Abstract
Hantavirus cardiopulmonary syndrome (HCPS) is a life-threatening respiratory disease characterized by profound pulmonary edema and myocardial depression. Most cases of HCPS in North America are caused by Sin Nombre virus (SNV), which is carried asymptomatically by deer mice (Peromyscus maniculatus). The underlying pathophysiology of HCPS is poorly understood. We hypothesized that pathogenic SNV infection results in increased generation of reactive oxygen/nitrogen species (RONS), which contribute to the morbidity and mortality of HCPS. Human disease following infection with SNV or Andes virus was associated with increased nitrotyrosine (NT) adduct formation in the lungs, heart, and plasma and increased expression of inducible nitric oxide synthase (iNOS) in the lungs compared to the results obtained for normal human volunteers. In contrast, NT formation was not increased in the lungs or cardiac tissue from SNV-infected deer mice, even at the time of peak viral antigen expression. In a murine (Mus musculus) model of HCPS (infection of NZB/BLNJ mice with lymphocytic choriomeningitis virus clone 13), HCPS-like disease was associated with elevated expression of iNOS in the lungs and NT formation in plasma, cardiac tissue, and the lungs. In this model, intraperitoneal injection of 1400W, a specific iNOS inhibitor, every 12 h during infection significantly improved survival without affecting intrapulmonary fluid accumulation or viral replication, suggesting that cardiac damage may instead be the cause of mortality. These data indicate that elevated production of RONS is a feature of pathogenic New World hantavirus infection and that pharmacologic blockade of iNOS activity may be of therapeutic benefit in HCPS cases, possibly by ameliorating the myocardial suppressant effects of RONS.
In 1993, a previously unknown New World hantavirus, later named Sin Nombre virus (SNV) (15), was identified as the causative agent of an outbreak of severe, life-threatening respiratory disease in the Four Corners region of the southwestern United States (20). Subsequently, over 280 confirmed cases of this disease, now known as hantavirus cardiopulmonary syndrome (HCPS), have been identified in the United States andCanada (http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/caseinfo.htm), with a similar number occurring throughout South America. There is no known effective specific pharmacologic therapy for HCPS, and there is no available vaccine (13). Despite improvements in supportive therapy, the current mortality rate for HCPS remains at 30%.
Infection with SNV is followed by an incubation period of 1 to 4 weeks and a short prodrome of fever, headache, and myalgia (36). Subsequent progression to the cardiopulmonary phase of HCPS is rapid and is characterized by severe pulmonary edema and myocardial depression, without significant renal insufficiency or hemorrhagic manifestations (32). Clinical management in this phase is largely dependent upon hemodynamic and ventilatory support and, in the absence of aggressive supportive therapy, patients may die within a few hours of initial presentation. Death often results from progressive myocardial insufficiency, with the development of pulseless cardiac electrical activity (64).
At necropsy of fatal HCPS cases, rubbery, edematous, airless lungs show septal and severe intra-alveolar edema but, unlike adult respiratory distress syndrome (ARDS), no widespread hyaline membrane formation or neutrophilic infiltrate (57). Moreover, there is no light or electron microscopic evidence of damage to the respiratory epithelium (75). Likewise, and despite clinical evidence of myocardial dysfunction (32), there is no pathologic evidence of myocardial injury (57). The primary lesion in HCPS therefore appears to be functional rather than structural.
Most cases of HCPS in North America have been caused by SNV. The natural host and reservoir of this virus is the deer mouse (Peromyscus maniculatus) (14). Unlike hantavirus-infected humans, SNV-infected deer mice show no apparent ill effects (10). There is no evidence of gross lung pathology or respiratory dysfunction in SNV-infected deer mice caught in the wild (58), and lung histopathology is entirely absent in experimentally infected mice (10).
Currently, the pathogenesis of HCPS and other hantavirus infections is poorly understood. Reactive oxygen/nitrogen species (RONS) are attractive candidates as mediators of the pathogenic effects of SNV in humans. During the inflammatory response to lung insult, alveolar macrophages and other inflammatory cells can generate and release RONS, particularly nitric oxide (·NO) and superoxide (O2−) (42). Most of the injurious effects of RONS have been attributed to the formation of peroxynitrite (ONOO−) from ·NO and O2−, which react at a nearly diffusion-limited rate (40). ONOO− is a potent oxidizing and nitrating agent that damages a wide spectrum of biological molecules, including nucleic acids, lipids, and proteins (7).
The abilities of alveolar macrophages and pneumocytes to produce RONS and to contribute to both viral killing and damage to pulmonary tissues in viral pneumonitis have been alluded to in a number of studies (17); however, the vast majority of such studies have used mouse models. Since there are known differences in the regulation of inducible nitric oxide synthase (iNOS) gene expression between rodents and primates (55), the relevance of RONS to the pathogenesis of viral pneumonitis in humans has remained unclear; in addition, their involvement in the pathogenesis of any form of human respiratory tract viral pneumonitis has not been directly demonstrated to date.
We hypothesized that the detrimental effects of SNV on human cardiopulmonary function may not be solely or directly mediated by the proinflammatory cytokines generated as a consequence of the inflammatory response to SNV infection of the pulmonary endothelium. Rather, we proposed that the pathologic effects of these proinflammatory cytokines on the heart and the pulmonary endothelium are mediated at least in part by RONS generated by alveolar macrophages (and possibly alveolar epithelial cells) as a result of activation by these proinflammatory cytokines. Furthermore, we proposed that apathogenic infection of the natural host, the deer mouse, may, in contrast, be associated with an absence of RONS generation in response to infection. Finally, we hypothesized that blockade of nitric oxide (·NO) generation by 1400W, which is a specific inhibitor of iNOS (24), may attenuate HCPS-like disease in a heterologous animal model system of HCPS that uses adult NZB/BLNJ (NZB) mice (Mus musculus) infected intravenously with the lymphocytic choriomeningitis virus (LCMV) clone 13 strain (LCMV-13) (60).
MATERIALS AND METHODS
Patient population.
HCPS cases were stratified by severity as previously described (9). Class 0 patients have no pulmonary disease but prodromal symptoms (e.g., fever, headache, chills, nausea or vomiting, and myalgia). Class I patients have pulmonary disease (as diagnosed by chest radiography) but do not require intubation. Class II patients are those who have been intubated. This class is subdivided into patients who do or do not receive extracorporeal membrane oxygenation (ECMO) therapy. Class III patients are those in whom HCPS is fatal. Plasma samples from 6 patients with class I and 15 patients with class II confirmed HCPS and convalescent-phase plasma samples from 9 patients who recovered from HCPS were analyzed (mean time for convalescent-phase samples, 42 days after admission), although there was not always a sufficient amount of sample to allow analysis of more than one sample parameter. None of the class I cases analyzed progressed to class II disease, and none of the class II cases analyzed was fatal. Plasma samples were also available from 13 healthy controls, derived from populations at both the University of Alabama at Birmingham and the University of New Mexico.
At autopsy of fatal (class III) HCPS cases, representative pieces of lung, heart, and kidney tissues were fixed in 10% neutral buffered formalin, paraffin embedded, cut into serial thin sections (5 μm), and mounted on Colorfrost Plus slides. For routine histopathologic evaluation, sections were stained with hematoxylin and eosin. Lung tissues from 11 fatal cases of HCPS were available for this study. Two of the 11 fatal cases were patients (from Argentina) infected with Andes virus (a South American hantavirus) rather than SNV. The Tissue Procurement Facility at the University of Alabama at Birmingham provided sections of formalin-fixed, paraffin-embedded normal lung tissues from four subjects.
All plasma and autopsy-derived tissue samples were numerically coded and maintained as anonymous. General and limited demographic information was requested at the end of all experiments. Data on patient ethnicity was not available for any plasma samples. The institutional review boards at the University of Alabama at Birmingham and the University of New Mexico approved the relevant aspects of the study. All clinical investigations were conducted in accordance with the principles of the Declaration of Helsinki.
Animals and infection with SNV.
Deer mice (P. maniculatus) were bred and housed in an outdoor quarantine facility under standard, non-pathogen-free conditions as previously described (11). Infections were carried out by intramuscular injection of 4- to 6-week-old P. maniculatus rufinus with 5 50% animal infective doses of passage 2 strain SN77734, consisting of 100 μl of pooled tissue homogenate diluted 1:1,500 in minimal essential medium (10). This line of SN77734 virus had been maintained solely through deer mouse passage. For controls, sham-infected deer mice were injected with a similar dilution of tissue homogenate from an uninfected deer mouse. Infected and uninfected deer mice were maintained in individual cages equipped with insulated nest boxes in 0.04-ha (20- by 20-m) outdoor enclosures. We adhered strictly to the biosafety recommendations of the U.S. Centers for Disease Control and Prevention in all aspects of this work (53). Workers wearing respirators and personal protective gear carried out all inoculations and manipulations of infected animals in the colony as described previously (11).
For blood sampling, rodents were removed from the artificial burrows in Sherman live traps and were anesthetized with methoxyfluorane before samples were obtained. An intracardiac blood sample was collected from P. maniculatus specimens by retro-orbital puncture at the time of sacrifice for hantavirus serologic testing. To confirm infection, a strip immunoblot assay was used to detect antibodies to SNV nucleocapsid antigen as previously described (10). Infection was also confirmed by reverse transcription-PCR detection of the viral small genome in all infected specimens (10). Euthanasia was performed with tribromoethanol. For histopathologic and immunohistochemical analyses, we placed tissues in 10% neutral buffered formalin for at least 24 h before embedding them in paraffin (27).
