Abstract
Helper-dependent (HD) adenovirus vectors devoid of all viral coding sequences have a large cloning capacity and provide long-term transgene expression in vivo with negligible toxicity, making them attractive vectors for gene therapy. Currently, the most efficient means of producing HD vectors involves coinfecting 293 cells expressing Cre with the HD vector and a helper virus bearing a packaging signal flanked by loxP sites. Cre-mediated packaging signal excision renders the helper virus genome unpackageable but still able to replicate and provide helper functions for HD vector propagation. Typically, helper virus contamination is ≤1% pre- and ≤0.1% postpurification by CsCl banding. While these contamination levels are low, further reduction is desirable. However, this objective has not been realized since the Cre/loxP system was first developed. This lack of progress is due, at least in part, to our lack of understanding of the origins of the contaminating helper virus, thus rendering its reduction or elimination difficult to achieve. This study was designed to investigate the possible sources of contaminating helper virus persisting during HD vector amplification. The results revealed that Cre is limiting in helper virus-infected Cre-expressing 293 cells, thereby permitting helper viruses to escape packaging signal excision and propagate. The results of this study should provide a foundation for developing rational strategies to further reduce or possibly eliminate the contaminating helper virus.
Helper-dependent (HD) adenovirus vectors are devoid of all adenovirus coding sequences, retaining only ∼500 bp of adenovirus sequences required in cis for HD vector propagation. These comprise the inverted terminal repeats (ITRs) necessary for DNA replication and the packaging signal (ψ) necessary for viral DNA encapsidation (20, 26, 28). Like first-generation adenovirus vectors (7, 17, 18), HD vectors can efficiently transduce a wide variety of cell types from numerous species in a cell cycle-independent manner but, unlike first-generation adenovirus vectors, have the added advantage of increased cloning capacity (∼37 kb), reduced toxicity, and prolonged stable transgene expression in vivo (5, 9, 10, 19, 21, 22, 23, 24, 27, 31, 35).
The first efficient and currently most practical means of generating HD vectors is the Cre/loxP system developed by Graham and coworkers (29). In this system, Cre-expressing 293 cells are coinfected with the HD vector and a helper virus bearing a packaging signal flanked by loxP sites. Cre-mediated packaging signal excision renders the helper virus genome unpackageable but able to provide all functions needed in trans for HD vector propagation. HD vector titer is increased by serial coinfection of Cre-expressing 293 cells with the HD vector and the helper virus. Finally, the HD vector is purified by CsCl ultracentrifugation. Typically, about 1010 to 1011 vector particles are produced per 107 coinfected cells with a helper virus contamination level of ≤1% and ≤0.1% pre- and postpurification by CsCl ultracentrifugation (25, 29, 30). Recently, analogous systems for generating HD vector based on the yeast site-specific recombinase FLP have been developed (25, 32).
While helper virus contamination levels are low using this system, further reduction is desirable, considering the safety issues associated with high doses of HD vector that may be required as well as the inconvenience of CsCl ultracentrifugation for large-scale production of clinical-grade vectors. However, no significant reduction in helper virus contamination has been achieved since the Cre/loxP system was first reported over 5 years ago (29). A major factor in this lack of progress is likely our lack of understanding of the nature of the residual helper virus that escapes Cre-mediated recombination. The objective of this study was to determine the sources of the contaminating helper virus during HD vector amplification so that rational strategies may be developed to further reduce, if not eliminate, the contaminating helper virus.
MATERIALS AND METHODS
Viruses and cell lines.
The helper virus AdLC8cluc bears a packaging signal flanked by loxP sites (Fig. 1A) (29). The HD vector AdC4HSULacZ bears a lacZ reporter expression cassette (Fig. 1B) (25). The helper virus AdNG114R is identical to AdLC8cluc except that the region of DNA encompassing the loxP sites and the packaging signal is in the opposite orientation (Fig. 1C) and restriction enzyme site polymorphisms have been introduced to flank the loxP sites and the packaging signal (see Fig. 5). Details regarding the sequence of AdNG114R and its construction can be obtained from the authors upon request.
FIG. 1.
Adenoviruses and HD vector. (A) The helper virus AdLC8cluc has a packaging signal flanked by loxP sites. (B) The HD vector AdC4HSULacZ contains a lacZ reporter gene driven by the murine CMV immediate-early promoter. (C) The helper virus AdNG114R is identical to AdLC8cluc except that the loxP sites and the packaging signal are in the inverted orientation and restriction enzyme site polymorphisms have been introduced flanking the loxP sites and the packaging signal (see Fig. 5). (D) The adenovirus vector AdCreM1 contains a Cre expression cassette in place of the E1 region. Small black arrows represent the adenovirus ITRs. Triangles represent loxP sites. The sizes of the relevant restriction enzyme fragments and the positions of probe SB and probe H are shown. The adenovirus pIX-specific probe SB is the 496-bp SalI-BglI fragment from plasmid pDC411 (Microbix Biosystems, Inc.), and the lacZ-specific probe H is the 624-bp HpaI fragment from plasmid pCA36 (2).
FIG. 5.
