Abstract
Protective immunization against rotavirus (RV) can be achieved with heterologous RV, i.e., virus isolated from another species, and with heterologous inner core VP2 and VP6 proteins assembled as virus-like particles (VLP). Although the antigenically conserved VP6 protein does not induce in vitro-neutralizing antibodies, it may, however, elicit immunoglobulins (Ig) involved in heterologous protection, as some IgA against VP6 prevent RV infection in a backpack mouse model. The protective role of Ig directed to the RV inner core proteins VP2 and VP6 was investigated in J-chain-deficient mice (J chain−/−), which have a defect in the polymeric Ig receptor (pIgR)-mediated transcytosis of IgA and IgM. J chain−/− mice and wild-type (WT) mice were intranasally vaccinated with bovine RV-derived VLP2/6 and then challenged with highly infectious murine ECw RV. Whereas WT mice were totally protected, immunized J chain−/− mice shed RV for several days. In addition, naïve J chain−/− mice exhibited a 2-day delay in clearing RV compared with WT mice. The immunized J chain−/− mice displayed unaltered VLP2/6-specific B-cell numbers in spleen and in mesenteric nodes and similar levels of serum anti-VLP2/6 Ig, confirming that the adaptive B-cell response is preserved in J chain−/− mice. These results indicate that J-chain-mediated transcytosis of Ig participates in the clearance of RV and that epithelial pIgR-mediated transport of Ig is involved in the heterologous protection induced by VLP2/6.
Rotaviruses (RV) are ubiquitous pathogens that infect mature enterocytes of the intestinal villi, subsequently leading to gastrointestinal disease and severe diarrhea in young animals and children (10). RV infections are responsible for over 600,000 infant deaths world wide, mainly in developing countries (20). In industrialized countries, the majority of the children get infected before the age of three, with a great proportion developing symptomatic infections. As the social and economical burden due to RV infections is important, an efficient vaccine is urgently needed (3). However, the recently licensed vaccine RotaShield, a vaccine based on a live attenuated simian RV, was withdrawn from the market due to an increased incidence of intussusceptions during the first 2 weeks postimmunization (5). Further efforts in the vaccine field are needed in order to develop safe and efficient protection against RV.
Several successful vaccination strategies against RV involving laboratory scale experiments and clinical trials have been used. Vaccination with heterologous RV (virus isolated from a different species) (42), with live heterotypic RV (virus with a distinct serotype) (12), or with heterologous virus-like particles (VLP) (30) have conferred either total or partial protection. These findings suggest that common antigenic structures in different viral isolates generate a protective immunity. A Jennerian approach using rhesus or bovine RV against a murine RV challenge (ECw) indicated that protection was correlated with fecal immunoglobulin A (IgA) levels to the antigenically conserved group-specific VP6 protein, and not with serum IgG responses (12). Since antibodies to the inner capsid protein VP6 are not neutralizing (4, 34), the mechanism by which they would exert an antiviral effect is unclear. Burns et al. reported that two murine hybridomas producing an IgA directed to the VP6 protein and implanted in a backpack model completely protected adult mice from a murine RV challenge (4). The authors suggested that the anti-VP6 IgA probably blocks crucial steps of the viral cycle inside the infected enterocyte during the transcytosis of dimeric IgA via the polymeric Ig receptor (pIgR). However, Ruggeri et al. reported findings that are discordant with those of Burns et al. (34). They showed that backpack-implanted hybridomas secreting IgA against the external capsid VP4 protein, but not against the internal VP6 protein, were protective against RV-induced diarrhea in a neonatal mouse model of infection (34). The discrepancy of those observations and those of Burns et al. may be explained by biological differences between the adult and the neonatal mouse models, or more likely by the VP6 epitopes recognized by the different IgA-producing hybridomas. However, these works did not address the question of whether the mucosal anti-VP6 antibodies elicited by vaccination play a determining role in protection and whether Ig transcytosis via the pIgR is actually involved in protection.
Mucosal pIgA and pIgM transcytose through epithelial cells after binding to pIgR, which is expressed at the basolateral cellular pole of crypt epithelial cells (2). The pIg-pIgR interaction is strictly dependent on the Ig disulfide-mediated covalent link with the 15-kDa polypeptide J chain (41). The pIg-pIgR complex is then transported via a vesicular pathway inside the epithelial cells. At the luminal cell surface, the pIgR is proteolytically cleaved, with a portion known as secretory component remaining associated with the pIgs in secretions (40). J-chain-deficient (J chain−/−) mice that are impaired in mucosal IgA and IgM transport have been generated. They exhibit serum IgA accumulation and lack pIgA in their intestinal secretions (23) and in feces (17, 19).
In order to demonstrate that pIgR-mediated transcytosis of antibodies directed to the inner capsid (VP2 and VP6) proteins prevents RV infection, we assessed whether the protection conferred by heterologous VLP2/6 is altered in J chain−/− mice. We found that whereas adult wild-type (WT) mice nasally vaccinated with VLP2/6 were protected against infection by a high-dose murine ECw virus challenge, immune J chain−/− mice got readily infected and shed virus for several days. These results indicate that nasal vaccination with heterologous VLP2/6 can protect normal mice against infection with a high-viral-dose challenge, and that transport of nonneutralizing anti-VP2 or -VP6 mucosal Ig via the J chain/pIgR-dependent pathway is mandatory to achieve protection.
MATERIALS AND METHODS
Viruses and cells.
A stock of WT murine RV which cause epizootic diarrhea of infant mice (EDIM) (Cambridge ECw, P[16], G3) was prepared as intestinal homogenates from 4-day-old BALB/c neonates. The titer of ECw in 6-week-old adult mice was determined as previously described (11) and was estimated to be 108 shedding dose 50 (SD50) per ml.
Recombinant baculoviruses used in this study were BacRF2A, encoding for bovine strain RF VP2 (22), Bac RF6, encoding for bovine strain RF VP6 (39), and DsRedJA16, which expresses the DsRed fluorescent protein fused to VP2 (6).