Animals were handled according to University of New Mexico Animal Research Facility guidelines and by using a protocol approved by the Main Campus Animal Care and Use Committee at the University of New Mexico.
Animals and infection with LCMV.
NZB mice (M. musculus) were obtained from Jackson Laboratory, Bar Harbor, Maine. Mice were maintained in autoclaved microisolator cages (Lab Products, Maywood, N.J.) and provided with autoclaved food (Agway, Syracuse, N.Y.) and water ad libitum. All mice were used in studies at 6 to 8 weeks of age. Mice were inoculated with 4 × 106 PFU of LCMV-13 by intravenous injection. Mice were anesthetized for euthanasia by injection with ketamine (8.7 mg/100 g of body weight; Aveco, Fort Dodge, Iowa) and xylazine (1.3 mg/100 g of body weight; Haver, Shawnee, Kans.). Euthanasia was performed by exsanguination following transection of brachial vasculature; a blood sample was taken from the transected brachial vessels of each mouse and placed in an individual microcentrifuge tube containing 30 μl of 7.5% EDTA solution to prevent clotting. Plasma was separated by centrifugation and stored at −20°C for subsequent determination of RONS content. All NZB mouse experiments were performed in ABSL-2/3 facilities at the University of Alabama at Birmingham by using procedures approved by its Institutional Animal Care and Use Committee and in accordance with federal guidelines and biosafety recommendations for working with LCMV.
Preparation of LCMV-13 stocks.
LCMV-13 was kindly provided by Rafi Ahmed (Emory University, Atlanta, Ga.) (1). Plaqued, picked viral isolates were propagated in BHK-21 cells, and titers of viral stocks were determined on Vero cell monolayers (see below).
LCMV plaque assays.
Titers of viral stocks and spleen homogenates were determined by plaque assays with Vero cell monolayers as previously described (1). After 4 days, plaques were visualized by overnight staining with neutral red.
Detection of nitrate (NO3−) and nitrite (NO2−).
Plasma NO2− plus NO3− (NOx) concentrations were measured with Greiss reagents after the reduction of NO3− to NO2− by nitrate reductase. Briefly, 50 μl of each sample was incubated with an equal volume of 0.2 mM NADPH in 50 mM phosphate buffer (pH 7.5) at room temperature for 2 h in the presence of 0.2 U of nitrate reductase/ml. Samples were then mixed with equal volumes of 0.5% sulfanilamide and 0.05% N-(1-naphthyl)ethylenediamine dihydrochloride and incubated for 10 min. The absorbance was measured at 550 nm, and the NO2− concentration was determined with NaNO2 and NaNO3 standards.
NT ELISA.
Plasma nitrotyrosine (NT) content was determined by an enzyme-linked immunosorbent assay (ELISA) with a polyclonal anti-NT antibody (Upstate Biotechnology, Lake Placid, N.Y.) and nitrated bovine serum albumin (BSA) as a standard as previously described (76). Briefly, a nitrated protein solution was prepared for use as a standard by incubating BSA (10 mg/ml in 50 mM KH2PO4 [pH 7.4]) with 100 μM tetranitromethane (a very efficient nitrating agent) in 50 mM KH2PO4 (pH 8) for 30 min at 37°C. After the pH was adjusted to 10 with 3 M NaOH, the amount of NT present in this solution was measured at 430 nm and expressed as nanomoles of NT per milligram of BSA (using a ɛM of 4,400 M−1 cm−1). Diluted standards and serial dilutions of plasma samples were bound to Immulon2 96-well ELISA plates. After nonspecific antibody binding was blocked, bound NT was detected with a mouse immunoglobulin G (IgG) monoclonal anti-NT primary antibody (Upstate Biotechnology) diluted 1:200 and subsequently a peroxidase-conjugated goat anti-mouse IgG secondary antibody (Kirkegaard & Perry Laboratories) diluted 1:1,000. Peroxidase activity was detected by using a tetramethylbenzidine colorimetric substrate; the absorbance was read at 650 nm. The NT content in samples was then calculated per milligram of protein (as measured by a standard bicinchoninic acid [BCA] assay; Pierce). All samples were analyzed in a blinded fashion.
Immunohistochemical analysis.
Paraffin-embedded lung sections were deparaffinized and rehydrated through a series of ethanols to phosphate-buffered saline (PBS). For tissue sections that were subsequently to be subjected to a peroxidase reaction, endogenous peroxidases were blocked by treatment with 0.3% hydrogen peroxide in PBS for 30 min at room temperature, followed by washing with PBS. Nonspecific protein binding in all sections was then blocked with a ready-to-use serum-free protein block (Dako, Carpinteria, Calif.) for 1 h at room temperature. Tissue NT was detected with a rabbit anti-NT polyclonal IgG antibody kindly provided by J. S. Beckman and L. Estevez (8). The specificity of the NT reaction was confirmed by preincubating the antibody with 10 mM free NT prior to use. iNOS protein was detected by using Transduction Laboratories clone 3 anti-human/mouse iNOS antibody (after antigen unmasking by boiling in citrate buffer). All primary antibodies were diluted to working strength in Dako antibody diluent, which contains background-reducing components.
Primary antibody binding was performed by overnight incubation at 4°C. Bound primary antibodies were detected by single-color immunohistochemical analysis with the Dako Envision peroxidase and alkaline phosphatase systems in accordance with the manufacturer's instructions. Staining was developed with 3′,3′-diaminobenzidine hydrochloride (which produces an insoluble brown precipitate) and Fast Red chromogen (which produces a soluble red precipitate) for peroxidase-conjugated and alkaline phosphatase-conjugated secondary antibodies, respectively. Sections were counterstained with methyl green (0.5% [wt/vol] in 0.1 M sodium acetate) for peroxidase preparations or hematoxylin for alkaline phosphatase preparations. Negative control reactions with normal rabbit serum (at a protein concentration equivalent to that of the anti-NT antibody) or monoclonal antibodies to irrelevant antigens were performed simultaneously with all immunostaining reactions to confirm the specificity of these reactions. Likewise, samples from normal human volunteers, SNV-infected deer mice, and mock-infected NZB mice (all of which were NT negative) were stained concurrently with samples from HCPS patients and LCMV-infected NZB mice to confirm that NT immunostaining reactions were successful. We should note that, because of concerns expressed by van der Vliet et al. that the hydrogen peroxide blocking step used in peroxidase-based immunostaining reactions can result in artifactual formation of NT, via the formation of RONS through the peroxidase-catalyzed oxidation of NO2− (73), all peroxidase-based NT immunostaining results were confirmed by repeat staining with an alkaline phosphatase detection system.
Measurement of the wet lung/dry lung weight ratio.
Measurement of the wet lung/dry lung weight ratio was performed as previously described (51). Briefly, mice were euthanatized at 6.25 days after infection and exsanguinated, and their lungs were removed, weighed, and dried in an oven at 55°C for 7 days. After being dried, the lungs were weighed again. The wet lung/dry lung weight ratio was then calculated as an index of intrapulmonary fluid accumulation.
Modulation of lung damage by blockade of RONS production.
The specific iNOS inhibitor 1400W {N-[3-(aminomethyl)benzyl]acetamidine} was used to block RONS generation in LCMV-13-infected NZB mice. 1440W was administered intraperitoneally at a dose of 10 mg/kg every 12 h for 36 h prior to infection and throughout its course to groups of seven mock-infected and eight LCMV-13-infected NZB mice. An equivalent volume (150 μl) of sterile normal saline was administered at the same frequency and by the same route in two control groups of mock-infected and LCMV-13-infected mice (four and five mice per group, respectively). Mice were sacrificed at 6.25 days after infection. Lungs were removed for measurement of the wet lung/dry lung weight ratio, spleens were removed for LCMV plaque assays, and plasma was removed for the NT ELISA as described above.
Statistical analyses.
Data were analyzed by using Instat (GraphPad software). Pre- and postinfection data were analyzed by an analysis of variance and the two-tailed Student t test. The alternate Welch t test was used when group variances differed significantly, as determined by Bartlett's test. The method of Kolmogorov and Smirnov was used to confirm that data were sampled from populations that follow Gaussian distributions. Correlations were calculated by Pearson's linear correlation analysis. A P value of <0.05 was considered statistically significant. All data are presented as the mean and standard error of the mean (SEM).
RESULTS
Demographics of HCPS case samples.
Of all persons ill with HCPS to date, 60% have been male and 40% have been female (http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/caseinfo.htm). The mean age of patients with confirmed cases is 37 years (range, 10 to 75 years). In the present study, patients from whom plasma samples were available had a mean age of 36 years (range, 14 to 56 years) and were 22% male and 78% female. Control subjects had a mean age of 37 years (range, 24 to 83 years) and were 69% male and 31% female. Stratification of these HCPS cases by case severity revealed no significant difference in age between any groups or between HCPS cases and controls. For the nine patients who died from HCPS caused by SNV and for whom tissue samples were analyzed, the mean age was 34 years (range, 16 to 64 years), 33% were male, and 66% were female. Of these patients, six were Native Americans and the remainder were Caucasians. Thus, samples from both experimental and control groups were representative of the HCPS case population as a whole, in terms of both mean age and age range, although there was some skewing of the gender ratio in our HCPS case population.
Plasma NOx levels in HCPS cases.
