Abstract
Adenovirus infection and expression of E1A induces both proliferation and apoptosis, the latter of which is blocked by the adenovirus Bcl-2 homologue E1B 19K. The mechanism of apoptosis induction and the role that it plays in productive infection are not known. Unlike apoptosis mediated by death receptors, infection with proapoptotic E1B 19K mutant viruses did not induce cleavage of Bid but nonetheless induced changes in Bak and Bax conformation, Bak-Bax interaction, caspase 9 and 3 activation, and apoptosis. In wild-type-adenovirus-infected cells, in which E1B 19K inhibits apoptosis, E1B 19K was bound to Bak, precluding Bak-Bax interaction and changes in Bax conformation. Infection with E1B 19K mutant viruses induced apoptosis in wild-type and Bax- or Bak-deficient baby mouse kidney cells but not in those deficient for both Bax and Bak. Furthermore, Bax and Bak deficiency dramatically increased E1A expression and virus replication. Thus, Bax- and Bak-mediated apoptosis severely limits adenoviral replication, demonstrating that Bax and Bak function as an antiviral response at the cellular level.
The replication strategy of DNA viruses invariably requires that the infected quiescent cell enter the S-phase of the cell cycle, facilitating replication of the viral genome. The members of the Adenoviridae initiate this process by expression of the E1A oncoprotein, which binds the p300 transcriptional coactivator and retinoblastoma protein family members, stimulating deregulation of cell cycle progression and apoptosis (40). Although E1A can cause apoptosis through the stabilization of the p53 tumor suppressor protein during oncogenic transformation (6, 17, 39), in productively infected cells p53 is degraded through ubiquitin-mediated proteolysis by a complex containing the adenovirus E1B 55K and E4orf6 proteins (26, 30). Therefore, E1A induces apoptosis in a p53-independent manner during infection (4).
Adenovirus normally prevents this E1A-mediated apoptosis by expressing the adenoviral Bcl-2 homologue E1B 19K (42). Infection with viral mutants lacking E1B 19K results in the fragmentation of viral and cellular DNA and, in some cases, premature death of the infected cell and a reduction in viral titer (24, 31, 34, 44). It has been difficult to distinguish whether diminution of virus production may be due to a failure to inhibit apoptosis or to a missing alternate function of E1B 19K. Similarly, the precise role in infection and mechanism of action of the inhibitors of apoptosis encoded by many DNA viruses remain to be determined (28).
The mechanisms that regulate apoptosis in response to a variety of stimuli have been studied in several different systems. In general, the onset of apoptosis initiates signaling cascades that are mediated by pro- and antiapoptotic members of the Bcl-2 family of proteins and by a class of cysteine proteases known as the caspases, which converge on a checkpoint in which mitochondria play a central role. The critical event in apoptotic signaling through mitochondria is a conformational change in the proapoptotic Bcl-2 family members Bak and Bax (7, 20, 23, 33, 38) and the formation of high-molecular-weight Bak-Bax heterocomplexes (32).
The signal that causes the conformational change of Bak and Bax is clearly understood in the case of death receptor (i.e., tumor necrosis factor alpha [TNF-α] and Fas ligand) signaling, where activated caspase 8 processes the Bcl-2 family member Bid to the truncated form tBid by a proteolytic cleavage event near the amino terminus (10, 16, 18). tBid is a powerful proapoptotic factor that functions by causing the conformational change in Bak and Bax through a direct interaction (23, 38). Currently, no other factor is known to influence the conformational status of Bak and Bax. These events correlate with the release of cytochrome c and Smac/DIABLO from the intermembrane space of mitochondria (23, 33, 38), resulting in activation of the downstream caspases 9 and 3 and proteolytic cleavage of many target proteins. The events that result from caspase activation are probably intended to kill abnormal cells and thus restrict the proliferation of a neoplastic phenotype or perhaps the progression of a viral infection.
In an effort to better understand the regulation of apoptosis in the adenovirus-infected cell, we have studied the molecular events brought about by E1A expression during infection. To this end, we have identified the essential regulators of E1A-mediated apoptosis in virus infection, the precise role played by apoptosis in the viral life cycle, and the mechanism by which E1B 19K blocks this process. Previous work has suggested that the onset of apoptosis limits the intracellular replication of adenovirus (3). Here, we demonstrate that Bak and Bax are the central mediators of apoptosis mediated by E1A expression during infection, establishing the role of the mitochondrial checkpoint in the induction of apoptosis by adenovirus. Interestingly, the activation of Bak and Bax under these circumstances appears to be mediated by a novel mechanism rather than the currently known pathway requiring tBid. In addition, we present evidence suggesting that apoptosis mediated by Bak and Bax is a significant limiting factor in the efficiency of adenoviral replication, indicating that the apoptotic response of the cell to infection has indeed evolved as an antiviral response. To compensate, the virus has developed the antiapoptotic activity of E1B 19K. Thus, Bak and Bax regulate the host cell's response to infection and are therefore targeted by the virus to counteract apoptosis.
MATERIALS AND METHODS
Virus infection and Western blotting.
HeLa (human cervical carcinoma) cells were mock infected or infected with the wild-type adenovirus type 5 (Ad5) Ad5dl309 (13) or the deletion mutant Ad5dl337 (24) or Ad5E1B− (2) at a multiplicity of infection (MOI) of 100 by standard methods (41). Attached and floating cells were harvested at 0 (for mock only), 12, 24, 36, 48, and 72 h postinfection by scraping and centrifugation. Uninfected HeLa cells treated with tumor necrosis factor alpha (2,000 U/ml) and cycloheximide (30 μg/ml) (TNF/CHX) for 12 h were processed in parallel. All cell pellets were resuspended in 2× Laemmli buffer and vortexed briefly. Each lysate sample was subjected to sodium dodecyl sulfate-17% polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting (22) with the following primary antibodies: anti-caspase 9 rabbit polyclonal (Stratagene, La Jolla, Calif.); anti-caspase 3 rabbit polyclonal (PharMingen, San Diego, Calif.); anti-caspase 8 rat monoclonal (Zymed Laboratories, South San Francisco, Calif.); anti-Bid mouse monoclonal (Junying Yuan, Harvard Medical School, Boston, Mass.); and anti-poly(A)-ribopolymerase (PARP) mouse monoclonal (Guy Poirier, Centre de Recherche du Chul, Quebec, Canada) antibodies.
Immunoprecipitation.