Strategy to investigate the contribution of the reverse Cre reaction to the contaminating helper virus. 293Cre4 cells were coinfected with AdLC8cluc and AdNG114R. Cre-mediated exchange of packaging signals between the two viruses would produce two novel recombinants (type I and II), and detection of these would provide evidence for the reverse Cre reaction. Restriction enzyme site polymorphisms flanking the loxP site and packaging signal in AdLC8cluc and AdNG114R permit parental and recombinant viruses to be distinguished (a, BamHI; b, ScaI; c, BsrGI; d, XbaI; e, EcoRI; f, BspEI; g, SpeI; h, HindIII).
AdCreM1 (M. Anton and F. L. Graham, unpublished data; available from Microbix Biosystems Inc.) is a first-generation adenovirus vector bearing a Cre expression cassette in place of the E1 region (Fig. 1D). 293 cells (12) and the Cre-expressing 293-derived cell line 293Cre4 (8) were maintained as previously described. The Cre expression cassette used to generate the 293Cre4 cell line, described in detail elsewhere (8), contains the Cre coding sequence modified to include a nuclear localization signal at the 5′ end and is driven by the human cytomegalovirus (CMV) promoter.
Adenovirus infections and analyses.
Unless otherwise indicated, all infections were performed as follows. The indicated cell lines in 60-mm dishes were infected with the indicated virus at various multiplicities of infection (MOIs) as described elsewhere (26). At the indicated times postinfection, the infected cells were scraped into the medium and processed as follows: 80% of the cell suspension was centrifuged and 0.4 ml of sodium dodecyl sulfate (SDS)-pronase solution (0.5 mg of pronase per ml, 0.5% SDS) was added to the cell pellet and incubated overnight at 37°C. Total intracellular DNA was recovered from the lysed cells by ethanol precipitation for Southern blot hybridization analyses. The remaining 20% of the cell suspension was centrifuged, and the cell pellet was resuspended in minimal essential medium (MEM) supplemented with 5% fetal bovine serum (FBS) and 1/10 volume of 40% sucrose. Intracellular virus was release from the infected cells by freezing and thawing and counted by plaque assay on 293 or 293Cre4 cells as indicated. HD vector titer, expressed as blue-forming units (BFU) per milliliter, was determined by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining of transduced 293 cells as described elsewhere (26).
Western analysis.
Total protein was extracted by incubating cells with 0.5 ml of radioimmunoprecipitation assay buffer supplemented with aprotinin (10 μg/ml; Roche) and leupeptin (10 μg/ml; Roche) for 30 min on ice. Total protein was quantitated by a colorimetric assay (Micro BCA assay; Pierce), and 20 μg of protein was fractionated through a 10% polyacrylamide gel and transferred to an Immobilon P polyvinylidene difluoride membrane (Millipore). The Cre protein expressed from AdCreM1 is 38 kDa, and the Cre protein expressed by 293Cre4 cells is 39 kDa due to the presence of a seven-amino-acid nuclear localization signal at the N terminus. Both species of Cre were detected using a rabbit anti-Cre polyclonal antibody (3) and horseradish peroxidase-conjugated goat anti-rabbit polyclonal antibody (PharMingen) by chemiluminescence (Amersham Pharmacia Biotech). The Full Range Rainbow Molecular Weight Marker (Amersham Pharmacia Biotech) was included in each Western as a size standard.
RESULTS
Numerous mechanisms can be postulated to explain the origin of the contaminating helper virus in HD vector preparations. The objective of this study was to identify the sources of helper virus contamination so that improved strategies may be devised to further reduce, if not eliminate, the contaminating helper virus.
Cre-mediated packaging signal excision is not complete.
Inhibition of helper virus propagation relies on efficient Cre-mediated packaging signal excision. To investigate this in detail, three series of infections (denoted I, II, and III) were performed. Infection I involved infecting 60-mm dishes of 293 cells with AdLC8cluc at an MOI of 1 PFU/cell and served as a control for helper virus propagation in the absence of Cre. Infection II involved infecting 60-mm dishes of 293Cre4 cells with AdLC8cluc at an MOI of 1 PFU/cell to determine the effect of Cre on helper virus propagation. Infection III involved coinfecting 60-mm dishes of 293Cre4 with AdLC8cluc at an MOI of 1 PFU/cell and the HD vector AdC4HSULacZ at an MOI of 10 BFU/cell to determine the extent of helper virus propagation during HD vector amplification. For all three infections, total intracellular DNA and intracellular virus were isolated at various times postinfection for Southern blot hybridization and quantitation by titration, respectively.