Spodoptera frugiperda 9 (Sf9) insect cells were maintained in Hink's medium (Gibco-BRL, Grand Island, N.Y.) containing 10% fetal calf serum (FCS).
Animals.
The J chain−/− mice were generated as previously described (17) and bred for 10 to 12 generations into the BALB/c background (Taconic Laboratories). Age-matched WT BALB/c mice (Taconic Laboratories) were used as controls. Mice were systematically confirmed to be RV antibody free prior to vaccination by enzyme-linked immunosorbent assay (ELISA) on serum samples. They were housed in microisolator cages within a barrier facility during the vaccination period. The infectious challenge was performed in an A3 animal facility (Unité d'Expérimentation Animale Rongeurs, Jouy-en-Josas, France).
VLP.
VLP2/6 were prepared in Sf9 cells coinfected with recombinant baculoviruses (five particle-forming units per cell) expressing the bovine RF VP2 and VP6 genes and purified as previously described (22). Briefly, infected cells were collected 5 days postinfection and then extracted with Freon 113, and the aqueous phase containing VLP was subjected to an isopycnic cesium chloride gradient in a 20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer with 10 μM CaCl2 (pH 6.6). Purified VLP2/6 were then desalted with RPMI medium-equilibrated Sephadex G-25 columns. The desalted VLP2/6 were then subjected to a Bradford assay for protein quantitation using bovine serum albumin as a standard.
DsRed fused to VP2 was coexpressed with VP6 in Sf9 cells to produce particles designated DsRed-VLP2/6 (6).
Nasal immunizations and sample collection.
Six-week-old mice were anesthetized by intraperitoneal administration of a mixture of ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg). The immunogen was given in a 20-μl volume by gradual inoculation in the nostrils of the mice on days 0, 21, and 42. Groups of six to eight mice received 9 μg of VLP2/6 mixed with 5 μg of cholera toxin (CT) (Sigma, St. Louis, Mo.) or phosphate-buffered saline (PBS) mixed with 5 μg of CT as a control. Blood was collected 21 days after the last immunization under general anesthesia. Stools were harvested from individual mice placed in individual cages on absorbent paper on the day of the challenge and on days 2, 3, 4, 7, and 8 after the challenge. Both the serum and the feces specimens were kept at −20°C before analysis.
Virulent murine RV challenge.
WT and J chain−/− mice (eight mice per group) were challenged at the same time, 21 days after the last immunization. They were orally gavaged with 104 SD50 of ECw murine virus after receiving 100 μl of 1.33% sodium bicarbonate to neutralize stomach acid.
Detection of RV antigens.
Shedding of RV in feces samples was examined. Feces were suspended as a 10% suspension in 10 mM Tris (pH 7.4)-140 mM NaCl-10 mM CaCl2-25 mM EDTA-0.05% Tween 20-1% protease inhibitor cocktail (Complete Mini; Roche, Switzerland). After 45 min of incubation on ice, the feces suspension was frozen at −80°C, thawed, and spun down at 13,000 × g, and the crude supernatant was tested for the presence of RV antigens. Two monoclonal mouse anti-RV VP6 antibodies (R50 and 138) were used to coat an ELISA 96-well plate (Probind 3915; Becton Dickinson, San Jose, Calif.) at a 2-μg/ml dilution in carbonate buffer (pH 8.3) overnight at 4°C. The plates were washed twice in PBS containing 0.05% Tween 20 (PBS-T) and saturated in PBS plus 5% FCS (saturation buffer) for 2 h at 37°C. Undiluted feces supernatant was added to plates and incubated for 2 h at 20°C. After six washes in PBS-T, a 1:1,000 dilution of rabbit anti-RV serum diluted in saturation buffer was applied onto the well for 1 h at 20°C, washed, and followed by a peroxidase-conjugated goat anti-rabbit serum (Biosys S.A., Compiègne, France). After six washes, the Sigma Fast o-phenylenediamine dihydrochloride (OPD) (Sigma Chemical Co.) was used for color development, which was stopped by HCl. The plates were read at 492 nm by a microplate reader (Dynex, Chantilly, Va.). Stools from noninfected mice were included as control samples and gave a mean optical density at 492 nm (OD492) value of 0.08. Samples with OD492 above 0.2 were considered positive for RV shedding. The total fecal virus shedding for each mouse was calculated by integrating the area under the curve defined by the OD492 of each feces sample (y axis) over the 8-day period of feces collection (x axis) with the Microcal Origin 5.0 software. The day of viral clearance was deduced from a mathematical formula derived from the viral shedding curve and corresponded to the time point at which the OD492 value reached 0.2 (11).
Detection of RV-specific fecal Ig.
ELISA wells were coated overnight with 200 ng of VLP2/6 in carbonate buffer (pH 8.3) at 4°C. They were washed with PBS-T and saturated for 2 h at 37°C in saturation buffer. Feces dilutions in saturation buffer were incubated for 2 h at 20°C. Peroxidase-conjugated anti-mouse IgA (Caltag), IgG (Jackson Laboratories), or IgM (Jackson Laboratories) was added to the plate for 1 h and washed with PBS-T, and the OPD reagent was used for revelation. Antigen-specific IgA, IgM, or IgG titers in fecal samples were expressed as the reciprocal of the highest feces dilution giving an OD492 reading above 0.2. The mean OD492 value obtained with the lower fecal dilution (1:5) from unvaccinated control mice was 0.1.
Detection of RV-specific serum Ig.
ELISA wells were coated overnight with 200 ng VLP2 and VLP6 in carbonate buffer pH 8.3 at 4°C. They were washed with PBS-T and saturated for 2 h at 37°C in saturation buffer. Serum dilutions in saturation buffer were incubated for 2 h at 20°C. A peroxidase conjugated anti mouse heavy and light chain antibody (Biosys, Compiègne, France) or peroxidase conjugated anti mouse IgA, G or M were added to the plate for one hour, washed with PBS-T and the OPD reagent was used for revelation. Antigen-specific Ig titers were expressed as the reciprocal of the highest serum dilution giving a OD492 reading above 0.15. The mean OD492 value obtained with the lower serum dilution (1:100) from unvaccinated control mice was 0.075.
Isolation of splenic and mesenteric lymph node cells.
Spleen and mesenteric nodes were aseptically removed from three mice per group. Single-cell suspensions were prepared by mechanical dissociation and filtered on 70-μm-pore-size nylon meshes and counted. Two million cells were stained with a fluorescein isothiocyanate-conjugated anti B220 antibody (Becton Dickinson) that specifically labels B cells in mice and with 3 μg of DsRed-VLP2/6 per ml. After an incubation in PBS containing 4% FCS for 30 min at 4°C, the cells were washed twice and analyzed on a FACScan with the CELLQuest software (Becton Dickinson). DsRed-VLP are excited by a 488-nm argon laser, and the emitted fluorescence is detected in the FL2 channel. A control of background fluorescence without DsRed-VLP2/6 was included and attested for the specificity of the labeling. A total of 2 × 105 viable cells were analyzed per sample.
Statistical analyses.
A paired two-tailed Student t test was done to estimate statistically significant differences between the experimental values obtained from groups of mice, such as total viral shedding and antibody titers.
RESULTS
Impaired secretion of RV-specific IgA in J chain−/− mice intranasally vaccinated with bovine RF VLP2/6.
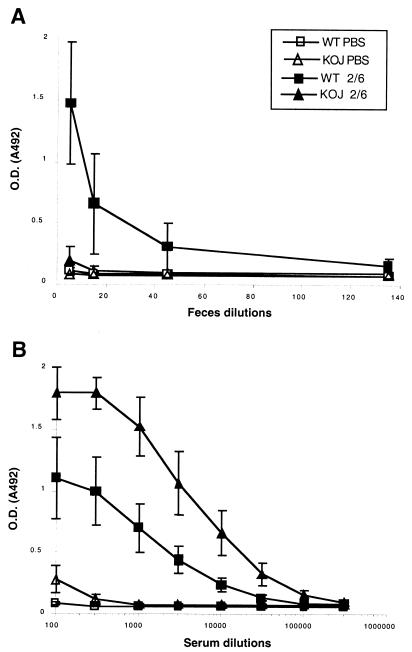
J chain−/− mice exhibit altered intestinal and biliary IgA transport leading to an increase in serum IgA and a reduction of IgA in intestinal lavage fluids (23) or in feces samples (17). These results were obtained with J chain−/− mice in a 129/SvJ background. As most previously published data regarding mucosal vaccination against RV have been obtained with mice in a BALB/c background, we used J chain−/− mice back-crossed with BALB/c mice. We then evaluated whether our BALB/c J chain−/− mice presented the expected alterations in RV-specific IgA in serum and in feces after intranasal vaccination with bovine strain RF-derived VLP2/6 plus CT. Twenty-one days after the last immunization, WT mice showed RV-specific IgA in their feces, with a mean IgA titer value of 83 ± 53 (standard deviation) and a mean OD492 value of 1.47 ± 0.5 at the 1:5 stool dilution (Fig. 1A; see also Table 2). In contrast, J chain−/− mice showed little to undetectable specific IgA under our experimental conditions, although three of six J chain−/− mice presented a slight but detectable level of anti VLP2/6 fecal IgA at the 1:5 stool dilution (OD492, 0.3, 0.26, and 0.3). On the other hand, anti-VLP2/6 serum IgA titers were significantly higher in the vaccinated J chain−/− mice than in the WT mice (114,000 ± 24,000 versus 21,000 ± 7,000, respectively; P < 0.005 [Fig. 1B; Table 2]). This observation indicates that after nasal vaccination with VLP2/6, a reduced level of fecal RV-specific IgA and an accumulation of RV-specific IgA are detected in BALB/c J chain−/− mice, a finding in accordance with the lack of efficient intestinal and biliary Ig transport in these mice.
FIG. 1.
Detection of IgA in feces (A) and serum (B) samples from vaccinated WT and J chain−/− (KOJ) mice. VLP2/6 (9 μg) and CT (5 μg) were intranasally administered to six WT (▪) and J chain−/− (▴) mice on days 0, 21, and 42. Mock-treated WT(□) and J chain−/− (▵) groups of mice were intranasally inoculated with 5 μg of CT in PBS. Feces and serum specimens were collected on day 63. Feces (A) and serum (B) samples were tested by ELISA for specific IgA against VLP2/6; the reverse dilutions are reported on the x axis, and the corresponding OD492 mean values (± standard errors of the means) are shown on the y axis. The cutoff OD492 for positive value is 0.2 (A) and 0.15 (B).
TABLE 2.
Serum and fecal anti-RV Ig in WT and J chain−/− micea
| Mouse genotype | Serum anti-RV Ig titers
|
Fecal anti-RV Ig titers
|
|||||
|---|---|---|---|---|---|---|---|
| All Ig (106) | IgM (103) | IgG (106) | IgA (105) | IgM | IgG | IgA | |
| WT | 1.6 ± 0.2 | 2.9 ± 0.08 | 1.3 ± 0.2 | 0.21 ± 0.07 | NDb | 19 ± 10 | 83 ± 53 |
| J chain−/− | 1.45 ± 0.12 | 3.6 ± 0.3 | 1.2 ± 0.08 | 1.14 ± 0.24 | NDb | 40 ± 20 | NDc |
Groups of six mice were analyzed.
ND, not detectable.
Detectable at the 1:5 dilution in three of six samples.
Nasal vaccination with heterologous bovine RF VLP2/6 does not protect J chain−/− mice against infection with a murine RV ECw challenge.
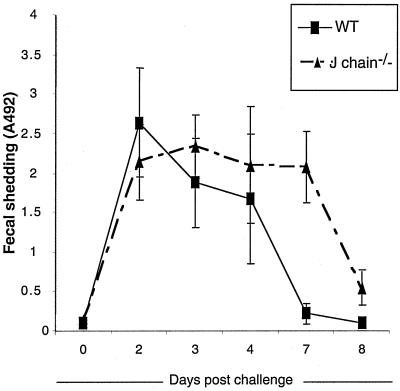
After having confirmed that transport of anti-RV-specific IgA is impaired in the J chain−/− mice, we tested whether protection against an oral murine RV challenge is affected by the constitutive lack of J chain. Groups of eight WT and J chain−/− mice were either nasally inoculated with PBS plus CT or with RF VLP2/6 plus CT. They then were infected at the same time with a high ECw challenge dose (104 SD50), and the presence of RV antigen in fecal samples was detected by ELISA. Both the mock-vaccinated WT and J chain−/− mice were all infected following challenge, with a peak level of viral shedding occurring between 2 to 3 days postinfection (Fig. 2). The OD492 values corresponding to the maximal viral shedding indicated the same order of magnitude in the two types of mice. However, the overall amounts of viral shedding over an 8-day period, calculated from the integrated area under the viral shedding curve, were quite different for the two types of mice (Table 1). As shown in Table 1, the mean of the total viral antigen shedding was higher in J chain−/− mice (13.9 ± 2.8) than in WT mice (8.8 ± 2.1, P < 0.005 from data of individual mice). This increased viral shedding in the J chain−/− mice was related to a delay in clearing infection, reaching 8.4 ± 0.2 days in the J chain−/− mice compared to only 6.3 ± 0.6 days in the WT mice (P < 0.001 from data of individual mice).
FIG. 2.
Fecal viral antigen shedding curves of control WT (▪) and J chain−/− mice (▴). Control mice were infected with 104 SD50 ECw. Feces were collected, solubilized, and tested as crude supernatants for RV antigens by ELISA; the results are expressed as OD492 readings, and the cutoff for positive value is 0.2. Each time point represents the mean OD492 from eight mice ± standard error of the mean. The experiment was done twice with similar results. The results of one experiment are presented.
TABLE 1.
Total viral antigen shedding over 8 days
| Mouse groupa | Viral shedding | Day of clearance |
|---|---|---|
| WT PBSb | 8.8 ± 2.1 | 6.3 ± 0.6 |
| J chain−/− PBS | 13.9 ± 2.8 | 8.4 ± 0.2 |
| WT 2/6c | 0.095 ± 0.07 | NRd |
| J chain−/− 2/6 | 5.5 ± 2.2 | 7.2 ± 0.28 |
Groups of eight mice were analyzed.
Control mice nasally vaccinated with PBS and CT.
Mice nasally vaccinated with VLP2/6 and CT.
NR, not relevant.
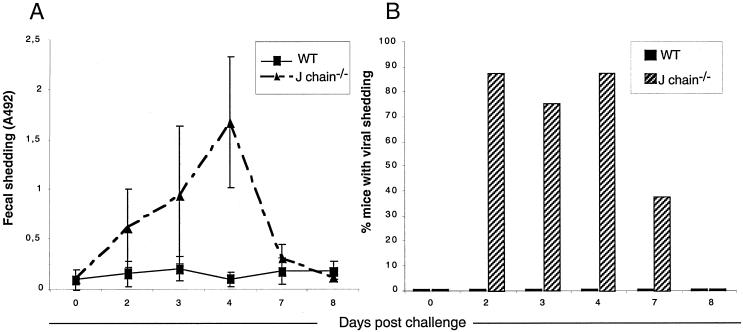
The VLP2/6-vaccinated WT and J chain−/− mice were then orally challenged with ECw. The VLP2/6-immunized WT mice did not show any detectable viral shedding in their feces; in contrast, 87.5% of the VLP2/6-vaccinated J chain−/− mice had detectable RV in their feces (Fig. 3B). Although most of the J chain−/− mice were not protected against the viral challenge, vaccination with VLP2/6 reduced the total viral antigen shedding by 61% compared to the respective groups mock vaccinated with PBS (P < 0.005 [Table 1]). Immunized J chain−/− mice thus develop an immune response independent of J chain that only partially controls RV infection. These results indicate that protection against RV infection by heterologous VLP2/6 is strongly impaired by the lack of J chain.
FIG. 3.
J chain−/− mice are not protected against an RV ECw challenge by nasal vaccination with VLP2/6. Eight WT(▪) and J chain−/− mice (▴) were nasally inoculated with 9 μg of VLP2/6 plus 5 μg of CT on days 0, 21, and 42. They were challenged with 104 SD50 of ECw on day 63. Feces were collected at the indicated days, solubilized, and tested as crude supernatants for RV antigen detection by ELISA. (A) Mean shedding of RV antigens (expressed as OD492); the cutoff for positive value is 0.2. (B) Percentages of mice with detectable RV shedding. ▪, WT; ▨, J chain−/−. The experiment was done twice with similar results. The results of one experiment are presented.
J chain−/− and WT mice have similar systemic and local RV-specific B-cell responses.
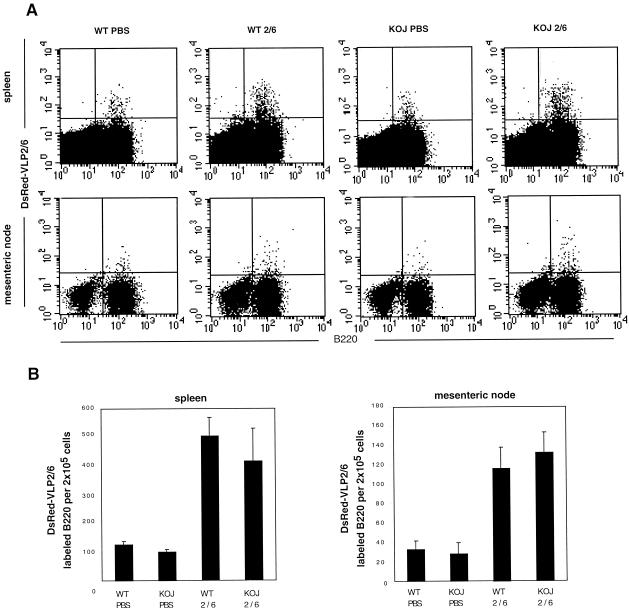
In order to rule out that the lack of protection in J chain−/− mice resulted from an altered B-cell response to VLP2/6, the B-cell responses to VLP2/6 in J chain−/− and WT mice were comparatively analyzed. The systemic and local distribution of B cells specific for VLP2/6 was estimated by a recently described fluorescence-activated cell sorter (FACS)-based technique that allows the identification of B cells reacting with fluorescent VLP2/6 (21). Double labeling of 2 × 105 splenic cells with anti-B220 and DsRed-tagged VLP2/6 showed that 417 ± 133 J chain−/− B cells and 505 ± 54 WT B cells reacted with DsRed-VLP2/6 21 days after vaccination (P = 0.17) (Fig. 4B). Mock-treated WT and J chain−/− mice had 127 ± 10 and 101 ± 5 splenic B cells that spontaneously reacted with VLP2/6. J chain−/− and WT mice had similar numbers of total spleen cells (95 × 106 ± 8 × 106 and 92 × 106 ± 3.5 × 106, respectively) and percentages of B cells (54% ± 5% and 57% ± 2%, respectively). The high number of B cells reactive to VLP2/6 in the nonimmune mice is surprising; among hypotheses, it is suggested that VLP2/6 may interact with a wide Ig preimmune repertoire, with polyreactive Ig, or with a non-Ig surface molecule expressed on some B cells. In the mesenteric node, 133 ± 21 and 117 ± 21 B cells reacted with DsRed-VLP among 2 × 105 FACS-analyzed cells from J chain−/− and WT mice, respectively, whereas mock-treated mice showed 35 ± 10 and 30 ± 12 reacting B cells, respectively (P = 0.8) (Fig. 4). These data show that nasal vaccination induces similar increases in WT and J chain−/− mice of VLP2/6-specific B cells in spleen and intestinal draining lymph node. Finally, antigen-specific Ig levels were assessed in sera from VLP2/6 and PBS-inoculated mice. In WT and J chain−/− immune mice, anti-RV Ig titers in serum were 1.6 × 106 ± 0.2 × 106 and 1.45 × 106 ± 0.12 × 106, respectively (P = 0.3) (Table 2). Similar anti-RV serum IgG titers were found in the two types of mice (Table 2). Slightly higher levels of anti-RV IgM were identified in the VLP2/6-vaccinated J chain−/− mice (3.6 × 103 ± 0.3 × 103 versus 2.9 × 103 ± 0.08 × 103, P < 0.05). Immune J chain−/− mice presented a tendency for higher levels of fecal IgG than those of WT mice (40 ± 20 versus 19 ± 10, P = 0.14). Fecal IgM could not be detected under our experimental conditions in either type of mice.
FIG. 4.
B-cell reactivity with VLP2/6 in nasally vaccinated WT and J chain−/− mice in spleen and mesenteric nodes. (A) Dot plot representations of FACS analyses from 2 × 105 splenic and mesenteric cells labeled with fluorescein isothiocyanate-conjugated anti B-220 and DsRed-VLP2/6 from representative mice. Nine micrograms of VLP2/6 containing 5 μg of CT was instilled into WT (WT2/6) and J chain−/− (KOJ 2/6) via the nasal route on days 0, 21, and 42. WT and J chain mice that had been inoculated with PBS and CT were used as controls (WT PBS, KOJ PBS). Ten days after the last injection, cells from the spleen and mesenteric nodes were harvested and labeled. (B) Graphic representation of the number of B220-positive cells specific for VLP2/6 out of 2 × 105 total cells (y axis) from three individual mice per group.
Hence, specific B-cell labeling and serum and fecal Ig analyses show that the B-cell response does not seem to be reduced in J chain−/− mice.
DISCUSSION
The B-cell arm of the immune response plays a major role in controlling RV infection. This inference comes from many reports of experimental and clinical observations. For example, in RV infections of children (8, 24, 32), mice (11), and piglets (44), serum and intestinal antibodies correlate with protection. Furthermore, infection experiments using B-cell-deficient mice indicate that B cells are necessary for protection (14, 27). Interestingly, chronic RV infection in Rag-2-deficient mice is resolved by transfer of mucosally targeted α4β7high B cells, indicating that local B cells have a potent role in the control of RV infection (43). However, the mechanisms by which B cells act against RV are unclear. Among unsolved issues is whether and how the immunodominant antibody response against the inner capsid VP2 and VP6 proteins plays a determining role in the defense against RV. Several experimental studies suggest that anti-VP6 and/or -VP2 antibodies can be involved in protection. Indeed, double-layer EDIM virus, i.e., EDIM virus without the external capsid, induces better protection in WT mice than in B-cell-deficient μMT mice (28). Furthermore, in the mouse backpack model, a hybridoma producing anti-VP6 IgA protected the mice against an RV ECw challenge (4). In this report, we further show that adult mice with an inherent block in IgA and IgM transcytosis via the pIgR demonstrate a delay in clearing RV and are not protected against a murine RV challenge by immunization with heterologous bovine VLP2/6. The J chain−/− mice's failure to control RV infection cannot be attributed to known altered B- or T-cell responses. In fact, we verified that the anti-RV-specific B-cell distributions in spleen and mesenteric lymph node of the two types of mice were similar. In addition, anti-RV IgA-secreting cells were detected by the enzyme-linked spot assay in both immune WT and J chain−/− mouse intestine (data not shown). FACS analysis of splenocytes from the J chain−/− mice using monoclonal antibodies to CD3, CD4, and CD8 revealed staining patterns indistinguishable from those of WT littermates (17). In addition, T-cell-dependent IgG responses were comparable in J chain−/− mice and WT controls (unpublished data).
The significant delay in clearance of RV infection in J chain−/− mice in the context of preserved B-cell responses supports the role of mucosal immunoglobulins transported by the pIgR in the control of RV primary infection. This finding suggests that secretion of mucosal IgA and IgM directed to the whole viral particle participates in the clearance of RV infection, either in the lumen of the intestine or possibly inside the epithelial cells, as suggested for human immunodeficiency virus (1), influenza virus (25), and Sendai virus (26) infections. Previous works using genetically engineered mice have shown that B cells are major players in the protection against RV reinfection but minor actors in the resolution of infection (14, 27). However, these findings were obtained with mice bred on a C57BL/6 × 129 (14) or on a C57BL/6 (26) background that probably emphasizes the impact of CD8 T cells on viral clearance, as C57BL/6 mice are genetically biased towards potent cytotoxic responses.
VLP2/6 has been shown to induce protection against RV infection in mouse (30) and rabbit models (7) using intranasal and parenteral vaccination routes, respectively. In this study, nasally delivered VLP2/6 induced protection in the adult mouse against infection with a 104 SD50 challenge dose of ECw virus, which is 103 higher than the dose previously used by O'Neal et al. (30). By contrast, immunization with VLP2/6 did not protect J chain−/− mice under these challenge conditions. This finding strongly suggests that transcytosis of IgA and IgM directed to VLP2/6 is required to fully protect mice against a high virus dose challenge. As for other viruses (1, 25, 26), intracellular neutralization of RV has been proposed by Burns et al. (4). Conceivably, transcytosis of anti-VP2 or -VP6 polymeric Ig associated to pIgR may allow intracellular interaction between the Ig and the corresponding structural protein VP6 or VP2, inhibiting a crucial step of the viral cycle such as transcription or assembly. It has been recently shown that some protective IgA in a backpack mouse model inhibits the assembly of the RV shell by preventing association of the outer capsid VP7 protein (15). The subcellular compartment where the transcytosing IgA interacts with the RV particle has yet to be identified.
Whereas the intracellular neutralization is an attractive mechanistic hypothesis, it still remains possible that the mucosal secretory anti-VP6 antibodies act inside the intestinal lumen, after their intracellular transit. Although monoclonal and polyclonal anti-VP6 antibodies have never been found to be neutralizing in in vitro assays, some secretory antibodies to VP6 may be able to prevent viral infection in vivo. Actually, secretory IgA exhibits higher stability and avidity as well as an increased neutralization potential compared to monomeric IgA or IgG (33, 38). In addition, due to a high sugar moiety content, immune complexes made of secretory IgA are highly hydrophilic and interact efficiently with mucins, leading to an efficient elimination of undesired antigens through the mucus layer (40). Thus, another possible explanation of our data is that secretory IgA (or IgM) directed to VP6-accessible epitopes may actually bind to infectious RV particles efficiently enough to lead to the viral immune exclusion within the mucus layer. This mechanism would be particularly efficient in rodents, as huge amounts of secretory IgA are found in the intestinal lumen due to the biliary excretion of IgA.
The role of anti-VP6 antibodies in protection could not be demonstrated with anti-VP6 IgA-producing hybridomas in a neonatal mouse infection model (34). The discordant findings between studies regarding the protective capacities of anti-VP6 hybridomas may be due to different biological conditions between the adult and the neonatal mouse models of infection. The protective mechanisms involved, such as pIgR transcytosis, secretory IgA interaction with the intestinal milieu, or complementation with undefined immune factors, may be modified with age. In addition, the lack of protection observed in the neonatal mouse model of infection with the chosen anti-VP6 IgA-producing hybridomas may have resulted from anti-VP6 IgA unable to interfere with the viral life cycle, in either an extracellular or an intracellular compartment. Actually, the epitopes that are recognized by the anti-VP6 IgA are probably essential to achieving antiviral effects. As vaccination with VLP2/6 elicits a wide panel of anti-VP6 and -VP2 antigenic specificities, it is likely that anti-VP2 or -VP6 IgA (or IgM) of adequate reactivity, efficient at blocking the viral cycle, was produced in our immune mice.
The major role of mucosal Ig in protection against RV that can be deduced from the J chain−/− model is not in accordance with the observation that lack of IgA synthesis in IgA−/− mice does not impair protection against secondary RV infection (31). This discrepancy may result from the fact that J chain−/− mice have a defect in secretion of both IgM and IgA whereas IgA−/− mice can produce secretory IgM. In fact, IgM from intestinal human plasma B cells shows accumulations of somatic mutations in the variable region to the same extent as IgA, implying that IgM could functionally replace IgA in mucosa (13). A compensatory role of IgM in IgA−/− mice immunized against reovirus (37) and against CT (16) has been suggested, as these mice showed increased specific IgM levels in their feces compared to WT mice. However, RV-specific IgM levels could not be detected in the IgA−/− feces (31), nor were they found in our study (Table 2). This lack of detection could result from the stability of secretory IgM being lower than that of secretory IgA, rendering IgM detection in feces unreliable (35). Intestinal IgG secretion was suggested to compensate for IgA absence in the IgA−/− mice (31). However, although immune J chain−/− mice had higher levels of anti-inner core fecal IgG than WT mice, they were unable to efficiently control the viral challenge. Finally, infection of the IgA−/− mice, which were bred onto the C57BL/6 genetic background, could elicit immune effectors that compensate for the lack of IgA in the IgA−/− mice and that may not be elicited in our J chain−/− BALB/c mice. Although CD4 and CD8 T cells did not seem to be involved in the protection of immune IgA−/− mice (31), subpopulations of intraepithelial T cells or γ/δ T cells could have played a determining compensatory role.
J chain−/− mice have been used in several infectious models to assess whether secretory polymeric immunoglobulins play a dominant role in protection against pathogens. J chain is not required for the cross-protective immunity against influenza A virus (9) or for protection against herpes simplex virus type 2 disease following immunization with an attenuated virus (18). Thus, in some instances, lack of J chain does not interfere with the establishment of an efficient immune response to ensure protection of mucosa. By contrast, lack of J chain was associated with a marked decrease in the resistance to a CT challenge in small intestinal ligated loops of orally immunized mice (23). This finding indicates that epithelial transport of specific IgA and/or IgM can prevent toxin-induced symptoms, i.e., loss of epithelial barrier integrity. In our study, we further show that the epithelial transport of IgM and/or IgA can actually control RV spreading through the intestine.
Other pathways of IgA secretion through the epithelial layer of the intestine have been suggested. J chain−/− mice show large amounts of monomeric IgA in milk and in nasal and intestinal washes (19), although the extent of IgA representation in intestinal secretions was not consistent between studies (23). The accumulation of monomeric IgA in the intestinal lumen may result from passive leakage or from alternative intraepithelial routing of IgA, as several IgA receptors that are not specific for dimeric IgA have been described (29, 36). In any event, the possible alternative pathways of monomeric IgA secretion in the intestine were not efficient at controlling RV infection, nor were they efficient at preventing CT-induced damages.
Overall, our data support that pIgR-mediated transcytosis of IgA and/or IgM directed to the inner capsid proteins plays a major role in protection against RV infection in an adult mouse model. This finding encourages the development of mucosal vaccination strategies to ensure an optimal defense of the intestine through secretory immunoglobulins. Our study also presents additional data in favor of heterotypic virus-based strategies in the field of RV vaccine design.
Acknowledgments
We thank Annie Charpilienne, Christine Hervé, and Cynthia Jaeger for excellent technical help. We thank the technicians from the Unité d'Expérimentation Animale Rongeurs du centre INRA de Jouy-en-Josas for assistance with the animal work and help in establishing adequate mouse housing conditions. We are grateful to Morgane Bomsel and Manuel Franco for stimulating discussions.
This work was supported in part by a 5th PCRD grant from UE (QLRT 1999-00634).
REFERENCES
- 1. Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. [DOI] [PubMed] [Google Scholar]
- 2.Brandzaeg, P., P. Krajci, M. E. Lamm, and C. S. Kaetzel. 1994. Epithelial and hepatobiliary transport of polymeric immunoglobulins, p. 113-126. In P. L. Ogra, M. E. Lamm, J. R. Mcghee, J. Mestecky, W. Strober, and J. Bienenstock (ed.), Handbook of mucosal immunology. Academic Press Inc., New York, N.Y.
- 3.Bresee, J. S., S. El Arifeen, T. Azim, J. Chakraborty, A. W. Mounts, G. Podder, J. R. Gentsch, R. L. Ward, R. Black, R. I. Glass, and M. Yunus. 2001. Safety and immunogenicity of tetravalent rhesus-based rotavirus vaccine in Bangladesh. Pediatr. Infect. Dis. J. 20:1136-1143. [DOI] [PubMed] [Google Scholar]
- 4.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104-107. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1999. Withdrawal of rotavirus vaccine recommendation. Morb. Mortal. Wkly. Rep. 48:1007.. [PubMed] [Google Scholar]
- 6.Charpilienne, A., M. Nejmeddine, M. Berois, N. Parez, E. Neumann, E. Hewat, G. Trugnan, and J. Cohen. 2001. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J. Biol. Chem. 276:29361-29367. [DOI] [PubMed] [Google Scholar]
- 7.Ciarlet, M., S. E. Crawford, C. Barone, A. Bertolotti-Ciarlet, R. F. Ramig, M. K. Estes, and M. E. Conner. 1998. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J. Virol. 72:9233-9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulson, B. S., K. Grimwood, I. L. Hudson, G. L. Barnes, and R. F. Bishop. 1992. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J. Clin. Microbiol. 30:1678-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein, S. L., C. Y. Lo, J. A. Misplon, C. M. Lawson, B. A. Hendrickson, E. E. Max, and K. Subbarao. 1997. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J. Immunol. 158:1222-1230. [PubMed] [Google Scholar]
- 10.Estes, M. K., H. B. Greenberg, and R. L. Ward. 1998. Viral gastroenteritis vaccine, p. 282-305. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. R. McGhee, and J. Bienenstock (ed.), Mucosal immunology, 2nd ed. Academic Press, San Diego, Calif.
- 11.Feng, N., J. W. Burns, L. Bracy, and H. B. Greenberg. 1994. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J. Virol. 68:7766-7773. (Erratum, 69:3246, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, N., P. T. Vo, D. Chung, T. V. Vo, Y. Hoshino, and H. B. Greenberg. 1997. Heterotypic protection following oral immunization with live heterologous rotaviruses in a mouse model. J. Infect. Dis. 175:330-341. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, M., and R. Kuppers. 1998. Human IgA- and IgM-secreting intestinal plasma cells carry heavily mutated VH region genes. Eur. J. Immunol. 28:2971-2977. [DOI] [PubMed] [Google Scholar]
- 14.Franco, M. A., and H. B. Greenberg. 1995. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J. Virol. 69:7800-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert, J. M., N. Feng, J. T. Patton, and H. B. Greenberg. 2001. Rotavirus assembly—interaction of surface protein VP7 with middle layer protein VP6. Arch. Virol. 146:1155-1171. [DOI] [PubMed] [Google Scholar]
- 16.Harriman, G. R., M. Bogue, P. Rogers, M. Finegold, S. Pacheco, A. Bradley, Y. Zhang, and I. N. Mbawuike. 1999. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol. 162:2521-2529. [PubMed] [Google Scholar]
- 17.Hendrickson, B. A., D. A. Conner, D. J. Ladd, D. Kendall, J. E. Casanova, B. Corthesy, E. E. Max, M. R. Neutra, C. E. Seidman, and J. G. Seidman. 1995. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. J. Exp. Med. 182:1905-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrickson, B. A., J. Guo, I. Brown, K. Dennis, D. Marcellino, J. Hetzel, and B. C. Herold. 2000. Decreased vaginal disease in J-chain-deficient mice following herpes simplex type 2 genital infection. Virology 271:155-162. [DOI] [PubMed] [Google Scholar]
- 19.Hendrickson, B. A., L. Rindisbacher, B. Corthesy, D. Kendall, D. A. Waltz, M. R. Neutra, and J. G. Seidman. 1996. Lack of association of secretory component with IgA in J chain-deficient mice. J. Immunol. 157:750-754. [PubMed] [Google Scholar]
- 20.Kapikian, A. Z., and R. M. Chanock. 1996. Rotaviruses, p. 1657-1708. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, New York, N.Y.
- 21.Kuklin, N. A., L. Rott, N. Feng, M. E. Conner, N. Wagner, W. Muller, and H. B. Greenberg. 2001. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J. Immunol. 166:1894-1902. [DOI] [PubMed] [Google Scholar]
- 22.Labbe, M., A. Charpilienne, S. E. Crawford, M. K. Estes, and J. Cohen. 1991. Expression of rotavirus VP2 produces empty corelike particles. J. Virol. 65:2946-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lycke, N., L. Erlandsson, L. Ekman, K. Schon, and T. Leanderson. 1999. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J. Immunol. 163:913-919. [PubMed] [Google Scholar]
- 24.Matson, D. O., M. L. O'Ryan, I. Herrera, L. K. Pickering, and M. K. Estes. 1993. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J. Infect. Dis. 167:577-583. [DOI] [PubMed] [Google Scholar]
- 25.Mazanec, M. B., C. L. Coudret, and D. R. Fletcher. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 69:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazanec, M. B., C. S. Kaetzel, M. E. Lamm, D. Fletcher, and J. G. Nedrud. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 89:6901-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeal, M. M., K. S. Barone, M. N. Rae, and R. L. Ward. 1995. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology 214:387-397. [DOI] [PubMed] [Google Scholar]
- 28.McNeal, M. M., M. N. Rae, J. A. Bean, and R. L. Ward. 1999. Antibody-dependent and -independent protection following intranasal immunization of mice with rotavirus particles. J. Virol. 73:7565-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moura, I. C., M. N. Centelles, M. Arcos-Fajardo, D. M. Malheiros, J. F. Collawn, M. D. Cooper, and R. C. Monteiro. 2001. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J. Exp. Med. 194:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neal, C. M., S. E. Crawford, M. K. Estes, and M. E. Conner. 1997. Rotavirus virus-like particles administered mucosally induce protective immunity. J. Virol. 71:8707-8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neal, C. M., G. R. Harriman, and M. E. Conner. 2000. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A. J. Virol. 74:4102-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Ryan, M. L., D. O. Matson, M. K. Estes, and L. K. Pickering. 1994. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J. Infect. Dis. 169:504-511. [DOI] [PubMed] [Google Scholar]
- 33.Renegar, K. B., G. D. Jackson, and J. Mestecky. 1998. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J. Immunol. 160:1219-1223. [PubMed] [Google Scholar]
- 34.Ruggeri, F. M., K. Johansen, G. Basile, J. P. Kraehenbuhl, and L. Svensson. 1998. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J. Virol. 72:2708-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell, M. W., M. Kilian, and M. E. Lamm. 1999. Biological activities of IgA, p. 225. In P. L. Ogra, M. E. Lamm, J. R. McGhee, J. Mestecky, W. Strober, and J. Bienenstock (ed.), Mucosal immunology. Academic Press, San Diego, Calif.
- 36.Sakamoto, N., K. Shibuya, Y. Shimizu, K. Yotsumoto, T. Miyabayashi, S. Sakano, T. Tsuji, E. Nakayama, H. Nakauchi, and A. Shibuya. 2001. A novel Fc receptor for IgA and IgM is expressed on both hematopoietic and non-hematopoietic tissues. Eur. J. Immunol. 31:1310-1316. [DOI] [PubMed] [Google Scholar]
- 37.Silvey, K. J., A. B. Hutchings, M. Vajdy, M. M. Petzke, and M. R. Neutra. 2001. Role of immunoglobulin A in protection against reovirus entry into murine Peyer's patches. J. Virol. 75:10870-10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stubbe, H., J. Berdoz, J. P. Kraehenbuhl, and B. Corthesy. 2000. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J. Immunol. 164:1952-1960. [DOI] [PubMed] [Google Scholar]
- 39.Tosser, G., M. Labbe, M. Bremont, and J. Cohen. 1992. Expression of the major capsid protein VP6 of group C rotavirus and synthesis of chimeric single-shelled particles by using recombinant baculoviruses. J. Virol. 66:5825-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Underdown, B., and J. Mestecky. 1994. Mucosal immunoglobulins, p. 79-97. In P. Ogra, J. Mestecky, M. Lamm, W. Strober, J. McGhee, and J. Bienenstock (ed.), Handbook of mucosal immunology. Academic Press, Inc., New York, N.Y.
- 41.Vaerman, J. P., A. Langendries, D. Giffroy, P. Brandtzaeg, and K. Kobayashi. 1998. Lack of SC/pIgR-mediated epithelial transport of a human polymeric IgA devoid of J chain: in vitro and in vivo studies. Immunology 95:90-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vesikari, T. 1993. Clinical trials of live oral rotavirus vaccines: the Finnish experience. Vaccine 11:255-261. [DOI] [PubMed] [Google Scholar]
- 43.Williams, M. B., J. R. Rose, L. S. Rott, M. A. Franco, H. B. Greenberg, and E. C. Butcher. 1998. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, alpha4beta7. J. Immunol. 161:4227-4235. [PubMed] [Google Scholar]
- 44.Yuan, L., L. A. Ward, B. I. Rosen, T. L. To, and L. J. Saif. 1996. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 70:3075-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]