cytokine expression following the administration of AAV and adenovirus vectors may originate from transduced hepatocytes, Kupffer cells, or infiltrating inflammatory cells. Kupffer cells have been shown to play an instrumental role in the innate immune response to adenovirus vectors (22, 40). Therefore, we tested the abilities of both viral vectors to induce chemokine and cytokine expression and to recruit leukocytes in the absence of Kupffer cells. DBA/2 mice were depleted of Kupffer cells by using a modified GdCl3 protocol (15). Mice were injected intravenously with GdCl3 (10 mg/kg) 48 and 24 h prior to the intravenous administration of vehicle or 2.5 × 1011 particles of AdlacZ or AAVlacZ. At 24 h following the last injection of GdCl3, liver Kupffer populations were reduced by >90%. This value was determined by using the Kupffer cell-specific antibody F4/80 in immunohistochemical analysis of the samples (Fig. 7A). Blinded quantification of liver Kupffer cells revealed 0.9 ± 1 F4/80-positive cells/hpf (×40) in GdCl3-treated animals compared to 98 ± 22 F4/80-positive cells/hpf in normal animals. In vehicle-treated animals, liver chemokine and cytokine mRNA expression was not increased over baseline at 1, 6, and 24 h as determined by RNase protection assay. This finding confirmed that GdCl3 did not affect basal gene expression in the liver. Kupffer cell depletion did not affect AdlacZ-induced chemokine mRNA expression in the liver at 1 h following intravenous administration (Fig. 7B). In contrast, the induction of chemokines following intravenous administration of AAVlacZ was completely abolished in Kupffer cell-depleted animals at 1 h (Fig. 7B). Additionally, the expression of LTB-β and TNF-α mRNA was absent following AAVlacZ but not AdlacZ administration in Kupffer cell-depleted animals (Fig. 7C). Consistent with these results, Kupffer cell depletion also reduced AAVlacZ-induced hepatic leukocyte recruitment at 1 h to baseline (Fig. 5). Adenovirus vector-induced leukocyte recruitment to the liver was only slightly reduced in Kupffer cell-depleted animals. These results show that AAV but not adenovirus vector-induced chemokine and cytokine expression and leukocyte recruitment in the liver are dependent solely on Kupffer cells.
FIG. 7.
Adenovirus and AAV vector-induced chemokine and cytokine expression in Kupffer cell-depleted mice. (A) Kupffer cell immunohistochemistry in mouse liver following gadolidium chloride treatment. Liver Kupffer cells are reduced over 90% in mice receiving GdCl3. (B) RNase protection assay of mouse liver RNA, 1 h following intravenous administration of 2.5 × 1011 particles of AdlacZ or AAVlacZ in normal mice (+KC) or GdCl3-treated mice (−KC). Kupffer cell depletion abolishes AAVlacZ-induced chemokine mRNA expression at 1 h. VH, vehicle. Data are representative samples of experiments performed with four animals per group (results for two representative animals are shown). (C) LTB-β and TNF-α mRNA expression in GdCl3-treated mice (−KC) 1 h following the administration of 2.5 × 1011 particles of AdlacZ or AAVlacZ.
To determine whether the reduction in chemokine and cytokine expression following Kupffer cell depletion in AAVlacZ-infected mice was due to decreased liver transduction, viral genomes were assessed by slot and Southern blot analysis of total liver DNA. Probing for the LacZ transgene, slot blot analysis revealed similar amounts of AAVlacZ and AdlacZ genomes in the livers of Kupffer cell-depleted animals at 1 h (Fig. 8A). Southern blot analysis of total liver DNA again revealed multiple species of AAV genomes, confirming its intracellular location (Fig. 8B). These data confirm that Kupffer cell depletion does not substantially reduce the transduction of liver by AAVlacZ.
FIG. 8.
Analysis of adenovirus and AAV vector transduction in Kupffer cell-depleted mice. (A) Slot blot analysis of mouse liver total DNA 1 h following the administration of 2.5 × 1011 particles of AdlacZ or AAVlacZ to normal (+KC) and GdCl3-treated (−KC) animals. Probing for the lacZ reporter gene reveals equivalent amounts of AAV and adenoviral genome DNA in transduced mouse livers. Data are representative samples of experiments performed with four animals per group (results for two representative animals are shown). (B) Southern blot analysis of mouse liver total DNA, 1 h following the administration of 2.5 × 1011 particles of AdlacZ or AAVlacZ to Kupffer cell-depleted mice. Probing for the lacZ gene reveals a 7,501-bp fragment of adenovirus DNA in AdlacZ-transduced mice. AAVlacZ-transduced livers demonstrate multiple genome conformations, characteristic of early AAV infection. SS, single-stranded vector genome; M, monomer; VH, vehicle.
