Abstract
Neurons of the mammalian central nervous system (CNS) are an essential and largely nonrenewable cell population. Thus, virus infections that result in neuronal depletion, either by virus-mediated cell death or by induction of the cytolytic immune response, could cause permanent neurological impairment of the host. In a transgenic mouse model of measles virus (MV) infection of neurons, we have previously shown that the host T-cell response was required for resolution of infection in susceptible adult mice. In this report, we show that this protective response did not result in neuronal death, even during the peak of T-cell infiltration into the brain parenchyma. When susceptible mice were intercrossed with specific immune knockout mice, a critical role for gamma interferon (IFN-γ) was identified in protection against MV infection and CNS disease. Moreover, the addition of previously activated splenocytes or recombinant murine IFN-γ to MV-infected primary neurons resulted in the inhibition of viral replication in the absence of neuronal death. Together, these data support the hypothesis that the host immune response can promote viral clearance without concomitant neuronal loss, a process that appears to be mediated by cytokines.
CD8+ cytotoxic T lymphocytes (CTL) mediate their antiviral effects by recognizing viral peptides presented by class I major histocompatibility complex (MHC) molecules on infected target cells. Engagement of the T-cell receptor with the peptide-loaded MHC leads to perforation of the target cell membrane, delivery of cytolytic granules, and induction of apoptotic death of the infected target cell (37, 56). Thus, CTL serve a crucial role in restricting viral spread by selectively eliminating infected cells. In most tissues, the loss of infected cells is accompanied by enhanced uninfected cell division to replenish the cell population. However, immune-mediated lysis of infected cells may not be an optimal strategy for clearance of all virus infections, especially those that involve tissues with little capacity for renewal, such as the central nervous system (CNS) (38, 42).
To limit immune cell entry into the CNS and to restrict T-cell-neuron interactions, multiple anatomical and biochemical barriers exist within the brain, including the presence of the blood-brain barrier, limited lymphatic drainage from the CNS, and the paucity of class I MHC molecules on resident brain cells (38, 49). These properties collectively contribute to an “immune-privileged” environment under normal conditions. Nevertheless, activated T lymphocytes patrol the CNS parenchyma (17, 53) and are recruited into the brain after infection by many neurotropic viruses (2, 19, 30, 41). In some circumstances, including herpes simplex virus encephalitis, such inflammatory responses are associated with marked CNS damage (19). In others, however, the immune response appears able to resolve an infection without apparent damage to the host, although the basis of such virus clearance remains poorly understood.
Previous work from our laboratory and others suggested that the host immune response may mediate viral clearance via noncytolytic strategies. For example, immune-mediated clearance of a persistent lymphocytic choriomeningitis virus (LCMV) infection within the CNS is noncytopathic (33, 42, 50) and requires the presence of T-cell-elaborated cytokines such as gamma interferon (IFN-γ) (51). A similar dependence on noncytolytic immune clearance has also been shown for Sindbis virus (2), mouse hepatitis virus (26, 34, 48), and vesicular stomatitis virus (22-24). Noncytolytic viral clearance is not restricted to the CNS: in a transgenic mouse model of hepatitis B virus infection of the liver, cytokines were also shown to be crucial for reduction of viral load (14, 15). However, in each of these model systems, either the immune response must be adoptively transferred into persistently infected or otherwise tolerant mice, or the infection occurs in multiple cell types in which different clearance mechanisms may be operative (5, 12, 27).
To define the role of the host antiviral immune response in the resolution of a neuron-restricted virus infection, we employed a transgenic mouse model in which a human measles virus (MV) receptor, CD46, was expressed specifically in CNS neurons under the transcriptional control of the rat neuron-specific enolase (NSE) promoter (39). We have previously shown that these mice support infection by MV, Edmonston strain (MV-Ed), but that the pathogenic outcome of infection is age dependent (39). Neonatal NSE-CD46+ mice develop an unrestricted neuronal infection and fatal CNS disease, whereas adult mice mount a rapid and robust T-cell response which protects them from neurological impairment and death (25). We show here, by using primary neuron cultures and knockout mice deficient in components of the host response, that the adult immune response noncytolytically protects against MV infection and CNS disease. This process appears to be dependent on cytokines such as IFN-γ.
MATERIALS AND METHODS
Cells and virus.
Vero fibroblasts were maintained in Dulbecco modified Eagle medium (Life Technologies, Grand Island, N.Y.), supplemented with 5% fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 ng of streptomycin per ml. Primary hippocampal neurons were prepared from day 16 embryonic mice, as described previously (1, 35, 40), except that the cells were maintained in Neurobasal Medium (Life Technologies) containing 4 μg of glutamate/ml in the absence of an astrocyte feeder layer. These cultures are routinely >95% MAP-2+ (data not shown). All cells were maintained at 37°C in a humidified incubator with 5% CO2.
MV-Ed was purchased from American Type Culture Collection (Manassas, Va.) and passaged and titered in Vero fibroblasts. LCMV Armstrong (LCMV-Arm; a gift from Michael Oldstone, The Scripps Research Institute) was passaged on BHK-21 fibroblasts and plaque purified, and titers were determined on Vero fibroblasts.
Mice.
Inbred C57BL/6 (H-2b) and homozygous NSE-CD46 transgenic mice (line 18; H-2b) (39) were maintained in the closed mouse breeding colony of The Fox Chase Cancer Center. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Homozygous NSE-CD46+ and haplotype-matched homozygous immune knockout (KO) mice with targeted gene deletions were intercrossed for three generations to obtain CD46+ mice on the desired KO background and maintained by backcrossing to the appropriate KO mice. Genotypes of all mice used in these experiments were confirmed prior to infection. CD4 KO (21), β-2-microglobulin KO (β-2-mic KO [55]), and RAG-2 KO (44) progeny were identified by flow cytometry on peripheral blood lymphocytes immunostained with fluorophore-conjugated antibodies to mouse CD4 and CD8 antigens. Genotypes of IFN-γ KO (6) and perforin KO (20) mice were determined by PCR analysis of DNA isolated from tail biopsy samples.
Mice were infected with 104 PFU of MV-Ed. The inoculum was diluted in phosphate-buffered saline (PBS) and delivered intracerebrally to metofane-anesthetized mice along the midline, in a volume of 20 μl, by using a sterile 27-gauge needle. Additional mice were challenged with 2 × 105 PFU of LCMV-Arm, delivered intraperitoneally in a volume of 200 μl.
RNA hybridization.