For each immunoprecipitation reaction, HeLa cells were infected and harvested at 24 and 48 h postinfection by scraping and lysed in 2.0% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS)-containing lysis buffer (23). Procedures for immunoprecipitation have been described elsewhere (23, 32, 33). Immunoprecipitations in Fig. 2A were carried out with the following primary antibodies: anti-BRCA2 rabbit polyclonal (Santa Cruz Biotechnology, Santa Cruz, Calif.); anti-Bak(23-37) rabbit polyclonal (Upstate Biotechnology, Lake Placid, N.Y.); anti-Bak(Ab-1) mouse monoclonal (Oncogene Research, San Diego, Calif.); and anti-Bak minus transmembrane domain (−TM) rabbit polyclonal (PharMingen) antibodies. Immunoprecipitations in Fig. 2B were carried out with the following antibodies: anti-Myc rabbit polyclonal (Santa Cruz); anti-Bax(11-30) rabbit polyclonal (Santa Cruz); anti-Bax(150-165) rabbit polyclonal (Oncogene Research); and anti-Bax(43-61) rabbit polyclonal (PharMingen) antibodies. Immunoprecipitated material was resuspended in 2× Laemmli buffer, and a sample of each immunoprecipitation reaction was subjected to SDS-17% PAGE. Western blotting (23) was carried out with the following primary antibodies: anti-Bax(11-30) rabbit polyclonal (Santa Cruz); anti-Bak(23-37) (Upstate Biotechnology); and anti-E1B 19K rabbit antiserum described previously (33).
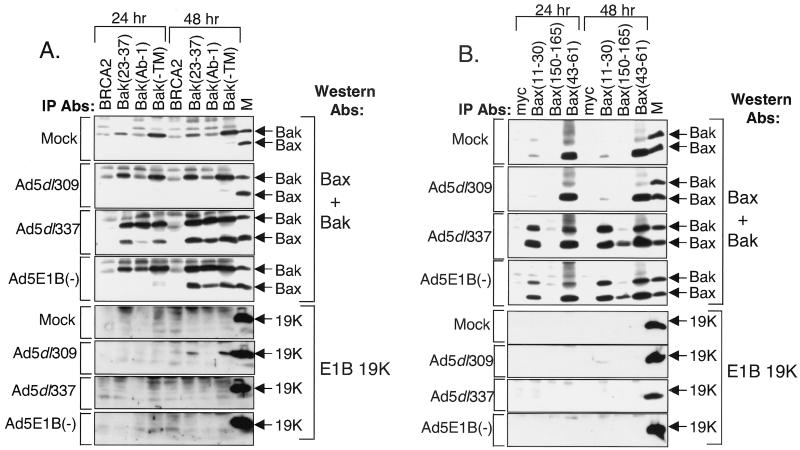
FIG. 2.
Conformational changes in Bak and Bax and Bak-Bax interaction in Ad5dl337- and Ad5E1B−-infected HeLa cells. (A) Immunoprecipitation (IP) of Bak from mock-, Ad5dl309-, Ad5dl337-, and Ad5E1B−-infected cells. Immunoprecipitations were carried out with anti-BRCA-2, anti-Bak(23-37), anti-Bak(Ab-1), and anti-Bak(−TM) antibodies (Abs) from the soluble fraction of cells lysed in CHAPS-containing buffer at 24 and 48 h postinfection. Ad5dl309-infected HeLa cell lysate (M) was used as a marker for Bak, Bax, and E1B 19K. Western blotting was carried out on precipitated material with a combination of anti-Bak and anti-Bax antibodies (top four panels) or with anti-E1B 19K antibody (bottom four panels). (B) Immunoprecipitation of Bax from mock-, Ad5dl309-, Ad5dl337-, and Ad5E1B−-infected cells. Experiment was executed as for panel A except that immunoprecipitation was carried out with anti-Myc, anti-Bax(11-30), anti-Bax(150-165), and anti-Bax(43-61) antibodies. Western blotting was carried out as for panel A.
Subcellular fractionation.
For each fractionation condition, HeLa cells were mock or virus infected, and at 24 and 48 h postinfection, cells were harvested by scraping and lysed by mechanical disruption. The fractionation procedure has been described elsewhere (32). A sample of total lysate from each condition was retained and analyzed as an indicator of total protein levels. Cells were lysed by membrane disruption through a syringe and centrifuged at 1,000 × g, yielding a pellet that contained intact (unlysed) cells, nuclei and a varying fraction of the mitochondria. The supernatant was centrifuged at 10,000 × g, and the pellet was retained as the mitochondrial fraction. The resulting supernatant was centrifuged at 100,000 × g, and the soluble portion was retained as the cytosolic fraction.
Each total lysate and the mitochondrial and cytosolic fractions were mixed with equal volumes of 2× Laemmli buffer and subjected to SDS-17% PAGE. Western blotting for cytochrome c, cytochrome oxidase subunit II (COXII), and Smac/DIABLO was carried out as previously described (32), with the following primary antibodies: anti-denatured cytochrome c monoclonal mouse (PharMingen), Smac/DIABLO rabbit polyclonal (32), and anti-COXII mouse monoclonal (Molecular Probes, Leiden, The Netherlands) antibodies.
Analysis of apoptosis in infected BMK cells.
Baby mouse kidney (BMK) cells derived from wild-type, Bax-deficient, Bak-deficient, or Bax- and Bak-deficient mice and transformed by a genomic construct of adenovirus type 5 E1A driven by the cytomegalovirus (CMV) promoter (42) and CMV-driven dominant negative (C-terminal fragment, termed p53DD) mouse p53 have been described elsewhere (6a). Equal numbers of BMK cells of each line were infected with Ad5dl309, Ad5dl337, or Ad5E1B− at an MOI of 100. Cells were observed throughout the infection by phase-contrast microscopy and photographed.
Fragmentation of DNA was assayed by harvesting cells at 48 h postinfection, and Hirt supernatants were prepared and analyzed by Tris-acetate-EDTA-agarose gel electrophoresis as previously described (44). For assessment of viral replication in BMK cell lines, infection was carried out with Ad5dl309 and Ad5dl377 at an MOI of 10, and floating and attached cells were collected by scraping at 3 days and 7 days postinfection. The cell suspension was frozen and thawed for three cycles, and the titer of PFU was determined by serial dilution of cell supernatants and infection of 293 cell monolayers under nutrient agar overlay.
For Western blotting analysis, equal numbers of cells of each BMK cell line were infected and harvested at 24 and 48 h postinfection by scraping, and each cell pellet was resuspended in 2× Laemmli buffer. Each lysate was subjected to SDS-17% PAGE, and Western blotting was performed for Bax and Bak as described above. Blotting for E1A was performed with anti-E1A monoclonal mouse antibody (Oncogene Research), and polypeptide III was detected with an anti-adenovirus 2 goat polyclonal antibody (Accurate Chemical and Scientific, Westbury, N.Y.).
Indirect immunofluorescence.
Wild-type and Bax- and Bak-deficient BMK cells were grown on glass coverslips and infected with Ad5dl309, Ad5dl337, or Ad5dlE1B− or mock infected for up to 3 days (72 h) at an MOI of 10. Every 24 h, a sample of coverslips was fixed in 4% paraformaldehyde, and indirect immunofluorescence was performed as described previously (23), except that coverslips were blocked with 4% bovine serum albumin-phosphate-buffered saline (PBS) for 1 h at 37°C. Coverslips were stained with either anti-adenovirus 2 goat polyclonal antibody (Accurate Chemical and Scientific, Westbury, N.Y.) diluted 1:60 or anti-Ad5 E1B 55K rat monoclonal antibody (Oncogene Research) diluted 1:60. Staining was visualized by epifluorescence microscopy as described previously (23), and the percentages of cells positive for either E1B 55K or adenovirus structural proteins were determined by scoring 100 to 200 cells on each coverslip.