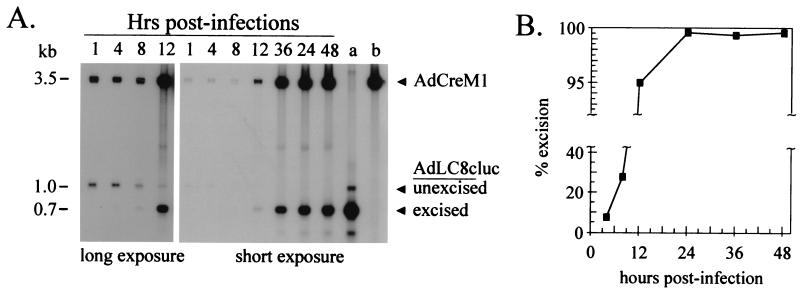
The results of the Southern analyses for infections I, II, and III are presented in Fig. 2A, 2B, and 2C, respectively. In the case of infection I, onset of DNA replication of the packaging signal-bearing viral genome (1.0-kb band) was observed after 8 h postinfection, and packaging signal excision was not observed, as expected (Fig. 2A). In contrast, infection II resulted in packaging signal excision, as expected (Fig. 2B). Excision was first detected after 1 h postinfection and increased until 24 h postinfection, after which there was little further increase in the amount of excision. Viral DNA replication of both the packaging signal-bearing and excised genomes was observed after 8 h postinfection. The results obtained for infection III (Fig. 2C) were similar to those obtained for infection II (Fig. 2B) with respect to the replication and packaging signal excision of the helper viral genome. As expected, efficient replication of the HD vector genome (2.1-kb band) was observed (Fig. 2C).
FIG. 2.
(A) Southern blot analysis of 293 cells infected with AdLC8cluc (denoted infection I in the text). Total intracellular DNA was extracted from infected cells at the indicated time postinfection, digested with BglI, and hybridized with probe SB. (B) Southern blot analysis of 293Cre4 cells infected with AdLC8cluc (denoted infection II). Total intracellular DNA was digested with BglI and hybridized with probe SB. Lane a contains the same DNA sample as the 48-h lane in A. (C) Southern blot analysis of 293Cre4 cells coinfected with AdLC8cluc and AdC4HSULacZ (denoted infection III). Total DNA was digested with BglI and hybridized simultaneously with probe SB and probe H. Lane a contains the HD vector plasmid pC4HSULacZ (25) digested with BglI and PmeI. Lane b contains the same DNA sample as the 48-h lane in panel B. Lane c contains plasmid pCA36 digested with AvaII (2), resulting in a 2,326-bp fragment which encompasses probe SB and a 3,126-bp fragment which encompasses probe H.
The results presented in Fig. 2B and 2C revealed that while Cre-mediated packaging signal excision was efficient, it was incomplete. To determine the fraction of viral genomes that had undergone packaging signal excision over time, phosphorimager analysis was performed on the Southern blots presented in Fig. 2B and 2C to quantify the packaging signal-bearing and packaging signal-excised helper viral genomes. The results revealed that for infection II, approximately 8% of the genomes had undergone packaging signal excision by 4 h postinfection, about 25% by 8 h, and 90% by 12 h, after which a plateau of 93 to 94% excision was reached from 24 to 48 h (Fig. 3A). Similar results were obtained in the case of infection III and revealed that approximately 35% of the helper viral genomes had undergone excision by 8 h postinfection and about 90% by 12 h postinfection, after which a plateau of about 95% was reached from 24 to 48 h (Fig. 3B).
FIG. 3.
Efficiency of Cre-mediated packaging signal excision. Percent packaging signal excision for infection II (A) and infection III (B) was calculated by comparing the intensity, as determined by PhosphorImager analysis, of the 1.0-kb and the 0.7-kb bands in each lane of the Southern blots presented in Fig. 2B and 2C, respectively. The source of the band visible below the 0.7-kb band in this and other Southern blots is unknown, but this species is not packaged (unpublished results).
In summary, the results presented above revealed that packaging signal excision, while efficient, was incomplete, and DNA replication was observed for the helper viral genomes retaining the packaging signal.
Contaminating helper virus has propagated in 293Cre4 cells.
To determine whether DNA replication of the packaging signal-bearing helper viral genome corresponded to an increase in intracellular infectious helper virus, the samples were titrated on 293 cells. As expected, in the case of infection I, propagation of the helper virus was observed and exhibited a typical adenovirus growth curve (Fig. 4A, solid squares). In the case of infection II, growth of the helper virus was also observed, as determined by titration on 293 cells (Fig. 4B). However, the amount of infectious progeny virus produced by infection II was reduced about 10-fold compared to infection I. These results indicated that Cre-mediated inhibition of helper virus propagation was about 90% but was clearly not complete, consistent with the results of the Southern blot hybridization analysis presented in Fig. 2B and 3A.
FIG. 4.
Yield of infectious virus at various times postinfection from infection I (A), infection II (B), and infection III (C) as titrated on 293 cells (solid squares) and on 293Cre4 cells (open circles). For infection III (C), the yield of HD vector at various times postinfection was also determined as BFU by X-Gal staining of transduced 293 cells (solid triangles).
In the case of infection III, propagation of the helper virus was also observed, as determined by titration on 293 cells. However, the amount of progeny virus produced was reduced another 10-fold (Fig. 4C). This further decrease in helper virus yield may be explained by the added competition in packaging provided by the HD vector (25). Amplification of the HD vector was also measured for infection III by titration on 293 cells followed by X-Gal staining. The results revealed that the HD vector was very efficiently amplified, exhibiting a typical adenovirus growth curve similar to that observed in Fig. 4A and yielding about 5,000 BFU/cell.
In summary, the results presented in Fig. 4 indicated that the helper virus is capable of propagating, albeit with reduced efficiency, following infection of 239Cre4 cells. Additionally, helper virus propagation is further reduced in the presence of the HD vector.
Contaminating helper virus is still sensitive to Cre.
One possible explanation for the growth of helper virus in 293Cre4 cells is that viral genomes have acquired Cre-resistant mutations. To test this hypothesis, the progeny viruses generated from infections I, II, and III were titrated on 293Cre4 cells and the results were compared to the titers on 293 cells. The difference in plaque-forming efficiencies of AdLC8cluc on 293Cre4 compared to 293 cells was first established as a control. As shown in Fig. 4A, the titer of AdLC8cluc was approximately 10-fold lower on 293Cre4 than 293 cells.
Therefore, if the progeny viruses generated from infections II and III were parental (i.e., Cre sensitive), then their plaque-forming ability should be about 10-fold lower on 293Cre4 cells than 293 cells, as in the case of infection I. In contrast, if they were Cre-resistant mutants, then their plaque-forming ability would be expected to be the same on 293Cre4 cells and 293 cells. Instead, the titers of the helper viruses obtained from both infections II and III, like those obtained from infection I, were approximately 10-fold lower on 293Cre4 cells than on 293 cells, suggesting that the helper viruses were as sensitive to Cre as the parental virus grown on 293 cells.
To confirm this, helper viral DNA was extracted from 50 plaques isolated by titration on 293 cells, 25 from infection II, and 25 from infection III and sequenced. The results revealed that none of the 50 isolates bore a Cre-resistant mutation, i.e., all had the expected packaging signal flanked by loxP sites identical in sequence to that of AdLC8cluc (data not shown).
In summary, these results indicated that the ability of the contaminating helper virus to escape Cre-mediated packaging signal excision and propagate following infection of 293Cre4 cells was not due to mutations that resulted in resistance to Cre.
Reverse Cre reaction is insignificant.
Another possible explanation for the ability of the helper virus to propagate following infection of 293Cre4 cells may be the reversibility of the Cre reaction. In addition to efficiently catalyzing intramolecular site-specific recombination (resulting in packaging signal excision), Cre is also capable of catalyzing the reverse intermolecular reaction, which would reinsert the excised packaging signal, albeit less efficiently than the intramolecular reaction (1). As described above (Fig. 3A and B), following infection of 293Cre4 cells, only a small proportion (about 5 to 6%) of the helper viral genomes remained unexcised. Perhaps this small but persistent population resulted from the low-efficiency reverse, intermolecular Cre reaction.
This possibility was investigated by using the experimental strategy shown in Fig. 5 in which 293Cre4 cells were coinfected with two helper viruses, AdLC8cluc and AdNG114R. These viruses are identical except for the orientations of the segment of DNA encompassing the packaging signal and loxP sites and except for restriction enzyme site polymorphisms that were introduced into this region of DNA to distinguish the packaging signals of the two viruses. Packaging signal exchange between these two helper viruses would generate two novel recombinants distinguishable from each other as well as from the parental viruses by the unique linkage relationship of the restriction enzyme site polymorphisms (Fig. 5). To simplify interpretation of the results, the loxP sites and the packaging signal of the two parental helper viruses are in opposite orientations. This precluded the generation of infectious recombinants by direct homologous (between packaging signals) or Cre-mediated recombination between the two parental genomes because such recombinants would be unpackageable.
Therefore, assuming that the excised packaging signal is not preferentially reinserted into the parental molecule from which it was excised, the frequency with which infectious recombinants are generated would be a measure of the reverse Cre reaction. If the reverse Cre reaction is responsible for the majority of the contaminating helper virus, then about half of the progeny virus should be parental, 25% being AdLC8cluc and 25% being AdNG114R, and the remaining progeny should be recombinants I and II (about 25% each). However, if the reverse reaction contributes insignificantly to the contaminating helper virus, then essentially all the progeny should be parental, 50% being AdLC8cluc and 50% being AdNG114R.
The experiment was performed by coinfecting 293Cre4 cells with the two parental viruses, each at an MOI of 5 PFU/cell, ensuring that every cell was infected by both viruses. At 48 h postcoinfection, intracellular progeny viruses were isolated by plaquing on 293 cells, and viral DNA was extracted and sequenced to determine the linkage relationship of the restriction enzyme site polymorphisms. A total of 70 plaque isolates were sequenced, and the results revealed that all were parental with respect to the linkage relationship of the polymorphic markers. Specifically, 38 of 70 (56%) were AdNG114R and 32 of 70 (46%) were AdLC8cluc. Therefore, these results suggest that the reverse Cre reaction does not contribute significantly to the contaminating helper virus. These results also support the conclusion presented above that the contaminating helper viruses are not Cre-resistant mutants, since no loxP mutations were observed in any of the 70 isolates.
Helper viral DNA is accessible to Cre but Cre is limiting.
It is possible that the helper virus has escaped Cre-mediated packaging signal excision because Cre is limiting in 293Cre4 cells. Another, not mutually exclusive possibility is that a proportion of the helper viral genome is inaccessible to Cre. To address these possibilities, the packaging signal excision efficiency was determined following coinfection of 293 cells with AdLC8cluc at an MOI of 1 PFU/cell and AdCreM1 at an MOI of 10 PFU/cell and compared to the results obtained following single infection of 293Cre4 cells with AdLC8cluc (Fig. 2B and 3A).
AdCreM1 is a first-generation adenovirus vector bearing a Cre expression cassette capable of directing very high levels of Cre expression in infected cells (see below). At various times postcoinfection, total intracellular DNA was isolated and analyzed by Southern blot hybridization. As expected, the 3.5-kb AdCreM1-specific band increased in intensity during the time course of infection (Fig. 6A). More importantly, the results revealed that while the kinetics of excision were similar to those in Fig. 2A at early times postinfection, the absolute amount of excision appeared much higher after 12 h, with the unexcised 1.0-kb band barely detectable even after prolonged exposures (Fig. 6A). Phosphorimager analysis comparing the intensities of the 1.0-kb band and the 0.7-kb band revealed that >99% of the helper viral genomes had undergone packaging signal excision by 24 h postcoinfection (Fig. 6B). Comparable results were obtained following coinfection of 293Cre4 cells with AdLC8cluc and AdCreM1 (Fig. 7).
FIG. 6.
(A) Southern blot analysis of 293 cells coinfected with AdLC8cluc and AdCreM1. Total intracellular DNA was extracted from the coinfected cells at the indicated times postcoinfection, digested with BglI, and hybridized with probe SB. Lane a contains BglI-digested DNA from 293 cells extracted at 48 h postinfection with AdLC8cluc. Lane b contains total intracellular DNA from 293 cells extracted at 48 h postinfection with AdCreM1 digested with BglI. (B) Percent packaging signal excision was calculated by comparing the intensity of the 1.0-kb and the 0.7-kb bands, as determined by PhosphorImager analysis, in each lane of the Southern blot presented in panel A.
FIG. 7.
(A) Southern blot analysis of 293Cre4 cells coinfected with AdLC8cluc and AdCreM1. Total intracellular DNA was extracted from the coinfected cells at the indicated times postcoinfection, digested with BglI, and analyzed with probe SB. Lane a contains total intracellular DNA from 293Cre4 cells extracted at 48 h postinfection with AdLC8cluc digested with BglI. Lane b contains total intracellular DNA from 293Cre4 cells extracted at 48 h postinfection with AdCreM1 digested with BglI. (B) Percent packaging signal excision was calculated by comparing the intensity of the 1.0-kb and the 0.7-kb bands, as determined by PhosphorImager analysis, in each lane of the Southern blot presented in A.
It is interesting that the lag in Cre expression in the experiment presented in Fig. 6 compared to that in Fig. 7 did not have an effect on the efficiency of excision, suggesting that preexisting intracellular Cre provided no advantage. The observation that the packaging signal excision efficiency is dramatically increased due to high levels of Cre expression from AdCreM1 suggests that Cre expression in helper virus-infected 293Cre4 cells is limiting. In addition, these results suggest that the majority of the contaminating helper virus is not a subpopulation that is inaccessible to Cre. Finally, these results are consistent with our previous finding that the reverse Cre reaction is not a significant contributing factor, since increasing the Cre activity would be expected to increase both the forward and reverse reactions equivalently. The results also provide additional evidence that acquisition of Cre-resistant mutations is not a major factor, since in this case increasing Cre activity would not increase the efficiency of excision.
Level of Cre decreases in helper virus-infected 293Cre4 cells.
Based on the observation that Cre is limiting with respect to packaging signal excision, the level of Cre protein in 293Cre4 cells was investigated. To accomplish this, total intracellular protein was extracted at various times postinfection with the indicated virus and subjected to Western blot analysis for Cre. The Cre coding sequence in 293Cre4 cells has been modified to include a seven-amino-acid nuclear localization signal, resulting in a 39-kDa recombinant protein (8). Western analysis of mock-infected 293Cre4 cells revealed that, in the absence of adenovirus infection, Cre was present at similar levels from 1 to 48 h post-mock infection (Fig. 8A). In contrast, following infection of 293Cre4 cells with AdLC8cluc, the level of Cre appeared to decrease after 12 h postinfection (Fig. 8B).
FIG. 8.
Western analysis for Cre at various times postinfection. (A) Proteins extracted from mock-infected 293Cre4 cells. Lane a, proteins extracted from 293 cells. (B) Proteins extracted from 293Cre4 cells infected with AdLC8cluc at an MOI of 5 PFU/cell. Lane a, proteins extracted from 293 cells. (C) Proteins extracted from 293 cells infected with AdCreM1 at an MOI of 10 PFU/cell. Lane a, proteins extracted from 293 cells. Lane b, proteins extracted from 293Cre4 cells. (D) Proteins extracted from 293Cre4 cells infected with AdCreM1 at an MOI of 10 PFU/cell. Lane a, proteins extracted from 293 cells. Lane b, proteins extracted at 48 h postinfection of 293 with AdCreM1 at an MOI of 10 PFU/cell. Lane c, proteins extracted from 293Cre4 cells. The Cre protein expressed from AdCreM1 is 38 kDa. The Cre protein expressed by 293Cre4 cells is 39 kDa (see Materials and Methods). Extraneous bands are due to nonspecific binding of the polyclonal primary antibody.
The level of Cre was also investigated following infection of 293 or 293Cre4 cells with AdCreM1. In 293 cells infected with AdCreM1, the 38-kDa Cre protein was first detected 4 h postinfection and increased by 8 h postinfection to a high level, which was maintained throughout the course of infection (Fig. 8C). In the case of 293Cre4 cells infected with AdCreM1, the level of Cre expressed from 293Cre4 cells (39 kDa) decreased as in Fig. 8B, while the amount of Cre expressed from AdCreM1 (38 kDa) increased to a high level by 8 h postinfection and persisted at high levels for the duration of the infection, as was seen in Fig. 8C.
In summary, the results indicated that the amount of Cre is significantly higher in AdCreM1-infected cells than 293Cre4 cells. Furthermore, these results also indicated that expression of Cre from 293Cre4 cells decreased over the time course of an adenovirus infection (AdLC8cluc or AdCreM1) but that the amount of Cre expression from an adenovirus vector (AdCreM1) increased and was maintained at a constant high level late in infection. These results are consistent with the phenomenon of adenovirus-mediated host cell shutoff in which expression of cellular proteins but not those encoded by the adenovirus is inhibited in infected cells (4, 6, 11, 15, 33, 34). The significance of these results will be addressed in the Discussion.
DISCUSSION
Because cell lines that express the full array of adenovirus genes are currently not available, a helper virus is required to provide all necessary functions in trans for HD vector propagation. The first efficient and currently most widely used system for generating HD vectors relies on Cre-mediated packaging signal excision to inhibit helper virus propagation (29). While the helper virus contamination levels are low using this system, further reduction is desirable due to safety concerns and production issues. However, this objective has remained elusive owing to our lack of understanding of the origin of the contaminating helper virus. The objective of this study was to determine the mechanisms responsible for the contaminating helper virus so that novel, rational strategies may be developed to further reduce, if not eliminate, helper virus contamination.
It is clear that the contaminating helper virus must have somehow escaped Cre-mediated packaging signal excision. Numerous mechanisms can be postulated to account for this. We began first by investigating in detail the kinetics and efficiencies of Cre-mediated packaging signal excision in helper virus-infected 293Cre4 cells. The results revealed that while Cre-mediated packaging signal excision was efficient, reaching 94 to 95%, it was incomplete, and replication of the packaging signal-bearing helper viral genome was observed (Fig. 2 and 3). Not surprisingly, this resulted in an increase in the production of infectious progeny virus (Fig. 4), indicating that the vast majority of the contaminating helper virus had propagated and therefore did not simply represent unabsorbed virus or virus that had absorbed but had not uncoated. It is interesting that output of infectious helper virus is greater following single infection of 293Cre4 cells than following coinfection with the HD vector (Fig. 4B and 4C) despite comparable amounts of packageable genomes (Fig. 2B and 2C). This may be explained by the reported competition in packaging between the AdLC8cluc and AdC4HSULacZ genomes (25).
Three lines of evidence indicated that the contaminating helper viruses were not Cre-resistant mutants. First, like the parent virus, their plaque-forming ability was about 10-fold lower in 293Cre4 cells compared to 293 cells. Second, no mutations in loxP were found in a total of 120 contaminating helper virus isolates, as determined by sequence analyses of the relevant DNA region. Third, increasing the amount of Cre in the producer cells, accomplished by coinfection with AdCreM1, resulted in increased packaging signal excision (Fig. 6 and 7). This result is inconsistent with Cre-resistant mutations as a major source of contaminating helper virus because in this case no increase in excision should have been observed by increasing Cre activity. These results are in contrast to those obtained by other investigators, who have observed emergence of Cre-resistant mutants leading to high levels of helper virus contamination (14, 16). The reason for this discrepancy remains to be determined. However, acquisition of Cre-resistant mutations resulting in the outgrowth of helper virus and high contamination levels may be dependent on the HD vector. In our experience, HD vectors based on the C4HSU backbone (30), like AdC4HSULacZ, have not been observed to result in the outgrowth of helper viruses when amplified using 293Cre4 cells and AdLC8cluc (unpublished results). Other variables, such as differences in the helper viruses, may also play a role.
Two lines of evidence suggest that the reverse Cre reaction does not contribute significantly to the contaminating helper virus. First, packaging signal exchange, evidence of the reverse Cre reaction, was not observed between two marked helper viruses following coinfection of 293Cre4 cells (Fig. 5). Second, increasing the amount of Cre in the producer cells, accomplished by coinfection with AdCreM1, resulted in increased packaging signal excision (Fig. 6 and 7). This result is inconsistent with the reverse Cre reaction as a major source of the contaminating helper virus because the reinsertion reaction would also be expected to increase with increasing amounts of Cre and result in no net improvement in excision efficiency. These results imply that use of alternative site-specific recombinases which only catalyze unidirectional reactions, such as the integrase from the Streptomyces phage φC31 (13), are unlikely to significantly reduce helper virus contamination levels.
The observation that the packaging signal excision efficiency in 293Cre4 cells can be improved from 95 to >99% by coinfection with AdCreM1 indicated that Cre was limiting in 293Cre4 cells. This was consistent with Western analyses which showed that the amount of Cre produced by AdCreM1 was greater than that produced by 293Cre4. Furthermore, the Western analyses also revealed that the amount of Cre in adenovirus-infected 293Cre4 cells decreased over the course of infection. This decrease was first observed shortly after the onset of viral DNA replication. In contrast, the amount of Cre in AdCreM1-infected cells was not only higher but also did not decrease late in infection.
These results are consistent with the phenomenon of adenovirus-mediated host cell shutoff in which production of cellular proteins, but not those encoded by adenovirus vectors, is inhibited following adenovirus infection. This well-documented phenomenon coincided with the onset of adenovirus DNA replication and has been attributed to a block in cytoplasmic accumulation of cellular mRNAs and preferential translation of viral mRNA (4, 6, 11, 15, 33, 34). However, since maximum excision was first observed 24 h postinfection (Fig. 2B and 2C), coinciding with the first observed drop in Cre levels (Fig. 8B, 8C, and 8D), incomplete excision is probably due primarily to the relatively low endogenous levels of Cre in 293Cre4 cells, and this situation may be exacerbated by the further decrease in Cre levels as a consequence of adenovirus-mediated host cell shutoff (Fig. 8). That the sustained high levels of Cre expressed from AdCreM1 resulted in greater excision also suggests that there does not exist a significant fraction of helper viral genomes that are inaccessible to Cre in 293Cre4 cells.
This study showed that about 5% of the helper viral genomes escape Cre-mediated packaging signal excision following infection of 293Cre4 cells. These genomes replicate and are packaged, resulting in helper virus contaminations levels of ≤1% in HD vector preparations before purification by CsCl ultracentrifugation. Our results suggest that a significant further reduction in helper virus contamination levels may be achieved by increasing the amount of Cre in the producer cells. Exactly how much of an increase will be required remains to be determined empirically. However, the phenomenon of adenovirus-mediated host cell shutoff may need to be avoided or addressed for such a strategy to be completely successful.
Acknowledgments
This work was supported by grants from the National Institutes of Health, the Canadian Institutes of Health Research (CIHR), and the National Cancer Institute of Canada (NCIC). P.N. was supported by a CIHR postdoctoral fellowship.
REFERENCES
- 1.Abremski, K., and R. Hoess. 1984. Bacteriophage P1 site-specific recombination. J. Biol. Chem. 259:1509-1514. [PubMed] [Google Scholar]
- 2.Addison, C. L., M. Hitt, D. Kunsken, and F. L. Graham. 1997. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J. Gen. Virol. 78:1653-1661. [DOI] [PubMed] [Google Scholar]
- 3.Anton, M., and F. L. Graham. 1995. Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: a molecular switch for control of gene expression. J. Virol. 69:4600-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babich, A., C. T. Feldman, J. R. Nevins, J. E. Darnell, and C. Weinberger. 1983. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol. Cell. Biol. 3:1212-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balague, C., J. Zhou, Y. Dai, et al. 2000. Sustained high-level expression of full-length human factor VIII and restoration of clotting activity in hemophilic mice using a minimal adenovirus vector. Blood 95:820-828. [PubMed] [Google Scholar]
- 6.Beltz, G. A., and S. J. Flint. 1979. Inhibition of HeLa cell protein synthesis during adenovirus infection. J. Mol. Biol. 131:353-373. [DOI] [PubMed] [Google Scholar]
- 7.Berkner, K. L. 1988. Development of adenovirus vectors for expression of heterologous genes. BioTechniques 6:616-629. [PubMed] [Google Scholar]
- 8.Chen, L., M. Anton, and F. L. Graham. 1996. Production and characterization of human 293 cell lines expressing the site-specific recombinase Cre. Somat. Cell Mol. Genet. 22:477-488. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H.-H., L. M. Mack, R. Kelly, M. Ontell, S. Kochanek, and P. R. Clemens. 1997. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc. Natl. Acad. Sci. USA 94:1645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cregan, S. P., J. MacLaurin, T. F. Gendron, S. Callaghan, D. S. Park, R. J. Parks, F. L. Graham, P. Morley, and R. S. Slack. 2000. Helper-dependent adenovirus vectors: their use as a gene delivery system to neurons. Gene Ther. 14:1200-1209. [DOI] [PubMed] [Google Scholar]
- 11.Gaynor, R. B., D. Hillman, and A. J. Berk. 1984. Adenovirus early region 1A protein activates transcription of a nonviral gene introduced into mammalian cells by infection or transfection. Proc. Natl. Acad. Sci. USA 81:1193-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus 5. J. Gen. Viol. 36:59-72. [DOI] [PubMed] [Google Scholar]
- 13.Groth, A. C., E. C. Olivares, B. Thyagarajan, and M. P. Calos. 2000. A phage integrase directs efficient site-specific integration in human cells. Proc. Natl. Acad. Sci. USA 97:5995-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy, S., M. Kitamura, T. Harris-Stansil, Y. Dai, and M. L. Phipps. 1997. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 71:1842-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hearing, P., and T. Shenk. 1985. Sequence-independent autoregulation of adenovirus type 5 E1A transcription unit. Mol. Cell. Biol. 5:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillgenberg, M., F. Schneider, P. Löser, and M. Strauss. 2001. System for efficient helper-dependent minimal adenovirus construction and rescue. Hum. Gene Ther. 12:643-657. [DOI] [PubMed] [Google Scholar]
- 17.Hitt, M., C. L. Addison, and F. L. Graham. 1997. Human adenovirus vectors for gene transfer into mammalian cells. Adv. Pharmacol. 40:137-206. [DOI] [PubMed] [Google Scholar]
- 18.Hitt, M. M., R. J. Parks, and F. L. Graham. 1999. Structure and genetic organization of adenovirus vectors, p. 61-86. In T. Friedman (ed.), The development of human gene therapy. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Kim, I. H., A. Jozkowicz, P. A. Piedra, K. Oka, and L. Chan. 2001. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. USA 98:13282-13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochanek, S. 1999. High-capacity adenoviral vectors for gene transfer and somatic gene therapy. Hum. Gene Ther. 10:2451-2459. [DOI] [PubMed] [Google Scholar]
- 21.Maione, D., M. Wiznerowicz, P. Delmastro, R. Cortese, G. Ciliberto, N. La Monica, and R. Savino. 2000. Prolonged expression and effective readministration of erythropoietin delivered with a fully deleted adenoviral vector. Hum. Gene Ther. 11:859-868. [DOI] [PubMed] [Google Scholar]
- 22.Morral, N., W. O'Neal, K. Rice, M. Leland, J. Kaplan, P. A. Piedra, H. Zhou, R. J. Parks, R. Velji, E. Aguilar-Córdova, S. Wadsworth, F. L. Graham, S. Kochanek, K. D. Carey, and A. L. Beaudet. 1999. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver directed gene transfer in baboons. Proc. Natl. Acad. Sci. USA 96:12816-12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morral, N., R. J. Parks, H. Zhou, C. Langston, G. Schiedner, J. Quinones, F. L. Graham, S. Kochanek, and A. L. Beaudet. 1998. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of α1-antitrypsin with negligible toxicity. Hum. Gene Ther. 9:2709-2716. [DOI] [PubMed] [Google Scholar]
- 24.Morsy, M. A., M. Gu, S. Motzel, J. Zhao, J. Lin, Q. Su, H. Allen, L. Franlin, R. J. Parks, F. L. Graham, S. Kochanek, A. J. Bett, and C. T. Caskey. 1998. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA 95:7866-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng, P., C. Beauchamp, C. Evelegh, R. Parks, and F. L. Graham. 2001. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol. Ther. 3:809-815. [DOI] [PubMed] [Google Scholar]
- 26.Ng, P., R. J. Parks, and F. L. Graham. 2001. Methods for the preparation of helper-dependent adenoviral vectors. Methods Mol. Med. 69:371-388. [DOI] [PubMed] [Google Scholar]
- 27.Oka, K., L. Pastore, I.-H. Kim, A. Merched, S. Nomura, H.-J. Lee, M. Merched-Sauvage, C. Arden-Riley, B. Lee, M. Finegold, A. Beaudet, and L. Chan. 2001. Long-term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation 103:1274-1281. [DOI] [PubMed] [Google Scholar]
- 28.Parks, R. J. 2000. Improvements in adenoviral vector technology: overcoming barriers for gene therapy. Clin. Genet. 58:1-11. [DOI] [PubMed] [Google Scholar]
- 29.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adeonvirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 93:13565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandig, V., R. Youil, A. J. Bett, L. L. Franlin, M. Oshima, D. Maione, F. Wang, M. L. Metzker, R. Savino, and C. T. Caskey. 2000. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc. Natl. Acad. Sci. USA 97:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiedner, G., N. Morral, R. J. Parks, Y. Wu, S. C. Koopmans, Langston, F. L. Graham, A. L. Beaudet, and S. Kochanek. 1998. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 18:180-183. [DOI] [PubMed] [Google Scholar]
- 32.Umaña, P., C. A. Gerdes, D. Stone, J. R. E. Davis, D. Ward, M. G. Castro, and P. R. Lowenstein. 2001. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat. Biotechnol. 19:582-585. [DOI] [PubMed] [Google Scholar]
- 33.Yoder, S. S., B. L. Robberson, E. J., Leys, A. G. Hook, M. Al-Ubaidi, C. Y.Yeung, R. E. Kellems, and S. M. Berget. 1983. Control of cellular gene expression during adenovirus infection: induction and shut off of dihydrofolate reductase gene expression by adenovirus type 2. Mol. Cell. Biol. 3:819-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zang, Y., and R. Schneider. 1993. Adenovirus inhibition of cellular protein synthesis and the specific translation of late viral mRNA. Semin. Virol. 4:229-236. [Google Scholar]
- 35.Zou, L., X. Yuan, H. Zhou H., Lu, and K. Yang. 2001. Helper-dependent adenoviral vector-mediated gene transfer in aged rat brain. Hum. Gene Ther. 12:181-191. [DOI] [PubMed] [Google Scholar]