Brains were removed from infected mice at 1, 3, 5, 7, and 13 days postinfection (three mice/group), quick-frozen in liquid nitrogen, and homogenized in Tri-Reagent (Sigma Chemical Co., St. Louis, Mo.) by using a tissue homogenizer (Virtis, Gardiner, N.Y.). Total RNA was isolated according to the manufacturer's instructions and resuspended in diethyl pyrocarbonate (DEPC)-treated water. Then, 5-μg RNA samples were mixed to a final concentration of 48% formamide and 6.6% formaldehyde and incubated at 68°C for 15 min. The samples were then chilled on ice and diluted twofold with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The RNA samples were applied to a nylon membrane by using a slot blot apparatus; the membrane was UV cross-linked and hybridized with a radioactive probe at 68°C for 1 h (QuikHyb; Stratagene, La Jolla, Calif.). DNA probes were labeled by random priming (Prime-It II; Stratagene) by using [32P]dCTP (Dupont-NEN, Boston, Mass.). A DNA fragment from peF1 (4) was used as a template for hybridization to F as a representative MV RNA. The signal intensity of radioactive slots was quantified by phosphorimager analysis (Fuji).
Immunohistochemical analysis of mouse tissues.
Brains from 4 to 5 mice/time point postinfection were removed, immersed in tissue embedding compound (Fisher Scientific, Pittsburgh, Pa.), snap-frozen in a dry ice-isopentane bath, and stored at −70°C. Horizontal cryosections (10 μm) were air dried and stored at −70°C. On the day of staining, sections were fixed in ice-cold 95% ethanol, rehydrated in PBS, and blocked for 20 min with 0.1% bovine serum albumin-PBS (Sigma), followed by an avidin and biotin block (Vector Laboratories, Burlingame, Calif.). To detect MV-infected cells, a human immune serum (a gift from Michael Oldstone) was used at a dilution of 1:2,000, followed by a biotinylated anti-human secondary antibody (1:300; Vector). For LCMV immunostaining, a polyclonal mouse anti-LCMV antibody (American Type Culture Collection) was used at a dilution of 1:750, followed by a biotinylated, anti-mouse immunoglobulin G secondary antibody. Rat anti-mouse CD4 (clone RM4-5; 1:100; Pharmingen, San Diego, Calif.) or rat anti-mouse CD8α/CD8β antibodies (clones 53-6.7 and 53-5.8, respectively; 1:100 each; Pharmingen) were used to identify CD4+ and CD8+ T lymphocytes. To detect B cells, rat anti-mouse B220 (Pharmingen) was used at a dilution of 1:1,000. These sections were then incubated for 1 h at room temperature with a biotinylated anti-rat immunoglobulin G secondary antibody at a 1:200 dilution (Vector). Sections labeled with biotinylated antibodies were treated for 30 min with a streptavidin-peroxidase conjugate (ABC Elite; Vector), followed by visualization with diaminobenzidine (0.7 mg/ml in 60 mM Tris) and urea-H2O2 (0.2 mg/ml), purchased as preweighed tablets (Sigma). All cells were counterstained with hematoxylin (Sigma) and mounted with an aqueous mounting medium (Crystal Mount; Fisher). Uninfected tissues or omission of the primary antibody served as negative controls. For all histological analyses, at least three sections per brain were examined from three different horizontal levels, and at least four mice per experimental group were assessed.
TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assays.
Cryosections were fixed in 10% neutral buffered formalin, postfixed in ethanol-acetic acid (2:1), and quenched for endogenous peroxidase activity with 3% hydrogen peroxide in PBS. Sections were labeled with a terminal deoxynucleotidyltransferase (TdT)-digoxigenin-deoxynucleoside triphosphate mixture (Intergen, Purchase, N.Y.). In order to end label, slides were equilibrated, covered with working-strength TdT in reaction buffer, and incubated in a humidified chamber at 37°C for 1 h. Reactions were washed in stop buffer. An anti-digoxigenin peroxidase conjugated-antibody was used to detect end-labeled DNA fragments. This step was followed by visualization with diaminobenzidine as previously described. All tissues were counterstained with methyl green (Sigma) and mounted in Crystal Mount (Fisher).
Sickness assays.
Mice were weighed throughout infection by using a digital balance, and the weights were recorded in grams to the first decimal place. In addition, mice were assessed by using two subjective measures of health. Balance was assayed by placing the infected mouse on a three-quarter-inch wooden dowel rod and scored as follows: 0, mouse balanced on rotating rod for >20 s; 1, mouse balanced on rod for 5 to 20 s; 2, mouse balanced on rod for <5 s; and 3, mouse demonstrated no ability to grip rod. Movement was also documented following placement of infected mice into an 8-in. circle imprinted in the base of a 2-in. by 2-in. opaque plastic container. Scores were assigned as follows: 0, movement out of the circle occurred in <5 s; 1, movement out of the circle occurred between 5 to 20 s; 2, movement out of the circle took >20 s and/or evidence of seizures or tremors were noted during a 1-min observation period; and 3, hindlimb paralysis.
Ex vivo viral clearance assays.
Primary hippocampal neurons were infected with either MV-Ed or LCMV-Arm at a multiplicity of infection (MOI) of 3. Either immediately after infection or 24 h postinfection (hpi), lymphocytes or recombinant IFN-γ was added to the cultures. Red blood cell-depleted, bulk splenocytes were isolated from both perforin-deficient mice and C57BL/6 mice that had been challenged with LCMV 7 days previously or that were uninfected. Splenocytes were added to MV- or LCMV-infected primary hippocampal neurons at effector/target ratios of 25:1 for cell death assays and 10:1 for viral RNA clearance assays. In separate studies, recombinant mouse IFN-γ, heat-inactivated mouse IFN-γ (65°C, 30 min), or PBS-0.2% bovine serum albumin was also added to MV- or LCMV-infected neurons. After an extensive washing, neurons were harvested 24 h later by scraping them in Tri-Reagent (Sigma). Total RNA was isolated according to the manufacturer's instructions and resuspended in DEPC-treated water. The RNA samples were then analyzed by slot blot as described above. LCMV RNA was hybridized with a radiolabeled probe derived from the cloned LCMV nucleoprotein gene. To determine the proportion of infected cells in some experiments, cells on coverslips included in the cultures were fixed at the time of harvesting and immunostained for the presence of viral antigen, and the immunopositive cells were counted.
RESULTS
Kinetics of immune-mediated resolution of neuronal MV infection.
We previously demonstrated (25) that the adaptive immune response of adult NSE-CD46+ transgenic mice was essential for protection against MV infection, since CD46+ mice backcrossed to T- and B-cell-deficient RAG-2 KO mice developed unrestricted neuronal infection and CNS disease after MV inoculation. To explore the basis of this protection, we performed an extensive time course analysis of brain tissues collected from immunocompetent, NSE-CD46+ adults (6 to 8 weeks of age) after MV challenge.