RESULTS
E1B 19K blocks apoptosis upstream of caspase 9 activation.
As E1A-mediated apoptosis during infection is blocked by a pan-inhibitor of caspase activity (3), we identified the caspases that are activated by adenovirus infection. HeLa cells, the most commonly used cells in the study of adenovirus replication and apoptosis, were used to characterize the molecular events in death signaling during infection. Although HeLa cells are essentially p53 null, their p53 status is inconsequential to the adenovirus life cycle due to the expression of E1B 55K, which normally inactivates p53. HeLa cells were infected with the E1B 19K-expressing, wild-type adenovirus Ad5dl309, the E1B 19K gene deletion mutant Ad5dl337, and the E1B gene deletion mutant Ad5E1B−. These mutant adenoviruses have a similar apoptosis phenotype, although Ad5E1B− is somewhat more impaired than Ad5dl337 in the efficiency of replication owing to the loss of E1B 55K's function in facilitating the nuclear export of viral mRNA (1, 25).
Mock-infected and virus-infected cultures were harvested at the indicated time points and analyzed for caspase 9, 3, and 8 processing, which is indicative of activation, and the cleavage substrates Bid and PARP (Fig. 1). In mock- and Ad5dl309-infected cells, procaspase 9 remained largely unprocessed compared to HeLa cells treated with TNF/CHX. A background level of activation was seen very late in the time course, which is probably attributable to some cells' undergoing apoptosis due to overcrowding, particularly in the mock-infected sample. In contrast, infection with the proapoptotic viruses Ad5dl337 and Ad5E1B− resulted in the progressive (though not complete) loss of procaspase 9 and accumulation of the p35 and p36 caspase 9 cleavage products (Fig. 1), starting at 24 h postinfection.
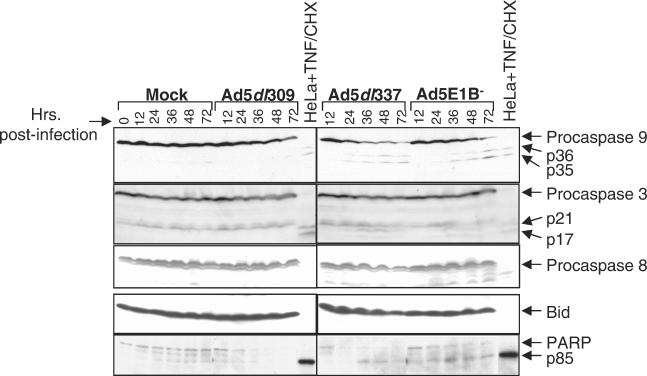
FIG. 1.
E1B 19K expression during adenovirus infection blocks activation of caspases 9 and 3. Cells were mock, Ad5dl309, Ad5dl337, or Ad5E1B− infected for 72 h, and representative samples were collected at 0 (for mock only), 12, 24, 36, 48, and 72 h postinfection and analyzed by Western blotting with antibodies against caspase 9, caspase 3, caspase 8, Bid, and PARP. Arrows indicate unprocessed (pro-) and processed forms (where visible) of caspases 9 and 3 and PARP. Lysates of TNF/CHX-treated HeLa cells were located in each panel as controls for caspase processing and substrate cleavage.
Probing for caspase 3 revealed a similar pattern, with no sign of processing in mock- or Ad5dl309-infected cells. However, infection with both E1B mutant viruses caused the appearance of the p17 and p21 caspase 3 cleavage products (Fig. 1). Despite activation, the proform of caspase 3 (and, to a lesser extent, caspase 9) appears undiminished, perhaps due to the continued synthesis of caspase proforms during infection. Concurrent with the processing of caspase 3, cleavage of PARP into the 85-kDa caspase-generated product occurred only in the E1B mutant virus-infected cells, although degradation of full-length PARP did occur in the mock and wild-type infections, as evidenced by the appearance of non-caspase-generated cleavage products.
Blotting for procaspase 8 or its physiological target Bid revealed almost no processing of either protein in cells infected with either the wild-type or mutant viruses, as ascertained by the abundance of the unprocessed forms compared to the TNF/CHX-treated controls (Fig. 1). The anti-caspase 8 and anti-Bid antibodies did not recognize the caspase-generated products of either protein in TNF/CHX-treated HeLa cells. Lower-molecular-weight bands seen in the caspase 8 Western blot do not correspond to the sizes of activation products and probably represent nonspecific degradation of the full-length protein. Thus, adenovirus infection in the absence of E1B 19K resulted in the activation of caspases 9 and 3, while caspase 8 was not activated, indicating that E1B 19K blocks apoptosis upstream of caspase 9 activation. These observations are in agreement with previous work (23) showing the absence of caspase 8 activation and Bid cleavage in wild-type- and E1B mutant virus-infected HeLa cells.
Death signaling during virus infection induces conformational changes in Bak and Bax and Bak-Bax interaction.
In apoptosis mediated by death receptors, cleavage of Bid to tBid (10, 16, 18) results in tBid-Bax and tBid-Bak protein interactions (23, 38), conformational changes in Bax and Bak (7, 23, 32, 38), Bak-Bax interaction and oligomerization (32, 33, 36), cytochrome c and Smac/DIABLO release from mitochondria (23, 32, 38), caspase 9 and 3 activation, and apoptosis. E1B 19K expression blocks death receptor signaling by binding to both Bak and Bax after induction of tBid-induced conformational changes in the amino termini of both Bak and Bax (23, 32, 33).
To test if death signaling by E1A resembled that of death receptors, we investigated the conformational status of Bak and Bax by conformation-specific immunoprecipitation from infected HeLa cells. An antibody that recognizes amino acid residues 23 to 37 precipitated Bak preferentially in cells infected with either the wild-type or E1B mutant virus at both 24 and 48 h postinfection, compared to mock-infected cells (Fig. 2A). In contrast, the anti-Bak(Ab-1) monoclonal antibody, whose epitope has not been mapped, precipitated more Bak from cells infected with the E1B mutant viruses than from cells infected with wild-type virus (Fig. 2A). An antibody that recognizes Bak regardless of conformational status [Bak(−TM)] precipitated Bak at approximately equal levels in mock- and virus-infected cells at both 24 and 48 h postinfection (Fig. 2A). Bax was coprecipitated by Bak(23-37), Bak(Ab-1), and Bak(−TM) antibodies, but only from cells infected with E1B mutant viruses (Fig. 2A).
These observations suggested that adenovirus infection stimulated a conformational change in the amino terminus of Bak, even in the absence of tBid, which occurred independently of E1B 19K expression. The interaction between Bak and Bax was, however, blocked by E1B 19K expression (Fig. 2A). The conformational change in Bak and the Bak-Bax interaction did not occur in cells infected with the viral mutant Ad5E1A−, which lacks E1A, or PAC3, which lacks both E1A and E1B, indicating that the Bak alteration, Bak-Bax interaction, and apoptosis were E1A dependent (data not shown).
At 24 and 48 h postinfection, an antibody which recognizes residues 11 to 30 precipitated Bax poorly from mock- and Ad5dl309-infected cells. The same antibody displayed robust immunoprecipitation of Bax from Ad5dl337- and Ad5E1B−-infected cells (Fig. 2B). Conversely, an antibody that recognizes residues 150 to 165 of Bax, comprising the BH2 region near the carboxy terminus, precipitated a significant amount of Bax only in mutant virus-infected cells and only at 48 h postinfection (Fig. 2B). An antibody which recognizes an epitope in the unstructured loop region of Bax encompassing residues 43 to 61 and which recognizes Bax regardless of conformational status (33) immunoprecipitated equal amounts of Bax at 24 and 48 h in either uninfected or infected cells (Fig. 2B).
Bak coprecipitated with Bax only in the E1B mutant virus-infected cells at 24 and 48 h postinfection under the same conditions in which the Bax amino terminus was exposed (Fig. 2B). These data indicate that adenovirus infection induced the sequential exposure of the amino terminus followed by the carboxy terminus of Bax, both of which were blocked by E1B 19K expression. As in the case of Bak, the conformational changes in Bax were independent of tBid. In addition, the Bak-Bax interaction is concurrent with the amino-terminal change but prior to the carboxy-terminal change in Bax, suggesting that binding of Bak to Bax may prompt exposure of the Bax carboxy terminus.
E1B 19K binds Bak and prevents Bak-Bax interaction.
When E1B 19K levels in the immunoprecipitations were analyzed, we observed scant levels of E1B 19K coprecipitating with Bax at 48 h postinfection with Ad5dl309 (Fig. 2B). This finding is consistent with the interaction of E1B 19K with Bax requiring exposure of the Bax amino-terminal epitope (23, 33). Thus, it is unlikely that that the binding of the Bax(11-30) and Bax(150-165) antibodies with Bax is affected by the presence of E1B 19K, indicating that the failure of these antibodies to immunoprecipitate Bax from Ad5dl309-infected cells reflects the unavailability of the corresponding epitopes. E1B 19K coprecipitated with Bak using the Bak(23-37) and Bak(−TM) antibodies at 48 h postinfection with Ad5dl309 (Fig. 2A). The Bak(Ab-1) antibody did not precipitate a Bak-E1B 19K complex, perhaps because E1B 19K may interfere with recognition of this Bak epitope. The interaction of E1B 19K with Bak and the loss of the Bak-Bax interaction in Ad5dl309-infected cells indicated that E1B 19K may function by binding to Bak directly and abrogating the Bak-Bax interaction that is induced by E1A expression during infection.
E1B 19K prevents release of proapoptotic mitochondrial proteins.
As Bax and Bak function is linked to translocation to the cytosol of mitochondrial proteins critical to caspase activation, the localization of cytochrome c and Smac/DIABLO was analyzed by subcellular fractionation in HeLa cells that were mock, Ad5dl309, Ad5dl337, or Ad5E1B− infected. As a marker for the recovery of mitochondria from the intact cell/nuclear fraction, the samples were also analyzed for the presence of the mitochondrial protein COXII. Mitochondria were apparently more easily recovered once infection had progressed, as evidenced by the appearance of a strong signal for COXII in the mitochondrial fractions either in late infection with Ad5dl309 or in infection with the viral mutants; this effect may be due to disruption of the cytoskeleton.
In mock-infected HeLa cells, the levels of both cytochrome c and Smac/DIABLO in the cytosolic fraction were nearly undetectable at both 24 and 48 h. Cytosolic cytochrome c was not observed at 24 h in the Ad5dl309 infection, although low levels were detectable in the cytosol at 48 h postinfection (Fig. 3). In the Ad5dl337- and Ad5E1B−-infected cells, cytochrome c was present in the cytosol at both 24 h and 48 h postinfection, compared to mock- and Ad5dl309-infected cells (Fig. 3). In addition, the total signal for cytochrome c was reduced in the mitochondrial fraction of Ad5dl337-infected cells, despite efficient recovery of the mitochondria. Concurrently, we observed a loss of Smac/DIABLO from both the mitochondrial fraction and total extract in Ad5dl337-infected cells, although recovery of mitochondria was fairly efficient.
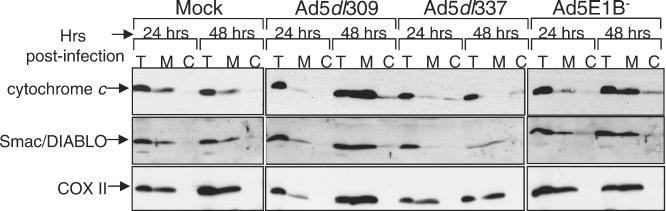
FIG. 3.
E1B 19K prevents the release and degradation of cytochrome c and Smac/DIABLO from mitochondria during adenovirus infection. HeLa cells that were mock, Ad5dl309, Ad5dl337, or Ad5E1B− infected were harvested at 24 and 48 h postinfection. The total lysate (T) and mitochondrial (M) and cytosolic (C) fractions from each sample were analyzed by Western blotting with antibodies against cytochrome c (top panels), Smac/DIABLO (middle panels), or the mitochondrial marker protein COXII (bottom panels).
In p53-mediated apoptosis, a reduction of both cytochrome c and Smac/DIABLO levels accompanies their release from the mitochondria and may correspond to their degradation (11). In Ad5E1B−-infected cells, loss of cytochrome c and Smac/DIABLO was less pronounced, but release into the cytosolic fraction is more apparent, indicating a slower progression of mitochondrial release that is probably due to the slower replication of the more defective Ad5E1B− virus. As mentioned previously, the lack of E1B 55K's nuclear export function may be responsible for the slower onset of apoptosis in infection with Ad5E1B− (1, 25). Thus, the loss of Smac/DIABLO signal from Ad5dl337-infected cells and the lack of translocation and degradation of both cytochrome c and Smac/DIABLO in early infection with Ad5dl309 compared to the E1B mutant viruses demonstrate that expression of E1B 19K retards the release of these factors from the intermembrane space of mitochondria, as is the case in p53 and TNF-α-mediated apoptosis (11, 23, 32). As Bak and Bax function is required for cytochrome c release in other signaling pathways (37), binding and/or inhibition of Bak and Bax by E1B 19K may be the means to block release of mitochondrial proteins and apoptotic signaling.
Bak and Bax both signal apoptosis during adenovirus infection.
To investigate the functional necessity of Bax and Bak in infection-mediated apoptosis, we used baby mouse kidney (BMK) cell lines transformed with E1A and dominant negative p53 that were generated from newborn mice that were either wild type (W2) or deficient in Bax (X1), Bak (K1), or both Bax and Bak (D2) (6a). Although rodent cells are semipermissive to adenovirus type 5 replication, they are capable of supporting replication at a reduced capacity. As is the case with the HeLa cell line, the inactivation of p53 in the BMK cell lines is not expected to affect adenoviral replication due to the normal activity of the E1B 55K protein in neutralizing p53.