DISCUSSION
The relative lack of inflammatory responses in AAV-transduced hosts compared to the acute toxicity and inflammation induced by adenovirus vectors prompted us to embark on the studies in this report (17). Although these observations have been documented in numerous studies in vivo (13, 18, 37), the potential molecular mechanism(s) underlying these divergent responses is not clear. The results of our studies in vitro showed that adenovirus but not AAV vectors induced the expressions of various chemokines in two epithelium-derived cell lines, REC and HeLa. The differential induction of chemokines was evident in vitro despite exposure of cells to AAV titers that were 40-fold higher than the lowest titer of adenovirus required to initiate the response. Since cells cultured in vitro do not reflect the response in the whole animal, mice were used to examine whether findings in culture extended to an in vivo model. We found that AAV vectors in vivo induced only transient chemokine and cytokine expression and leukocyte recruitment in the liver. This response was not associated with significant toxicity or inflammation. In contrast, the chemokine and cytokine induction following exposure of mice to adenovirus vectors was rapid, marked, and sustained. These features stand in direct contrast to what is observed with AAV and confirm the differences in host responses to these two vectors.
The early host response to viral infection involves interaction between two components, the infected target cell and the innate immune system (14). We and others have previously demonstrated with epithelium-derived target cells in vitro that adenovirus vectors induce the expression of chemokines (1, 5, 27). The molecular basis for adenovirus vector induction of host inflammatory genes has been studied in nonmacrophage target cells. For example, adenovirus transduction of HeLa cells activates Raf-1 and the extracellular signal-regulated kinase signaling pathway. These events trigger expression of the C-X-C chemokine IL-8 (6). It was previously shown in epithelium-derived REC cells that the nuclear translocation of NF-κB is an important component of adenovirus vector induction of the C-X-C chemokine IP-10 (5). These findings illustrate the ability of adenovirus vectors to induce the expression of host inflammatory genes.
Unlike the situation with adenovirus vectors, the impact of AAV vector transduction in target cells has not been extensively studied. Recently, Stilwell and Samulski reported the effects of AAV-2 and adenovirus vectors on cellular gene expression in lung fibroblasts in studies using DNA microarray technology (J. Stilwell and R. J. Samulski, •••, Mol. Ther., p. 131, 2001.). Their findings parallel the results in this report. AAV demonstrated minimal effects on cellular gene expression in transduced cells, while adenovirus vectors increased the mRNA levels of many genes including those encoding cytokines and chemokines and stress response genes. Unlike the adenovirus, AAV vectors may lack a feature or features that enable them to activate signal transduction pathways following viral cell entry. It is possible that differences in surface receptor usage, virus internalization, or intracellular trafficking may result in differential activation of signaling pathways by these two vectors. For example, there is evidence suggesting that AAV particles enter the nucleus prior to uncoating and this pathway differs from the entry of adenovirus particles (2). Furthermore, AAV virions may need to be routed as far as the late endosome before penetration into the cytosol. This route contrasts with the early endosome escape during adenovirus infection (10). The inability of AAV vectors to induce the expression of host genes in target cells may underlie the truncated inflammatory response induced by these agents in vivo.
The target cell response acts in collaboration with the innate immune system in the early host response to invading pathogens, including viruses (14). Effector cells of the innate immune system include macrophages, neutrophils, and natural killer cells that serve as the primary defense to infection. Adenovirus vectors efficiently activate the innate immune system, a response that leads to acute inflammation of transduced tissues and reduced gene transfer efficiency (22, 32, 35, 41). Resident macrophages such as Kupffer cells in the liver and alveolar macrophages in the lung are significant components of the innate immune system that mediate the acute inflammatory response to adenovirus vectors (32, 35, 41). In addition to performing important phagocytic and antigen presenting functions, macrophages secrete cytokines and chemokines in response to viral infection, which in turn trigger multiple effects (22, 40). These effects include leukocyte recruitment to infected tissues and the participation of other effector cells of the immune system, resulting in a cascade of events that propagate the inflammatory response (30). The results of our studies in vivo show a Kupffer cell-dependent activation of innate immune responses following AAV vector administration. While this outcome is unexpected, it is not surprising given the role of Kupffer cells in the first line of defense against invading pathogens in the liver (20). Although AAV vectors induced transient chemokine and cytokine expression and leukocyte recruitment to the liver, this response did not result in detectable tissue damage or inflammation. In contrast, the induction of chemokines and cytokines by adenovirus vectors was greater in magnitude, prolonged, and associated with widespread liver inflammation. These findings suggest that a sustained increase in chemokine and cytokine expression appears to be required to trigger more-severe inflammation in the liver.
