Abstract
Both the RNase H domain of Moloney murine leukemia virus (Mo-MLV) reverse transcriptase (RT) and Escherichia coli RNase H possess a positively charged α-helix (C helix) and a loop that are not present in the RNase H domains of human immunodeficiency virus (HIV) RT or avian sarcoma virus RT. Although a mutant Mo-MLV RT lacking the C helix (ΔC RT) retains DNA polymerase activity on homopolymeric substrates and partial RNase H activity, reverse transcription of the viral RNA genome in vivo is defective. To identify the essential features of the C helix, a panel of Mo-MLV RT mutants was generated. Analyses of these mutant viruses revealed the importance of residues H594, I597, R601, and G602. The mutants were tested for their ability to synthesize viral DNA after acute infections and to form proper 5′ and 3′ viral DNA ends. The mutant RTs were tested in vitro for exogenous RT activity, minus-strand strong-stop DNA synthesis in endogenous RT reactions, nonspecific RNase H activity, and finally, proper cleavage at the polypurine tract-U3 junction. The R601A mutant was the most defective mutant both in vivo and in vitro and possessed very little RNase H activity. The H594A, I597A, and G602A mutants had significant reductions in RNase H activity and in their rates of viral replication. Many of the mutants formed improper viral DNA ends and were less efficient in PPT-U3 recognition and cleavage in vitro. The data show that the C helix plays a crucial role for overall RNase H cleavage activity. The data also suggest that the C helix may play an important role in polypurine tract recognition and proper formation of the plus-strand DNA's 5′ end.
Reverse transcriptase (RT) of retroviruses synthesizes a double-stranded DNA copy of the single-stranded viral RNA genome (2, 33). RT contains two enzymatic domains: a DNA polymerase domain that can use either RNA or DNA as a template and an RNase H domain that is required for degradation of genomic RNA in RNA-DNA hybrids. Mutations that disrupt the functions of either domain render the virus incapable of replication (29, 32). During the course of reverse transcription, the minus-strand DNA is primed by a host tRNA annealed to the primer binding site (PBS) while the plus-strand DNA is primed by the polypurine tract (PPT), a fragment of the genome produced by RNase H action.
Nonspecific RNase H cleavage of the viral genome serves to free minus-strand DNA, which can then be used as a template for plus-strand DNA synthesis. Specific RNase H cleavages, however, occur in two regions of the genome. Cleavage between the PBS of the tRNA primer and the minus-strand U5 DNA occurs after minus-strand synthesis to remove the primer. This cleavage event defines the 5′ end of the minus-strand DNA and ultimately the right end of the double-stranded viral DNA. Second, specific cleavages are used to produce the PPT to serve as a primer for plus-strand synthesis. After plus-strand synthesis initiation, the PPT primer is released from plus-strand DNA to define the 5′ end of the plus strand and ultimately the left end of the viral DNA. Correct termini at both ends of the viral DNA have been shown to be important for successful integration of the DNA into the host genome (6, 7, 11).
Alignments show that Moloney murine leukemia virus (Mo-MLV) RNase H and Escherichia coli RNase H contain a positively charged α-helix (the C helix) and loop that are absent from human immunodeficiency virus (HIV) RNase H and avian sarcoma-leukosis virus RNase H (see Fig. 1) (8, 14, 15, 18, 35). Modeling with the E. coli enzyme suggests that the C helix is in a position to contact the RNA-DNA substrate. Functional studies of E. coli RNase H confirm that the C helix contributes to nucleic acid binding (16). While the isolated HIV RNase H domain is not enzymatically active, insertion of the C helix into an independently expressed minimal HIV RNase H domain will activate the protein (19, 28). It is believed that the HIV polymerase and connection domains normally compensate for the substrate binding function of the missing helix (13).
FIG. 1.
Amino acid sequence alignment of E. coli RNase H C helix (as indicated) with homologous regions from Mo-MLV, Rous sarcoma virus (RSV), and HIV. Every 10th residue is indicated by a dot above the sequence. Residues 593 to 603 in Mo-MLV are lacking in the ΔC mutant.
Many different regions in retroviral RTs are probably important in determining the specificities of RNase H cleavages. The Mo-MLV RNase H domain requires regions from the polymerase domain for tRNA primer removal and proper PPT primer formation (24). Likewise, the thumb and connection subdomains of the polymerase, provided in cis or trans, can activate HIV RNase H and allow the specific removal of tRNALys3 from minus-strand viral DNA (26). An extended HIV RNase H domain and an HIV RNase H with the E. coli C helix also retain activity and cleavage specificity for tRNALys3 removal, presumably because both modifications confer nucleic acid binding ability (26-28).
Deletion of the C helix in Mo-MLV (ΔC Mo-MLV) results in a replication-defective virus and an enzyme (ΔC RT) with impaired polymerase and RNase H activity (31). While an in situ gel assay showed ΔC RT to have significant RNase H activity (31), more quantitative assays showed ΔC RT's RNase H activity to be very low (5). ΔC RT's DNA polymerase activity on heteropolymeric RNA templates is also reduced, as was revealed both by in vitro assays and by reactions on the endogenous viral RNA in purified virions (3, 5, 31). To further explore the role of the C helix, a panel of mutants with alterations in the helix was generated and the phenotypes of the defective mutant viruses were analyzed. In addition, the corresponding mutant RTs were expressed in bacteria, purified, and assayed in vitro. Here we report that in addition to facilitating nucleic acid binding for both the polymerase and RNase H domains, the C helix is important for PPT recognition and cleavage efficiency at the PPT-U3 junction.
MATERIALS AND METHODS
Plasmids and mutant plasmid construction.
The pNCA plasmid is an infectious clone of Mo-MLV (7), and pNCS is the same clone with a simian virus 40 origin of replication within the plasmid backbone (9). Mutant Mo-MLV RTs were constructed with oligonucleotides by PCR site-directed mutagenesis. Briefly, each oligonucleotide contained a silent AatII site within L604 and T605 (see “Oligonucleotides” below). A 139-bp BlpI-AatII fragment was amplified and ligated with a 485-bp, AatII-HindIII fragment into pNCA and with a 543-bp, AatII-SacII fragment into pNCS. The I593A, H594A, G595A, E596A, I597A, Y598A, R599A, R600A, R601A, G602A, and L603A mutants contain point mutations in the RNase H domain of Mo-MLV RT; the nomenclature describes the amino acid found in wild-type (WT) RT, the residue number, and the amino acid found in the mutant RT at that position. ΔC Mo-MLV contains an 11-amino-acid deletion from residues I593 to L603, contains the silent AatII restriction site at codons 604 and 605, and was previously constructed in this lab by Alice Telesnitsky and her colleagues (31). The BlpI-AatII region of all mutants was sequenced to verify the desired mutation and to ensure the absence of PCR-induced mutations.
The bacterial expression plasmid pRT-HIS-30-2 encodes a Mo-MLV RT with 6 histidine residues attached at the C-terminal end (His tag). A 230-bp, BstEII-BglII fragment was used to clone the H594A, I597A, R601A, G602A, and ΔC Mo-MLV mutants into pRT-HIS-30-2. The D524N mutant was previously constructed by Stacy Blain and her colleagues, cloned into pRT30-2 (no His tag), and purified as previously described (3, 4). The BstEII-BglII region was sequenced to verify the cloning of desired C helix mutations and deletion.
Oligonucleotides.
The primers used to construct C helix point mutants were as follows: (i) forward primer Blp 1 (5′-ACATCCGCTCAGCGGGCTGAACTG-3′) and (ii) a reverse primer for each mutant that included 12 bp downstream of T605 (except for the oligonucleotides with the I597T mutation, the H593A and G602A mutations, and E. coli residues Q80 to W90, which each had only 6 extra bp downstream of T605), a silent AatII restriction site (at L604 and T605), the desired C helix point mutation, and 18 bp upstream of the 5′-most point mutation. Table 1 displays all mutagenic oligonucleotides used. The I597T oligonucleotide contained an additional silent SalI site, while the H594A G602A oligonucleotide contained an additional silent SacI site.
TABLE 1.