Infection with Ad5dl309 resulted in some cytopathic effect (CPE), consistent with a partial loss of viability in the W2, X1, and K1 cells compared to mock infection (Fig. 4A). E1B 19K is less effective at inhibiting apoptosis in mouse cells compared to human cells (9), and this finding is consistent with that observation. Infection with Ad5dl337 and Ad5E1B− resulted in rapid CPE resembling apoptosis in the W2, X1, and K1 cells starting at 24 h postinfection (data not shown) and culminating in nearly complete destruction of the monolayer by 48 h (Fig. 4A). In contrast, infection of the D2 cells with Ad5dl309, Ad5dl337, or Ad5E1B− caused negligible apoptotic CPE up to 48 h postinfection (Fig. 4A).
FIG. 4.
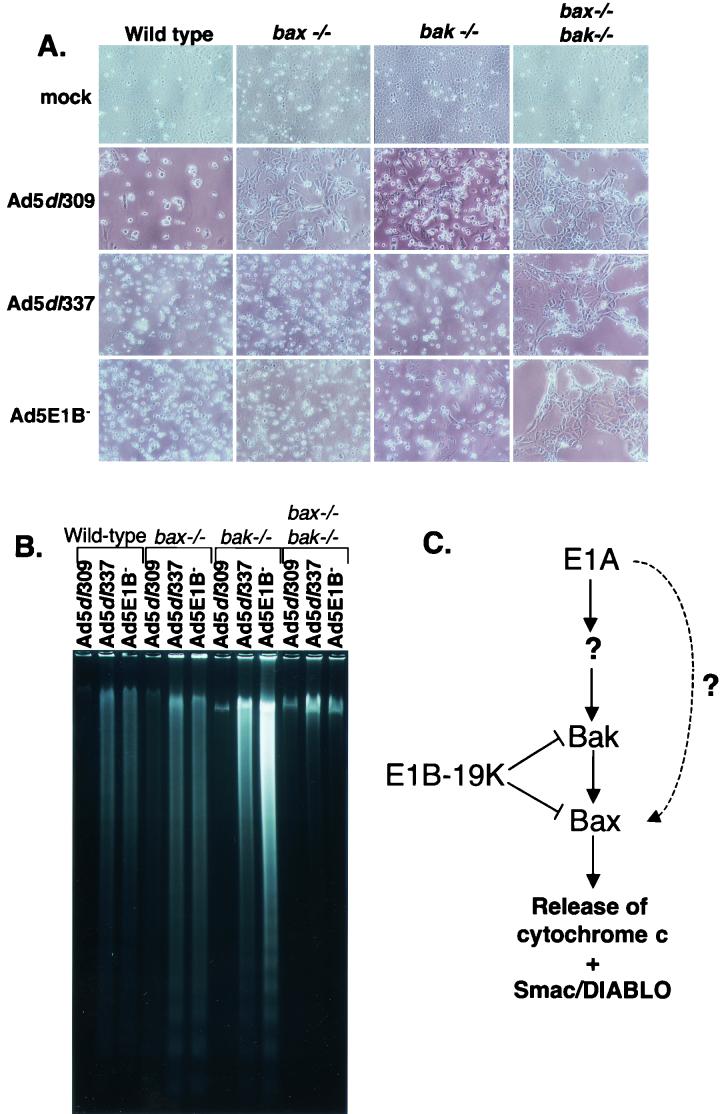
Cells deficient in both Bak and Bax are resistant to E1A-induced apoptosis. (A) Bak and Bax deficiency eliminates morphological features of apoptosis. BMK cells that were wild type (W2), deficient in Bax (bax−/−, or X1), deficient in Bak (bak−/−, or K1), or deficient in both Bax and Bak (bax−/− bak−/−, or D2) were either mock infected or infected with Ad5dl309, Ad5dl337, or Ad5E1B−. At 48 h postinfection, attached and floating cells were examined by phase-contrast microscopy and photographed. (B) Bax and Bak deficiency prevents apoptotic DNA fragmentation induced by E1A during infection with proapoptotic E1B 19K mutant viruses. Low-molecular-weight DNA was isolated in Hirt supernatants from W2, X1, K1, and D2 cells infected as for panel A. (C) Schematic of apoptotic regulation during adenovirus infection. See text for details.
Apoptosis of infected BMK cells was assessed by monitoring the fragmentation of cellular DNA. In the W2, X1, and K1 cells, infection with Ad5dl309 caused minimal DNA fragmentation, as expected, whereas infection with the E1B mutant viruses resulted in highly fragmented cellular DNA in the characteristic apoptotic nucleosome ladder (Fig. 4B). The D2 cells, however, exhibited almost no DNA fragmentation when infected with either Ad5dl337 or Ad5E1B− (Fig. 4B). These observations suggest that either Bax or Bak is required for E1A-mediated apoptosis during infection and that binding to and inhibiting Bak and Bax can entirely account for the mechanism of apoptotic inhibition by E1B 19K.
Absence of Bak and Bax inhibits apoptosis and enhances virus production.
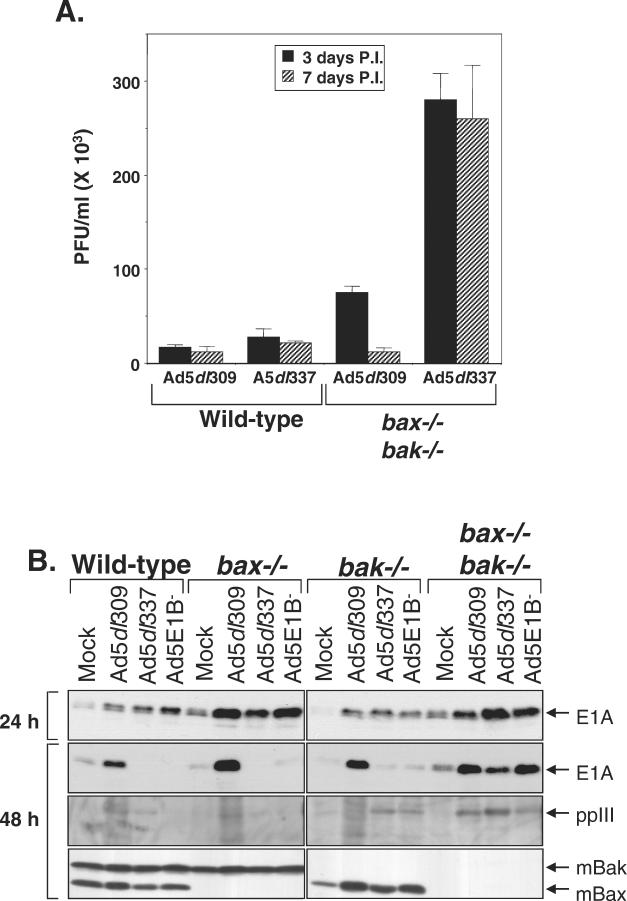
To address the role of apoptosis in adenovirus infection and replication, we assessed virus production in W2 and D2 cells infected with Ad5dl309 and Ad5dl337. Infection of the W2 cells with both viruses yielded roughly equivalent titers. However, the yield of Ad5dl337 from the D2 cells was approximately 10-fold higher than from the W2 cells. An increase in virus production was also observed with Ad5dl309-infected D2 cells compared to infection of W2 cells but was less substantial than that observed with Ad5dl337 infection (Fig. 5A). These data indicate that the increased viability that results from the loss of death signaling due to Bak and Bax deficiency significantly enhances virus production and suggest that the apoptotic response to infection by DNA viruses has evolved as an antiviral mechanism. Although virus yield is less in the D2 cell line than in human cells, the dramatic increase in yield over the W2 cells suggests that apoptosis may account in part for the reduced permissiveness of the mouse cells to adenovirus type 5 replication.