The depletion of Kupffer cells abolished AAV but not adenovirus vector-induced chemokine and cytokine expression in the liver. Yet, AAV vector transduction of the liver was not affected by Kupffer depletion, suggesting that AAV vectors did not activate other cells in the liver, such as hepatocytes. The differential induction of chemokines in vivo by AAVlacZ and AdlacZ in the absence of Kupffer cells is consistent with our in vitro data demonstrating a lack of chemokine induction by AAV vectors in nonmacrophage target cells. While macrophages may simply be more sensitive than epithelial cells to AAV transduction, these results raise the possibility that AAV vectors interact with macrophage and nonmacrophage cells via different mechanisms.
The induction of chemokines and cytokines by AAV vectors in vivo is likely not due to viral gene or transgene expression. Recombinant AAV vectors lack wild-type viral genes, and furthermore, lacZ expression in AAVlacZ-transduced livers was not detectable within 24 h (data not shown). The differential activation of chemokines and cytokines by adenovirus and AAV vectors is also not likely the result of adenovirus gene expression. Transcription-defective adenovirus particles have been shown to induce chemokine and cytokine expression in vitro and induce acute inflammation in vivo (5, 24, 27, 32).
Viral vector-induced immunity and toxicity limit the success of human gene therapy. Our results demonstrate, for the first time, the involvement of the innate immune system in the host response to AAV vectors. While AAV vectors transiently activate cells of the innate immune system, they have a limited capacity to trigger the expression of proinflammatory genes and induce inflammation in transduced tissues. The impact of the innate immune system on AAV-mediated gene transfer remains to be determined. Since the innate immune system significantly reduces adenovirus gene transfer efficiency (35), the activation of innate immune responses may also affect AAV-mediated gene delivery. Understanding the molecular mechanism underlying the differential activation of innate immune responses between adenovirus and AAV vectors is expected to facilitate the design of safer, more-effective vectors for human gene therapy.
Acknowledgments
We thank Suranga Wijesuriya and Susan Hui for their technical assistance.
This study was funded by a grant from the Canadian Institutes of Health Research (CIHR). D.A.M. is a recipient of a Clinical Investigator award from the Alberta Heritage Foundation for Medical Research and a Scholarship award from CIHR.
REFERENCES
- 1.Amin, R., R. Wilmott, Y. Schwarz, B. Trapnell, and J. Stark. 1995. Replication-deficient adenovirus induces expression of interleukin-8 by airway epithelial cells in vitro. Hum. Gene Ther. 6:145-153. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. S., R. Wilcher, and R. J. Samulski. 2000. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 74:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, T. C., H. BeltrandelRio, R. J. Noel, J. H. Johnson, and C. B. Newgard. 1994. Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J. Biol. Chem. 269:21234-21238. [PubMed] [Google Scholar]
- 4.Becker, T. C., R. J. Noel, W. S. Coats, A. M. Gomez-Foix, T. Alam, R. D. Gerard, and C. B. Newgard. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43:161-189. [DOI] [PubMed] [Google Scholar]
- 5.Borgland, S. L., G. P. Bowen, N. C. Wong, T. A. Libermann, and D. A. Muruve. 2000. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-κB. J. Virol. 74:3941-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bueler, H. 1999. Adeno-associated viral vectors for gene transfer and gene therapy. Biol. Chem. 380:613-622. [DOI] [PubMed] [Google Scholar]
- 8.Carter, P. J., and R. J. Samulski. 2000. Adeno-associated viral vectors as gene delivery vehicles. Int. J. Mol. Med. 6:17-27. [DOI] [PubMed] [Google Scholar]
- 9.Clark, K. R., X. Liu, J. P. McGrath, and P. R. Johnson. 1999. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum. Gene Ther. 10:1031-1039. [DOI] [PubMed] [Google Scholar]
- 10.Douar, A. M., K. Poulard, D. Stockholm, and O. Danos. 2001. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J. Virol. 75:1824-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari, F. K., T. Samulski, T. Shenk, and R. J. Samulski. 1996. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 70:3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher, K. J., G. P. Gao, M. D. Weitzman, R. DeMatteo, J. F. Burda, and J. M. Wilson. 1996. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 70:520-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher, K. J., K. Jooss, J. Alston, Y. Yang, S. E. Haecker, K. High, R. Pathak, S. E. Raper, and J. M. Wilson. 1997. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 3:306-312. [DOI] [PubMed] [Google Scholar]
- 14.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 15.Hardonk, M. J., F. W. Dijkhuis, C. E. Hulstaert, and J. Koudstaal. 1992. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J. Leukoc. Biol. 52:296-302. [DOI] [PubMed] [Google Scholar]
- 16.Herz, J., and R. D. Gerard. 1993. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc. Natl. Acad. Sci. USA 90:2812-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kay, M. A., J. C. Glorioso, and L. Naldini. 2001. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat. Med. 7:33-40. [DOI] [PubMed] [Google Scholar]
- 18.Kay, M. A., C. S. Manno, M. V. Ragni, P. J. Larson, L. B. Couto, A. McClelland, B. Glader, A. J. Chew, S. J. Tai, R. W. Herzog, V. Arruda, F. Johnson, C. Scallan, E. Skarsgard, A. W. Flake, and K. A. High. 2000. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 24:257-261. [DOI] [PubMed] [Google Scholar]
- 19.King, J. A., R. Dubielzig, D. Grimm, and J. A. Kleinschmidt. 2001. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 20:3282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laskin, D. L., B. Weinberger, and J. D. Laskin. 2001. Functional heterogeneity in liver and lung macrophages. J. Leukoc. Biol. 70:163-170. [PubMed] [Google Scholar]
- 21.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik, A. K., P. E. Monahan, D. L. Allen, B. G. Chen, R. J. Samulski, and K. Kurachi. 2000. Kinetics of recombinant adeno-associated virus-mediated gene transfer. J. Virol. 74:3555-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy, R. D., B. L. Davidson, B. J. Roessler, G. B. Huffnagle, S. L. Janich, T. J. Laing, and R. H. Simon. 1995. Pulmonary inflammation induced by incomplete or inactivated adenoviral particles. Hum. Gene Ther. 6:1553-1560. [DOI] [PubMed] [Google Scholar]
- 25.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monahan, P. E., and R. J. Samulski. 2000. AAV vectors: is clinical success on the horizon? Gene Ther. 7:24-30. [DOI] [PubMed] [Google Scholar]
- 27.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 28.Nemerow, G. R., and P. L. Stewart. 1999. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 63:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qing, K., C. Mah, J. Hansen, S. Zhou, V. Dwarki, and A. Srivastava. 1999. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 5:71-77. [DOI] [PubMed] [Google Scholar]
- 30.Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217-242. [DOI] [PubMed] [Google Scholar]
- 31.Schluger, N. W., and W. N. Rom. 1997. Early responses to infection: chemokines as mediators of inflammation. Curr. Opin. Immunol. 9:504-508. [DOI] [PubMed] [Google Scholar]
- 32.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708-722. [DOI] [PubMed] [Google Scholar]
- 33.Summerford, C., J. S. Bartlett, and R. J. Samulski. 1999. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat. Med. 5:78-82. [DOI] [PubMed] [Google Scholar]
- 34.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao, N., G. P. Gao, M. Parr, J. Johnston, T. Baradet, J. M. Wilson, J. Barsoum, and S. E. Fawell. 2001. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 3:28-35. [DOI] [PubMed] [Google Scholar]
- 36.Verma, I. M., and N. Somia. 1997. Gene therapy--promises, problems and prospects. Nature 389:239-242. [DOI] [PubMed] [Google Scholar]
- 37.Wang, L., K. Takabe, S. M. Bidlingmaier, C. R. Ill, and I. M. Verma. 1999. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc. Natl. Acad. Sci. USA 96:3906-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wickham, T. J. 2000. Targeting adenovirus. Gene Ther. 7:110-114. [DOI] [PubMed] [Google Scholar]
- 39.Wuthrich, R. P., L. H. Glimcher, M. A. Yui, A. M. Jevnikar, S. E. Dumas, and V. E. Kelley. 1990. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 37:783-792. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]
- 41.Zsengeller, Z., K. Otake, S. A. Hossain, P. Y. Berclaz, and B. C. Trapnell. 2000. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J. Virol. 74:9655-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]