Reverse primers used for PCR cloning of mutant Mo-MLV RTs
| Mo-MLV mutant | Primer sequencea |
|---|---|
| I593A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTGTATATTTCTCCATGGGCATGGGCAGTAGCAAAAGC-3′ |
| H594A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTGTATATTTCTCCAGCGATATGGGCAGTAGCAAA-3′ |
| G595A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTGTATATTTCTGCATGGATATGGGCAGTAGC-3′ |
| E596A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTGTATATTGCTCCATGGATATGGGCAGT-3′ |
| I597A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTGTATGCTTCTCCATGGATATGGGC-3′ |
| Y598A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTGGCTATTTCTCCATGGATATG-3′ |
| R599A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTGCGTATATTTCTCCATGGAT-3′ |
| R600A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCGCTCTGTATATTTCTCCATG-3′ |
| R601A | 5′-TTTGCCTTCTGACGTCAGCAACCCAGCCCTTCTGTATATTTCTCC-3′ |
| G602A | 5′-TTTGCCTTCTGACGTCAGCAACGCACGCCTTCTGTATATTTC-3′ |
| L603A | 5′-TTTGCCTTCTGACGTCAGCGCCCCACGCCTTCTGTATAT-3′ |
| I597T | 5′-TTCTGACGTCAGCAACCCACGTCGACGGTACGTTTCTCCATGGATATGGGCAGT-3′ |
| Y598R | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTCCTTATTTCTCCATGGATATG-3′ |
| Y598W | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTCCATATTTCTCCATGGATATG-3′ |
| H594A G602A | 5′-TTCTGACGTCAGCAGAGCTCGCCTTCTGTATATTTCTCCTGCGATATGGGCAGTAGCAAAAGC-3′ |
| R599E | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTTCGTATATTTCTCCATGGAT-3′ |
| R600E | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCTCTCTGTATATTTCTCCATG-3′ |
| R601E | 5′-TTTGCCTTCTGACGTCAGCAACCCTTCCCTTCTGTATATTTCTCC-3′ |
| R599A R600A | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCGCTGCGTATATTTCTCCATGGAT-3′ |
| R600A R601A | 5′-TTTGCCTTCTGACGTCAGCAACCCAGCCGCTCTGTATATTTCTCCATG-3′ |
| R599A R601A | 5′-TTTGCCTTCTGACGTCAGCAACCCAGCCCTTGCGTATATTTCTCCATGGAT-3′ |
| R599A R600A R601A | 5′-TTTGCCTTCTGACGTCAGCAACCCAGCCGCTGCGTATATTTCTCCATGGAT-3′ |
| R599E R600E | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCTCTTCGTATATTTCTCCATGGAT-3′ |
| R600E R601E | 5′-TTTGCCTTCTGACGTCAGCAACCCTTCCTCTCTGTATATTTCTCCATG-3′ |
| R599E R601E | 5′-TTTGCCTTCTGACGTCAGCAACCCTTCCCTTTCGTATATTTCTCCATGGAT-3′ |
| R599E R600E R601E | 5′-TTTGCCTTCTGACGTCAGCAACCCTTCCTCTTCGTATATTTCTCCATGGAT-3′ |
| Del 1 | 5′-TTTGCCTTCTGACGTCAGCAACCCACGCCTTCTGTATAT------------ATGGGCAGTAGCAAAAGC-3′ |
| Del 2 | 5′-TTTGCCTTCTGACGTCAGCAACCCACG------------TTCTCCATGGATATGGGCAGTAGCAAAAGC-3′ |
| Del 3 | 5′-TTTGCCTTCTGACGTCAG---------CCTTCTGTATATTTCTCCATGGATATGGGCAGTAGCAAAAGC-3′ |
| E. coli Q80-W90b | 5′-TTCTGACGTCAGCCACCCACGCTTCTTCCAATTATGTATCCATTGATGGGCAGTAGCAAAAGC-3′ |
Boldfaced and underlined nucleotides represent mutated sequence. The E. coli C helix is italicized (E. coli Q80 to W90). Gaps indicating deleted sequence in Del 1, 2, and 3 are marked by hyphens.
The mutant in which E. coli Q80 to W90 is substituted for the Mo-MLV C helix.
Primers for the in vitro PPT recognition and RNase H assay were as follows: RNA primer RNA I (5′-AGAAAAAGGGGGGAAUGAAA-3′) and DNA primer DNA II (5′-GGGGTCTTTCATTCCCCCCTTTTTCTGG-3′).
Primers used for PCR analysis of reverse transcription in vivo were as follows. Primers to amplify the minus-strand strong-stop region were forward primer ss-ps (5′-GCGCCAGTCCTCCGATTGACT-3′) and reverse primer ss-as (5′-CGGGTAGTCAATCACTCAG-3′). Primers to amplify long minus strands were forward primer RTSN18 (5′-GAGGGATCAGGAGCTCAG-3′) and reverse primer Bcl-rev (5′-GAGGTATGATCAGAGGAG-3′). Primers to amplify plus-strand strong-stop jumping and extension were forward primer ss-ps (see above) and reverse primer MψM5 (5′-ACAGAGCTCGGCCCCCGAAGTCCCT-3′) (generously given by Matt Evans).
Primers used in the amplification of Mo-MLV RT for I597A reversion cloning were forward primer RTSN (5′-GAGGGATCAGGAGCT-3′) and reverse primer IntZ (5′-GATCTCCCAATGAGTGCC-3′).
Primers used in circle junction analysis were as follows. Primers to amplify R-U5-U3 were forward primer ss-ps (see above) and reverse primer U3-rev (5′-CCGAGTGAGGGGTTGTGGGCT −3′). All oligonucleotides were synthesized on Applied Biosystems model 394 and 3948 DNA synthesizers at the Columbia University Protein Chemistry Core Facility, Howard Hughes Medical Institute.
Protein purification.
The expression plasmid pRT-HIS-30-2 and its relevant mutant plasmids were used to transform a BL-21-CodonPlus E. coli strain (Stratagene). A 300-ml culture was grown overnight for each mutant, centrifuged, and resuspended in 10 ml of 50 mM Tris (pH 8)-100 mM NaCl-1 mM phenylmethylsulfonyl fluoride. Lysozyme was added to 1 mg/ml, and the suspension was incubated on ice for 30 min. The suspension was next sonicated and ultracentrifuged at 100,000 × g for 25 min. NaCl was added to 500 mM, and 250 μl of Ni-agarose beads (QIAGEN) was gently mixed in the suspension for 1 h at 4°C. The Ni-agarose beads were spun down and washed twice with 500 mM NaCl-50 mM Tris (pH 8) and then washed three times with 50 mM NaCl-50 mM Tris (pH 8)-10 mM imidazole. Bound protein was then eluted with 8 ml of 50 mM NaCl-50 mM Tris (pH 8)-100 mM imidazole. The protein solution was dialyzed into 50 mM NaCl-25 mM sodium phosphate (pH 7.4)-2 mM EDTA-5 mM β-mercaptoethanol-5% glycerol. The protein was purified over a mono-S, cation-exchange chromatography column (Pharmacia) by using a gradient of 0 to 500 mM NaCl. Protein solutions were concentrated and dialyzed with Centricon 30 concentrators (Amicon) in 50 mM NaCl-20 mM sodium phosphate (pH 7.5)-1 mM EDTA-5 mM β-mercaptoethanol-5% glycerol. Proteins were quantitated by Bradford assays, and glycerol was then added to 50%. Protein concentrations were then also checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis by using bovine serum albumin as protein concentration standards. Protein preparations were stored at −20°C.
Cell culture, viral-spread assay, transformation of 293T cells for viral preparations, and acute infections of NIH 3T3 and Rat2-2 cells.
NIH 3T3 and Rat2-2 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum and penicillin-streptomycin. 293T cells were grown in DMEM supplemented with 10% fetal calf serum and penicillin-streptomycin. The 293T cells had been previously selected for a combination of superadherence to tissue culture plates and overexpression of transfected genes (293T-SAO cells). The cells were a generous gift from the laboratory of R. Axel, Columbia University. All cells were grown at 37°C and 5% CO2.
Assays for viral spread were performed as previously described (1, 30).About 70%-confluent 293T cells in 100-mm-diameter plates were washed three times with phosphate-buffered saline. Twenty micrograms of each WT or mutant pNCA or pNCS plasmid was then used to transiently transfect the cells by the calcium phosphate method (34). Culture medium was harvested 48 h posttransfection, filtered through a 0.45-μm-pore-size filter, and buffered with 25 mM HEPES (pH 7). A portion of each culture supernatant was further purified by ultracentrifugation through sucrose cushions as previously described (30). The virus concentration for each preparation was quantitated by Western blot analysis, using an anti-RT antibody, chemiluminescence (ECL), and a Molecular Dynamics densitometer with ImageQuant analysis software. The H594A, I597A, R601A, G602A, and ΔC mutant viral preparations and the WT were also quantitated by exogenous reverse transcription assays since all of these viruses had equivalent levels of polymerase activity. Viral preparations were aliquoted and stored at −70°C. After dilution and normalization of all viral preparations, 15 U of DNase I (Boehringer Mannheim) per ml was added for 30 min at 37°C to degrade contaminating plasmid DNA. Aliquots of undiluted mutant and WT viruses were incubated for 15 min at 70°C for heat inactivation. Polybrene was added to 8 μg/ml to 2 ml of each viral preparation, which was then used to infect NIH 3T3 or Rat2-2 cells in 100-mm-diameter plates. Infected NIH 3T3 cells were then split 1:10 every three days, at which times aliquots of supernatants were collected to monitor RT activity. Low-molecular-weight DNA was collected 16 h postinfection from Rat2-2 cells (12). The DNA was then used for PCR analysis of reverse transcription and circle junction long terminal repeat (LTR)-LTR analysis.
Exogenous and endogenous RT (polymerase) assays.
Virion production was detected (and quantitated for the H594A, I597A, R601A, G602A, and ΔC mutant viruses and the WT) by exogenous oligo(dT)·poly(rA) primer-template assays in the presence of radiolabeled dTTP (Pharmacia) and Mn2+ as previously described (10, 30). Bacterially expressed RTs previously quantitated by Bradford assays were also used in the exogenous RT assay.
Endogenous RT assays measure activity in vitro in purified, ultracentrifuged virions on an endogenous viral RNA template in the presence of radiolabeled dTTP as previously described (30).
I597A reversion cloning.
Four separate viral supernatants were harvested from NIH 3T3 cells actively replicating virus from acute infections with the I597A mutant virus. Supernatants were diluted 1 to 50 and repassaged through uninfected Rat2-2 cells four times. Sixteen hours after the fourth passage, low-molecular-weight DNA was collected. All four preparations were used as templates for PCR amplification of the Mo-MLV RT gene with the primers RTSN and IntZ. PCR products (2,438 bp) were digested with BclI and HindIII, resulting in a 2,154-bp digestion product, which was ligated back into pNCA. Unique proviral DNA clones from all four viral stocks were used to transform NIH 3T3 cells. A number of clones (8 of 12 analyzed) from each of four viral stocks displayed viral-spread kinetics similar to that of the WT. Sequencing revealed all reverted clones to have valine instead of alanine at position 597, a single nucleotide change from GCA to GTA.
RNase H assays.
The nonspecific, in vitro RNase H assay on the heteropolymeric template was previously described by Schultz and Champoux (24). The in vitro PPT recognition and RNase H assay was also previously described (24).
PCR analysis of reverse transcription in vivo.
Low-molecular-weight DNA from acutely infected Rat2-2 cells was used for analysis of progression through different steps of reverse transcription. The PCR primers ss-ps and ss-as were used to amplify the R-U5 region. The PCR primers RTSN18 and Bcl-rev were used to amplify the C terminus of protease and N terminus of RT for detection of the synthesis of long minus-strand DNA. The primers ss-ps and MψM5 were used to amplify R-U5-PBS-psi to detect plus-strand strong-stop jumping and elongation. Reaction conditions were 94°C for 15 s, 50°C for 30 s, and 72°C for 45 s. The PCR was performed with native Taq polymerase (PerkinElmer) for 20 cycles, followed by a 7-min cycle at 72°C in a GeneAmp PCR system 9700 (PerkinElmer).
Circle junction analysis.
LTR-LTR circle junction analysis was performed by a modification of a previously described protocol (25), which was utilized in this lab (1). Here primers ss-ps and U3-rev were used to amplify an R-U5-U3 region within circle junctions from low-molecular-weight DNA collected from acutely infected Rat2-2 cells. The fragment was cloned directly from the PCR with a TOPO TA cloning kit (Invitrogen) according to the manufacturer's protocol. Clones were sequenced on both strands.
RESULTS
C helix mutant viruses exhibit various defects in virus replication.
The Mo-MLV RNase H C helix includes Mo-MLV RT residues H594 to R601, corresponding to E. coli RNase H residues W81 to R88 (Fig. 1). It has been hypothesized that the conserved string of positively charged residues binds the negatively charged phosphate backbone of RNA-DNA hybrids (16). To explore the role of the Mo-MLV RNase H C helix, a panel of mutations affecting residues from I593 to L603 was generated and inserted into an infectious, proviral DNA clone of Mo-MLV (Fig. 1 and Table 2). The panel included complete deletion of the helix (ΔC), smaller deletions, substitution of alanine for each residue, substitution of threonine for I597, and substitution of arginine or tryptophan for Y598. Additional mutations were focused on arginine residues 599 to 601. Substitution of glutamate for each arginine was introduced as a reversal in electrostatic charge. Double and triple alanine and glutamate substitutions were introduced for all arginine residues. Finally, the E. coli RNase H C helix sequence (Q80 to W90) was substituted for the Mo-MLV RNase H C helix (I593 to L603).
TABLE 2.
Summary of C helix mutant phenotypes
| RT mutation | Viral replicationa | Polymerase activityb | Minus-strand strong-stop DNA synthesisc |
|---|---|---|---|
| I593A | + | ||
| H594A | 2- to 3-day delay | +++ | + |
| G595A | + | ||
| E596A | + | ||
| I597A | 14-day delay | +++ | + |
| Y598A | + | ||
| R599A | + | ||
| R600A | + | ||
| R601A | − | +++ | + |
| G602A | 4 to 5-day delay | +++ | + |
| L603A | + | ||
| I597T | 5- to 6-day delay | + | NDd |
| Y598R | − | +++ | ND |
| Y598W | + | + | + |
| H594A G602A | − | + | ND |
| R599E | + | + | + |
| R600E | + | + | + |
| R601E | − | +++ | + |
| R599A R600A | − | +++ | ND |
| R600A R601A | − | ND | ND |
| R599A R601A | − | + | + |
| R599A R600A R601A | − | + | + |
| R599E R600E | − | + | +/− |
| R600E R601E | − | + | +/− |
| R599E R601E | − | + | +/− |
| R599E R600E R601E | − | + | − |
| E. coli Q80-W90 for Mo-MLV I593-L603 | − | + | − |
| Del 1 (ΔI593-E596) | − | + | + |
| Del 2 (ΔI597-R600) | − | + | + |
| Del 3 (ΔR601-L603) | − | ++ | − |
| ΔC (I593-L603) | − | +++ | + |
| WT | + | +++ | + |
Mo-MLV proviral plasmids were used to transform NIH 3T3 cells. Culture supernatants were harvested every few days and tested for RT activity. +, RT activity detected by 3 days (WT viral replication kinetics). Defective but viable viruses are indicated by the number of days beyond three when RT activity was detected. −, no RT activity ever detected.
Mutant Mo-MLV proviral plasmids were used to transform 293T cells. Virus was harvested, normalized by anti-RT Western blot analysis, and used in exogenous reactions to assay polymerase activity. +++, WT RT polymerase levels of activity; ++, two-thirds of the level of WT RT polymerase activity; +, one-third of the level of WT RT polymerase activity. Single-alanine-substitution mutants with WT kinetics of viral replication were not utilized in this assay or any further assays.
Viral supernatants were concentrated through sucrose cushions, normalized by anti-RT Western blot analysis, and utilized in endogenous-RT reactions. Reverse-transcribed DNA was purified and analyzed on an 8% nondenaturing polyacrylamide gel. +, detectable minus-strand strong-stop DNA (close to WT levels); +/−, very little detectable minus-strand strong-stop DNA (less than 5% of WT levels); −, no detection of minus-strand strong-stop DNA.
ND, not determined.
Mutant proviral DNAs were used to transform NIH 3T3 cells, and culture supernatants were collected over the course of several days and assayed for RT activity as an indicator of viral replication and spread (Table 2 and Fig. 2 [not all data are shown]). WT gave rise to a spreading infection, and RT could be detected within 3 days. Many of the various mutant viruses showed either no viral replication or a delayed appearance of RT activity. The ΔC mutant never produced replicating virus, as was shown previously (Table 2 and Fig. 2) (31). Mutants Del 1, Del 2, and Del 3, each containing smaller deletions within the helix, also failed to replicate. Mutants with a single substitution of alanine or glutamate for either of the first two arginines (R599A, R600A, R599E, or R600E) replicated with kinetics similar to that of the WT virus. Mutants with double substitutions of both R599 and R600 with either alanine or glutamate (R599A R600A and R599E R600E), however, were incapable of replication. Of 31 new mutant viruses analyzed, all the mutants with an alteration or deletion of R601 were replication defective. These results suggest that it is essential to retain at least one of the first two arginines and also crucial to retain the third arginine residue.
FIG. 2.
Viral spread of the C-helix point mutant and WT virus in NIH 3T3 cells. NIH 3T3 cells were transiently transfected with mutant proviral DNAs cloned into pNCS. Cultures were passaged at a 1:10 dilution every 3 days. An exogenous assay was performed on supernatants collected on the indicated days after transfection. A negative control transfection was performed without plasmid DNA (mock). Here the I597A and G602A mutant viruses displayed viral spread slightly earlier (by 1 to 2 days) than was normally observed.
Several of the substitution mutants showed partial defects in replication. The alanine substitution I597A virus consistently showed a 14-day delay in the appearance of a detectable RT signal relative to that of the WT, while the I597T virus showed a 5- to 6-day delay in detectable viral spread. The better replication of the threonine substitution virus relative to that of the alanine substitution virus may be due to the beta-branched structure of threonine or simply a requirement for a gamma-carbon at position 597. Of the other mutants, the G602A mutant had a 5-day and the H594A mutant had a 3-day delay (Table 2 and Fig. 2; the I597A and G602A mutants arose slightly early in this figure). The H594A G602A double mutant was replication incompetent (Table 2), further confirming the importance of these two residues. Mutant viruses with a substitution at residue I593, G595, E596, or L603 were fully replication competent.
Previous work has shown that substitution of valine for Y598 significantly attenuates the ability of the virus to spread and reduces minus-strand strong-stop DNA strand transfer (3). Here we found that substitution of arginine for Y598 resulted in a replication-incompetent virus but that substitution of alanine or tryptophan did not affect viral-spread kinetics (Table 2). Kanaya et al. showed that in E. coli RNase H, substitution of alanine for W85 (the homologous residue of Y598) resulted in decreased catalytic efficiency (decrease in Vmax), most likely due to changes in tertiary structure.
E. coli residues K86, K87, and R88 correlate with Mo-MLV RT residues R599, R600, and R601, respectively (Fig. 1). The substitution of the E. coli C helix for the Mo-MLV C helix resulted in a mutant RT with exogenous polymerase activity, suggesting overall proper folding of the polymerase domain (Table 1). The mutant virus, however, was incapable of replication. This may be due to reduced efficiency of polymerase activity in vivo, improper folding of the RNase H domain, or possibly the insufficient substitution of lysine residues for R599 and R600 and substitution of intolerable residues for other critical Mo-MLV C helix residues (i.e., H594, I597, and G602).
The I597A mutant virus reverts to valine.
The I597A mutant virus was able to give rise to spreading virus, but there was a very long delay, compared to the initiation of spread of the WT virus. Thus, selective pressure for reversion of this mutation was likely to be very high. To test for the appearance of revertants, four separate viral supernatants were harvested from acutely infected NIH 3T3 cells actively spreading I597A mutant virus. Supernatants were diluted 1 to 50 and repassaged through uninfected Rat2-2 cells four times. Sixteen hours after the fourth passage, low-molecular-weight DNAs were collected from the four cultures and used as templates for PCR amplification of the Mo-MLV RT gene. PCR products were used to replace the corresponding WT fragment of the full-length Mo-MLV genome. Three independent proviral DNA clones from each of four viral harvests (a total of 12 clones) were used to transform NIH 3T3 cells. A number of clones produced virus capable of replicating with WT kinetics (Fig. 3; 6 of 12 clones are shown). DNA sequence analysis revealed that all reverted clones encoded valine instead of alanine at position 597, due to a single nucleotide change from GCA (Ala) to GTA (Val). It is interesting that valine is the only other hydrophobic amino acid besides the parental isoleucine with a beta-branched carbon atom. These results emphasize the importance of residue 597.
FIG. 3.
Viral spread of unique clones of I597 revertants in NIH 3T3 cells. Six unique clones of I597A revertants (Rev 1 to 6), the I597A and ΔC mutants, and the WT and no viral DNA (mock) were used to transform NIH 3T3 cells. Supernatants were collected on the indicated days and tested for RT activity. All viable I597A revertants (Rev 1, 3, 4, and 5) were sequenced and found to have valine instead of alanine in position 597.
C-helix mutant RTs retain polymerase activity and vary in their abilities to synthesize minus-strand strong-stop DNA in endogenous RT reactions.
To test other functions of the mutant RTs, we prepared virions by transient transfection of 293T cells with the mutant viral DNAs, such that secondary viral spread was prevented. Viral stocks were prepared by harvesting the supernatants 48 h after transfection. After normalization by Western blot analysis using anti-RT antibodies (data not shown), virions were lysed and used in RT assays on homopolymeric templates to quantitate RNA-dependent DNA polymerase activity (data not shown). All virions assayed displayed levels of DNA polymerase activity that were within threefold of each other (Table 2). All single-alanine-substitution mutant viruses analyzed (the H594A, I597A, R601A, and G602A mutants) as well as the ΔC mutant, had WT levels of DNA polymerase activity. To confirm these data, a number of mutant RTs expressed in bacteria were compared with one another. His-tagged versions of the H594A, I597A, R601A, G602A, and ΔC mutant RTs and WT RT were expressed in bacteria and purified (see Materials and Methods). Protein concentrations were normalized, and the purified proteins were utilized in exogenous RT assays. The DNA polymerase specific activities of all the purified proteins were equivalent to that of WT RT.
To test for the ability of the mutant RTs to reverse transcribe endogenous viral RNA, virions were purified by centrifugation through sucrose step gradients. After normalization by Western blot analysis using anti-RT antibodies, equal amounts of the purified virions were used in endogenous RT reactions to assay minus-strand strong-stop synthesis (Table 2 and Fig. 4). WT virus efficiently reverse transcribed viral RNA to form minus-strand strong-stop DNA of 145 nucleotides and accumulated DNAs of larger sizes migrating throughout the entire lane of the gel (Fig. 4, lane 6). The C helix H594A, I597A, R601A, G602A, and ΔC mutants produced minus-strand strong-stop DNA but did not synthesize longer DNA products as efficiently as WT virus. This is evident by the reduction in the amounts of DNA products larger than the 145-nucleotide minus-strand strong-stop DNA compared to the amounts of the WT DNA products (Fig. 4, compare lanes 1 to 5 to lane 6). In this particular experiment, the ΔC virus produced some DNAs smaller than 145 nucleotides (Fig. 4, lane 5). These DNAs have been previously hypothesized to be prematurely terminated, tRNA-primed minus-strand products (31). Such products were occasionally observed with the other alanine substitution mutant viruses (data not shown). The reduced levels of long DNA suggest that the mutant RTs make minus-strand strong-stop DNA but specifically fail to perform first-strand transfers for synthesis of longer minus-strand DNA.
FIG. 4.
Endogenous RT assays. Supernatants from 293T cells transiently producing mutant or WT virus were collected. Virions were purified through 25 to 45% sucrose step gradients, followed by an additional purification through 25% sucrose. Virus preparations were quantitated and normalized by exogenous-RT assay. The endogenous RT assay was performed in the presence of 0.05% NP-40 for 30 min to detect synthesis of the 145-bp minus-strand strong-stop DNA in an 8% nondenaturing polyacrylamide gel. Reaction mixtures with the supernatants from the ΔC mutant, the WT, and mock-transfected 293T cells were run in a separate gel.
Those mutants with multiple changes of the conserved arginine residues showed very little or no minus-strand strong-stop DNA synthesis (Table 2). Thus, although they exhibited DNA polymerase activity on exogenous templates, they were not active on endogenous viral RNA, which suggests that the three arginines played an important role. While a charge reversal of one arginine was not sufficient to completely disrupt RT activity, a charge reversal of two or more arginines did. Del 3 and the mutant that substituted the E. coli C helix for the Mo-MLV C helix also displayed no observable minus-strand strong-stop DNA. All these mutants that displayed no minus-strand strong-stop DNA may have major defects in minus-strand initiation, possibly from inefficient priming from tRNAPro bound to the PBS region.
The H594A, I597A, R601A, G602A, and ΔC mutant RTs differ in levels of RNase H activity on heteropolymeric RNA-DNA hybrids in vitro.
A subset of the mutants was selected for further characterization. The H594A, I597A, R601A, and G602A mutant RTs were further analyzed in comparison to ΔC and WT RTs. His-tagged versions of the mutant RTs were expressed in bacteria and purified on Ni-agarose beads. The isolated enzymes were then utilized on a heteropolymeric RNA-DNA substrate containing radiolabeled RNA. The substrate was incubated with equivalent amounts of mutant and WT RTs. Aliquots of each reaction mixture were collected, and the release of acid-soluble, low-molecular-weight, radiolabeled RNA was measured (Fig. 5). The H594A and G602A mutant RTs exhibited roughly 50% of the level of WT RNase H activity. The R601A and ΔC mutant RTs retained only approximately 8 and 4% of the WT level of RNase H activity, respectively. The low activity of ΔC is in accordance with data of Boyer and his colleagues (5). Except with the I597A mutant, these results are consistent with the replication behavior of mutant viruses containing these mutations and suggest that the reduced RNase H activity could be responsible for the replication defects seen in the mutants. The I597A mutant displayed high activity, 90% of that of the WT, and thus the replication defect of this mutant cannot be explained by a simple loss of RNase H activity. Further analysis to explain replication defects for the I597A mutant is presented below.
FIG. 5.
Nonspecific RNase H activity of WT and mutant RTs on a heteropolymeric RNA-DNA substrate. Radiolabeled M13 RNA was annealed to single-stranded DNA and incubated with 200 ng of protein. Aliquots were taken at the indicated times, and the substrate was precipitated with trichloroacetic acid. Unprecipitated, short-length RNA was collected and measured in a scintillation counter.
PCR analysis of viral DNAs reveals that the H594A, I597A, and G602A mutant viruses successfully reverse transcribe low levels of long viral DNA.
Since C helix H594A and G602A point mutant viruses are capable of slow replication and the I597A point mutant reverts to a replication-competent virus, these mutants presumably were able to synthesize at least low levels of viral DNA. PCR was utilized as a sensitive assay to detect viral-DNA synthesis. Low-molecular-weight DNAs were collected from Rat2-2 cells infected with various mutant viruses and the WT virus. The DNA was used as a template for PCRs to amplify products arising at three separate points during reverse transcription. Rat2-2 mitochondrial DNA primers were used for all infections to confirm that comparable numbers of cells were used for all DNA preparations (data not shown). To be sure that amplified products were derived from reverse-transcribed DNA and not contaminating plasmid DNA in the virus, undiluted viral supernatants were heat inactivated for 15 min at 70°C and used for infections. No viral DNA was detected in the low-molecular-weight DNAs collected from Rat2-2 cells infected with heat-inactivated viruses (Fig. 6, all lanes labeled HI).
FIG. 6.
PCR amplification of viral DNA reverse transcribed in vivo by the C helix mutant viruses and WT virus. Rat2-2 cells were infected with the mutant and WT viruses and serial dilutions (no dilution [ND], 1:10, and 1:100). Low-molecular-weight DNAs were collected 16 h postinfection and used as templates for PCRs amplifying products at different points in reverse transcription. Heat inactivated virus (HI) and no virus (mock) were used as a negative controls for confirming virus-mediated reverse transcription, while plasmid DNA (pNCS) was used as a positive control for the PCR primers. (A) PCR amplification of minus-strand strong-stop DNA. Primers that amplify a 126-bp stretch of the R-U5 region were used. Dilutions were carried out to 100-fold (1:100). (B) PCR amplification of long minus-strand DNA. Primers that amplify a 202-bp region of protease and RT were used to detect synthesis of long minus-strand DNA. (C) PCR amplification of plus-strand DNA synthesis. Primers that amplify a 394-bp stretch with R-U5-PBS-psi were used to confirm plus-strand jumping and elongation.
Primers amplifying the R-U5 region were used to monitor the formation of minus-strand strong-stop DNA. The H594A, I597A, and G602A mutant viruses displayed minus-strand strong-stop DNA synthesis, albeit at an efficiency lower than that of the WT virus (especially evident in the 1:10 dilutions) (Fig. 6A). The R601A mutant virus displayed only exceedingly small amounts of minus-strand strong-stop DNA synthesis, while ΔC showed no detectable minus-strand strong-stop DNA synthesis. These results for the R601A virus and ΔC differed from the results of endogenous reactions assaying minus-strand strong-stop DNA synthesis in vitro, in which the levels of minus-strand strong-stop DNA synthesis were similar to those seen with the other mutant viruses (Fig. 4, compare lanes 3 and 5 to lanes 1, 2, and 4).
Primers amplifying a region of the protease and RT genes within polymerase were used to assay the synthesis of long minus-strand DNA. Only low levels of minus-strand DNA were synthesized by the H594A, I597A, and G602A point mutant viruses (Fig. 6B). The H594A point mutant virus synthesized slightly less minus-strand DNA than the I597A and G602A mutant viruses. There was almost no detectable synthesis of long minus-strand DNA for these point mutant viruses at the 1:10 dilution and much less than was seen with WT virus. The R601A mutant had very little detectable minus-strand synthesis, while mutant virus ΔC had no detectable minus-strand synthesis. These results suggest that the mutant viruses are strongly deficient in processive synthesis after first-strand transfer. Overall, the data for the H594A, I597A, and G602A mutants confirm the results observed previously for endogenous reactions (Fig. 4) and viral-spread kinetics (Table 1). Defects in RT processivity would certainly contribute to delays in the kinetics of mutant virus spread. The I597A mutant virus was the most delayed in viral spread but was no more deficient in minus-strand DNA synthesis than the H594A and G602A mutants. This suggests that other undetected defects may be responsible for the I597A mutant's longer delay in viral spread.
Finally, primers were used to detect plus-strand-DNA synthesis by amplifying a region that contained the R, U5, PBS, and psi regions. Such amplification was possible only after plus-strand strong-stop translocation had occurred and polymerization had extended into the psi region. The R601A and ΔC mutant viruses exhibited no detectable plus-strand synthesis for all concentrations of viral infections. Synthesis of plus strands clearly occurred in the H594A, I597A, and G602A mutant viruses (Fig. 6C). As with minus-strand DNA synthesis, there was much less plus-strand synthesis detected than with the WT virus. Levels were slightly higher for the H594A and I597A mutant viruses than for the G602A mutant. These results were in accordance with the results observed for minus-strand synthesis. The findings also suggest that there is no major block after minus-strand synthesis and before plus-strand synthesis; that is, plus-strand priming is not specifically impaired.
Sequence analysis of LTR-LTR junctions in circular DNAs reveals improper viral DNA ends for the H594A, I597A, and G602A mutants.
Although the alanine substitution viruses had a significant reduction in their ability to synthesize DNA, differences in levels of DNA detected by PCR did not always correlate with the differences observed in viral-spread kinetics. One possible explanation for the varied rates of replication is that the mutants may differ in their abilities to cleave specific bases needed for correct DNA synthesis. The RNase H domain must cleave specific bases to define the proper 5′ DNA ends needed for integration (6, 7). After formation of the linear DNA, a portion of the DNA is circularized by host enzymes; thus, it is possible to determine both termini of the linear DNA by sequencing the LTR-LTR junction of the circular DNAs. PCR primers were used to amplify the LTR-LTR junctions, the amplified DNAs were cloned into a plasmid vector, and individual clones were sequenced. The left LTR of the viral DNA contributes the observed U3 sequences and is determined by plus-strand priming from the PPT and PPT primer removal. The right LTR of the viral DNA contributes the observed U5 sequences and is determined by minus-strand priming from tRNAPro and its removal from the minus strand.
Previous analysis of mutant viruses with a deletion near the PPT revealed four different classes of LTR-LTR junctions (1). Group 1 consisted of the normal 5′-U5-U3-3′ junction derived from ligation of WT termini, containing a perfect inverted repeat. Group 2 junctions contained deletions in either the 5′ end or the 3′ end of the proviral DNA. Group 3 consisted of insertions between U5 and U3 of foreign or unknown sequences. Group 4 consisted of insertions of viral DNA that arose by either mispriming or failure to remove tRNAPro or the PPT primers. WT virus gave rise to groups 1, 2, and 3 LTR-LTR junctions but did not result in group 4 junctions (Fig. 7A). In this experiment, 9 of 14 clones from WT virus were in group 1. This result was consistent with previous findings in which roughly half of WT viral LTR-LTR junctions are of the normal group 1 type (1).
FIG. 7.
Schematic representation of LTR-LTR circle junction analysis. Low-molecular-weight DNAs were collected from Rat2-2 cells 16 h after acute infections with mutant and WT viruses and were used as templates for PCRs. Primers were used to amplify an R-U5-U3 fragment from LTR-LTR junctions in circularized forms of proviral DNA. Circle junctions from WT virus (A) and viruses with the mutations H594A (B), I597A (C), and G602A (D) are listed here.
Of eight clones analyzed for the H594A mutant virus, only one was of the normal group 1 type (Fig. 7B). Two of the clones were of group 2 (deletions). No clones were of the group 3 type. There were, however, five clones with viral sequence insertions within group 4. Three of these had an insertion of TG or TGG. The insertions of TG or TGG are most simply explained as a result of RNase H cleavage 2 or 3 bp 59 from the end of tRNA Pro during primer removal (24, 25). Two of the viral insertion clones for group 4 included sequences from the PPT and are explained by plus-strand mispriming just upstream of the PPT or by the failure to remove the PPT primer. For the I597A mutant virus, two of the eight clones gave rise to normal group 1 junctions (Fig. 7C). Five of the clones, however, gave rise to group 4 junctions with viral sequence insertions. Three of these group 4 insertions contained sequences from the PPT, while the remaining two had viral sequences from the 59 end of the viral genome (PBS and/or gag). Finally, the G602A mutant virus gave rise to three clones in group 1, four clones in group 2, one clone in group 3, and three clones in group 4 (Fig. 7D). All three of the group 4 clones were single-base-pair insertions of T that were probably a result of cleavage 1 bp 59 from the end of the tRNA Pro primer (24, 25). Three of the four group 2 clones revealed large deletions (.90 bp) in the 59 end of U3. Such large deletions were not observed for the WT virus.
These results are most readily explained by the reduction in RNase H activity as observed for the C helix mutant RTs in vitro. Complete removal of PPT and PBS RNA sequences may require multiple, nonspecific cleavage events that the mutant RTs are not able to perform efficiently, resulting in retention of primer sequences on the DNA termini. In addition, the lower RNase H activities of the mutant RTs may particularly disrupt PPT primer formation, a process that requires at least one discrete cleavage at its 39 end for the proper positioning of plus-strand synthesis initiation. If the reduced RNase H activity of the mutants results in incorrect or nonfunctional PPT primers, then mispriming of plus-strand synthesis upstream of the PPT region would result in PPT sequence additions to the 59 viral DNA terminus. Priming downstream of the PPT region, however, would result in deletions in the 59 viral DNA terminus into U3. An alternative explanation for these deletions in the 59 viral DNA terminus is the possibility of incomplete synthesis of full-length minus strands through the entire LTR after second-strand transfer.