FIG. 5.
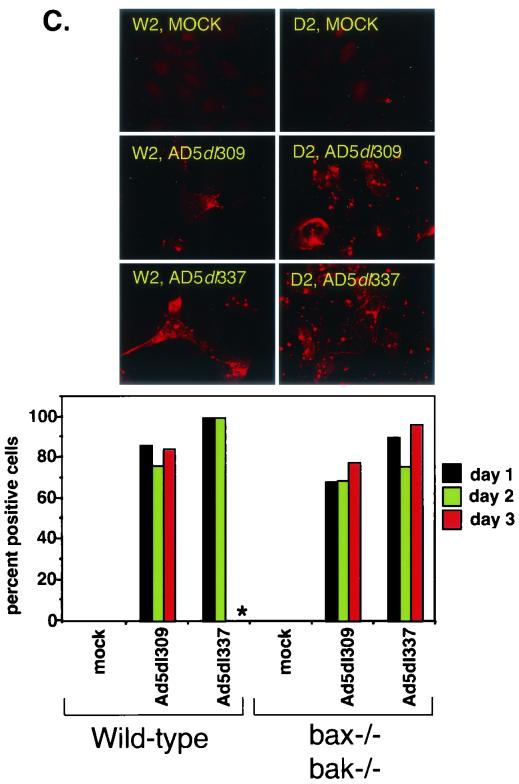
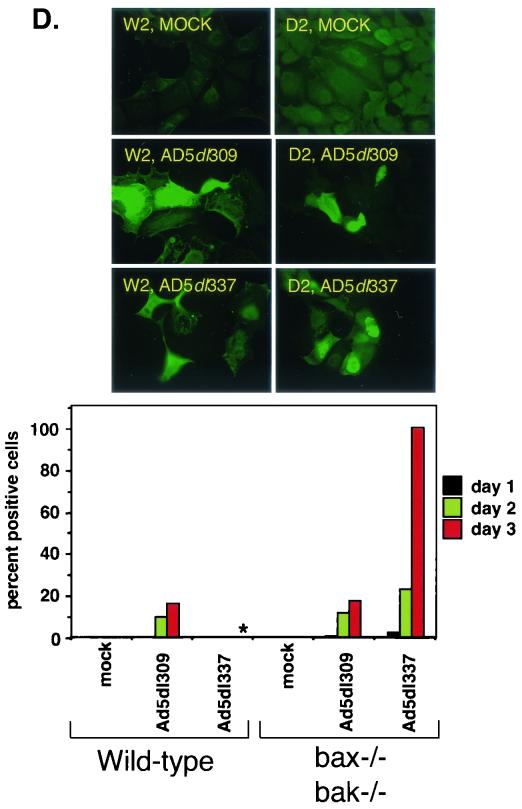
Deficiency of Bax and Bak enhances the efficiency of viral replication during adenovirus infection. (A) Titers of progeny virus from Ad5dl309- or Ad5dl337-infected wild-type (W2) or Bax- and Bak-deficient (D2) BMK cells. Infected cells were harvested at 3 and 7 days postinfection. Solid bars indicate titer at 3 days postinfection (P.I.), whereas patterned bars indicate titer at 7 days postinfection. Bars represent the mean titer, and error bars represent the standard variation from the mean of duplicate samples. (B) Expression of E1A, polypeptide III (ppIII), Bax, and Bak in W2, X1, K1, and D2 BMK cells infected with Ad5dl309, Ad5dl337, or Ad5E1B−. Cells were harvested at 24 and 48 h postinfection and analyzed at the time points indicated by Western blotting with antibodies against E1A (top panels), adenovirus structural proteins (polypeptide III [ppIII], middle panel), and a combination of anti-Bak and anti-Bax antibodies (bottom panel). (C) Indirect immunofluorescence staining for adenovirus E1B 19K in W2 and D2 cells at 1 to 3 days postinfection with Ad5dl309 or Ad5dl337 or mock infection. Photographs shown are from day 2 postinfection. Asterisk (∗) indicates a sample that was impossible to quantify due to loss of viability. (D) Indirect immunofluorescence staining for adenovirus structural proteins in W2 and D2 cells at 1 to 3 days postinfection with Ad5dl309 or Ad5dl337 or mock infection. Photographs shown are from day 1 postinfection. Asterisk (∗) indicates a sample that was impossible to quantify due to loss of viability.
To ensure that BMK cells were infected and expressing viral proteins, infected cell lysates were analyzed for E1A and adenovirus structural proteins as well as endogenous levels of Bak and Bax. At 24 h postinfection, viral E1A expression was equivalent in all infected samples (Fig. 5B). At 48 h postinfection, however, E1A levels dropped significantly in Ad5dl337- and Ad5E1B−-infected W2, X1, and K1 although high levels were observed in D2 cells and all Ad5dl309-infected cells (Fig. 5B). Similarly, levels of the structural protein polypeptide III (ppIII or penton base) dropped under the same conditions as did E1A (Fig. 5B). This loss of E1A under apoptotic conditions probably contributed to the severe limitation of viral replication, evidenced by the loss of structural protein synthesis. As expected, Bak was detected only in the W2 and X1 cells, while Bax was detected only in the W2 and K1 cells (Fig. 5B).
To examine the efficiency of infection and replication in the BMK cells, we performed indirect immunofluorescence on W2 and D2 cells that were mock, Ad5dl309, or Ad5dl337 infected for 1 to 3 days, using antibodies directed against the early protein E1B 55K or against the structural proteins of adenovirus. Cells that stained positive for either antigen were scored by determining their percentage against the total number of cells. Staining for E1B 55K was present in the majority of cells from 1 day postinfection, indicating that infection of the BMK cells with adenovirus is efficient and allowed expression of early proteins (Fig. 5C). Infection with Ad5dl337 was slightly more efficient at inducing E1B 55K expression than with Ad5dl309, particularly in the W2 cells (Fig. 5C).
As would be expected of proteins that are expressed late in the infection cycle, staining for adenovirus structural proteins was almost undetectable at 1 day postinfection but increased with time (Fig. 5D). However, staining for structural proteins was never detectable in the Ad5dl337-infected W2 cells, which stained very strongly for E1B 55K (Fig. 5D). The lack of staining for structural proteins was concomitant with a loss in viability of the cells, with no cells surviving past day 2. Similar to the results shown in Fig. 5B, these observations suggest that infection of the W2 cells with Ad5dl337 resulted in apoptosis prior to expression of viral late proteins. In contrast, infection of the D2 cells with Ad5dl337 resulted in 100% of the cells' expressing structural proteins by day 3 (Fig. 5D). These observations indicate that BMK cells become efficiently infected but only progress to the late stages of the viral life cycle when apoptosis is inhibited.
DISCUSSION
The molecular events that allow propagation of the apoptotic signal by E1A, which culminates in caspase activation, include a conformational alteration of Bak, which may facilitate an interaction with Bax and elicit completion of a conformational change in the Bax carboxy terminus. The resulting Bak-Bax complex formation and oligomerization (32, 33, 38) correlate with the mitochondrial release and degradation of cytochrome c and Smac/DIABLO in the cytoplasm. The requirement for Bak or Bax as the sole mediators of adenovirus infection-induced apoptosis was demonstrated by infection of Bak- and Bax-deficient BMK cells, where inactivation of the mitochondrial checkpoint led to abrogation of apoptosis, sustained E1A expression, and more efficient viral replication. Although a late onset of apoptosis may indeed play a role in allowing release of progeny particles from the host cell (3, 28, 35), our observations indicate that intracellular replication of adenovirus proceeds most efficiently in the absence of apoptosis.
Apoptotic signaling from E1A utilizes an unknown mediator, functionally analogous to tBid in the death receptor signaling pathway, to alter the conformation of Bak and possibly Bax, predominantly through Bak. Expression of E1B 19K largely precludes the conformational changes in Bax but not Bak. Thus, it is likely that Bak alteration occurs first, resulting in altered Bax conformation through Bak-Bax complex formation. Oligomerization of Bak and Bax, either independently or cooperatively, may facilitate the release of proapoptotic proteins from the mitochondria (Fig. 4C). Indeed, Bax oligomerization was observed in Ad5dl337- but not wild-type-infected cells (33). Apoptosis in infected cells, however, was not exclusively dependent on Bak, in that low levels of conformationally altered Bax and Bax-E1B 19K complexes were detected and Bak deficiency did not abrogate apoptosis. The most efficient death signaling may occur in the presence of both Bax and Bak, perhaps through cooperative oligomerization, but either protein remains able to compensate for the loss of the other.
Given the observation that E1B 19K predominantly binds Bak but not Bax during infection, it is probable that Bak is the primary target for E1B 19K antiapoptotic function. In contrast to the ability of E1B 19K to bind Bax that has been conformationally altered by tBid (23), in adenovirus-infected cells Bax is probably not a direct target of E1B 19K because Bax remains largely in its inactive conformation. Thus, we propose that the mechanism by which E1B 19K inhibits apoptosis during infection involves its binding to conformationally altered Bak, thereby preventing interaction with Bax, exposure of the Bax amino and carboxy termini, and abrogating formation of the high-molecular-weight complex. However, the absence of Bak may facilitate Bax conformational change in K1 cells, which may help explain the functional conservation of E1B 19K Bax-binding activity.
The consequences of the regulation of virus-induced apoptosis have been demonstrated in Sindbis virus, a member of the Alphavirus genus, for which the establishment of persistent infection was found to depend on the inhibition of apoptosis by the exogenous overexpression of Bcl-2 (15). Consistent with this finding, herpesviruses have evolved Bcl-2-like activities, such as the BHRF1 protein of Epstein-Barr virus, presumably to forestall infection-induced apoptosis and establish latency (28). In infection with wild-type adenovirus, apoptosis is blocked due to the expression of the Bcl-2 homologue E1B 19K. Therefore, viral inhibition of apoptosis at the mitochondrial checkpoint has evolved as a central requirement to the establishment of a productive infection. Seen in this context, the Bak- and Bax-mediated apoptotic response to infection must have evolved as an antiviral mechanism, which acts to destroy the viability of the infected host cell before viral replication has reached completion.
Several instances of persistent and/or latent infection by adenovirus have been documented. Infected individuals may shed virus for years after the onset of symptoms, and renal transplant recipients have been known to contract adenovirus infections through probable reactivation of latent virus in the donor organ (12). Chronic airway obstruction has been correlated with long-term adenoviral replication in some cases of bronchiolitis (19), and adenovirus has been isolated from tissue fragment cultures of tonsil and adenoid specimens up to 15 days after initial culture (36). In addition, continuous infection has been observed in a B-cell lymphoma line isolated from a patient (8) and in isolated human monocyte cell lines (5). The exact mechanism by which this would occur has not been elucidated. As in the case of Sindbis virus, the possibility arises that the lack of an apoptotic response during infection may be a key mechanism behind allowing replicating adenovirus to linger in infected tissues, causing chronic persistent infection or allowing the establishment of latency.
The lower levels of replication of the wild-type virus compared to the E1B 19K mutant in the D2 cells are unexplained but have been observed before under circumstances in which apoptosis is blocked (3) or in specific cell types (43). This effect may be due to unknown functions of E1B 19K. The ability of the E1B 19K mutant viruses to replicate most efficiently in cells that have lost proapoptotic regulation at the mitochondrial checkpoint raises the possibility that such viruses may replicate selectively in tumor cells, where death signaling is blocked at the mitochondria. Such tissues would include B-cell lymphomas, which overexpress Bcl-2 (21, 27), and metastatic melanomas, which are deficient for the caspase 9 coactivator Apaf-1 (29). One possible advantage of a tumoricidal agent that targets cell types with a downstream apoptotic defect would be the lack of dependence on either the p53 status or the proliferative phenotype of the tumor, which are the two main criteria for selective replication employed by virotherapies currently in development (14). However, the degree of selectivity for such tumor cells and the oncolytic efficiency of E1B 19K mutants remain to be determined.
Acknowledgments
We thank Holly Henry and Deirdre Nelson for critical reading of the manuscript, Guanghua Chen for technical assistance, Juying Yuan for the anti-Bid antibody, and Guy Poirier for the anti-PARP antibody.
This work was funded by NCI grant R01-CA53370 and the Howard Hughes Medical Institute.
REFERENCES
- 1.Babiss, L. E., H. S. Ginsberg, and J. E. Darnell. 1985. Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol. Cell. Biol. 5:2552-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiou, S.-K., C. C. Tseng, L. Rao, and E. White. 1994. Functional complementation of the adenovirus E1B 19K protein with Bcl-2 in the inhibition of apoptosis in infected cells. J. Virol. 68:6553-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiou, S.-K., and E. White. 1998. Inhibition of ICE-like proteases inhibits apoptosis and increases virus production during adenovirus infection. Virology 244:108-118. [DOI] [PubMed] [Google Scholar]
- 4.Chiou, S.-K., and E. White. 1997. p300 binding by E1A cosegregates with p53 induction but is dispensable for apoptosis. J. Virol. 71:3515-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu, Y., K. Sperber, L. Mayer, and M.-T. Hsu. 1992. Persistent infection of human adenovirus type 5 in human monocyte cell lines. Virology 188:793-800. [DOI] [PubMed] [Google Scholar]
- 6.Debbas, M., and E. White. 1993. Wild-type p53 mediates apoptosis by E1A which is inhibited by E1B. Genes Dev. 7:546-554. [DOI] [PubMed] [Google Scholar]
- 6a.Degenhardt, K., R. Sundararajan, T. Lindsten, C. B. Thompson, and E. White. Bax and Bak independently promote cytochrome c release from mitochondria. J. Biol. Chem., in press. [DOI] [PubMed]
- 7.Desagher, S., A. Osen-Sand, A. Nichols, R. Eskes, S. Montessuit, S. Lauper, K. Maundrell, B. Antonsson, and J. Martinou. 1999. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release and apoptosis. J. Cell Biol. 144:891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flomenberg, P., V. Piaskowski, J. Harb, A. Segura, and T. Casper. 1996. Spontaneous, persistent infection of a B-cell lymphoma with adenovirus. J. Med. Virol. 48:267-272. [DOI] [PubMed] [Google Scholar]
- 9.Gooding, L. R., L. Aquino, P. J. Duerksen-Hughes, D. Day, T. M. Horton, S. Yei, and W. S. M. Wold. 1991. The E1B-19K protein of group C adenoviruses prevents cytolysis by tumor necrosis factor of human cells but not mouse cells. J. Virol. 65:3083-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross, A., X.-M. Yin, K. Wang, M. C. Wei, J. Jockel, C. Milliman, H. Erdjument-Bromage, P. Tempst, and S. J. Korsmeyer. 1999. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274:1156-1163. [DOI] [PubMed] [Google Scholar]
- 11.Henry, H., A. Thomas, Y. Shen, and E. White. 2002. Regulation of the mitochondrial checkpoint in p53-mediated apoptosis confers resistance to cell death. Oncogene 21:748-760. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz, M. 2001. Adenoviruses, p. 2301-2326. In B. N. Fields (ed.), Fields virology, vol. 2, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 13.Jones, N., and T. Shenk. 1979. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc. Natl. Acad. Sci. USA 76:3665-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirn, D., R. L. Martuza, and J. Zwiebel. 2001. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 7:781-787. [DOI] [PubMed] [Google Scholar]
- 15.Levine, B., Q. Huang, J. T. Isaacs, J. C. Reed, D. E. Griffin, and J. M. Hardwick. 1993. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature (London) 361:739-742. [DOI] [PubMed] [Google Scholar]
- 16.Li, H., H. Zhu, C.-J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 17.Lowe, S., and H. E. Ruley. 1993. Stabilization of the p53 tumor suppressor is induced by adenovirus-5 E1A and accompanies apoptosis. Genes Dev. 7:535-545. [DOI] [PubMed] [Google Scholar]
- 18.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 19.Macek, V., J. Sorli, S. Kopriva, and J. Marin. 1994. Persistent adenoviral infection and chronic airway obstruction in children. Am. J. Respir. Crit. Care Med. 150:7-10. [DOI] [PubMed] [Google Scholar]
- 20.Nechushtan, A., C. L. Smith, Y.-T. Hsu, and R. J. Youle. 1999. Conformation of the Bax C terminus regulates subcellular location and cell death. EMBO J. 18:2330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pegoraro, L., A. Palumbo, J. Erikson, M. Falda, B. Giovanazzo, B. Emanuel, G. Rovera, P. Nowell, and C. Croce. 1984. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc. Natl. Acad. Sci. USA 81:7166-7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez, D., and E. White. 1998. E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE. J. Cell Biol. 141:1255-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez, D., and E. White. 2000. TNF-α signals apoptosis through a Bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol. Cell 6:53-63. [PubMed] [Google Scholar]
- 24.Pilder, S., J. Logan, and T. Shenk. 1984. Deletion of the gene encoding the adenovirus 5 early region 1B 21,000-molecular-weight polypeptide leads to degradation of viral and cellular DNA. J. Virol. 52:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilder, S., M. Moore, J. Logan, and T. Shenk. 1986. The adenovirus E1B 55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol. Cell. Biol. 6:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Querido, E., R. C. Marcellus, A. Lai, R. Charbonneau, J. G. Teodoro, G. Ketner, and P. E. Branton. 1997. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J. Virol. 71:3788-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed, J. C. 1997. Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin. Hematol. 34:9-19. [PubMed] [Google Scholar]
- 28.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 29.Soengas, M., P. Capodieci, D. Polsky, J. Mora, M. Esteller, X. Opitz-Araya, R. McCombie, J. Herman, W. Gerald, Y. Lazebnik, C. Cordón-Cardó, and S. Lowe. 2001. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 409:207-211. [DOI] [PubMed] [Google Scholar]
- 30.Steegenga, W. T., N. Riteco, A. G. Jochemsen, F. J. Fallaux, and J. L. Bos. 1998. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene 16:349-357. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian, T., M. Kuppuswamy, J. Gysbers, S. Mak, and G. Chinnadurai. 1984. 19-kDa tumor antigen coded by early region E1b of adenovirus 2 is required for efficient synthesis and for protection of viral DNA. J. Biol. Chem. 259:11777-11783. [PubMed] [Google Scholar]
- 32.Sundararajan, R., A. Cuconati, D. Nelson, and E. White. 2001. TNF-α induces Bax-Bak interaction and apoptosis which is inhibited by adenovirus E1B 19K. J. Biol. Chem. 276:45120-45127. [DOI] [PubMed] [Google Scholar]
- 33.Sundararajan, R., and E. White. 2001. E1B 19K blocks Bax oligomerization and tumor necrosis factor alpha-mediated apoptosis. J. Virol. 75:7506-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemori, N., C. Cladaras, B. Bhat, A. J. Conley, and W. S. M. Wold. 1984. cyt gene of adenoviruses 2 and 5 is an oncogene for transforming function in early region E1B and encodes the E1B 19,000-molecular-weight polypeptide. J. Virol. 52:793-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tollefson, A. E., A. Scaria, T. W. Hermiston, J. S. Ryerse, L. J. Wold, and W. S. M. Wold. 1996. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virol. 70:2296-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Der Veen, J., and M. Lambriex. 1973. Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect. Immun. 7:604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei, M., W.-X. Zong, E. Cheng, T. Lindsten, V. Panoutsakopoulou, A. Ross, K. Roth, G. MacGregor, C. Thompson, and S. Korsmeyer. 2001. Proapoptotic Bax and Bak: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei, M. C., T. Lindsten, V. K. Mootha, S. Weiler, A. Gross, M. Ashiya, C. B. Thompson, and S. J. Korsmeyer. 2000. tBid, a membrane-targeted death ligand, oligomerizes Bak to release cytochrome c. Genes. Dev. 14:2060-2071. [PMC free article] [PubMed] [Google Scholar]
- 39.White, E. 1994. Function of the adenovirus E1B oncogene in infected and transformed cells. Semin. Virol. 5:341-348. [Google Scholar]
- 40.White, E. 2001. Regulation of the cell cycle and apoptosis by the oncogenes of adenovirus. Oncogene 20:7836-7846. [DOI] [PubMed] [Google Scholar]
- 41.White, E., S. H. Blose, and B. Stillman. 1984. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol. Cell. Biol. 4:2865-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White, E., R. Cipriani, P. Sabbatini, and A. Denton. 1991. The adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J. Virol. 65:2968-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, E., B. Faha, and B. Stillman. 1986. Regulation of adenovirus gene expression in human WI38 cells by an E1B-encoded tumor antigen. Mol. Cell. Biol. 6:3763-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White, E., T. Grodzicker, and B. W. Stillman. 1984. Mutations in the gene encoding the adenovirus E1B 19K tumor antigen cause degradation of chromosomal DNA. J. Virol. 52:410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]