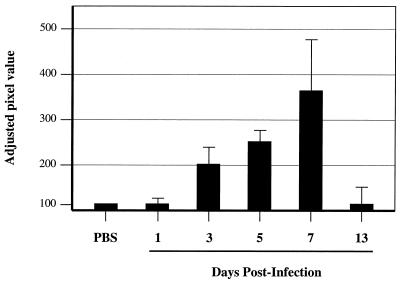
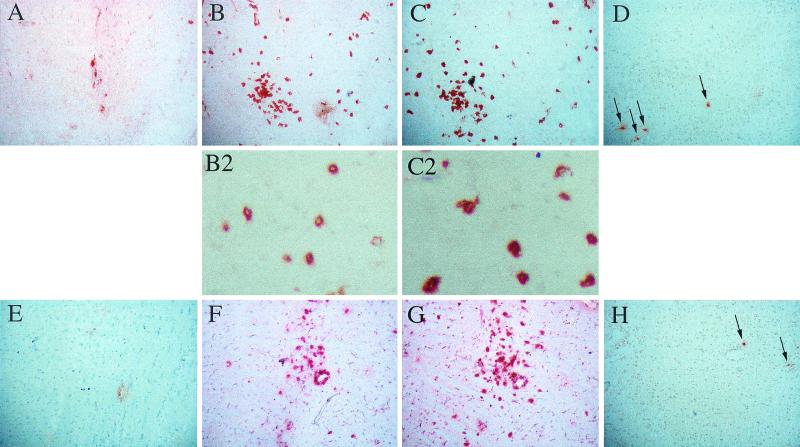
Mice were inoculated intracerebrally with 104 PFU of MV-Ed, and brains were collected at specific time points throughout infection for RNA and immunohistological analysis. Virus-infected neurons were first detectable at 3 to 4 days postinoculation (dpi) by both methods (Fig. 1; Table 1), reaching maximal infection at 7 to 10 dpi (Fig. 2A). The appearance of virus-infected neurons in the CNS correlated with the entry of both CD4+ and CD8+ T lymphocytes (Fig. 2B and C). As previously reported (25), B220+ cells were rarely observed in the infected CNS, representing maximally 3% of the immune cell infiltrate. After 13 dpi, virus was no longer detectable, and this was accompanied by a decreased number of intraparenchymal T cells, although the number of T cells present within the brains of recovering mice remained substantially higher than that observed in uninfected controls (Table 1; Fig. 2F and G).
FIG. 1.
MV RNA levels in brains of infected NSE-CD46+ adult mice. Immunocompetent NSE-CD46+ mice were inoculated intracerebrally with MV-Ed, and brains were harvested at 1, 3, 5, 7, and 13 dpi. Tissues were homogenized, and total RNA was hybridized with a radiolabeled probe specific for the MV fusion gene and quantified with a Fuji densitometer as described in Materials and Methods. The extent of MV-F RNA expression is shown as adjusted pixel values relative to a GAPDH (glyceraldehyde-3-phosphate dehydrogenase) internal control and compared to baseline (inoculation of mice with PBS). At least four mice/group were evaluated; the standard error is indicated.
TABLE 1.
Profile of lymphocyte infiltration and apoptosis in brains of MV-infected, NSE-CD46+ immunocompetent adultsa
| MV-Ed infection point (dpi) | n | No. of immunopositive cells/section (range)
|
|||||
|---|---|---|---|---|---|---|---|
| % Healthy | MV+ neurons | CD4+ T cells | CD8+ T cells | B cells | Cell death | ||
| 4 | 5 | 100 | 6 (0-20) | 51 (12-152) | 43 (8-133) | 3 (0-15) | 5 (0-9) |
| 7 | 5 | 100 | 13 (0-45) | 298 (205-417) | 243 (169-335) | 0 (0-3) | 2 (0-7) |
| 10 | 5 | 100 | 23 (6-58) | 254 (109-442) | 327 (209-503) | 20 (6-45) | 29 (15-48) |
| 16 | 5 | 83 | 0 | 196 (26-648) | 182 (117-766) | 0 (0-2) | 14 (0-62) |
| 21 | 5 | 100 | 0 | 251 (198-455) | 233 (188-326) | 7 (0-14) | 19 (6-30) |
| 29 | 5 | 100 | 0 | 247 (217-279) | 256 (113-392) | 5 (0-15) | 1 (0-3) |
| 37 | 4 | 100 | 0 | 114 (61-175) | 171 (29-268) | 0 | 5 (3-6) |
| Uninfected | 4 | 100 | NA | 20 (0-30) | 14 (3-30) | 0 | 9 (5-20) |
Four or five adult (>6 weeks old) NSE-CD46+ transgenic mice were infected intracerebrally with 104 PFU of MV-Ed. At the indicated days postinfection (dpi), mice were assessed for illness and sacrificed. Brains were removed, frozen, and cryosectioned. Serial sections were stained with specific antibodies for MV, CD4+ T cells, CD8+ T cells, B cells, and cell death by using a TUNEL stain approach. Horizontal 10-μm cryosections taken from equivalent regions of the brain were counted; values displayed are the number of immunopositive cells/horizontal section. The average of four or five mice is shown, with the range indicated in parentheses. NA, not applicable.
FIG.2.
Lymphocytic infiltration and cell death in brains of MV-infected NSE-CD46+ mice. Immunocompetent NSE-CD46+ mice were inoculated intracerebrally with MV-Ed, and brains were harvested throughout infection. Frozen tissues were sectioned and immunostained for the presence of MV antigens (A and E), CD4+ T cells (B and F), CD8+ T cells (C and G), and DNA ends by TUNEL assay (D and H; arrows indicate stained nuclei). Magnification, ×84. Higher-magnification images (×277) of T cells are shown in panels B2 and C2. (A to D) Serial sections taken from a representative mouse at 10 dpi; (E to H) serial sections taken from a mouse at 21 dpi. A dense region of infiltration was selected to emphasize TUNEL-positive cells at this time point.
While the majority (>95%) of infected mice remained healthy throughout the time course, a small number of immunocompetent adults consistently showed signs of sickness after viral challenge, including tremors, ataxia, and a hunched posture, as seen with one mouse in Table 1 at the 16-dpi time point and in two mice in Table 2, experiment 2. When examined histologically, no MV-immunopositive neurons were observed in brains of these mice, but all had high levels of T-cell infiltration and cell death. For example, the sick mouse in Table 1 had three to four times more CD4+ and CD8+ T cells within the parenchyma than matched healthy mice (648 versus 196 CD4+ T cells and 766 versus 182 CD8+ T cells) and the highest levels of TUNEL-positive nuclei observed in the time course. These data suggest that, in a small percentage of immunocompetent hosts, an overly aggressive immune response may be associated with neuropathology.
TABLE 2.
Consequence of MV infection in CD46+ transgenic mice on various immunodeficient backgroundsa
| Expt and genotype | CD46+
|
CD46−
|
||||
|---|---|---|---|---|---|---|
| n | % Mice sickb | Avg days to illness | n | % Mice sick | Avg days to illness | |
| 1 | ||||||
| Wild type | 42 | 2 | 19 | 7 | 0 | NAc |
| RAG-2 KO | 17 | 94 | 13.7 | 7 | 0 | NA |
| CD4 KO | 17 | 59 | 18.8 | 6 | 0 | NA |
| β-2-mic KO | 32 | 41 | 19 | 12 | 0 | NA |
| PFN KO | 17 | 18 | 15.6 | 5 | 0 | NA |
| IFN-γ KO | 40 | 68 | 14.9 | 11 | 0 | NA |
| 2 | ||||||
| Wild type | 8 | 13 | 9 | NDd | ND | ND |
| RAG-2 KO | 15 | 100 | 10.9 | 4 | 0 | NA |
| IFN-γ KO | 15 | 67 | 12.8 | 9 | 0 | NA |
CD46 transgenic (+) and nontransgenic (−) mice were intercrossed with the specified immunodeficient mice, and resulting adult progeny (>6 weeks of age) were infected intracerebrally with MV-Ed as described in Materials and Methods. Mice were monitored daily throughout infection. Mice that displayed signs of sickness for two consecutive days were sacrificed.