The H594A and G602A mutant viruses displayed defects with complete tRNA Pro removal, as was evidenced by insertions of T, TG, or TGG in LTR-LTR junctions. The H594A and I597A mutant viruses had insertions of regions near and including the PPT that could have resulted from improper plus-strand priming upstream of the normal PPT sequence or defective PPT primer removal. The G602A mutant virus displayed large deletions in the 59 viral DNA terminus that may have arisen from improper plus-strand priming downstream of the PPT or the failure to complete full-length, minus-strand polymerization. Although the I597A mutant RT had the highest level of in vitro RNase H activity of the mutant RTs (90% of the level of WT RT), the I597A mutant virus still produced a high proportion of defective circle junctions (six of eight clones) and had the longest delay in kinetics of viral spread. This necessitated the further analysis of the mutant RTs.
H594A, I597A, R601A, and G602A RTs demonstrate defects in PPT recognition and RNA cleavage in vitro.
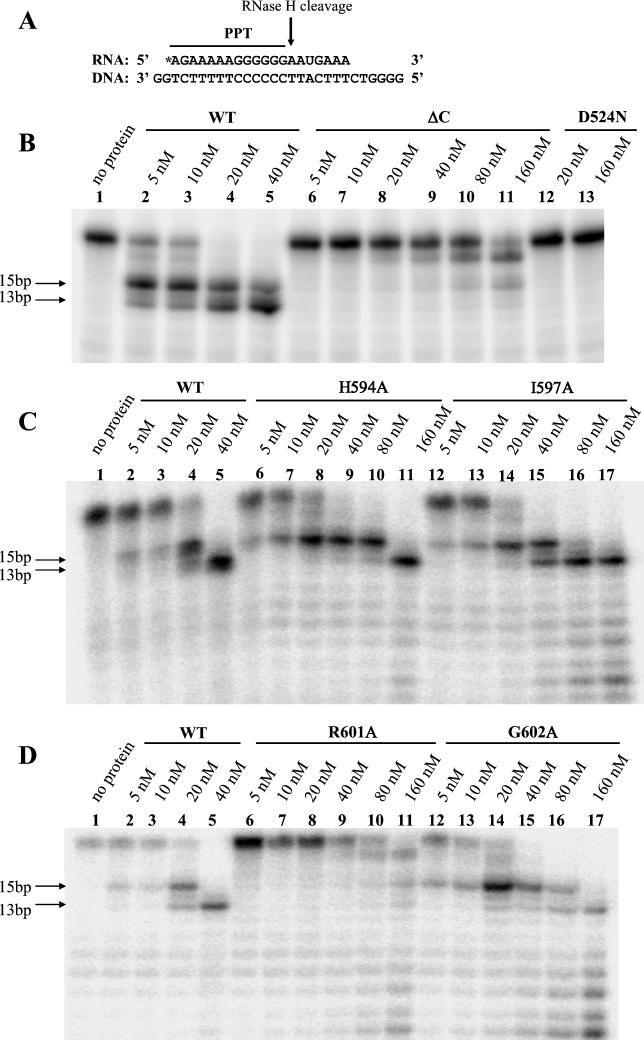
Five of seven LTR-LTR junctions from the various RNase H mutant RTs contained insertions of regions near and including the PPT. One explanation is that improper plus-strand priming or lack of PPT removal (or both events) resulted in the retention of PPT sequences at the 59 plus-strand viral DNA end. To further test this notion, a PPT-U3 recognition and cleavage assay was performed in vitro. A 59-end, radiolabeled oligoribonucleotide consisting of the 13 nucleotides of the PPT sequence plus the first 7 nucleotides of U3 (PPT-U3) was annealed to a complementary, single-stranded DNA with overhangs of 2 and 6 bp on the 59 and 39 ends, respectively (Fig. 8A). The substrate was incubated with increasing amounts of WT and mutant RTs (5 to 160 nM) in the presence of magnesium for 20 min. Reaction products were then purified and analyzed by electrophoresis through a denaturing polyacrylamide gel.
FIG. 8.
In vitro PPT recognition and cleavage assay for WT and mutant RTs. (A) Sequences of the RNA and DNA hybrid substrates used in the reactions. An asterisk represents the 5′ radiolabel. (B to D) The substrate was annealed, and then various concentrations of WT and mutant RTs from 5 to 160 nM were used in the presence of 6 mM magnesium. Reaction products were purified and electrophoresed through a 20% denaturing polyacrylamide gel. PPT recognition and RNase H cleavage specificity in the presence of magnesium leaves 13 bp intact (lower arrows). In panel B, lane 5, WT RT aberrantly left some 15-bp substrate uncleaved. PPT recognition and cleavage specificity are lost in the presence of manganese. The D524N RT is a point mutant RT without RNase activity.
WT RT first cleaved the radiolabeled RNA to a 15-nucleotide fragment and then to a final 13-nucleotide product (Fig. 8B to D, lanes 5). ΔC RT did not efficiently cleave the PPT-U3 substrate and did not make correct cleavages even at the highest protein concentration (Fig. 8B, lane 11). Mutant D524N RT has a mutation in a conserved aspartic acid residue that is part of the active site of all RNases H (3, 17). D524N RT does not possess RNase H activity and was used as a negative control to demonstrate that there was no E. coli RNase activity contaminating the protein preparations. D524N RT did not cleave the substrate at all (Fig. 8B, lanes 12 and 13).
H594A RT specifically cleaved the PPT-U3 substrate to the 13-nucleotide fragment only at the highest level of enzyme (Fig. 8C, lanes 6 to 11). I597A RT cleaved the PPT-U3 substrate to the 13-nucleotide fragment at a twofold-higher level of enzyme than did WT RT (80 versus 40 nM) (Fig. 8C, lanes 12 to 17). G602A RT cleaved the substrate to the 13-nucleotide PPT fragment only at the highest level of enzyme (Fig. 8D, lane 17). There was, however, stronger evidence of nonspecific PPT cleavage for G602A as seen in the intense ladder of cleavage products below the 13-nucleotide PPT fragment (Fig. 8D, lane 17). R601A RT was extremely defective and did not cleave the PPT-U3 substrate to the 13-nucleotide PPT fragment, similar to what occurred with ΔC RT (Fig. 8D, lanes 6 to 11). R601A RT, however, did cleave the substrate nonspecifically, as is seen in the ladder of cleavage products at the highest level of enzyme (Fig. 8D, lane 11). Such a distinctive and intense ladder of smaller RNA molecules was not seen in any of the WT RT reactions (Fig. 8B to D, lanes 5). The PPT-U3 cleavage tests of WT RT and all of the mutant RTs in the presence of manganese, however, showed nonspecific cleavage activity at levels similar to those of the previously described (Fig. 5), nonspecific assay performed with magnesium (data not shown).
These results suggest that the C-helix point mutants are less efficient in forming the proper PPT primer for plus-strand synthesis. The nonspecific, in vitro RNase H assay showed mutant ΔC and R601A RTs to have 4 and 8% of the WT RT RNase H activity, respectively (Fig. 5). In this in vitro PPT-U3 recognition and cleavage assay, ΔC and R601A RTs failed to properly form the PPT primer even at 160 nM (Fig. 8B and D, lanes 11). WT RT efficiently formed the PPT primer at 40 nM (Fig. 8C and D, lanes 5). The nonspecific, in vitro RNase H assay showed the H594A, I597A, and G602A mutant RTs to have 50, 90, and 50% of the level of WT RT RNase H activity, respectively (Fig. 5). The H594A, I597A, and G602A mutant RTs had 25, 50, and 25% of the level of WT RT RNase H activity, respectively, on the PPT-U3 hybrid substrate (Fig. 8C, lanes 11 and 16, and D, lane 17). Overall, compared to WT RT, the C-helix mutant RTs have even less RNase H activity on the PPT-U3 hybrid substrate than on nonspecific heteropolymeric substrates.
DISCUSSION
Previous analysis of Mo-MLV ΔC revealed normal DNA polymerase activity on homopolymer templates present in excess but a major decrease in ΔC RT's ability to process DNA synthesis activity on viral and nonviral RNA templates in vitro (5, 31). Here, we detected no minus-strand strong-stop DNA or longer DNA products for ΔC RT in vivo (Fig. 6). The discordance between assays in vitro and in vivo may have been due to the higher concentrations of deoxynucleoside triphosphates and the primer or template supplied in vitro versus in vivo or other differences between the conditions. With regard to RNase H activity, our studies showed ΔC RT to have only about 4% of the level of WT activity by a conventional enzyme assay (Fig. 5). Loss of the C helix, therefore, seems to dramatically disrupt RNase H function. Considering the data together, the absence of minus-strand strong-stop DNA synthesis in vivo and low RNase H activity in vitro for ΔC RT support the hypothesis that the Mo-MLV C helix is important for efficient activity for both the polymerase and RNase H domains.