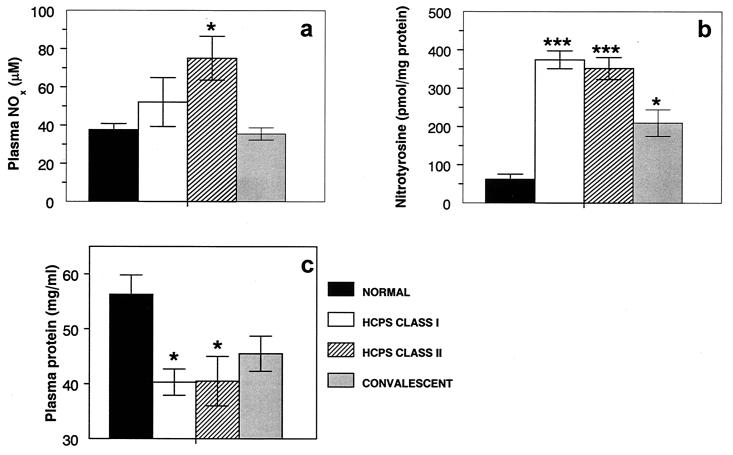
The final breakdown products of RONS are NO3− and NO2−. These products are easily measured in biological fluids by using Greiss reagents and are stable indicators of RONS generation. NOx levels in plasma samples from HCPS class I patients (52.1 ± 12.8 μM; n = 5) were not significantly elevated over control values (37.7 ± 3.2 μM; n = 6) (Fig. 1a). In contrast, levels of NOx in plasma samples from HCPS class II patients (75.0 ± 11.4 μM; n = 15) were significantly higher than control values (P < 0.01). When class II patients were divided into those who did (n = 8) and those who did not (n = 7) receive ECMO therapy, no effect of ECMO on NOx levels was detected. NOx levels in convalescent-phase plasma samples from patients who survived HCPS (35.5 ± 3.2 μM; n = 8) were very similar to control values but were significantly lower than those in class II HCPS patients (P < 0.005). For six patients, matched acute-phase (three patients each in HCPS classes I and II) and convalescent-phase plasma samples were available. For this group, plasma NOx levels were significantly (P < 0.05) higher during acute disease (74.4 ± 13.7 μM) than during recovery (37.7 ± 3.8 μM). Plasma NOx levels in acute disease for this group, which survived HCPS, did not differ significantly from those for nonsurvivors.
FIG. 1.
Plasma NOx (a), NT (b), and total protein (c) levels in HCPS patients. Plasma NO2− levels were determined by using Greiss reagents after conversion of NO3− to NO2− with Escherichia coli reductase. Plasma protein-associated NT content was determined by an ELISA with a polyclonal anti-NT antibody and nitrated BSA as a standard. NT values were normalized to plasma protein values measured by a standard BCA assay. A single asterisk indicates a significant difference from results for normal volunteers at a P value of <0.05; triple asterisks indicate a significant difference from results for normal volunteers at a P value of <0.0005. Results are expressed as the mean and SEM.
Plasma NT in HCPS cases.
NT residues are stable end products of RONS-mediated reactions (6). They serve as footprints of RONS action and are readily detectable by immunohistochemical analysis and ELISA. The NT content of plasma samples from HCPS cases was quantified by an ELISA. Significantly (P < 0.0005) increased levels of NT were found in the plasma of patients with HCPS classes I (374.4 ± 23.2 pmol/mg of protein; n = 6) and II (352.0 ± 28.9 pmol/mg of protein; n = 13) (Fig. 1b). When class II patients were divided into those who did (n = 8) and those who did not (n = 5) receive ECMO therapy, no effect of ECMO on NT levels was detected. The NT content of convalescent-phase plasma samples from patients who survived HCPS (209.7 ± 34.8 pmol/mg of protein; n = 9) declined toward but was still significantly (P < 0.05) higher than control values (62.1 ± 13.1 pmol/mg of protein; n = 6) and significantly (P < 0.005) lower than values for HCPS cases. There was no correlation between plasma NT and NOx levels. For seven patients (three in HCPS class I and four in HCPS class II), matched acute- and convalescent-phase plasma samples were available. For this group, plasma NT levels were significantly (P < 0.05) higher during acute disease (333.7 ± 45.5 pmol/mg of protein) than during recovery (167.6 ± 24.7 pmol/mg of protein). Plasma NT levels in acute disease for this group, which survived HCPS, did not differ significantly from those for nonsurvivors.
Plasma protein in HCPS cases.
Significant (P < 0.05) hypoproteinemia was noted for class I (40.3 ± 2.4 mg/ml; n = 6) and class II (40.5 ± 4.5 mg/ml; n = 13) HCPS cases compared to plasma protein levels in control samples (56.3 ± 3.5 mg/ml; n = 13) and convalescent-phase samples (45.5 ± 3.2 mg/ml; n = 9) (Fig. 1c). Plasma protein concentrations in control samples were within the normal range for humans.
Tissue NT in HCPS cases.
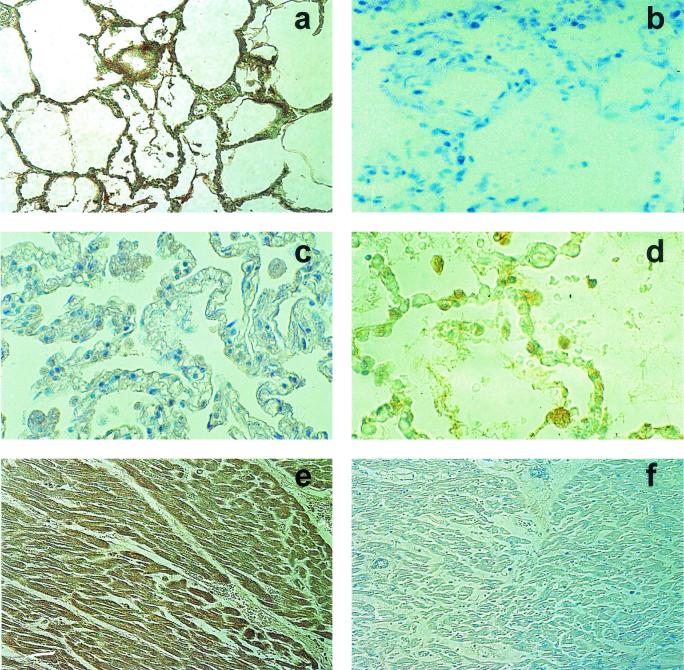
Since we found evidence of elevated NT adduct formation on plasma proteins in HCPS cases, we wished to determine whether NT adducts could also be detected in lung and cardiac tissues from patients who died of HCPS. Elevated NT immunoreactivity (brown) was detected by immunohistochemical analysis in lung tissues from 11 of 11 HCPS patients (Fig. 2a). NT immunoreactivity was particularly prominent in activated alveolar macrophages present in the alveoli (identified by immunoreactivity for human alveolar macrophage HAM-56; red in Fig. 2a) but was also present diffusely throughout the lung parenchyma, interstitial mononuclear cells, and interstitial connective tissue. Areas of lung tissues with more severe pathology appeared subjectively to have more intense parenchymal NT staining. No difference in NT reactivity in lung tissues was seen between the fatal HCPS cases caused by SNV (n = 9) and those caused by Andes virus (n = 2). The specificity of the NT reaction was confirmed by preincubation of the antibody with 10 mM free NT prior to use (Fig. 2b) and by substitution of normal rabbit serum for the primary antibody (data not shown). No NT immunoreactivity was detected in four of four samples of autopsy-derived normal human lung tissues (Fig. 2c).
FIG. 2.
NT adduct formation and iNOS expression in lungs from patients for whom HCPS was fatal. (a) NT immunoreactivity (brown) in lung tissue from an HCPS patient. NT immunoreactivity is particularly prominent in activated alveolar macrophages. (b) Absence of NT immunoreactivity in lung tissue from the same HCPS patient when antibody was preincubated with 10 mM free NT prior to use. (c) Absence of NT immunoreactivity in autopsy-derived normal human lung tissue. (d) Elevated iNOS immunoreactivity (brown) in infiltrating interstitial immunoblasts and alveolar macrophages in lung tissue from an HCPS patient. (e) NT immunoreactivity (brown) in cardiac tissue from an HCPS patient. (f) Absence of NT immunoreactivity in cardiac tissue from the same HCPS patient when antibody was replaced with normal rabbit serum. Paraffin-embedded sections from tissues fixed at autopsy were deparaffinized, rehydrated, and subjected to peroxidase and protein blocking. Tissue NT was detected with a rabbit anti-NT polyclonal IgG antibody. Human iNOS was detected by using clone 3 anti-human/mouse iNOS antibody (after antigen unmasking by boiling in citrate buffer).
For some patients, autopsy-derived samples of tissues other than lung tissues were also available for analysis. NT immunoreactivity was detected in cardiac myocytes from four of four patients (one was a patient infected with Andes virus) and was prominent in two of these patients (Fig. 2e; antibody control reaction, Fig. 2f). In addition, NT immunoreactivity was found in hepatocytes from one of two patients (although the patient may have had underlying hepatitis). NT immunoreactivity was also present in kidney tissues from one of three patients. No immunoreactivity was detected in splenic tissues or bone marrow (one sample of each was analyzed). Unfortunately, no normal samples of these tissues were available for analysis.
Expression of iNOS in HCPS cases.
In order to confirm that fatal HCPS was associated with elevated RONS generation, we evaluated the expression of iNOS in the lung parenchyma. The expression of iNOS is believed to be minimal in the normal lung (47). Elevated iNOS immunoreactivity (brown) in infiltrating interstitial immunoblasts and alveolar macrophages (red) was detected in lung tissues from 11 of 11 HCPS patients by immunohistochemical analysis (Fig. 2d). Although, as a result of incompatibility in staining pretreatments, costaining for iNOS and NT could not be performed, analysis of subjacent tissue sections indicated that iNOS staining was particularly prominent in areas with more severe pathology, in which NT immunoreactivity was also strongest. Indeed, many activated alveolar macrophages stained for both iNOS and NT. No iNOS immunoreactivity was detected in four of four samples of autopsy-derived normal human lung tissues (data not shown).
Plasma NOx and NT levels in NZB mice.
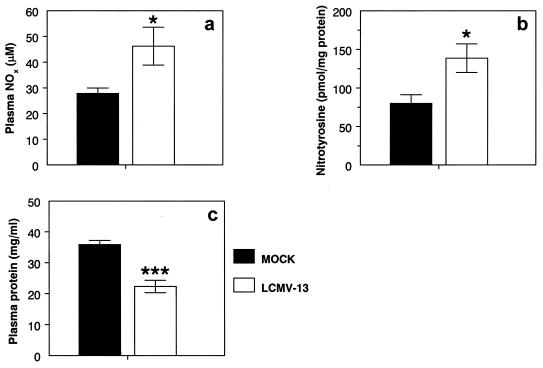
Adult NZB mice (M. musculus) infected intravenously with LCMV-13 develop acute lethal disease with pulmonary failure by 5 to 6 days after infection (60). At necropsy, lungs of infected mice show interstitial pneumonitis with mononuclear cell infiltrates, edema, and alveolar wall thickening, a pathology that clearly mimics that of HCPS. We wished to determine whether acute lethal disease in this model was associated with increased RONS generation. As in HCPS cases, significantly (P < 0.05) higher levels of NOx were found in the plasma of NZB mice sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13 (46.2 ± 7.4 μM; n = 10) than in the plasma of mock-infected controls (27.9 ± 2.0 μM; n = 6) (Fig. 3a). Likewise, significantly (P < 0.05) higher levels of NT were found in the plasma of NZB mice sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13 (138.7 ± 18.5 pmol/mg of protein; n = 13) than in the plasma of mock-infected controls (80.3 ± 11.1 pmol/mg of protein; n = 7) (Fig. 3b). Significant (P < 0.0005) hypoproteinemia was also detected in LCMV-13-infected mice (22.3 ± 2.0 mg/ml; n = 14) compared with mock-infected controls (35.9 ± 1.3 mg/ml; n = 18) (Fig. 3c).
FIG. 3.
Plasma NOx (a), NT (b), and total protein (c) levels in LCMV-13-infected NZB mice. NZB mice were sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13. Plasma NO2− levels were determined by using Greiss reagents after conversion of NO3− to NO2− with E. coli reductase. Plasma protein-associated NT content was determined by an ELISA with a polyclonal anti-NT antibody and nitrated BSA as a standard. NT values were normalized to plasma protein values measured by a standard BCA assay. A single asterisk indicates a significant difference from results for mock-infected animals at a P value of <0.05; triple asterisks indicate a significant difference from results for mock-infected animals at a P value of <0.0005. Results are expressed as the mean and SEM.
Tissue NT levels in SNV-infected deer mice and in LCMV-13-infected NZB mice.
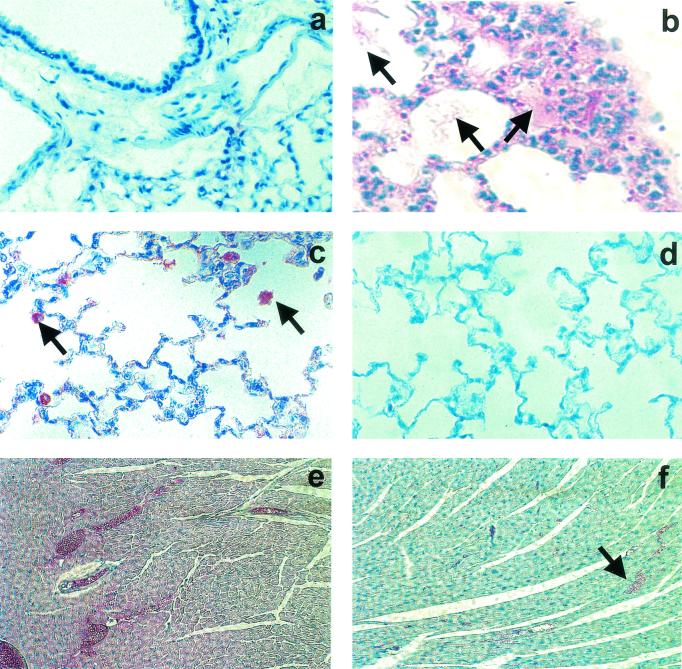
In contrast to the results for SNV-infected humans with fatal HCPS, no positive NT signal (red) was detected in 12 of 12 deer mice sacrificed 21 days after infection with SNV (time of peak viral replication in the lungs) (10) (Fig. 4a) or in 3 of 3 uninfected deer mice (data not shown). Additionally, we did not detect NT immunoreactivity in cardiac tissues from three of three SNV-infected deer mice (cardiac tissues were unavailable for other mice in this study; data not shown). However, when NZB mice were sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13, at which time significant alveolar flooding was evident histopathologically (Fig. 4b), NT immunoreactivity (red) was visible, particularly in alveolar macrophages, in lung tissues from 21 of 22 animals (Fig. 4c). NT immunostaining (red) was absent in lung tissues from three of three mock-infected NZB mice (Fig. 4d). Similarly, NT immunoreactivity was detected in cardiac myocytes from 18 of 19 LCMV-13-infected mice (Fig. 4e) (cardiac tissues were unavailable for other mice in this study) but not in myocardial tissues from two mock-infected animals (Fig. 4f).
FIG. 4.
Pulmonary NT in SNV-infected deer mice and LCMV-13-infected NZB mice. (a) Absence of NT immunoreactivity (red) in lung tissue from a deer mouse sacrificed at 21 days after infection with SNV (time of peak viral replication in the lungs). (b) Macrophage infiltration and alveolar flooding (arrows) in lungs of an NZB mouse sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13. (c) NT immunoreactivity (red) in lung tissue (particularly alveolar macrophages; arrows) from an NZB mouse sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13. (d) Absence of NT immunoreactivity (red) in lung tissue from a mock-infected NZB mouse. (e) NT immunoreactivity (red) in cardiac tissue from an NZB mouse sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13. (f) Absence of NT immunoreactivity (red) in cardiac tissue from a mock-infected NZB mouse (staining of erythrocytes is nonspecific; arrow). Paraffin-embedded lung sections were deparaffinized, rehydrated, and subjected to peroxidase and protein blocking. Tissue NT was detected with a rabbit anti-NT polyclonal IgG antibody. The specificity of the NT reaction was confirmed by preincubating the antibody with 10 mM free NT prior to use.
We were unable to detect iNOS in lung tissues from SNV-infected deer mice, but we were unable to determine whether this was the result of a lack of iNOS expression or a failure of the antibody to cross-react with deer mouse iNOS.
Wet lung/dry lung weight ratio.
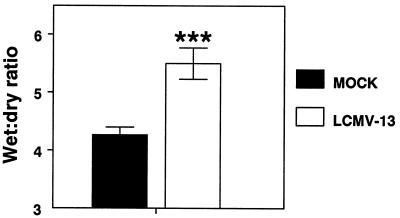
The wet lung/dry lung weight ratio is a reliable quantitative measure of extravascular lung water (66). Wet lung/dry weight ratios were significantly elevated in NZB mice 6.25 days after infection with 4 × 106 PFU of LCMV-13 (5.5 ± 0.27; n = 13) compared with mock-infected mice (4.27 ± 0.13; n = 12) (Fig. 5).
FIG. 5.
Wet lung/dry lung weight ratios in LCMV-13-infected NZB mice. NZB mice were sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13. Following exsanguination, the lungs were removed, weighed, and dried in an oven at 55°C for 7 days. After being dried, the lungs were weighed again. The wet lung/dry lung weight ratio was then calculated as an index of intrapulmonary fluid accumulation. Triple asterisks indicate a significant difference from results for mock-infected animals at a P value of <0.0005. Results are expressed as the mean and SEM.
Effect of 1400W on LCMV-13-induced disease.
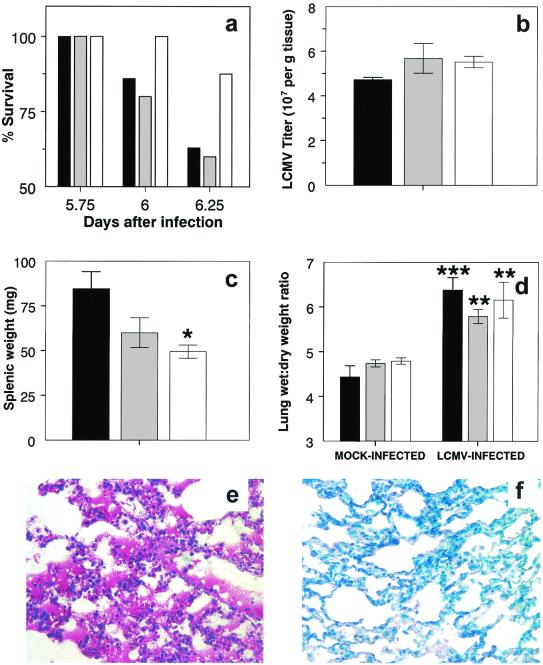
Since we hypothesized that RONS may play a significant role in the pathogenesis of both HCPS and HCPS-like disease in NZB mice, we wished to determine whether RONS blockade would improve morbidity and mortality in LCMV-13-infected mice. 1400W is a highly specific inhibitor of iNOS, with minimal effects on the other isoforms of NOS, and can be administered to mice by intraperitoneal injection every 12 h. Untreated and saline- or 1400W-treated LCMV-13-infected mice began to show signs of respiratory distress (hunched posture, labored breathing, and staring coat) between 5 and 5.5 days after infection, although signs were subjectively less severe in 1400W-treated animals. When examined at 5.5 days after infection, all mice were still alive, but by 6 days, LCMV-13-infected mice had begun to die; the experiment was immediately terminated on humane grounds. By this time, 10 of 27 LCMV-13-infected untreated mice (37%) and 2 of 5 infected saline-treated mice (40%) had died. In contrast, only one of eight LCMV-13-infected 1400W-treated mice (13%) had died (Fig. 6a). Mock-infected mice remained clinically normal throughout the study period, and neither saline nor 1400W treatment appeared to have any adverse effects on this group.
FIG. 6.
Effect of 1400W treatment on LCMV-13-infected NZB mice. (a) Increased survival of LCMV-13-infected 1400W-treated NZB mice. (b) 1400W treatment does not affect splenic virus titers. (c) 1400W treatment reduces splenic weight in LCMV-13-infected NZB mice. (d) 1400W treatment does not reduce wet lung/dry lung weight ratios. (e) Macrophage activation and alveolar flooding in lung tissue from an LCMV-13-infected 1440W-treated NZB mouse. (f) Absence of NT immunoreactivity (red) in lung tissue from an LCMV-13-infected 1400W-treated NZB mouse. Untreated (black bar), saline-treated (grey bar), and 1400w-treated (white bar) NZB mice were sacrificed at 6.25 days after intravenous infection with 4 × 106 PFU of LCMV-13. Virus recovered from spleens was titrated by standard LCMV plaque assays, and values were normalized to the splenic wet weight. Following exsanguination, the lungs were removed, weighed, and dried in an oven at 55°C for 7 days. After being dried, the lungs were weighed again. The wet lung/dry lung weight ratio was then calculated as an index of intrapulmonary fluid accumulation. Tissue NT was detected as described in the legend to Fig. 4. A single asterisk indicates a significant difference from results for mock-infected animals at a P value of <0.05; double asterisks indicate a significant difference from results for mock-infected animals at a P value of <0.005; triple asterisks indicate a significant difference from results for mock-infected animals at a P value of <0.0005. Results are expressed as the mean and SEM.
Despite the dramatic effect of 1400W treatment on the LCMV-13-induced mortality of NZB mice, treatment with either saline or 1400W had no significant effect on splenic virus titers in this group (1.1 × 103 ± 0.3 × 103/g [n = 6] versus 0.9 × 103 ± 0.1 × 103/g in untreated control animals [n = 6] and 1.3 × 103 ± 0.7 × 103/g in saline-treated animals [n = 3]) (Fig. 6b), although splenic weights after 1400W treatment were significantly (P < 0.05) lower than those in untreated animals (49.6 ± 4.6 mg versus 84.6 ± 9.5 mg in untreated control animals and 60.2 ± 8.4 mg in saline-treated animals) (Fig. 6c). Interestingly, 1400W treatment did not result in a significant reduction in the wet lung/dry lung weight ratios in LCMV-13-infected mice, indicating that iNOS blockade had no effect on the formation of pulmonary edema (6.2 ± 0.4 [n = 6] versus 6.4 ± 0.3 in untreated control animals [n = 6] and 5.8 ± 0.2 in saline-treated animals [n = 5]) (Fig. 6d). Neither saline nor 1400W treatment had any effect on the wet lung/dry lung weight ratios (4.8 ± 0.1 [n = 7] versus 4.4 ± 0.2 in untreated control animals [n = 6] and 4.7 ± 0.1 in saline-treated animals [n = 4]) (Fig. 6d) or plasma NT levels in mock-infected mice. Interestingly, 1400W treatment also had no effect on plasma NT levels in LCMV-13-infected 1400W-treated mice (although plasma was available only from three animals; data not shown). Moreover, although lungs from only two LCMV-13-infected 1400W-treated mice were examined histopathologically, both animals demonstrated significant pulmonary pathology (alveolar flooding, alveolar macrophage activation, and peribronchial edema) (Fig. 6e), despite a reduction in NT immunoreactivity (Fig. 6f).
DISCUSSION
To our knowledge, the data presented in this article provide the first direct ex vivo evidence of upregulated RONS generation in response to viral infection in the human lung and the first direct evidence of increased pulmonary RONS generation in response to a hantavirus. This is also the first study in which a direct comparison has been made between any aspect of the cellular immune responses of humans and deer mice to SNV. Moreover, the distinct association between elevated RONS generation and induction of disease in both human HCPS cases (caused by either North or South American hantaviruses) and LCMV-13-infected NZB mice, together with the lack of RONS generation in response to infection of deer mice with SNV, argues that RONS play a significant role in the pathogenesis of HCPS. Furthermore, because the difference in disease manifestations between hemorrhagic fever with renal syndrome (HFRS) and HCPS does not preclude the involvement of common pathophysiologic mechanisms (59), our study may provide further insight into the pathogenesis of HFRS, in which a direct role for RONS has not been established. Finally, the protective effect of a specific iNOS inhibitor against lethality induced by infection of NZB mice with LCMV-13 indicates that such inhibitors may be a useful addition to the sparse therapeutic armamentarium currently available to combat HCPS, a disease with a mortality rate that remains at more than 30%.
Understanding of the pathogenesis of HCPS has been hampered by the paradoxical nature of the disease pathology. The high protein content of pulmonary edema fluid from HCPS patients (12) suggests that alveolar flooding results from increased alveolar permeability (22), yet there is neither histopathologic nor ultrastructural evidence of any alteration in alveolar epithelial or endothelial cell integrity (57). Moreover, the capillary leak does not seem to result from direct cytopathicity of SNV for pulmonary endothelial cells, which appears to be minimal (41, 59). Because direct infusion of tumor necrosis factor alpha or interleukin 2 has been shown to induce plasma leakage in both experimental animals and human volunteers (4), it has been proposed that these cytokines may play a role in endothelial cell activation and dysfunction, leading to the capillary leak syndrome in both HCPS and HFRS (59). Nevertheless, the mechanism by which proinflammatory cytokines effect changes in capillary permeability remains unknown. In contrast, although the mechanisms by which RONS cause functional damage to proteins and lipids remain poorly understood, there is no doubt that RONS can directly cause such damage (7). Moreover, RONS have been shown to alter alveolar epithelial and endothelial cell permeability, in some cases in the absence of detectable ultrastructural injury (30, 51, 56). RONS are therefore much more likely to be the terminal effectors of damage to the lung than are proinflammatory cytokines, which nonetheless play a vital role as activators of alveolar macrophages and pneumocytes and triggers for RONS generation. Finally, it should be noted that HCPS patients generally die from myocardial suppression rather than as a direct consequence of alveolar flooding. It is possible that cardiac suppression is mediated by factors that are released into the pulmonary circulation and that affect the heart as the next organ downstream from the pulmonary bed. RONS have been proposed to be mediators of cardiac suppression in sepsis (see below), and we propose that they may similarly mediate myocardial suppression in HCPS.
Our study used multiple complementary approaches to confirm that human HCPS is associated with elevated RONS generation, including detection of NOx, the final breakdown product of RONS (26) in plasma; detection of NT in plasma, lung parenchyma, and cardiac tissue; and detection of iNOS immunoreactivity in lung tissue. In earlier studies, Groeneveld and coworkers (28)and Linderholm and coworkers (50)detected increased concentrations of NOx in plasma of patients with nephropathia epidemica (NE), a mild form of HFRS caused by Old World hantaviruses. (Similarly, in a recent, limited study of experimental NE in three cynomolgus macaques [Macaca fascicularis], an elevated level of plasma “NO” [presumably NOx] was detected after seroconversion [46]). Likewise, we found mild increases in plasma NOx levels in class I HCPS patients and significant elevations in class II HCPS patients which declined to control levels during convalescence. Plasma NOx levels in our normal human volunteer samples (37.7 ± 3.2 μM; n = 6) were very comparable to those reported by Groeneveld et al. (28) (33 ± 3 μM; n = 19) and by Zhu et al. (77) (26 ± 3 μM; n = 9). Likewise, plasma NOx levels in our class I and II HCPS patients were similar to those reported by Groeneveld et al. (28) for patients with NE (69 ± 17 μM; n = 7) but without significant impairment of renal function (serum creatinine level, <200 μM), which is not a prominent feature of HCPS. It would be informative to determine whether bronchoalveolar lavage fluid NOx levels are elevated in HCPS patients (as they are in patients with acute lung injury) (77), but such samples were unfortunately not available for this study.
NT levels in plasma from normal controls and HCPS patients were comparable to those reported for patients with ARDS (77). Indeed, Zhu et al. found that patients with acute lung injury or ARDS had a mean plasma NT level of about 400 pmol/mg of protein, very similar to that seen in class II HCPS patients. Although both plasma NOx and NT levels were elevated in HCPS patients and declined toward control values in convalescing patients, we did not find a significant correlation between plasma NOx and NT levels in individual patients. A similar discordance between plasma NOx and NT levels has been noted for patients with sepsis (67). These findings suggest that plasma NOx concentrations may not always accurately reflect ·NO production in inflammatory disease and that, where possible, both assays should be performed. The finding of hypoproteinemia in our cohort of class I and II HCPS patients is entirely consistent with the results of other studies (18). It should be noted that even after normalizing for this difference in plasma protein concentrations between controls and HCPS patients (by multiplying control NT values by 1.5), plasma NT levels remained fourfold higher in HCPS patients, indicating that this was a true elevation rather than an artifact of hypoproteinemia.
In a previous study, severe HCPS disease (class II or III) was associated with significantly (P < 3.4 × 10−5) reduced neutralizing antibody titers to SNV (9). We had hoped that plasma NOx and/or NT levels might similarly prove of predictive value for disease progression and/or survival. The NOx assay is relatively simple to perform and, based on our data, an increase in plasma NOx levels might suggest disease progression from class I to class II. However, sample sizes in both groups were relatively small, and we had no access to serial samples from individual patients. Moreover, based on our current data, plasma NOx levels during acute HCPS do not appear to be predictive of disease outcome. Unfortunately, and unlike plasma NOx levels, plasma NT levels in patients with class I or class II HCPS did not significantly differ, and so it is unlikely that plasma NT levels can be used as a predictor of HCPS disease progression (this assay is, in any case, difficult to perform and interpret).
Nitrated proteins have been detected in lung tissues from infants who died with respiratory failure or ARDS and adults with ARDS (30). Protein nitration and oxidation by RONS in vitro have been associated with diminished function of a variety of crucial proteins that are present in the alveolar space and plasma and that have been shown to be nitrated in the plasma of patients with ARDS (25, 31). The strong NT immunoreactivity of alveolar macrophages in lung sections from all fatal HCPS cases analyzed is very similar to that described for alveolar macrophages isolated from the bronchoalveolar lavage fluid of ARDS patients (65). Moreover, this finding is consistent with the elevations in plasma NT levels detected in HCPS cases. Finally, it appears that elevated lung NT immunoreactivity may be a common feature of HCPS caused by both North and South American hantaviruses.
Previous studies suggested that iNOS is constitutively expressed in human upper-airway epithelium (29) and occasional alveolar macrophages (47), but this expression may be the result of chronic exposure of these cells to inhaled pollutants and microbes. The expression of iNOS in other regions of the normal human lung is believed to be minimal and was found to be so in our normal lung tissue samples. In contrast, iNOS has been immunolocalized to human lung tissue and inflammatory cells obtained from patients with ARDS (65), bacterial pneumonia, or sepsis (72). Our finding of elevated iNOS expression in lung tissue from fatal HCPS cases, particularly for large, activated alveolar macrophages, is consistent with these earlier findings and is again the first demonstration of elevated iNOS expression in the human lung in response to a pulmonary viral pathogen. While elevated iNOS immunoreactivity does not directly imply enzymatic activity and ·NO generation, in conjunction with our finding of increased deposition of NT in the lung, it suggests that increased iNOS activity may be a component of HCPS pathogenesis (NT can be formed in the absence of iNOS activity [34], but only in the presence of functional neutrophils [35], which are by no means a prominent feature of the immune response to SNV in humans). The underlying mechanism of iNOS upregulation remains unclear but is likely to involve the action of proinflammatory cytokines. Gamma interferon and tumor necrosis factor alpha, both of which are known to be produced in increased amounts in patients with HCPS (21, 54; S. Q. Simpson, V. Mapel, F. T. Koster, J. M. Montoya, D. E. Bice, and A. J. Williams, abstract from Scientific Highlights, abstract of original investigation, 29 Oct.-2 Nov. 1995, New York, N.Y., Chest 108:97.S, 1995; S. Q. Simpson, V. Mapel, F. T. Koster, J. M. Montoya, D. E. Bice, and A. J. Williams, abstract from 25th Educational and Scientific Symposium, Society of Critical Care Medicine, New Orleans, La., Crit. Care Med. 24:A26, 1996), are potent inducers of iNOS expression (70) and O2− generation (3) in humans.
Until the very recent description of a novel Syrian hamster (Mesocricetus auratus) model of Andes virus infection (39), studies of HCPS pathogenesis have been particularly hampered by the lack of a suitable animal model. However, the high biosafety requirements severely restrict the utility of the hamster model, which also has the more minor limitation that it does not use a North American hantavirus. We therefore used two other complementary models in our study; both have particular advantages and limitations. The first animal model that we used is the deer mouse, which is the natural reservoir of SNV. This model has the advantages that it uses both deer mice and an SNV isolate from exactly the same geographic locale and that the virus has never been propagated in tissue cultures, only in deer mice. Thus, the model is as close to the natural infection as possible (10). However, its utility is somewhat limited by the absence of pulmonary pathology and dysfunction, and so it can serve only as a “negative model,” in which the absence of a pathogenic factor is more likely to be of significance than its presence. Although a direct comparison of the distributions of viral antigens has been made between infected deer mice and humans (27), characterization of immune responses to SNV in this model has not yet been performed, primarily because of a lack of cross-reactivity of immunologic reagents between the deer mouse and the more commonly used experimental animal species or humans. Because assays of NOx and NT formation are dependent on the detection of highly stable and non-species-specific end products, we were able to circumvent this problem. Our data obtained with this model are therefore the first to show (other than in terms of histopathology) discordance in the pulmonary inflammatory response to SNV between the natural host and humans. Our findings would indicate that RONS generation and NT formation in the lungs and heart are specific components of SNV-induced disease and not just a feature of SNV infection. Our findings again suggest that increased RONS generation is part of a human-specific response to SNV and that it may therefore play a role in HCPS pathogenesis.
The second animal model that we used, NZB mice infected with LCMV-13, reproduces human hantavirus disease pathology in a murine (M. musculus) system. Although the NZB mouse model has the disadvantage of using a heterologous virus, the pathology clearly reproduces that of HCPS (in our experiments, significant alveolar flooding could be found at 6 days after infection) and at a lower animal biosafety level than the hamster model. It therefore allows testing of therapeutic and experimental interventions to reduce disease, which the deer mouse model cannot do. It should be noted, however, that severe hypotension is a prominent feature of disease in this model; this factor rendered the collection of adequate blood volumes extremely difficult. Our finding of elevated plasma NOx and NT levels in LCMV-13-infected NZB mice is highly consistent with our findings for HCPS cases. Plasma NOx and NT levels in mock-infected NZB mice were comparable to those previously reported for C57BL/6 mice (23, 35). It will be interesting to determine whether there is similar evidence for elevated RONS and proinflammatory cytokine generation in the Andes virus-infected Syrian hamster model of HCPS.
The wet lung/dry lung weight ratio is a measure of extravascular lung water (66). Wet lung/dry lung weight ratios in mock-infected NZB mice were comparable to those reported for C57BL/6 mice (43), while ratios in LCMV-13-infected NZB mice were somewhat higher than those reported for C57BL/6 mice following endotoxin administration (48). We did not find a significant effect of 1400W on wet lung/dry lung weight ratios in LCMV-13-infected NZB mice. A trivial explanation for this finding would be that the dosages of 1400W administered to the mice were insufficient to fully inhibit iNOS activity in the pulmonary epithelium, although this explanation seems unlikely. More likely, this finding suggests that ·NO and ONOO− do not play a role in the formation of pulmonary edema and alveolar flooding, at least in the NZB mouse model of HCPS. Nevertheless, it does not preclude a role for reactive oxygen species (ROS) in mediating these effects. Although the administration of ONOO− can induce pulmonary edema experimentally (5), O2− and hydrogen peroxide in the absence of ·NO are equally capable of altering pulmonary endothelial cell permeability and inducing pulmonary edema without causing significant endothelial cell death (45, 68). Moreover, ROS have been shown to trigger damage to the alveolar epithelium, resulting in permeability-type alveolar edema (38, 51), consistent with the high levels of protein alveolar fluid seen in HCPS cases. Although we have not specifically demonstrated increased ROS generation in this study, it can be inferred from the presence of large amounts of NT found in the lungs and plasma of both HCPS patients and LCMV-13-infected NZB mice. While NT can be generated in an O2−-independent fashion, by the interaction of HOCl (the product of neutrophil myeloperoxidase) with NO2− (19), this reaction is unlikely to be of relevance to HCPS, since this disease is not associated with a significant granulocytic infiltrate in the lungs (57). Therefore, despite indicating a lack of involvement of RONS in the induction of pulmonary edema, our data in no way exclude a role for ROS in the formation of pulmonary edema in HCPS-like disease in NZB mice.
It is interesting, although not altogether surprising, that 1400W treatment of LCMV-13-infected NZB mice had no effect on splenic virus titers. Although RONS have been shown to have antiviral effects against influenza A and B viruses (61), herpes simplex virus type 1 (16), rhinovirus (62), and vaccinia virus (33) in vitro, administration of the iNOS inhibitor l-NMMA to influenza virus-infected mice had no effect on viral replication (2, 44). Like the situation for influenza virus, it appears that LCMV-13-induced disease in NZB mice is not directly related to viral burden but is instead a consequence of the immune response to infection. Similarly, the development and progression of HCPS do not appear to be directly related to viral load (71). Indeed, HCPS, when it is lethal, is usually rapidly so, and virus levels are usually declining by the time patients are hospitalized (37). We are currently unable to account for the reduction in splenic weights associated with 1400W treatment.
The finding of reduced mortality in LCMV-13-infected 1400W-treated NZB mice was surprising, particularly since pulmonary edema did not seem any less severe than that in untreated controls. However, it is possible that like HCPS patients, who generally die as a consequence of myocardial failure (64), LCMV-13-infected mice do not die as a result of pulmonary lesions or hypoxemia. Although we can only speculate about the underlying mechanism by which iNOS inhibition may reduce mortality, we propose that the protective effect of 1400W against mortality in LCMV-13-infected NZB mice results from the inhibition of myocardial depression. Results of several studies have demonstrated a role for iNOS and RONS in the suppression of cardiac myocyte contractility in sepsis (23, 49, 52, 63, 69). Our finding of increased NT immunoreactivity in cardiac myocytes in LCMV-13-infected NZB mice lends support to the hypothesis that myocardial suppression in this model of HCPS is RONS induced (unfortunately, cardiac tissue from LCMV-13-infected 1400W-treated NZB mice was not available for NT immunostaining). The cause of myocardial insufficiency in HCPS remains undefined, and we propose that ·NO and/or ONOO− similarly act as potent myocardial depressant factors in HCPS. Our finding of increased NT immunoreactivity in cardiac tissue samples from a limited number of fatal HCPS cases (caused by either North or South American hantaviruses) provides support for this hypothesis. While the issue of cardiac endothelial cell infection with SNV needs to be reexamined in humans (it is now well established that the cardiac endothelium is heavily infected in SNV-infected deer mice) (10), it is possible that myocardial suppression in HCPS results from locally generated rather than circulating RONS. In addition, our findings suggest that other scavengers of RONS or ROS, such as allopurinol or N-acetylcysteine, may be of therapeutic value in HCPS.
Despite the fact that we were unable to demonstrate a specific role for RONS in the pathogenesis of alveolar flooding in HCPS, the fact that RONS inhibition appears to impart a survival benefit in a mouse model of HCPS offers some hope for improvements in HCPS therapy, particularly if these findings can be reproduced in the Andes virus-infected hamster model of HCPS. From a clinical standpoint, RONS are much more amenable to therapeutic inhibition than are proinflammatory cytokines (74). 1440W is highly selective for iNOS and thus has few of the hypertensive effects associated with older NOS inhibitors. In contrast, cytokine action can be blocked only by the administration of large quantities of specific monoclonal antibodies, and such approaches have rarely been successful. To our knowledge, neither 1400W nor the less selective iNOS inhibitor aminoguanidine has been used to date in human clinical studies, but their relatively low toxicity in animal studies, their lack of effect on blood pressure, and the relative severity of HCPS indicate that such agents may be useful adjuncts to supportive therapy for HCPS. Nevertheless, it should be noted that further studies are necessary before findings with the LCMV model of HCPS can be extrapolated to the human disease.
In summary, we found that SNV infection of humans but not deer mice was associated with elevated RONS generation. Likewise, lethal infection of NZB mice with LCMV-13, which is a model for HCPS, was also associated with elevated RONS generation. Furthermore, we found that treatment of NZB mice with a specific iNOS inhibitor protected against lethality induced by LCMV-13, suggesting that iNOS inhibition strategies may be useful in the treatment of HCPS, possibly because of protective effects on the myocardium.
Acknowledgments
This study was supported by grants HL31197, HL51173, and AI41692 from the National Institutes of Health. I.C.D. is a Parker B. Francis Families Fellow in Pulmonary Research.
We gratefully acknowledge the excellent technical assistance of Glenda Davis, Marilyn Shackleford, Jane Hosmer, and Roger Harris.
REFERENCES
- 1.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaike, T., Y. Noguchi, S. Ijiri, K. Setoguchi, M. Suga, Y. M. Zheng, B. Dietzschold, and H. Maeda. 1996. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. USA 93:2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 4.Baluna, R., and E. S. Vitetta. 1997. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology 37:117-132. [DOI] [PubMed] [Google Scholar]
- 5.Beckman, D. L., P. Mehta, V. Hanks, W. H. Rowan, and L. Liu. 2000. Effects of peroxynitrite on pulmonary edema and the oxidative state. Exp. Lung Res. 26:349-359. [DOI] [PubMed] [Google Scholar]
- 6.Beckman, J. S., H. Ischiropoulos, L. Zhu, W. M. van der, C. Smith, J. Chen, J. Harrison, J. C. Martin, and M. Tsai. 1992. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 298:438-445. [DOI] [PubMed] [Google Scholar]
- 7.Beckman, J. S., and W. H. Koppenol. 1996. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271:C1424-C1437. [DOI] [PubMed]
- 8.Beckman, J. S., Y. Z. Ye, P. G. Anderson, J. Chen, M. A. Accavitti, M. M. Tarpey, and C. R. White. 1994. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe-Seyler 375:81-88. [DOI] [PubMed] [Google Scholar]
- 9.Bharadwaj, M., R. Nofchissey, D. Goade, F. Koster, and B. Hjelle. 2000. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J. Infect. Dis. 182:43-48. [DOI] [PubMed] [Google Scholar]
- 10.Botten, J., K. Mirowsky, D. Kusewitt, M. Bharadwaj, J. Yee, R. Ricci, R. M. Feddersen, and B. Hjelle. 2000. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus). Proc. Natl. Acad. Sci. USA 97:10578-10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botten, J., R. Nofchissey, H. Ahern, P. Rodriguez-Moran, I. A. Wortman, D. Goade, T. L. Yates, and B. Hjelle. 2000. Outdoor facility for quarantine of wild rodents infected with hantavirus. J. Mammal. 81:250-259. [Google Scholar]
- 12.Bustamante, E. A., H. Levy, and S. Q. Simpson. 1997. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest 112:1133-1136. [DOI] [PubMed] [Google Scholar]
- 13.Chapman, L. E., G. J. Mertz, C. J. Peters, H. M. Jolson, A. S. Khan, T. G. Ksiazek, F. T. Koster, K. F. Baum, P. E. Rollin, A. T. Pavia, R. C. Holman, J. C. Christenson, P. J. Rubin, R. E. Behrman, L. J. Bell, G. L. Simpson, R. F. Sadek, et al. 1999. Intravenous ribavirin for hantavirus pulmonary syndrome: safety and tolerance during 1 year of open-label experience. Antivir. Ther. 4:211-219. [DOI] [PubMed] [Google Scholar]
- 14.Childs, J. E., T. G. Ksiazek, C. F. Spiropoulou, J. W. Krebs, S. Morzunov, G. O. Maupin, K. L. Gage, P. E. Rollin, J. Sarisky, and R. E. Enscore. 1994. Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. J. Infect. Dis. 169:1271-1280. [DOI] [PubMed] [Google Scholar]
- 15.Chizhikov, V. E., C. F. Spiropoulou, S. P. Morzunov, M. C. Monroe, C. J. Peters, and S. T. Nichol. 1995. Complete genetic characterization and analysis of isolation of Sin Nombre virus. J. Virol. 69:8132-8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croen, K. D. 1993. Evidence for an antiviral effect of nitric oxide: inhibition of herpes simplex virus type 1 replication. J. Clin. Investig. 91:2446-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis, I., and S. Matalon. 2001. Reactive species in viral pneumonitis: lessons from animal models. News Physiol. Sci. 16:185-190. [DOI] [PubMed] [Google Scholar]
- 18.Duchin, J. S., F. T. Koster, C. J. Peters, G. L. Simpson, B. Tempest, S. R. Zaki, T. G. Ksiazek, P. E. Rollin, S. Nichol, E. T. Umland, et al. 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 330:949-955. [DOI] [PubMed] [Google Scholar]
- 19.Eiserich, J. P., M. Hristova, C. E. Cross, A. D. Jones, B. A. Freeman, B. Halliwell, and A. van der Vliet. 1998. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391:393-397. [DOI] [PubMed] [Google Scholar]
- 20.Elliott, L. H., T. G. Ksiazek, P. E. Rollin, C. F. Spiropoulou, S. Morzunov, M. Monroe, C. S. Goldsmith, C. D. Humphrey, S. R. Zaki, and J. W. Krebs. 1994. Isolation of the causative agent of hantavirus pulmonary syndrome. Am. J. Trop. Med. Hyg. 51:102-108. [DOI] [PubMed] [Google Scholar]
- 21.Ennis, F. A., J. Cruz, C. F. Spiropoulou, D. Waite, C. J. Peters, S. T. Nichol, H. Kariwa, and F. T. Koster. 1997. Hantavirus pulmonary syndrome: CD8+ and CD4+ cytotoxic T lymphocytes to epitopes on Sin Nombre virus nucleocapsid protein isolated during acute illness. Virology 238:380-390. [DOI] [PubMed] [Google Scholar]
- 22.Fein, A., R. F. Grossman, J. G. Jones, E. Overland, L. Pitts, J. F. Murray, and N. C. Staub. 1979. The value of edema fluid protein measurement in patients with pulmonary edema. Am. J. Med. 67:32-38. [DOI] [PubMed] [Google Scholar]
- 23.Feng, Q., X. Lu, D. L. Jones, J. Shen, and J. M. Arnold. 2001. Increased inducible nitric oxide synthase expression contributes to myocardial dysfunction and higher mortality after myocardial infarction in mice. Circulation 104:700-704. [DOI] [PubMed] [Google Scholar]
- 24.Garvey, E. P., J. A. Oplinger, E. S. Furfine, R. J. Kiff, F. Laszlo, B. J. Whittle, and R. G. Knowles. 1997. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 272:4959-4963. [DOI] [PubMed] [Google Scholar]
- 25.Gole, M. D., J. M. Souza, I. Choi, C. Hertkorn, S. Malcolm, R. F. Foust, I. I. I., B. Finkel, P. N. Lanken, and H. Ischiropoulos. 2000. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L961-L967. [DOI] [PubMed]
- 26.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 27.Green, W., R. Feddersen, O. Yousef, M. Behr, K. Smith, J. Nestler, S. Jenison, T. Yamada, and B. Hjelle. 1998. Tissue distribution of hantavirus antigen in naturally infected humans and deer mice. J. Infect. Dis. 177:1696-1700. [DOI] [PubMed] [Google Scholar]
- 28.Groeneveld, P. H., Colson, P., Kwappenberg, K. M. C., and J. Clement. 1995. Increased production of nitric oxide in patients infected with the European variant of hantavirus. Scand. J. Infect. Dis. 27:453-456. [DOI] [PubMed] [Google Scholar]
- 29.Guo, F. H., H. R. De Raeve, T. W. Rice, D. J. Stuehr, F. B. Thunnissen, and S. C. Erzurum. 1995. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc. Natl. Acad. Sci. USA 92:7809-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddad, I. Y., G. Pataki, P. Hu, C. Galliani, J. S. Beckman, and S. Matalon. 1994. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J. Clin. Investig. 94:2407-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad, I. Y., S. Zhu, H. Ischiropoulos, and S. Matalon. 1996. Nitration of surfactant protein A results in decreased ability to aggregate lipids. Am. J. Physiol. 270:L281-L288. [DOI] [PubMed]
- 32.Hallin, G. W., S. Q. Simpson, R. E. Crowell, D. S. James, F. T. Koster, G. J. Mertz, and H. Levy. 1996. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit. Care Med. 24:252-258. [DOI] [PubMed] [Google Scholar]
- 33.Harris, N., R. M. Buller, and G. Karupiah. 1995. Gamma interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. J. Virol. 69:910-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickman-Davis, J., J. Gibbs-Erwin, J. R. Lindsey, and S. Matalon. 1999. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc. Natl. Acad. Sci. USA 96:4953-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickman-Davis, J. M., J. R. Lindsey, and S. Matalon. 2001. Cyclophosphamide decreases nitrotyrosine formation and inhibits nitric oxide production by alveolar macrophages in mycoplasmosis. Infect. Immun. 69:6401-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hjelle, B., S. A. Jenison, D. E. Goade, W. B. Green, R. M. Feddersen, and A. A. Scott. 1995. Hantaviruses: clinical, microbiologic, and epidemiologic aspects. Crit. Rev. Clin. Lab. Sci. 32:469-508. [DOI] [PubMed] [Google Scholar]
- 37.Hjelle, B., C. F. Spiropoulou, N. Torrez-Martinez, S. Morzunov, C. J. Peters, and S. T. Nichol. 1994. Detection of Muerto Canyon virus RNA in peripheral blood mononuclear cells from patients with hantavirus pulmonary syndrome. J. Infect. Dis. 170:1013-1017. [DOI] [PubMed] [Google Scholar]
- 38.Holm, B. A., R. H. Notter, J. Siegle, and S. Matalon. 1985. Pulmonary physiological and surfactant changes during injury and recovery from hyperoxia. J. Appl. Physiol. 59:1402-1409. [DOI] [PubMed] [Google Scholar]
- 39.Hooper, J. W., T. Larsen, D. M. Custer, and C. S. Schmaljohn. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 289:6-14. [DOI] [PubMed] [Google Scholar]
- 40.Huie, R. E., and S. Padmaja. 1993. The reaction of NO with superoxide. Free Radic. Res. Commun. 18:195-199. [DOI] [PubMed] [Google Scholar]
- 41.Hutchinson, K. L., C. J. Peters, and S. T. Nichol. 1996. Sin Nombre virus mRNA synthesis. Virology 224:139-149. [DOI] [PubMed] [Google Scholar]
- 42.Ischiropoulos, H., L. Zhu, and J. S. Beckman. 1992. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 298:446-451. [DOI] [PubMed] [Google Scholar]
- 43.Kaner, R. J., J. V. Ladetto, R. Singh, N. Fukuda, M. A. Matthay, and R. G. Crystal. 2000. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 22:657-664. [DOI] [PubMed] [Google Scholar]
- 44.Karupiah, G., J. H. Chen, S. Mahalingam, C. F. Nathan, and J. D. MacMicking. 1998. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 188:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kevil, C. G., T. Oshima, and J. S. Alexander. 2001. The role of p38 MAP kinase in hydrogen peroxide mediated endothelial solute permeability. Endothelium 8:107-116. [DOI] [PubMed] [Google Scholar]
- 46.Klingstrom, J., A. Plyusnin, A. Vaheri, and A. Lundkvist. 2002. Wild-type Puumala hantavirus infection induces cytokines, C-reactive protein, creatinine, and nitric oxide in cynomolgus macaques. J. Virol. 76:444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobzik, L., D. S. Bredt, C. J. Lowenstein, J. Drazen, B. Gaston, D. Sugarbaker, and J. S. Stamler. 1993. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am. J. Respir. Cell Mol. Biol. 9:371-377. [DOI] [PubMed] [Google Scholar]
- 48.Kristof, A. S., P. Goldberg, V. Laubach, and S. N. Hussain. 1998. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am. J. Respir. Crit. Care Med. 158:1883-1889. [DOI] [PubMed] [Google Scholar]
- 49.Kumar, A., R. Brar, P. Wang, L. Dee, G. Skorupa, F. Khadour, R. Schulz, and J. E. Parrillo. 1999. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am. J. Physiol. 276:R265-R276. [DOI] [PubMed]
- 50.Linderholm, M., P. H. Groeneveld, and A. Tarnvik. 1996. Increased production of nitric oxide in patients with hemorrhagic fever with renal syndrome—relation to arterial hypotension and tumor necrosis factor. Infection 24:337-340. [DOI] [PubMed] [Google Scholar]
- 51.Matalon, S., and E. A. Egan. 1981. Effects of 100% O2 breathing on permeability of alveolar epithelium to solute. J. Appl. Physiol. 50:859-863. [DOI] [PubMed] [Google Scholar]
- 52.Mihm, M. J., C. M. Coyle, B. L. Schanbacher, D. M. Weinstein, and J. A. Bauer. 2001. Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc. Res. 49:798-807. [DOI] [PubMed] [Google Scholar]
- 53.Mills, J. N., Yates, T. L., Childs, J. E., Parmenter, R. R., Ksiazek, T. G., Rollin, P. E., and C. J. Peters. 1995. Guidelines for working with rodents potentially infected with hantavirus. J. Mammal. 76:716-722. [Google Scholar]
- 54.Mori, M., A. L. Rothman, I. Kurane, J. M. Montoya, K. B. Nolte, J. E. Norman, D. C. Waite, F. T. Koster, and F. A. Ennis. 1999. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J. Infect. Dis. 179:295-302. [DOI] [PubMed] [Google Scholar]
- 55.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nickerson, P. A., S. Matalon, and L. E. Farhi. 1981. An ultrastructural study of alveolar permeability to cytochrome C in the rabbit lung: effect of exposure to 100% oxygen at one atmosphere. Am. J. Pathol. 102:1-9. [PMC free article] [PubMed] [Google Scholar]
- 57.Nolte, K. B., R. M. Feddersen, K. Foucar, S. R. Zaki, F. T. Koster, D. Madar, T. L. Merlin, P. J. McFeeley, E. T. Umland, and R. E. Zumwalt. 1995. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathol. 26:110-120. [DOI] [PubMed] [Google Scholar]
- 58.O'Connor, C. S., J. P. Hayes, and S. C. Stjeor. 2002. Sin Nombre virus does not impair respiratory function of wild deer mice. J. Mammal. 78:661-668. [Google Scholar]
- 59.Peters, C. J., G. L. Simpson, and H. Levy. 1999. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu. Rev. Med. 50:531-545. [DOI] [PubMed] [Google Scholar]
- 60.Puglielli, M. T., J. L. Browning, A. W. Brewer, R. D. Schreiber, W. J. Shieh, J. D. Altman, M. B. Oldstone, S. R. Zaki, and R. Ahmed. 1999. Reversal of virus-induced systemic shock and respiratory failure by blockade of the lymphotoxin pathway. Nat. Med. 5:1370-1374. [DOI] [PubMed] [Google Scholar]
- 61.Rimmelzwaan, G. F., M. M. Baars, P. de Lijster, R. A. Fouchier, and A. D. Osterhaus. 1999. Inhibition of influenza virus replication by nitric oxide. J. Virol. 73:8880-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanders, S. P., E. S. Siekierski, J. D. Porter, S. M. Richards, and D. Proud. 1998. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J. Virol. 72:934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandirasegarane, L., and J. Diamond. 1999. The nitric oxide donors, SNAP and DEA/NO, exert a negative inotropic effect in rat cardiomyocytes which is independent of cyclic GMP elevation. J. Mol. Cell. Cardiol. 31:799-808. [DOI] [PubMed] [Google Scholar]
- 64.Simpson, S. Q. 1998. Hantavirus pulmonary syndrome. Heart Lung 27:51-57. [DOI] [PubMed] [Google Scholar]
- 65.Sittipunt, C., K. P. Steinberg, J. T. Ruzinski, C. Myles, S. Zhu, R. B. Goodman, L. D. Hudson, S. Matalon, and T. R. Martin. 2001. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 163:503-510. [DOI] [PubMed] [Google Scholar]
- 66.Staub, N. C. 1974. Pulmonary edema. Physiol. Rev. 54:678-811. [DOI] [PubMed] [Google Scholar]
- 67.Strand, O. A., A. Leone, K. E. Giercksky, and K. A. Kirkeboen. 2000. Nitric oxide indices in human septic shock. Crit. Care Med. 28:2779-2785. [DOI] [PubMed] [Google Scholar]
- 68.Tanita, T., C. Song, H. Kubo, Y. Hoshikawa, M. Chida, S. Suzuki, S. Ono, and S. Fujimura. 1999. Superoxide anion mediates pulmonary vascular permeability caused by neutrophils in cardiopulmonary bypass. Surg. Today 29:755-761. [DOI] [PubMed] [Google Scholar]
- 69.Tatsumi, T., S. Matoba, A. Kawahara, N. Keira, J. Shiraishi, Akashi k, M. Kobara, T. Tanaka, M. Katamura, C. Nakagawa, B. Ohta, T. Shirayama, K. