Abstract
The mechanisms by which Theiler's murine encephalomyelitis virus (TMEV) binds and enters host cells and the molecules involved are not completely understood. In this study, we demonstrate that the high-neurovirulence TMEV GDVII virus uses the glycosaminoglycan heparan sulfate (HS) as an attachment factor that is required for efficient infection. Studies based on soluble HS-mediated inhibition of attachment and infection, removal of HS with specific enzymes, and blocking with anti-HS antibodies establish that HS mediates GDVII virus entry into mammalian cells. Data from defined proteoglycan-deficient Chinese hamster ovary mutant cells further support the role of HS in GDVII infection and indicate that the extent of sulfation is critical for infection. Neuraminidase treatment of proteoglycan-deficient cells restores permissiveness to GDVII virus, indicating that sialic acid hinders direct access of virus to the protein entry receptor. A model of the potential steps in GDVII virus entry into mammalian cells involving HS is proposed.
Theiler's murine encephalomyelitis virus (TMEV), an enteric pathogen of mice, contains a single-stranded RNA genome of positive polarity and belongs to the Cardiovirus genus in the Picornaviridae family (30). TMEV can be divided into two groups based on neurovirulence following intracerebral inoculation of mice. High-neurovirulence strains, such as GDVII, produce fatal encephalitis in mice. Low-neurovirulence TMEV strains, such as BeAn and DA, cause a persistent infection in the central nervous system of mice, resulting in an inflammatory, demyelinating disease (21, 24). Analyses of recombinant TMEVs have mapped a persistence determinant(s) to the capsid, suggesting a role for a virus-cell receptor interaction in persistent infection (1, 18).
The mechanisms by which TMEV binds to and enters host cells are not completely understood. TMEV appears to use at least two molecules for attachment and cell entry (19). Low-neurovirulence BeAn and DA viruses have been shown to use cell surface sialic acid as an attachment factor (11, 33, 34). BeAn virus binds α2,3-linked sialic acid moieties on N-linked but not O-linked oligosaccharides or on glycolipids (A. H. Shah and H. L. Lipton, submitted for publication). Since nonenveloped viruses, such as picornaviruses, are unable to fuse with the cell lipid membrane bilayer, their internalization is thought to require a specific protein entry receptor. Although a TMEV cellular receptor has not been identified, a ligand-binding experiment, i.e., virus overlay protein blot assay, suggests the use of a 34-kDa protein entry receptor (20). The peripheral nerve protein PO has also been suggested as a TMEV protein entry receptor outside of the central nervous system (22), but this finding awaits confirmation.
Recently, Hertzler et al. (14) demonstrated that the UDP galactose transporter found in the trans-Golgi apparatus is required for GDVII virus binding and infection of a mutant receptor-negative BHK-21 cell variant (selected by resistance to BeAn virus infection), pointing to the need for galactose in this process. Recent studies indicate that the UDP galactose transporter is not the TMEV protein receptor (H. V. Reddi and H. L. Lipton, unpublished data). Examination of the role of various cell surface glycoconjugates containing galactose in binding and infection indicates that GDVII does not use either O-linked oligosaccharides or glycolipids for binding and infection. However, attachment to N-linked oligosaccharides accounts for a portion of GDVII binding (A. H. Shah and H. L. Lipton, submitted for publication). Since galactose is also a component of the tetrasaccharide linker of proteoglycans (PG) and heparan sulfate (HS), PG are coreceptors or attachment factors for a large number of RNA and DNA animal viruses (6), including the picornaviruses, echovirus-7 (13), and foot-and-mouth disease virus (FMDV) (12, 16), we asked whether HS plays a role in GDVII virus binding and infection of mammalian cells.
We report here that the high-neurovirulence GDVII virus uses the most ubiquitous cell surface PG, HS, as an attachment factor and that HS is required for efficient infection. A model of the potential steps in GDVII virus entry into mammalian cells with HS is proposed.
MATERIALS AND METHODS
Cells and virus stocks.
Baby hamster kidney (BHK-21) cells (CRL-10), Chinese hamster ovary (CHO) cells (CRL-1781), CHO sialic acid transporter-deficient mutant Lec-2 cells (CRL-1736), and PG synthesis-deficient mutants pgs A-745 (CRL-2242), D-677 (CRL-2244), C-605 (CRL-2245), and E-606 (CRL-2246) were obtained from the American Type Culture Collection. BHK-21 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM l-glutamine, 100 mg of streptomycin per ml, 100 U of penicillin per ml, 7.5% fetal bovine serum (FBS), and 6.5 mg of tryptose phosphate broth (Gibco-BRL) per ml at 37°C in a 5% CO2 atmosphere. CHO and Lec-2 cells were maintained in minimal essential medium supplemented with 2 mM l-glutamine, 100 mg of streptomycin per ml, 100 U of penicillin per ml, and 10% FBS. pgs A-745, D-677, C-605, and E-606 cells were maintained in DMEM/F-12 medium supplemented as above. BeAn and GDVII virus stocks were prepared as clarified lysates in BHK-21 cells with titers of >108 PFU/ml. BeAn-, GDVII-, and FA-infected suckling mouse brain stocks were prepared by brain-to-brain passages as 10% clarified homogenates in DMEM with 50% tissue culture infectious dose titers of 108.0, 108.0, and 107.2, respectively. GDVII and FA viruses were from brain stocks obtained from Larry Sturman (Albany, N.Y.) and the American Type Culture Collection, respectively, and had not been passaged in cells.
Reagents.
Chondroitin sulfate A from sturgeon notocord, heparan sulfate (HS) from bovine kidney, keratan sulfate from bovine cornea, chondroitinase ABC, heparinase, heparitinase II, and mouse anti-HS monoclonal antibody (MAb) F58-10E4 were obtained from Seikagaku America Inc. Heparin, heparinase I and III, neuraminidase, anti-mouse immunoglobulin G (IgG)-fluorescein isothiocyanate (FITC) conjugate, goat anti-mouse polyvalent Ig-FITC, and MTT reagent [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] were obtained from Sigma. Dermatan sulfate from porcine intestinal mucosa was obtained from Celsus Laboratories. Anti-TMEV VP1 MAb 6F7 was kindly provided by Raymond Roos at the University of Chicago.
Binding assay.
Binding of virus to cells was determined by fluorescence-activated cell sorting (FACS) as described before (14). Briefly, 106 cells were washed with phosphate-buffered saline (PBS) containing calcium and magnesium and incubated with virus for 45 min at 4°C. Unbound virus was washed off with PBS containing 5% FBS (PBS-FACS), and cells were blocked with 10 μl of normal goat serum for 15 min at 4°C, followed by incubation with 1 μg of anti-TMEV MAb for 30 min at 4°C. After three washes with PBS-FACS, cells were incubated with 1 μg of anti-mouse IgG-FITC for 30 min at 4°C, fixed with 1% paraformaldehyde, and analyzed on a FACSCalibur (Becton Dickinson). Mean fluorescence intensity of virus binding was calculated against that of cells incubated with the secondary antibody alone.
For analysis of virus binding to cells in the presence of soluble glycosaminoglycans (GAGs), virus was incubated with GAGs at 37°C for 1 h before binding to cells at 24°C for 45 min. To estimate the amount of HS on the cell surface, cells were incubated with anti-HS MAb F58-10E4 (binds HS cleaved by heparitinase I) or an IgM isotype control, followed by incubation with goat anti-mouse polyvalent Ig-FITC, and analyzed on a FACSCalibur.
Assays of infectivity.
For inhibition, virus was incubated with GAGs at 37°C for 1 h before adsorption to cells for 45 min at 24°C, which were then washed with PBS and incubated with appropriate medium. To examine the effect of enzymatic treatment of cells or the presence of anti-HS antibodies on infection, cells were incubated with either GAG lyases (0.02 U of heparinase or heparitinase or 1 U of chondroitinase ABC), 0.05 U of neuraminidase, or 1, 5, and 10 μg of HS antibody at 37°C for 1 h. Cells were washed with PBS, followed by adsorption of virus. Cell viability and percent cytopathic effect (CPE) were measured at 24 h postinfection as described before (9). Infections were carried out in 96-well plates in quadruplicate with uninfected or infected cells (with no treatment) as controls. Viability of cells was calculated as a percentage of uninfected cell controls, and percent protection was the difference in viability after infection with and without treatment.
CPE was also determined by crystal violet staining of cell monolayers at 24 h postinfection. Infected cell monolayers in 12-well plates were washed with PBS, fixed with absolute methanol, stained with 0.5% crystal violet in methanol for 10 min at 24°C, and photographed.
RESULTS
CHO and BHK-21 cells differ in GDVII virus binding and infection.
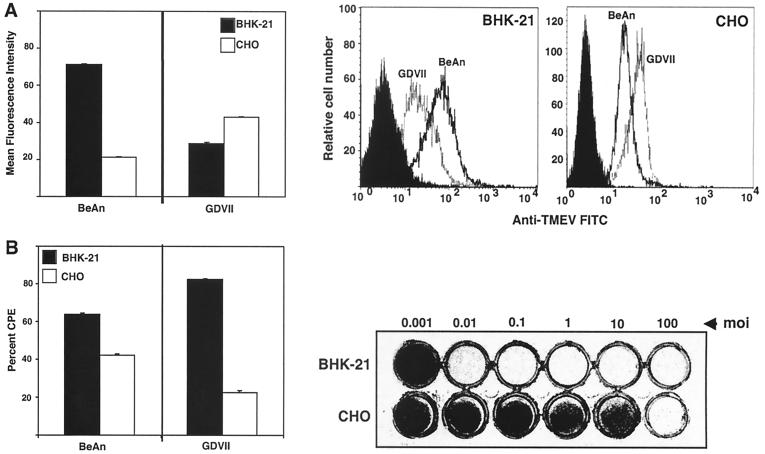
Observations of CPE development in GDVII virus-infected CHO cells compared to that in BHK-21 cells at a multiplicity of infection (MOI) of 10 revealed that a substantial portion of the CHO cell monolayer had survived at 24 h postinfection, while the BHK-21 cell monolayer showed complete CPE (not shown). GDVII virus binding and infection were therefore examined in both cell lines (MOI of 10) and contrasted with that of the low-neurovirulence BeAn virus.
BeAn virus binding and infection were comparable in both cell lines, whereas GDVII virus binding was twofold greater but infection was fourfold less in CHO cells than in BHK-21 cells (Fig. 1). The difference in GDVII virus infection of the two cell types was also clearly demonstrated when the MOI was varied 100,000-fold (Fig. 1B). The fact that GDVII virus binding increased at the same time that infection was markedly reduced suggests differences in the cell surface composition of the two cell lines and/or number of receptor molecules as well as a relative block in infection after the attachment step in CHO cells.
FIG. 1.
Comparison of GDVII and BeAn virus binding (A) and infection (B) in BHK-21 and CHO cells. (A) Cells (106) were incubated with the indicated virus (MOI of 10), anti-TMEV MAb 6F7, and goat anti-mouse Ig-FITC, each for 30 min at 4°C, and bound virus was detected with a FACSCalibur and plotted as mean fluorescence intensity (left). A representative flow cytometric analysis of virus binding to both cell lines is shown (right). (B) (Left) Cell lines were infected with GDVII and BeAn viruses at an MOI of 10, the extent of CPE was determined by the MTT assay, and percent CPE was calculated from the viability of uninfected cells at 24 h postinfection. (Right) Crystal violet-stained cell monolayers at 24 h postinfection with increasing MOIs of GDVII virus. Values represent means and standard deviations from three independent experiments.
GDVII virus requires galactose-containing molecules for binding and infection of mammalian cells (14); does not bind to O-linked oligosaccharides or glycolipids; and shows only limited binding to N-linked oligosaccharides (Shah and Lipton, submitted), suggesting a possible role for PG in GDVII virus attachment. Flow cytometry with anti-HS MAb F58-10E4 to examine the basis of the difference in GDVII virus binding to CHO and BHK-21 cells revealed half as much HS expression in CHO cells as in BHK-21 cells (mean fluorescence intensity, 23 versus 48), which does not correlate with either the binding or infection data and perhaps indicates a more complex process of binding and entry.
Soluble HS inhibits GDVII virus binding and infection.
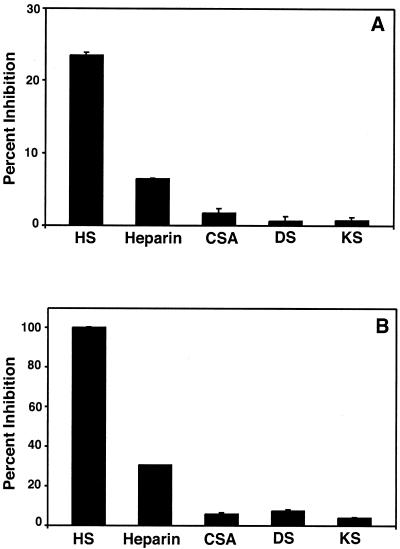
If GDVII infection is initiated through interaction with cell surface PG, one or more of the major GAGs found on membrane-associated PGs should act as a competitive inhibitor. To test this possibility, we used flow cytometry analysis and the MTT assay to examine the ability of soluble GAGs incubated with GDVII virus to block binding and infection, respectively. GDVII virus was incubated with HS, heparin, chondroitin sulfate A, keratan sulfate, and dermatan sulfate at a concentration of 250 μg/ml before binding and infection of CHO cells at an MOI of 10 and 100, respectively. The soluble GAGs chondroitin sulfate A, keratan sulfate, and dermatan sulfate showed no inhibitory effect on GDVII virus binding and infection (Fig. 2), even at a concentration of 1,000 μg/ml (not shown). In contrast, 250 μg of heparin per ml inhibited binding by 6% and infection by 30%, and 250 μg of HS per ml inhibited binding by 25% and infection by 100% (Fig. 2).
FIG. 2.
Competitive inhibition of GDVII virus binding (A) and infection (B) by GAGs. GDVII virus at an MOI of 100 was incubated with 250 μg of the indicated GAG per ml for 1 h at 37°C, and the mixture was adsorbed to CHO cells. Bound virus was analyzed by flow cytometry, and CPE was analyzed by the MTT assay. Inhibition of binding (A) and infection of cell monolayers (B) were calculated relative to binding and infection in the absence of GAGs. Values represent the means and standard deviations from three independent experiments. CSA, chondroitin sulfate A; DS, dermatan sulfate; KS, keratan sulfate.
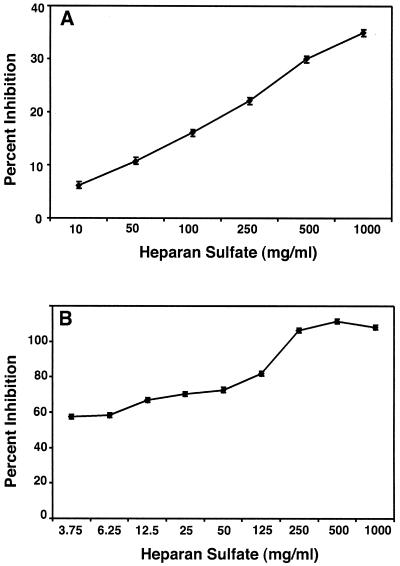
When GDVII virus was incubated with lower concentrations of HS, a dose-response effect was seen for binding and infection; while 1,000 μg of HS/ml inhibited GDVII virus binding by 40%, 3.75 μg of HS/ml provided 60% protection of monolayers (Fig. 3). The inhibition was not due to an effect on CHO cells, since incubation of the cells with increasing concentrations of HS followed by extensive washing and infection with GDVII virus at an MOI of 100 did not inhibit the infection (not shown). These data indicate that soluble HS has a more profound inhibitory effect on GDVII virus infection than on binding and that the effect of soluble HS is due to direct binding of HS by the virus.
FIG. 3.
Dose-dependent inhibition of GDVII virus binding (MOI of 10) (A) and infection (MOI of 100) (B) by soluble HS. GDVII virus was incubated with increasing concentrations of HS, and binding and infection of CHO cells were analyzed as described for Fig. 2.
GDVII virus requires cell surface HS for infection.
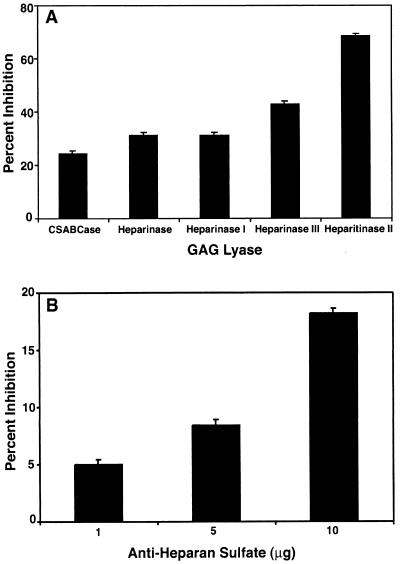
Since HS is known to bind to specific cell surface receptors, e.g., those of cytokines, growth factors, and chemokines (29), we tested the possibility that the observed effects of soluble HS represent competition for a common receptor in contrast to direct binding to virus. In light of our finding above that the greatest effect of soluble HS was on infection, we assessed GDVII virus infection of cell monolayers incubated with the following enzymes: heparinase, which cleaves the α-glucosaminide linkage to 2-O-sulfo-l-iduronic acid; heparinase I, which cleaves the 1,4-linked d-glucoronate or l-iduoronate residues in heparin; heparinase III (heparitinase I), which eliminates sulfate and cleaves α-N-acetyl-d-glucosaminidic linkages in HS; heparitinase II, which cleaves the α-N-acetyl-d-glucosaminidic linkages in HS and heparin; and chondroitinase ABC, which cleaves N-acetylhexosaminide linkages in all chondroitin sulfates, including dermatan sulfate. The two enzymes that act on HS, heparitinase II and heparinase III, showed the greatest inhibition of infection, 70 and 45%, respectively, whereas cells incubated with heparinase and heparinase I, enzymes specific for heparin, showed only slightly greater inhibition of infection than after chondroitinase ABC digestion (Fig. 4A). These data further support a role for HS in GDVII virus infection. Finally, infection at an MOI of 100 was blocked with 1 to 10 μg of anti-HS MAb F58-10E4/ml, resulting in partial protection from infection (Fig. 4B).
FIG. 4.
Inhibition of GDVII virus infection (MOI of 100) by treatment of CHO cells with GAG lyases (A) and anti-HS antibody (B). (A) CHO cells were treated with 1 U of chondroitinase ABC (CSABCase) or 0.02 U of heparinase, heparitinase II, heparinase I, or heparinase III for 1 h at 37°C prior to GDVII virus infection. CPE was determined by the MTT assay at 24 h postinfection and is expressed as percent inhibition. (B) CHO cells were incubated with increasing concentrations of anti-HS MAb F58-10E4 for 1 h at 37°C prior to virus infection, and percent inhibition was determined. Values indicate the means and standard deviations from four independent experiments.
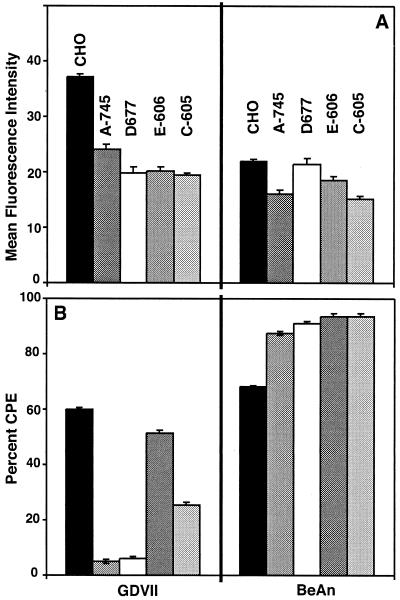
PG-deficient CHO mutants are resistant to infection by GDVII virus.
CHO cell mutants deficient in GAG synthesis were used to further define the role of HS and its component molecules in GDVII virus binding and infection. The following cell lines have specific deficiencies in GAG synthesis: pgs A-745 lacks xylosyl transferase, an enzyme required for initiation of the synthesis of GAGs, and produces no detectable levels of GAGs (2); pgs D-677 lacks galactosyl transferase I and is deficient in the ability to add HS disaccharide chains to the tetrasaccharide linker (23); pgs E-606 is partially defective in N-sulfotransferase activity; and pgs C-605 is completely defective in sulfate transporter activity. pgs E-606 synthesizes undersulfated HS. While only a modest reduction in GDVII virus binding was observed in pgs A-745 and pgs D-677 cells (Fig. 5A), infection in these mutants was markedly reduced (Fig. 5B). Only a small percentage of cells (<5%) in pgs A-745 and pgs D-677 monolayers stained positive for virus antigen after infection (not shown). Infection was also reduced in pgs C-605 and to a much lesser extent in pgs E-606 cells, indicating that sulfation but not N-sulfation is an important factor in GDVII virus infection. Binding or infection of BeAn virus was not affected in any of the cells deficient in GAG synthesis, confirming that HS is used only by the high-neurovirulence TMEV.
FIG. 5.
PG-deficient CHO cells are resistant to GDVII virus infection. Selected PG-deficient mutant CHO cells were examined for GDVII and BeAn virus binding (MOI of 10) and infection (MOI of 100). (A) Virus was bound to CHO and PG-deficient pgs A-745 and pgs D-677 cells and analyzed by flow cytometry, and data were plotted as mean fluorescence intensity. (B) Percent CPE of infected CHO, PG-deficient pgs A-745 and pgs D-677, and undersulfated pgs E-606 and pgs C-605 cells was determined by the MTT assay and calculated based on the viability of uninfected cells at 24 h postinfection. Values indicate the means and standard deviations from four independent experiments.
Cell culture adaptation of GDVII virus does not underlie HS use by the virus.
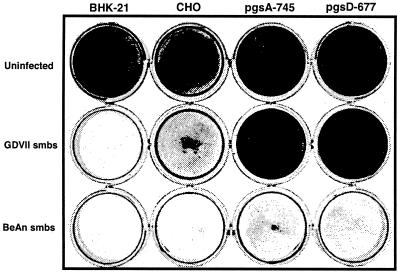
The use of HS as a coreceptor for FMDV and Sindbis virus was shown to be the result of cell culture adaptation of the viruses; field isolates of FMDV and mouse brain stocks of Sindbis virus did not use HS (7, 31). To examine the possibility that HS use by GDVII virus rests in passage of the virus in BHK-21 cells, we infected CHO mutants pgs A-745 and pgs D-677 with suckling mouse brain stocks of GDVII and BeAn viruses and stained the monolayers with crystal violet at 24 h postinfection. While the BeAn suckling mouse brain stock induced extensive CPE in both mutant cell lines, no CPE was observed with the GDVII suckling mouse brain stock (Fig. 6). A brain-derived stock of another high-neurovirulence TMEV, FA virus, did not infect these two CHO mutant cell lines (not shown). Infection of parental CHO cells with the GDVII suckling mouse brain stock incubated with 1 mg of soluble HS/ml also showed complete protection of monolayers (not shown). These results indicate that high-neurovirulence TMEV naturally uses HS for infection and that the observed effects are not an artifact of cell culture passage and adaptation of the virus.
FIG. 6.
Use of HS by GDVII virus to mediate infection does not rest on cell culture adaptation. CPE in PG-deficient pgs A-745 and pgs D-677 CHO cells was determined by crystal violet staining of cell monolayers after infection with suckling mouse brain stocks (smbs) of the BeAn and GDVII viruses.
Neuraminidase treatment of CHO cell mutants restores GDVII infection.
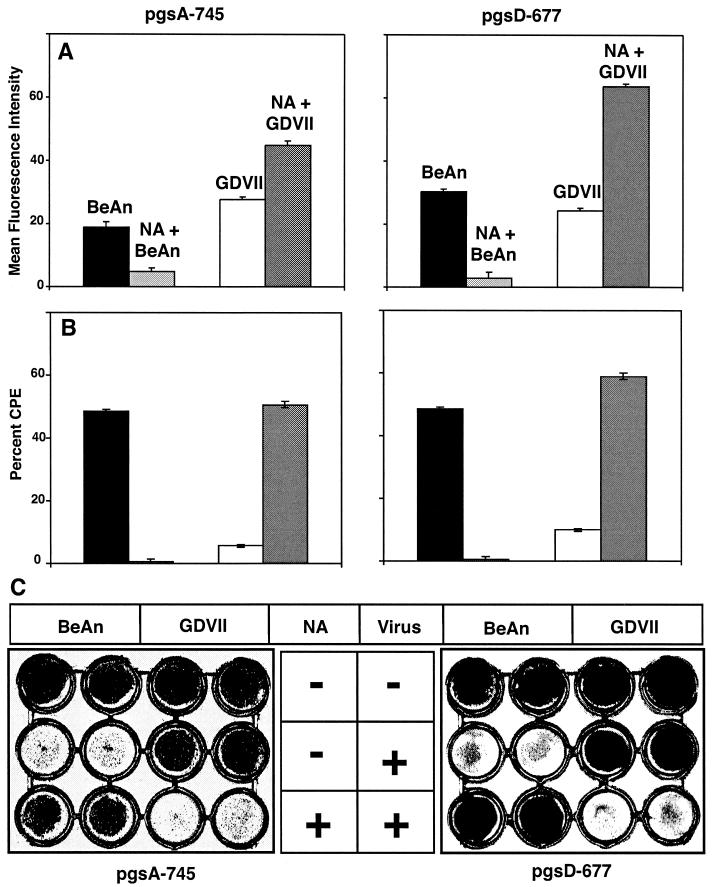
GDVII virus binding to and infection of Lec-2 cells, a CHO cell mutant lacking the CMP sialic acid transporter and deficient in sialic acid, was significantly higher than binding to and infection of parental cells (not shown), suggesting that the removal of sialic acid from the cell surface allows direct access of GDVII virus to the putative protein entry receptor. In fact, GDVII virus binding to pgs A-745 and D-677 cell monolayers treated with neuraminidase at a concentration that completely inhibited BeAn virus binding was 0.8- to 2-fold higher in comparison to binding levels observed with parental cells (Fig. 7A). Furthermore, removal of cell surface sialic acid rendered these mutant cells permissive to GDVII virus infection (Fig. 7B and C); neuraminidase treatment of parental CHO cells also dramatically increased GDVII virus binding and infection, comparable to that in Lec-2 cells (not shown), in contrast to the results shown in Fig. 1. Together, the data suggest that GDVII virus binds not only to HS but also to the putative protein receptor, direct access to which is sterically hindered by sialic acid.
FIG. 7.
GDVII virus uses another receptor, presumably a protein entry receptor, for infection after removal of sialic acid. Neuraminidase treatment of PG-deficient pgs A-745 and pgs D-677 CHO cells sufficient to inhibit BeAn virus binding and infection resulted in increased binding and restored infection by GDVII virus at an MOI of 10. (A) Virus was bound to PG-deficient pgs A-745 and pgs D-677 cells and analyzed by FACS, and data were plotted as the mean fluorescence intensity. Values indicate the means and standard deviations from three independent experiments. (B) Percent CPE of infected PG-deficient pgs A-745 and pgs D-677 was determined by the MTT assay and calculated based on the viability of uninfected cells at 24 h postinfection. Values indicate the means and standard deviations from four independent experiments. (C) Extent of CPE in neuraminidase-treated and untreated PG-deficient cell monolayers after infection was assessed by crystal violet staining.
DISCUSSION
In this study, we demonstrate for the first time that the high-neurovirulence GDVII virus uses the GAG HS as an attachment factor that is both highly specific and necessary for efficient infection of parental CHO cells. Analyses based on soluble HS inhibition of attachment and infection, enzymatic removal of HS with heparitinase II and heparinase III, and blocking with anti-HS antibodies established that GDVII virus uses HS for cell attachment to mediate infection. These experiments also excluded the involvement of other GAGs, e.g., CS, keratan sulfate, and dermatan sulfate, in GDVII virus infection. Data from defined PG-deficient CHO mutant cells (pgs A-745 and pgs D-677) further support the role of HS in GDVII infection, and reduced infection in undersulfated pgs E-606 and pgs C-605 cells indicated that the extent of sulfation, but not N sulfation is critical for infection. We also show that HS use by GDVII virus does not rest on cell culture adaptation of the virus, since a brain-derived stock passaged only in suckling mouse brain also required HS for infection. Since we also found that FA virus requires HS to infect CHO cells, we believe that all high-neurovirulence strains probably use HS as an attachment factor.
An increasing number of viruses that use distinct cell surface molecules for attachment and cell entry or internalization have been identified. These molecules represent both attachment factors and cell entry receptors that provide complementary functions and act sequentially to mediate viral entry. Attachment factors are thought to concentrate virus particles on the cell surface, fostering cell entry. Our results demonstrating reduced GDVII virus binding and lack of infection in the absence of HS indicate that HS is involved in facilitating entry of GDVII virus into cells.
Viruses that use HS for attachment and entry may interact with HS through heparin-binding domains (HBD). It has been proposed that there may be at least two major HBD consensus motifs in proteins, composed of a core of basic amino acids flanked by a second positively charged residue and separated by hydrophobic amino acids, e.g., XBBXBX or XBBBXXBX, where B is a basic amino acid and X is a hydrophobic residue (8). Heparin-binding proteins often contain more than one of these sequences.
Analysis of the 12 TMEV gene products revealed a putative HBD, YKKMKV, in VP1, a structural protein, and another in the 3D polymerase, a nonstructural protein. Only the VP1 domain in the viral coat would be available to bind to HS on the cell surface. The amino acids YKKMKV are conserved in all TMEVs and are located in the VP1 C terminus, the highest elevation on the virion surface, which forms a hook over the VP3 knob (25). The GDVII HBD is likely to be exposed, since a trypsin-sensitive site has been identified in the VP1 C terminus (28) in close proximity to the HBD. Furthermore, GDVII but not the low-neurovirulence DA virus reportedly loses infectivity with trypsin treatment (28), suggesting that the HBD could be used for infection. The fact that the HBD sequence is conserved in all of the 10 sequenced TMEV capsids but is not used by the low-neurovirulence strains suggests that the recognition of HS is conformation dependent, as is the recognition of sialic acid by low-neurovirulence strains (34). However, the role of the HBD in GDVII attachment awaits confirmation by site-directed mutagenesis of selected HBD residues.
Picornaviruses are believed to require a protein entry receptor for internalization. Four other nonenveloped viruses that use HS as an attachment factor are internalized by known protein entry receptors, including adenovirus 2 (coxsackievirus-adenovirus receptor) (4), adeno-associated virus 2 (α5 integrin) (32), FMDV (αvβ3, avβ6, and avβ1 integrins) (5, 17, 27), and human papillomavirus 16 (α6 integrin) (10). On the other hand, as discussed by Fry et al. (12) for FMDV, some HS PG alone might be able to internalize viruses undergoing endosomal cycling (15). Flexibility in receptor usage has been demonstrated for multiply passaged FMDV in cell culture under the selective pressure of neutralizing antibodies, with mutations in the RGD motif shifting preferential receptor use from integrin to HS (3, 26). However, this situation is artificial and might not reflect receptor use by nonenveloped viruses in nature.
We postulate that GDVII virus binding has two components, HS binding and direct binding to a protein entry receptor, based on retained binding and lack of infection in PG-deficient cells. In addition, infectivity in HS-deficient CHO cells is restored by removal of cell surface sialic acid with neuraminidase, suggesting that sialic acid sterically hinders full access to the protein entry receptor required for infection. Consistent with this notion, BeAn virus binds sialic acid and can competitively inhibit GDVII virus binding, whereas GDVII virus does not inhibit BeAn virus binding (11). Based on our data, we propose a model wherein high- and low-neurovirulence TMEVs bind different attachment factors, e.g., HS and sialic acid, but use a common protein entry receptor. Figure 8 depicts the proposed model of infection for the high-neurovirulence TMEV.
FIG. 8.
Postulated model for two components of GDVII virus binding and internalization by a protein entry receptor. BeAn virus has been previously shown to use sialic acid as an attachment factor and is not depicted here. (Left) GDVII virus binds to HS, which then mediates internalization via a protein entry receptor to enable infection of parental CHO, BHK-21, and other mammalian cells. The data suggest that GDVII virus also binds directly to a protein entry receptor, possibly at a site independent of the internalization site; however, in this case internalization is sterically hindered by sialic acid moieties located on the protein entry receptor. (Right, top panel) GDVII virus is unable to infect PG-deficient cells lacking HS. Although GDVII virus binds directly to the protein entry receptor, it is unable to be internalized due to steric hindrance by sialic acid. (Middle panel) Removal of sialic acid from PG-deficient cells with neuraminidase results in access to the protein entry receptor and thereby infection. (Bottom panel) Binding to and infection of Lec-2 cells which have HS but lack cell surface sialic acid are even more efficient than in the other cells.
Acknowledgments
We thank Patricia Kallio for technical assistance and Mary Lou Jelachich and Mark E. Peeples for helpful discussions.
This work was supported by NIH grants NS 21913 and NS 23349.
REFERENCES
- 1.Adami, C., A. E. Pritchard, T. Knauf, M. Luo, and H. L. Lipton. 1997. Mapping a determinant for central nervous system persistence in the capsid of Theiler's murine encephalomyelitis virus (TMEV) with recombinant viruses. J. Virol. 71:1662-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, X., G. Wei, A. Sinha, and J. D. Esko. 1999. Chinese hamster ovary cell mutants defective in glycosaminoglycan assembly and glucuronosyltransferase I. J. Biol. Chem. 19:13017-13024. [DOI] [PubMed] [Google Scholar]
- 3.Baranowski, E., C. M. Ruiz-Jarabo, N. Sevilla, D. Andreu, E. Beck, and E. Domingo. 2000. Cell recognition by foot-and-mouth disease virus that lacks the RGD integrin-binding motif: flexibility in aphthovirus receptor usage. J. Virol. 74:1641-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Berinstein, A., M. Roivainen, T. Hovi, P. V. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin αvβ3) inhibit binding and infection of foot-and-mouth disease to cultured cells. J. Virol. 69:2664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 7.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sinbis virus to cell surface heparan sulfate. J. Virol. 72:7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardin, A. D., and H. J. R. Weintraub. 1989. Molecular modeling of protein-glycosoaminoglycan interaction. Arteriosclerosis 9:21-32. [DOI] [PubMed] [Google Scholar]
- 9.Denizot, F., and R. Lang. 1986. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89:271-277. [DOI] [PubMed] [Google Scholar]
- 10.Evander, M., I. H. Frazer, E. Payne, Y. M. Qi, K. Hengst, and N. A. J. McMillan. 1997. Identification of the α6 integrin as a candidate receptor for papillomaviruses. J. Virol. 71:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fotiadis, C., D. R. Kilpatrick, and H. L. Lipton. 1991. Comparison of the binding characteristics to BHK-21 cells of viruses representing the two Theiler's virus neurovirulence groups. Virology 182:365-370. [DOI] [PubMed] [Google Scholar]
- 12.Fry, E. E., S. M. Lea, T. Jackson, J. W. Newman, F. M. Ellard, W. E. Blakemore, R. Abu Ghazaleh, A. Samuel, A. M. Q. King, and D. I. Stuart. 1999. The structure and function of a foot-and-mouth disease-virus oligosaccharide receptor complex. EMBO J. 18:543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodfellow, I. G., A. B. Sioofy, R. M. Powell, and D. J. Evans. 2001. Echoviruses bind heparan sulfate at the cell surface. J. Virol. 75:4918-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hertzler, S., P. Kallio, and H. L. Lipton. 2001. UDP-galactose transporter is required for Theiler's virus entry into mammalian cells. Virology 286:336-344. [DOI] [PubMed] [Google Scholar]
- 15.Iozzo, R. V. 1987. Turnover of heparan sulfate proteoglycan in human carcinoma cells: a quantitative biochemical and autoradiographic study. J. Biol. Chem. 262:1888-1900. [PubMed] [Google Scholar]
- 16.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. I. Newman, and A. M. Q. King. 1996. Efficient infection of cells in culture by type-O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson, T., A. P. Mould, D. Sheppard, and A. M. Q. King. 2001. Integrin αvβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarousse, N., R. A. Grant, J. M. Hogle, L. Zhang, A. Senkowski, R. P. Roos, T. Michiels, M. Brahic, and A. McAllister. 1994. A single amino acid change determines persistence of a chimeric Theiler's virus. J. Virol. 68:3364-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jnaoui, K., and T. Michiels. 1999. Analysis of cellular mutants resistant to Theiler's virus infection: differential infection of L929 cells by persistent and neurovirulent strains. J. Virol. 73:7248-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilpatrick, D. R., and H. L. Lipton. 1991. Predominant binding of Theiler's viruses to a 34-kilodalton receptor protein on susceptible cell lines. J. Virol. 65:5244-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehrich, J. R., B. G. W. Arnason, and F. H. Hochberg. 1976. Demyelinating myelopathy in mice induced by the DA virus. J. Neurol. Sci. 29:149-160. [DOI] [PubMed] [Google Scholar]
- 22.Libbey, J. E., I. J. McCright, I. Tsunoda, Y. Wada, and R. S. Fujinami. 2001. Peripheral nerve protein, PO, as a potential receptor for Theiler's murine encephalomyelitis virus. J. Neurovirol. 7:97-104. [DOI] [PubMed] [Google Scholar]
- 23.Lidholt, K., J. L. Weinke, C. S. Kiser, F. N. Lugemwa, K. J. Bame, S. Cheifetz, J. Massague, U. Lindahl, and J. D. Esko. 1992. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. USA 89:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton, H. L. 1975. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect. Immun. 11:1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo, M., K. S. Toth, L. Zhou, A. Pritchard, and H. L. Lipton. 1995. The structure of a highly virulent Theiler's murine encephalomyelitis virus (GDVII) and implications for determinants of viral persistence. Virology 220:246-250. [DOI] [PubMed] [Google Scholar]
- 26.Martinez, M. A., N. Verdaguer, M. G. Mateu, and E. Domingo. 1997. Evolution subverting essentiality: dispensability of the cell attachment Arg-Gly-Asp motif in multiply passaged foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 94:6798-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, L. C., W. Blakemore, D. Sheppard, A. Atakilit, A. M. Q. King, and T. Jackson. 2001. Role of the cytoplasmic domain of the β-subunit of integrin αvβ6 in infection by foot-and-mouth disease virus. J. Virol. 75:4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitayaphan, S., M. M. Toth, and R. P. Roos. 1985. Localization of a neutralization site of Theiler's murine encephalomyelitis viruses. J. Virol. 56:887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, P. W., O. Reizes, and M. Bernfield. 2000. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J. Biol. Chem. 275:29923-29926. [DOI] [PubMed] [Google Scholar]
- 30.Pevear, D. C., M. Calenoff, E. Rozhon, and H. L. Lipton. 1987. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J. Virol. 61:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sa-Carvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 71:5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Summerford, C., J. S. Bartlett, and R. J. Samulski. 1999. AlphaVbeta5 integrin: a coreceptor for adeno-associated virus type 2 infection. Nat. Med. 5:78-82. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, L., X. Lin, T. J. Green, H. L. Lipton, and M. Luo. 1997. Role of sialyloligosaccharide binding in Theiler's virus persistence. J. Virol. 71:9701-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou, L., Y. Luo, Y. Wu, J. Tsao, and M. Luo. 2000. Sialylation of the host receptor may modulate entry of demyelinating persistent Theiler's virus. J. Virol. 74:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]