Analysis of point mutations in the C helix has led to the identification of several crucial residues. We concentrated our studies on those alanine substitution point mutations that gave rise to attenuated or replication-defective viruses, the H594A, I597A, R601A, and G602A mutants. The defects of all four mutants are most likely not due to gross misfolding of RT since they all retain WT levels of DNA synthesis activity on homopolymeric substrates (Table 2). Consideration of the locations of these residues in the known structure of bacterial RNase H is informative. The implicated Mo-MLV C-helix residues H594, I597, R601, and G602 correspond to W81, N84, R88, and G89, respectively, in the E. coli sequence. The crystal structure of E. coli RNase H shows residues W81, N84, and R88 to lie on the same side of the helix facing solvent space that is proposed to make contact with the RNA-DNA hybrid substrate (Fig. 9) (20, 21, 35). In addition, recent crystal structure data of Mo-MLV RNase H from this lab and superimposition modeling upon the crystal structure of HIV RT bound to a substrate confirm that side chains of H594, I597, and R601 may point towards the substrate (unpublished data). It is possible that mutations H594A, I597A, and R601A have directly disrupted substrate interactions that result in the phenotypes observed. E. coli residue G89 resides just beyond the C terminus of the C helix and lies on the opposite side from residues H594, I597, and R601. Changes in G602 may affect C-helix stability or position and its normal interactions with the rest of the RNase H domain.
FIG. 9.
Worm diagram of the crystal structure of E. coli RNase H (35). The C helix is represented by the region in red and yellow. Alpha-carbon positions are shown for W81, N84, R88, and G89, which correspond to Mo-MLV RT residues H594, I597, R601, and G602, respectively. The recent crystal structure of Mo-MLV RNase H (unpublished data) and the crystal structure of E. coli RNase H superimposed upon an HIV RNase H bound to RNA-DNA show that the C helix may contact the substrate. Side chains for W81, N84, and R88 point to the side of the helix that is proposed to face the primer-template (toward the viewer), while G89 faces the opposite side (away from the viewer). The primer-template is proposed to lie roughly above the model from the top to bottom of the figure. This figure was made with MOLSCRIPT and Raster 3D (20, 21).
PCR with 20 cycles of amplification detected very little viral DNA made by the R601A mutant virus. This same analysis, however, showed the presence of at least some viral DNA in cells infected with the replication-competent H594A, I597A, and G602A mutants, albeit in amounts smaller than that of the WT virus (Fig. 6). The low levels of mutant viral DNAs may account for the requirement for PCR amplification for detection. PCR, however, does not reveal the possible heterogeneity of DNA which may have resulted from defective reverse transcription. The reduction in reverse transcription efficiency for these three mutant viruses certainly provides an explanation for the delays observed in the kinetics of mutant virus spread. Although the PCR analysis performed here may not accurately measure absolute quantities of viral DNA, the mutant viruses were qualitatively less efficient in viral DNA synthesis than the WT virus.
To analyze specific RNase H cleavage events, we sequenced circle junctions of reverse-transcribed viral DNAs from Rat2-2 cells infected with mutant and WT viruses. All the mutant viruses resulted in various defective LTR-LTR junctions not observed with WT virus (Fig. 7). Overall, the mutant viruses had a lower proportion of normal junctions (much less than the 50% ratio normally observed with WT virus). The H594A and G602A mutant viruses, but not the I597A mutant, resulted in viral sequence insertions that most likely resulted from RNase H cleavage within the minus-strand primer, tRNAPro, rather than at the DNA-primer junction (Fig. 7B and 8D). Linear DNAs containing extra bases could probably be integrated into the host DNA at a normal level of efficiency; mutant viruses carrying additions of a few nucleotides at this position were viable and replicated well (7). WT HIV RT has been observed to retain a terminal A ribonucleotide, both in vitro and in vivo, resulting from RNase H cleavage one base 5′ of the U5-tRNALys junction (22). While Mo-MLV RT apparently does not retain a terminal ribonucleotide A in vivo, experiments show that it does in vitro (25). The circle junction analysis suggests defects in complete tRNA primer removal from the minus-strand DNAs for H594A and G602A mutant RTs. Some of these mutant DNAs may retain viability; there were a total of 3 probably viable clones of 8 analyzed with the H594A mutation and 6 viable clones of 11 analyzed with the G602A mutation. The higher ratio of viable clones may possibly explain why mutants with the H594A and G602A mutations have shorter delays in viral-spread kinetics than do mutants with the I597A mutation, of which only two of eight clones analyzed were viable.
Other defects observed in circle junctions from mutant viruses included gross deletions in both the left and right LTRs and retention of the plus-strand primer or sequences from the PPT. Deletions may result from inappropriate priming downstream of either the PBS or the PPT or failure to complete minus- or plus-strand synthesis through the LTRs. Retention of the PPT sequence can result from inappropriate plus-strand priming upstream of the PPT or the failure to remove the PPT primer. The I597A mutant virus displayed many LTR-LTR junctions with viral sequence insertions (Fig. 7C). Three of these included the 13 nucleotides of the PPT, while the other two insertions most likely resulted from a combination of minus-strand mispriming and rearrangements. These retentions of longer sequences may also account for the increased delay in replication manifested by the I597A mutant.
Overall, the circle junction analysis revealed that the C-helix mutations caused defects in both minus- and plus-strand priming, complete strand synthesis, and primer removal. Since the PPT sequence was found to be retained in a number of LTR-LTR junctions, we tested the C helix mutants' abilities to make specific cleavages in assays performed on the critical PPT-U3 sequence. The assay mimics the step of plus-strand initiation. In the presence of magnesium, WT RT specifically cleaved the PPT-U3 to form the appropriate 13-nucleotide fragment at low enzyme concentrations (Fig. 8B). H594A, I597A, and G602A RTs all properly cleaved at the PPT-U3 junction but only at higher enzyme concentrations (Fig. 8C and D). Both R601A and ΔC RT did not cleave the PPT-U3 to the specific 13-nucleotide fragment (Fig. 8B and D). The mutant R601A and G602A RTs displayed nonspecific degradation of the PPT in the presence of magnesium as evidenced by the ladder of products shorter than 13 nucleotides (Fig. 9D). Such nonspecific cleavage of the PPT was not observed for WT RT (Fig. 8B to D). The inability of the C-helix point mutant RTs to form the 13-nucleotide PPT primer as efficiently as WT RT may account for the sequence defects observed at the 5′ termini of mutant viral DNAs. The mutant RTs had even less efficient RNase H activity upon the PPT-U3 substrate than upon a nonspecific substrate with respect to the WT RT. If the C helix mutant RTs do not initiate plus-strand synthesis from the PPT region, then priming may occur elsewhere. If priming occurs in the U3 region, U3 deletions within the 5′ terminus of the plus strand would result, as was observed with the H594A and G602A mutant viruses (Fig. 7B and D). If priming occurs further upstream, retention of the PPT and further upstream sequences on the 5′ terminus of the plus strand is expected, as was observed for the H594A and I597A mutant viruses (Fig. 7B and C).
Recently, the crystal structure of HIV RT bound to an RNA-DNA hybrid composed of the HIV PPT-U3 region was solved (23). The data show that regions in both the polymerase and RNase H domains bind and position the RNA-DNA substrate such that the unusually narrow minor groove of the PPT prevents the RNA strand from interacting with the catalytic site of RNase H. In addition, the primer-template normally adopts a 40° to 45° bend between the polymerase and RNase H active sites. Such a bent structure may not be physically possible for the sequences of the PPT. The “stiffness” of the PPT may induce a pause at the polymerase active site such that cleavage favorably occurs at the PPT-U3 junction. The structure suggests that if a C helix and following loop were added to the HIV RNase H domain, they would reside very close to the phosphate backbones and major groove of the RNA-DNA substrate. Such placement favors a role for the C helix and following loop of interacting with the substrate. Residues of the HIV connection domain of p66 make contacts with the substrate in this same region. The model suggests that, in Mo-MLV RT, the C helix may well make contact directly with the substrate and that alterations in the helix may well affect the overall enzymatic activities of both the polymerase and RNase H domains.