In these experiments, sickness was defined as a score of 2 or greater (as described in Materials and Methods) for two consecutive days.
NA, not applicable.
ND, not done.
Throughout the time course, the extent of cell death within infected brains was uniformly low, a finding consistent with the lack of illness observed in most infected mice. However, some cell death above the baseline level was seen: first observed at the peak of viral infection and immune cell infiltration (day 10; Fig. 2D; Table 1) and remaining elevated at later times postinfection (day 21; Fig. 2H) when no virus-infected cells were observed (Fig. 2E). Given the persistence of a small number of TUNEL-positive cells at late time points (when T cells were abundant but no MV-positive neurons were detectable) and the absence of cell death at early times postinfection (when MV-immunopositive neurons and T cells were present in the infected parenchyma), this low level of cell death likely reflects activation-induced cell death of infiltrating lymphocytes rather than immune-mediated death of MV-infected neurons.
IFN-γ is crucial for the resolution of neuronal infection.
To more precisely define components of the host response necessary for clearance, homozygous CD46+ mice possessing genetic immunodeficiencies were infected and monitored. To determine the contribution of individual T-cell populations, CD46+ mice were intercrossed with both CD4 KO mice (21) and β-2-mic KO mice that lack CD8+ T cells and class I MHC expression (55). In addition, CD46+ mice were also intercrossed with mice lacking either the pore-forming protein, perforin (20), or the cytokine IFN-γ (6), a Th1 cytokine produced by CD4+ T cells, CD8+ T cells, and NK cells. In addition to its well-established role in immune cell recruitment and activation, IFN-γ has also been implicated in the noncytolytic clearance of neurotropic virus infections (22, 34, 51).
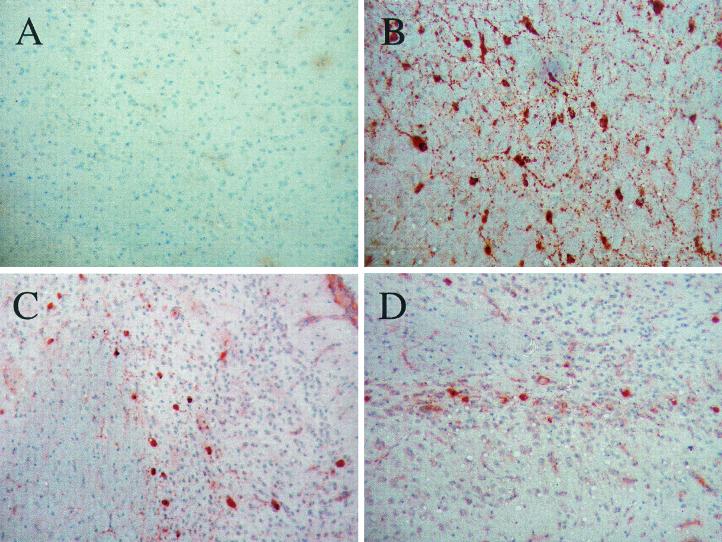
As shown in Table 2, MV-infected adults lacking either CD4+ T cells or CD8+ T cells were more susceptible to MV infection and CNS disease than immunocompetent adults (59 and 41% morbidity versus 2% morbidity in immunocompetent CD46+ mice). However, compared to immunodeficient CD46+/RAG-2 KO mice, both CD4 KO and β-2-mic KO mice showed reduced morbidity (Table 2, experiment 1) and reduced extent of CNS infection (Fig. 3). This was also correlated with a more extended interval between inoculation and illness (∼19 days versus ∼14 days). These data suggested that, while the CD4+ and CD8+ T-cell compartments are each involved in the anti-MV response within the brain, both are required for complete protection. In immunohistochemical assays of MV-infected CD46+/CD4 KO or CD46+/β-2-mic KO mice, the absence of one T-cell subset did not appreciably affect the degree of infiltration of the other subset into the brain parenchyma (data not shown).
FIG. 3.
Both T-cell subsets partially protect against MV infection in CD46+ mice. CD46+ mice on the indicated KO background were inoculated intracerebrally with MV-Ed, and brains were harvested at 20 dpi. Brains were sectioned and stained for the presence of MV antigens, and representative images are shown. (A) CD46+; (B) CD46+/RAG-2 KO; (C) CD46+/CD4 KO; (D) CD46+/β-2-mic KO. Magnification, ×100.
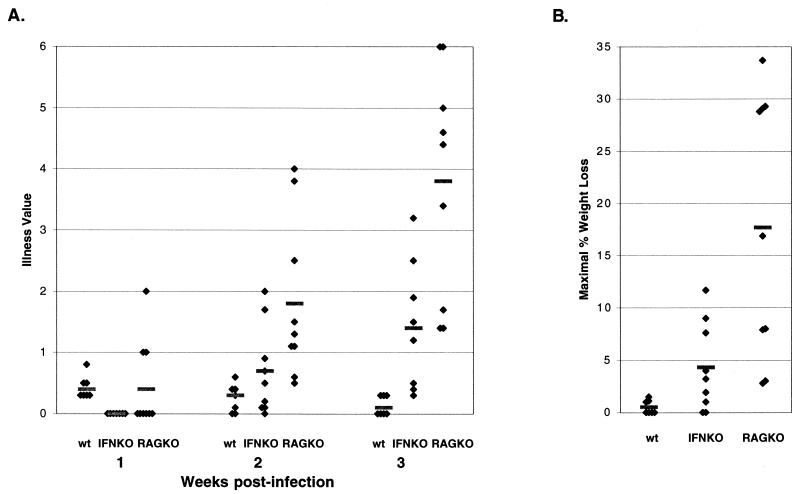
Consistent with our previous observation that immune-mediated resolution of MV from susceptible mice was associated with minimal cell lysis, the deletion of the perforin gene had a modest impact on the ability of mice to resolve an MV challenge (2% in CD46+ mice versus 18% in CD46+/perforin KO; Table 2, experiment 1). In sharp contrast, deletion of IFN-γ resulted in morbidity in ∼70% of infected mice compared to 94 to 100% in CD46+/RAG-2 KO mice. When immunocompetent CD46+ adults, CD46+/RAG-2 KO adults, and CD46+/IFN-γ KO adults were compared in sickness assays that assessed movement, coordination, and weight loss, MV-infected CD46+/IFN-γ KO mice showed marked motor problems that paralleled those seen in infected CD46+/RAG-2 KO with respect to kinetics, although the severity of CNS disease was less in infected CD46+/IFN-γ KO mice (Fig. 4). Moreover, the average interval to CNS disease was more similar to infected RAG-2 KO mice than previously observed with CD4 and β-2-mic KO mice.
FIG. 4.
CD46+/IFN-γ KO mice show progressive illness and weight loss after MV inoculation. Mice were subjected to three clinical analyses as described in Materials and Methods. (A) Balance and movement scores were combined and averaged from results obtained at 1 to 3 weeks postinoculation. wt, CD46+ immunocompetent mice; IFN KO, CD46+/IFN-γ KO; RAG KO, CD46+/RAG-2 KO. (B) Percent body weight loss plotted for CD46+ immunocompetent mice, CD46+/IFN-γ KO mice, and CD46+/RAG-2 KO mice. The maximum weight loss during the time course of infection is shown. For both panels, the mean values are represented by horizontal bars, and individual scores are represented as black diamonds. All of the infected mice from two experiments are included in this analysis.
One primary role of IFN-γ is the recruitment and activation of the adaptive immune response. It was therefore possible that the deletion of IFN-γ from these mice resulted in reduced T-cell infiltration, leading to infection and disease. To test this hypothesis, time-matched samples of immunocompetent CD46+ mice and CD46+/IFN-γ KO mice were collected throughout infection and stained for the presence of T cells within the brain. At no time point postinfection were substantial differences in intraparenchymal T-cell values noted between the groups (data not shown), indicating that the morbidity observed in infected CD46+/IFN-γ KO mice was not due to reduced T-cell infiltration.
IFN-γ exhibits a direct, antiviral role in MV-infected primary neurons.
The requirement for IFN-γ but not perforin in MV clearance from the infected CNS suggested that T-cell-elaborated cytokines may play a direct role in viral clearance, without necessitating the lysis of infected neurons. If true, the addition of either perforin-deficient, activated T cells or recombinant cytokines to infected neurons in culture should result in inhibition of viral replication.
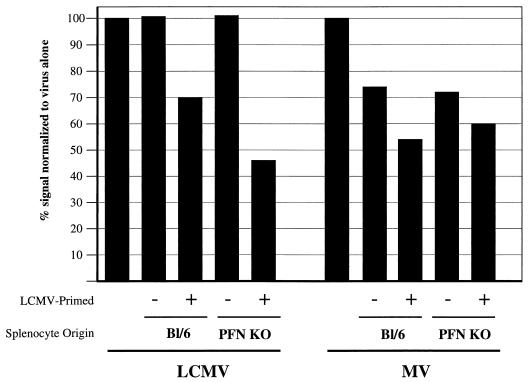
To test these hypotheses, virus-infected primary hippocampal neurons cultured from CD46+ embryos were incubated with splenocytes obtained from C57BL/6 mice or perforin KO mice. The donor mice were either naive or infected with LCMV 7 days previously. LCMV was chosen as an immunogen because of the robust CD8+ T-cell response generated in vivo after intraperitoneal inoculation. Total red blood cell-depleted splenocytes were isolated and immediately cultured with LCMV- or MV-infected primary neurons. In trypan blue cell viability studies, no neuronal loss was observed after a 6-h incubation with any of the effectors (data not shown), a finding consistent with the reported lack of class I MHC antigens on the neuronal cell surface (38). Interestingly, however, when RNA was isolated from infected neurons after a 16-h incubation with the effector splenocytes, loss of viral RNA was observed from both LCMV- and MV-infected neurons (Fig. 5). In the case of LCMV-infected neurons, addition of unprimed C57BL/6 or perforin KO splenocytes had no effect on viral RNA levels, whereas LCMV-primed lymphocytes resulted in a 30 to 50% loss of viral RNA. This effect was perforin independent. Interestingly, a similar loss of viral RNA was observed from MV-infected neurons, although in these cultures, the incubation of infected neurons with either primed or unprimed lymphocytes resulted in reduced viral RNA levels. Together, these data indicate that activated splenocytes can result in noncytolytic inhibition of neuronal viral replication; from these observations we hypothesized that cytokines elaborated by LCMV-specific splenocytes may suppress MV replication in infected primary neurons.
FIG. 5.
Splenocytes can inhibit viral replication ex vivo in the absence of neuronal loss. Primary hippocampal neurons obtained from embryonic day 16 NSE-CD46+ mice were infected with either LCMV-Arm or MV-Ed (MOI = 3) for 2 days prior to the assay. Wild-type, haplotype-matched (H-2b) splenocytes were obtained either from C57BL/6 mice or from perforin KO mice that were uninfected or that had been challenged with LCMV-Arm 7 days previously. Splenocytes were incubated with infected neurons at a ratio of 10:1 overnight. After incubation, total viral RNA loads were quantified as described in Materials and Methods.
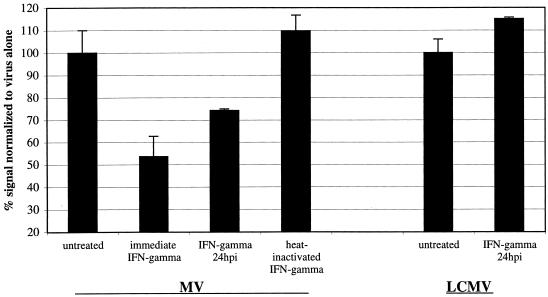
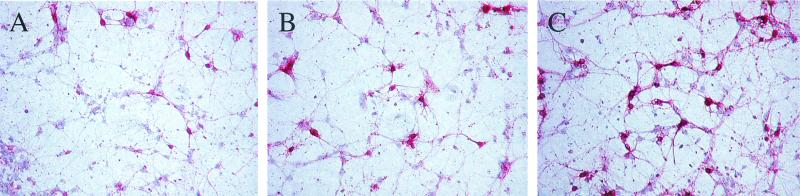
Finally, to establish if purified cytokines could inhibit viral replication in infected neurons, cultured CD46+ hippocampal neurons were infected with either MV-Ed or LCMV-Arm (MOI = 3), and recombinant murine IFN-γ (100 U/ml) was added either immediately after infection or 24 hpi. At 2 dpi, infected cells were harvested for immunochemistry and RNA analysis. As controls, PBS or heat-inactivated IFN-γ (65°C; 30 min) was also added to the infected cultures. As shown in Fig. 6, the addition of IFN-γ immediately after infection resulted in ∼50% inhibition in MV RNA load, compared to no effect seen with either PBS-treated or heat-inactivated, IFN-γ-treated cultures. Importantly, the addition of IFN-γ at 24 hpi (that is, after the establishment of an infection) also resulted in reproducibly reduced MV RNA levels. The addition of IFN-γ appeared to block subsequent viral spread: as shown in Fig. 7, the extent of MV infection in primary cultures treated with IFN-γ for 24 h and collected 48 hpi (Fig. 7B) paralleled that seen in cultures collected at 24 hpi (Fig. 7A). In contrast, untreated neurons collected at 48 hpi (Fig. 7C) showed extensive viral spread. Together, these data suggest that T-cell-elaborated cytokines, such as IFN-γ, play a direct role in the resolution of certain viral infections.
FIG. 6.
The effect of IFN-γ on MV infection in susceptible primary hippocampal neuron cultures. Primary hippocampal neurons were infected with MV-Ed at an MOI of 3. IFN-γ was added immediately, at 24 hpi, or as a heat-inactivated negative control (100 U/ml). Neurons were also infected with LCMV at an MOI of 1 and treated with IFN-γ. The percent RNA remaining at 48 hpi is shown compared to untreated samples, and the standard error is indicated.
FIG. 7.
Inhibition of MV spread in primary neurons after IFN-γ addition. MV-infected, primary hippocampal neurons were treated with IFN-γ or PBS and were subsequently fixed and immunostained as described in Materials and Methods. (A) Collected at 24 hpi. (B) IFN-γ added at 24 hpi; collected at 48 hpi. (C) PBS added at 24 hpi; collected at 48 hpi. Magnification, ×87.
DISCUSSION
In this study, a genetic approach was taken to determine the basis of immune-mediated clearance of MV from the CNS of infected, CD46+ transgenic mice. Our data indicate that clearance occurred without neuronal loss and that both CD4+ and CD8+ T lymphocytes contributed to this noncytolytic protection. While deletion of the perforin gene did not appreciably impact the ability of the immune response to resolve the infection, deletion of the IFN-γ gene had a significant effect which could not be attributed to reduced infiltration of T cells into the infected CNS parenchyma. In retrospect, if cytokines such as IFN-γ play a crucial role in viral clearance, the partial protective effect observed in CD46+ CD4 KO and β-2-mic KO mice is reasonable, since IFN-γ is produced by CD4+ T cells, CD8+ T cells, and NK cells. Thus, even on a RAG-2 KO background, a small number of mice can survive a persisting viral challenge, which may be due to the intact innate response in these animals. While the innate response per se may not be crucial for protection against MV-mediated neuropathology in this model (since CD46+/RAG-2 KO mice have a high morbidity rate after MV inoculation), the potential role of the innate response in the recruitment and/or education of the subsequent adaptive response is being considered in ongoing studies.
The ability of activated T cells to restrict viral replication in the absence of neuronal loss--and the importance of IFN-γ in this process--was further confirmed in ex vivo experiments with primary neuron cultures. Collectively, these data support and extend work from our laboratory and others that describes a novel, noncytolytic role of the antiviral T-cell response in the brain. The merits of such a strategy are especially clear in tissues in which cell renewal does not occur, as in the CNS.
It is likely that our ex vivo studies underestimate the impact of activated T cells and IFN-γ on MV replication, since the primary neuron cultures were obtained from a neuron-rich CNS structure (hippocampus at embryonic day 16) and were cultured in the absence of supporting glia (these cultures are routinely >95% MAP-2+). Thus, if MV infection of neurons induced elevated cytokine synthesis by adjacent glia or if glial contact is required for recognition of infected cells in vivo, highly purified neuronal cultures may not contain all of the appropriate cellular participants needed for maximal clearance. Nevertheless, the relatively modest effect of both activated T cells and recombinant cytokines on neuronal viral loads ex vivo and the fact that not all CD46+/IFN-γ KO mice succumb to CNS disease support the conclusion that, while IFN-γ plays a central role in protection, complete protection is multifactorial. Cytokines are known to act in concert (18); it is therefore likely that other Th1 cytokines, such as interleukin-2 and tumor necrosis factor alpha, also participate in the resolution of a neuron-restricted MV infection in vivo.
How cytokines such as IFN-γ mediate their protective effects in the resolution of established virus infections is not clear. At least two hypotheses exist. First, cytokines elaborated by infiltrating immune cells may exert their well-established effects by preventing viral spread, restricting infection to a small number of cells. If “classic” CTL-mediated cytolysis then occurs, it is unlikely that much damage would ensue, given the relatively small number of cells that would be targets for lysis. Our data do not contradict this hypothesis, since we did observe a modest degree of apoptosis in infected brains in vivo, and treatment of infected primary neurons with IFN-γ appeared to restrict viral spread (Fig. 7). However, (i) TUNEL-positive cells were detectable in the absence of virus-immunopositive neurons and (ii) at early stages of infection in vivo (when T cells were abundant in MV antigen-positive brains) no cell death was detected. We therefore hypothesize that the observed cell death was due to the loss of activated lymphocytes by activation-induced cell death at the later stages of the immune response (18) and not due to direct neuronal lysis. The absence of a correlation between neuronal infection and death therefore suggests that cytokines may play a more direct role in viral clearance than solely by inhibiting viral spread.
A second hypothesis is that T-cell-elaborated cytokines, such as IFN-γ and tumor necrosis factor alpha, participate directly in viral clearance. IFN-γ is crucial for the noncytopathic suppression of herpes simplex virus type 1 infections in trigeminal ganglia (3) and photoreceptor cells in the eye (10) and in the neuron infections caused by LCMV (51), Borna virus (16), mouse hepatitis virus (36), and Theiler's virus (7). Indeed, neurons express IFN-γ receptors (43, 52), providing support for the idea that neurons respond to this cytokine. For MV, the importance of IFN-γ has been noted in both humans (9) and mice (using a neuroadapted strain of MV) (8), although whether IFN-γ acts directly or indirectly (e.g., by activating T cells) to suppress viral replication could not be concluded from these studies. Both IFN-α/β and IFN-γ act by binding receptors on the cell surface, triggering recruitment of the JAK kinases and activation of STAT proteins (47). This results in a cascade of intracellular events that leads to the transcription of IFN response genes, including the 2′-5′ OAS system, the Mx proteins, inducible nitric oxide synthase, and the double-stranded RNA-PKR pathway. Collectively, activation of these genes contributes to the antiviral state (47). Whether such proteins are associated with viral clearance in established infections remains to be determined.
One surprising aspect of our work was the persistence of T cells in the brains of infected mice long after virus was no longer detectable by immunochemical and RNA hybridization methods (Table 1; Fig. 1). While we do not yet fully understand the basis for this T-cell retention, we speculate that the absence of CTL-mediated lysis of infected neurons may result in a “smoldering” infection; cytokine-dependent, noncytolytic clearance may take longer to result in complete virus loss. Our present efforts to test this hypothesis include use of more sensitive detection strategies to determine whether viral RNA persists in brain tissue after viral antigens are no longer detectable by immunohistochemistry.
The ability of T lymphocytes to eliminate virus that is restricted to CNS neurons is pertinent to an ongoing debate in neuroimmunology. Neurons are generally thought not to express either class I or class II MHC molecules (38); thus, how either CD4+ or CD8+ T cells mediate their protective effects is obscure. There are a number of possible explanations for this. (i) CD4+ and CD8+ T cells, educated in the periphery, may constitutively secrete cytokines; recruitment of these cells into the MV-infected parenchyma may, therefore, result in elevated localized cytokine concentrations. However, Whitton and coworkers (46) have shown that cytokine synthesis occurs only when T cells are engaged with their target; upon disengagement, cytokine secretion is rapidly turned off. These data suggest that infiltrating T cells in the NSE-CD46 model are not constitutively producing antiviral cytokines within the CNS and that this scenario is unlikely. (ii) Previous reports have shown that class I MHC can be induced on CNS neurons in response to injury (28, 29, 31, 32, 54) or virus infection, including MV (11, 13). Thus, while class I and II MHC expression may be below detection limits in the uninfected brain, MV infection may induce expression, allowing for MHC-T-cell receptor contact to occur. However, our inability to detect upregulated MHC on MV-infected neurons or in infected brains (unpublished observations), the ability of LCMV-specific splenocytes to restrict MV replication ex vivo (Fig. 5), and the survival of a substantial proportion of class I MHC-deficient β-2-mic KO mice after MV infection (Table 2) collectively indicate that T cells do not recognize virus-specific peptides in the context of class I MHC on neurons. (iii) A hypothesis that we favor is that, in vivo, infiltrating T cells interact with adjacent microglia that, though uninfected, may still present viral epitopes via class II MHC presentation to CD4+ T cells or via cross-presentation to CD8+ T cells (45). Elaboration of cytokines as a result of this interaction may then indirectly allow for cytokine-mediated, noncytolytic viral clearance from adjacent neurons to occur. A major focus of our present work is to determine whether such interactions occur, to establish the mechanism of viral clearance without concomitant neuronal loss, and to test whether this mode of viral clearance is applicable to other neurotropic pathogens.
Acknowledgments
We thank Melinda Vaughn, Donna Mscisz, Joan Cole, and Alec Belman for assistance with the experiments. We are also appreciative of the gifts of LCMV-Arm and human MV-reactive serum from Michael B. A. Oldstone of The Scripps Research Institute in La Jolla, Calif. Finally, we are grateful to Bill Mason and Luis Sigal for their invaluable comments regarding the manuscript.
G.F.R. was supported by a grant from the National Institute of Mental Health (MH-56951) and a grant from the F. M. Kirby Foundation. C.E.P. was supported by an individual NRSA award (NS-11100).
REFERENCES
- 1.Banker, G., and K. Goslin. 1991. Culturing nerve cells. MIT Press, Cambridge, Mass.
- 2.Binder, G. K., and D. E. Griffin. 2001. Interferon-gamma mediated site specific clearance of alphavirus from CNS neurons. Science 293:303-306. [DOI] [PubMed] [Google Scholar]
- 3.Cantin, E., B. Tanamachi, and H. Openshaw. 1999. Role for gamma interferon in control of HSV-1 reactivation. J. Virol. 73:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattaneo, R., A. Schmid, M. A. Billeter, R. D. Sheppard, and S. A. Udem. 1988. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a SSPE cell line. Virology 62:1388-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christian, A. Y., M. Barna, Z. Bi, and C. S. Reiss. 1996. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol. 9:195-205. [DOI] [PubMed] [Google Scholar]
- 6.Dalton, D., S. Pitts-Meek, S. Keshav, I. Figari, A. Bradley, and T. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1744. [DOI] [PubMed] [Google Scholar]
- 7.Fiette, L., C. Aubert, U. Muller, S. Huang, M. Aguet, M. Brahic, and J. F. Bureau. 1995. Theiler's virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J. Exp. Med. 181:2069-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finke, D., U. G. Brinckmann, V. ter Meulen, and U. G. Liebert. 1995. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J. Virol. 69:5469-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gans, H. A., Y. Maldonado, L. L. Yasukawa, J. Bewler, S. Audet, M. M. Rinki, R. DeHovitz, and A. M. Arvin. 1999. IL-12, IFN-γ, and T-cell proliferation to measles in immunized infants. J. Immunol. 162:5569-5575. [PubMed] [Google Scholar]
- 10.Geiger, K., D. Gurushanthaiah, E. Howes, G. Lewandowski, J. Reed, F. Bloom, and N. Sarvetnick. 1995. Cytokine-mediated survival from lethal HSV infection: role of programmed neuronal death. Proc. Natl. Acad. Sci. USA 92:3411-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gogate, N., P. Swoveland, T. Yamabe, L. Verma, J. Woyciechowska, E. Tarnowska-Dziduszko, J. Dymecki, and S. Dhib-Jalbut. 1996. MHC class I expression on neurons in SSPE and experimental subacute measles encephalitis. J. Neuropathol. Exp. Neurol. 55:435-443. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Dunia, D., C. Sauder, and J. C. de la Torre. 1997. Borna disease virus and the brain. Brain Res. Bull. 44:647-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopas, J., D. Itzhaky, Y. Segev, S. Salzberg, B. Trink, N. Isakov, and B. Rager-Zisman. 1992. Persistent measles virus infection enhances class I MHC expression and immunogenicity of murine neuroblastoma cells. Cancer Immunol. Immunother. 34:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidotti, L. G., K. Ando, M. V. Hobbs, T. Ishikawa, L. Runkel, R. D. Schreiber, and F. V. Chisari. 1994. CTL inhibit HBV gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl. Acad. Sci. USA 91:3764-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV clearance. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 16.Hallensleben, W., and P. Staeheli. 1999. Inhibition of Borna disease virus multiplication by interferon: cell line differences in susceptibility. Arch. Virol. 144:1209-1216. [DOI] [PubMed] [Google Scholar]
- 17.Hickey, W. F., B. L. Hsu, and H. Kimura. 1991. T-lymphocyte entry into the CNS. J. Neurosci. Res. 28:254-260. [DOI] [PubMed] [Google Scholar]
- 18.Janeway, C. A., P. Travers, M. Walport, and M. Shlomchik. 2001. Immunobiology, 5th ed. Garland, New York, N.Y.
- 19.Johnson, R. T. 1998. Viral infections of the nervous system. Lippincott-Raven, Philadelphia, Pa.
- 20.Kagi, D., B. Liedermann, K. Burki, P. Seller, B. Odermatt, K. J. Olsen, E. Podack, R. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and NK cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 21.Killeen, N., S. Sawada, and D. Littman. 1993. Regulated expression of human CD4 rescues helper T-cell development in mice lacking expression of endogenous CD4. EMBO J. 12:1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komatsu, T., Z. Bi, and C. S. Reiss. 1996. Interferon gamma-induced type I nitric oxide synthase activity inhibits viral replication in neurons. J. Neuroimmunol. 68:101-108. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu, T., D. D. Ireland, N. Chen, and C. S. Reiss. 1999. Neuronal expression of NOS-1 is required for host recovery from viral encephalitis. Virology 258:389-395. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu, T., N. Srivastava, M. Revzin, D. D. Ireland, D. Chesler, and C. S. Reiss. 1999. Mechanisms of cytokine-mediated inhibition of viral replication. Virology 259:334-341. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence, D. M. P., M. M. Vaughn, A. R. Belman, J. S. Cole, and G. F. Rall. 1999. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J. Virol. 73:1795-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, M. T., S. A. Stohlman, and D. R. Hinton. 1997. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J. Virol. 71:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipton, H. L., and M. L. Jelachich. 1997. Molecular pathogenesis of Theiler's murine encephalomyelitis virus-induced demyelinating disease in mice. Intervirology 40:143-152. [DOI] [PubMed] [Google Scholar]
- 28.Maehlen, J., I. Nennesmo, A. B. Olson, T. Olsson, M. D. Schröder, and K. Kristensson. 1989. Peripheral nerve injury causes transient expression of MHC class I antigens in rat motor neurons and skeletal muscles. Brain Res. 481:368-372. [DOI] [PubMed] [Google Scholar]
- 29.Maehlen, J., T. Olsson, A. Zachau, L. Klareskog, and K. Kristensson. 1989. Local enhancement of MHC class I and II expression and cell infiltration in experimental allergic encephalomyelitis around axotomized motor neurons. J. Neuroimmunol. 23:125-132. [DOI] [PubMed] [Google Scholar]
- 30.Nathanson, N. 1997. Viral pathogenesis. Lippincott-Raven, Philadelphia, Pa.
- 31.Neumann, H., A. Cavalie, D. E. Jenne, and H. Wekerle. 1995. Induction of MHC class I genes in neurons. Science 269:549-552. [DOI] [PubMed] [Google Scholar]
- 32.Neumann, H., H. Schmidt, A. Cavalie, D. Jenne, and H. Wekerle. 1997. MHC class I gene expression in single neurons of the central nervous system: differential regulation by interferon gamma and tumor necrosis factor alpha. J. Exp. Med. 185:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldstone, M. B. A., P. Blount, P. J. Southern, and P. W. Lampert. 1986. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature 321:239-243. [DOI] [PubMed] [Google Scholar]
- 34.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 35.Pasick, J. M., K. Kalicharran, and S. Dales. 1994. Distribution and trafficking of JHM coronavirus structural proteins and virions in primary neurons and the OBL-21 neuronal cell line. J. Virol. 68:2915-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce, B. D., M. V. Hobbs, T. S. McGraw, and M. J. Buchmeier. 1994. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J. Virol. 68:5483-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podack, E. R. 1995. Execution and suicide: CTL enforce Draconian laws through separate molecular pathways. Curr. Opin. Immunol. 7:11-16. [DOI] [PubMed] [Google Scholar]
- 38.Rall, G. F. 1998. CNS neurons: the basis and benefits of low MHC expression. Curr. Top. Microbiol. Immunol. 232:115-134. [DOI] [PubMed] [Google Scholar]
- 39.Rall, G. F., M. Manchester, L. R. Daniels, E. M. Callahan, A. R. Belman, and M. B. A. Oldstone. 1997. A transgenic mouse model for measles virus infection of the brain. Proc. Natl. Acad. Sci. USA 94:4659-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rall, G. F., L. Mucke, and M. B. A. Oldstone. 1995. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex-expressing neurons in vivo. J. Exp. Med. 182:1201-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rall, G. F., and M. B. A. Oldstone. 1997. Viral persistence in the CNS, p. 273-289. In P. K. Peterson and J. S. Remington (ed.), Defense of the brain. Blackwell Press, Cambridge, Mass.
- 42.Rall, G. F., and M. B. A. Oldstone. 1995. Virus-neuron-cytotoxic T lymphocyte interactions. Curr. Top. Microbiol. Immunol. 202:261-273. [DOI] [PubMed] [Google Scholar]
- 43.Robertson, B., G. Kong, Z. Peng, M. Bentivoglio, and K. Kristensson. 2000. Interferon-gamma responsive neuronal sites in the normal rat brain: receptor protein distribution and cell activation revealed by fos induction. Brain Res. Bull. 52:61-74. [DOI] [PubMed] [Google Scholar]
- 44.Shinkai, Y., G. Rathbun, and K. Lam. 1992. RAG-2 deficient mice lack mature lymphocytes owing to the inability to initiate V(D)J rearrangement. Cell 68:855-864. [DOI] [PubMed] [Google Scholar]
- 45.Sigal, L. J., S. Crotty, R. Andino, and K. L. Rock. 1999. Cytotoxic T cell immunity to virus-infected non-hematopoetic cells requires presentation of exogenous antigen. Nature 398:77-80. [DOI] [PubMed] [Google Scholar]
- 46.Slifka, M. K., F. Rodriguez, and J. L. Whitton. 1999. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature 401:76-79. [DOI] [PubMed] [Google Scholar]
- 47.Stark, G. R., I. M. Kerr, B. R. G. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 48.Stohlman, S. A., C. C. Bergmann, M. T. Lin, D. J. Cua, and D. R. Hinton. 1998. CTL effector function within the CNS requires CD4+ T cells. J. Immunol. 160:2896-2904. [PubMed] [Google Scholar]
- 49.Streilein, J. W. 1993. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Curr. Opin. Immunol. 5:428-432. [DOI] [PubMed] [Google Scholar]
- 50.Tishon, A., M. Eddleston, J. C. de la Torre, and M. B. A. Oldstone. 1993. CTL cleanse viral gene products from individually infected neurons and lymphocytes in mice persistently infected with LCMV. Virology 197:463-467. [DOI] [PubMed] [Google Scholar]
- 51.Tishon, A., H. Lewicki, G. Rall, M. von Herrath, and M. B. A. Oldstone. 1995. An essential role for type 1 interferon gamma in terminating persistent viral infection. Virology 212:244-250. [DOI] [PubMed] [Google Scholar]
- 52.Vikman, K., B. Robertson, G. Grant, A. Liljeborg, and K. Kristensson. 1998. Interferon-gamma receptors are expressed at synapses in the rat superficial dorsal horn and lateral spinal nucleus. J. Neurocytol. 27:749-759. [DOI] [PubMed] [Google Scholar]
- 53.Wekerle, M., C. Linington, H. Lassmann, and R. Meyermann. 1986. Cellular immune reactivity in the CNS. Trends Neurosci. 1985:271-277. [Google Scholar]
- 54.Wong, G. M. W., P. F. Bartlett, I. Clark-Lewis, F. Battye, and J. W. Schrader. 1984. Inducible expression of H-2 and Ia antigens on brain cells. Nature 310:688-691. [DOI] [PubMed] [Google Scholar]
- 55.Ziljstra, M., E. Li, F. Sajjadi, S. Subramani, and R. Jaenisch. 1989. Germline transmission of a disrupted β-2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature 342:435-439. [DOI] [PubMed] [Google Scholar]
- 56.Zinkernagel, R. M., and H. Hengartner. 1997. Antiviral immunity. Immunol. Today 18:258-259. [DOI] [PubMed] [Google Scholar]