We have demonstrated that disruption of the Mo-MLV C helix interfered with both the polymerase and RNase H functions in vivo. The R601A and ΔC mutants drastically reduced RNase H activity and the ability of the DNA polymerase to make long DNA, which accounted for their failure to replicate. The H594A, I597A, and G602A mutants retained both enzymatic activities at various levels, though all three mutant RTs synthesized less viral DNA than did WT RT. The reduced levels of the activities of both enzymes in the H594A and G602A mutants correlated with the observed delays in the kinetics of mutant-virus spread. While the I597A mutant possessed more RNase H activity and levels of viral DNA polymerase activity equivalent to those of the H594A and G602A viruses, the I597A mutant possessed the slowest kinetics in viral replication. We therefore speculated that the I597A virus may suffer grosser defects with specific cleavage events. In support of this notion, the I597A mutant did have a greater proportion of viral DNAs with severely defective termini than the H594A and G602A viruses. While the Mo-MLV C helix probably plays a role in cleavage efficiency at both the U5-PBS and PPT-U3 junctions, our mutants displayed grosser defects with PPT-related events. The in vitro PPT-U3 recognition and cleavage assay used here revealed that the mutant RTs had poor RNase H efficiency at the PPT-U3 junction compared to that of WT RT. The I597A mutant, however, was no more defective than the H594A and G602A mutants in this assay. Therefore, we cannot rule out the possibility that other undetected defects may explain the I597A mutant's longer delay in its kinetics of viral replication. These results support the model of the C helix playing a role in primer-template binding and contributing to PPT sequence recognition and efficient cleavage at the PPT-U3 junction.
Acknowledgments
We thank Craig Bingman for producing the worm diagram of E. coli RNase H and Wayne Hendrickson for his insight and generosity with his laboratory equipment and reagents.
This work was partially supported by Public Health Service grant CA 30488 from the NCI. S.P.G. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Bacharach, E., J. Gonsky, D. Lim, and S. P. Goff. 2000. Deletion of a short, untranslated region adjacent to the polypurine tract in Moloney murine leukemia virus leads to formation of aberrant 5′ plus-strand DNA ends in vivo. J. Virol. 74:4755-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltimore, D. 1970. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 226:1209-1211. [DOI] [PubMed] [Google Scholar]
- 3.Blain, S. W., and S. P. Goff. 1995. Effects on DNA synthesis and translocation caused by mutations in the RNase H domain of Moloney murine leukemia virus reverse transcriptase. J. Virol. 69:4440-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blain, S. W., and S. P. Goff. 1993. Nuclease activities of Moloney murine leukemia virus reverse transcriptase. Mutants with altered substrate specificities. J. Biol. Chem. 268:23585-23592. [PubMed] [Google Scholar]
- 5.Boyer, P. L., H. Q. Gao, P. Frank, P. K. Clark, and S. H. Hughes. 2001. The basic loop of the RNase H domain of MLV RT is important both for RNase H and for polymerase activity. Virology 282:206-213. [DOI] [PubMed] [Google Scholar]
- 6.Colicelli, J., and S. P. Goff. 1985. Mutants and pseudorevertants of Moloney murine leukemia virus with alterations at the integration site. Cell 42:573-580. [DOI] [PubMed] [Google Scholar]
- 7.Colicelli, J., and S. P. Goff. 1988. Sequence and spacing requirements of a retrovirus integration site. J. Mol. Biol. 199:47-59. [DOI] [PubMed] [Google Scholar]
- 8.Davies, J. F., II, Z. Hostomska, Z. Hostomsky, S. R. Jordan, and D. A. Matthews. 1991. Crystal structure of the ribonuclease H domain of HIV-1 reverse transcriptase. Science 252:88-95. [DOI] [PubMed] [Google Scholar]
- 9.Gao, G., and S. P. Goff. 1998. Replication defect of Moloney murine leukemia virus with a mutant reverse transcriptase that can incorporate ribonucleotides and deoxyribonucleotides. J. Virol. 72:5905-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff, S., P. Traktman, and D. Baltimore. 1981. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J. Virol. 38:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff, S. P. 1990. Integration of retroviral DNA into the genome of the infected cell. Cancer Cells 2:172-178. [PubMed] [Google Scholar]
- 12.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 13.Hostomsky, Z., Z. Hostomska, G. O. Hudson, E. W. Moomaw, and B. R. Nodes. 1991. Reconstitution in vitro of RNase H activity by using purified N-terminal and C-terminal domains of human immunodeficiency virus type 1 reverse transcriptase. Proc. Natl. Acad. Sci. USA 88:1148-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobo-Molina, A., J. Ding, R. G. Nanni, A. D. Clark, Jr., X. Lu, C. Tantillo, R. L. Williams, G. Kamer, A. L. Ferris, P. Clark, et al. 1993. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 Å resolution shows bent DNA. Proc. Natl. Acad. Sci. USA 90:6320-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, M. S., M. A. McClure, D. F. Feng, J. Gray, and R. F. Doolittle. 1986. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc. Natl. Acad. Sci. USA 83:7648-7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanaya, S., C. Katsuda-Nakai, and M. Ikehara. 1991. Importance of the positive charge cluster in Escherichia coli ribonuclease HI for the effective binding of the substrate. J. Biol. Chem. 266:11621-11627. [PubMed] [Google Scholar]
- 17.Kanaya, S., A. Kohara, Y. Miura, A. Sekiguchi, S. Iwai, H. Inoue, E. Ohtsuka, and M. Ikehara. 1990. Identification of the amino acid residues involved in an active site of Escherichia coli ribonuclease H by site-directed mutagenesis. J. Biol. Chem. 265:4615-4621. [PubMed] [Google Scholar]
- 18.Katayanagi, K., M. Miyagawa, M. Matsushima, M. Ishikawa, S. Kanaya, M. Ikehara, T. Matsuzaki, and K. Morikawa. 1990. Three-dimensional structure of ribonuclease H from E. coli. Nature 347:306-309. [DOI] [PubMed] [Google Scholar]
- 19.Keck, J. L., and S. Marqusee. 1995. Substitution of a highly basic helix/loop sequence into the RNase H domain of human immunodeficiency virus reverse transcriptase restores its Mn2+-dependent RNase H activity. Proc. Natl. Acad. Sci. USA 92:2740-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraulis, P. J. 1991. “MOLSCRIPT”: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24:946-950. [Google Scholar]
- 21.Merritt, E. A., and D. J. Bacon. 1997. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277:505-524. [DOI] [PubMed] [Google Scholar]
- 22.Pullen, K. A., L. K. Ishimoto, and J. J. Champoux. 1992. Incomplete removal of the RNA primer for minus-strand DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 66:367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarafianos, S. G., K. Das, C. Tantillo, A. D. Clark, Jr., J. Ding, J. M. Whitcomb, P. L. Boyer, S. H. Hughes, and E. Arnold. 2001. Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. EMBO J. 20:1449-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz, S. J., and J. J. Champoux. 1996. RNase H domain of Moloney murine leukemia virus reverse transcriptase retains activity but requires the polymerase domain for specificity. J. Virol. 70:8630-8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, C. M., W. B. Potts III, J. S. Smith, and M. J. Roth. 1997. RNase H cleavage of tRNAPro mediated by M-MuLV and HIV-1 reverse transcriptases. Virology 229:437-446. [DOI] [PubMed] [Google Scholar]
- 26.Smith, J. S., K. Gritsman, and M. J. Roth. 1994. Contributions of DNA polymerase subdomains to the RNase H activity of human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 68:5721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, J. S., and M. J. Roth. 1993. Purification and characterization of an active human immunodeficiency virus type 1 RNase H domain. J. Virol. 67:4037-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahl, S. J., J. D. Kaufman, S. Vikic-Topic, R. J. Crouch, and P. T. Wingfield. 1994. Construction of an enzymatically active ribonuclease H domain of human immunodeficiency virus type 1 reverse transcriptase. Protein Eng. 7:1103-1108. [DOI] [PubMed] [Google Scholar]
- 29.Tanese, N., and S. P. Goff. 1988. Domain structure of the Moloney murine leukemia virus reverse transcriptase: mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc. Natl. Acad. Sci. USA 85:1777-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telesnitsky, A., S. Blain, and S. P. Goff. 1995. Assays for retroviral reverse transcriptase. Methods Enzymol. 262:347-362. [DOI] [PubMed] [Google Scholar]
- 31.Telesnitsky, A., S. W. Blain, and S. P. Goff. 1992. Defects in Moloney murine leukemia virus replication caused by a reverse transcriptase mutation modeled on the structure of Escherichia coli RNase H. J. Virol. 66:615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telesnitsky, A., and S. P. Goff. 1993. Two defective forms of reverse transcriptase can complement to restore retroviral infectivity. EMBO J. 12:4433-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temin, H. M., and S. Mizutani. 1970. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 226:1211-1213. [DOI] [PubMed] [Google Scholar]
- 34.Wigler, M., R. Sweet, G. K. Sim, B. Wold, A. Pellicer, E. Lacy, T. Maniatis, S. Silverstein, and R. Axel. 1979. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell 16:777-785. [DOI] [PubMed] [Google Scholar]
- 35.Yang, W., W. A. Hendrickson, R. J. Crouch, and Y. Satow. 1990. Structure of ribonuclease H phased at 2 Å resolution by MAD analysis of the selenomethionyl protein. Science 249:1398-1405. [DOI] [PubMed] [Google Scholar]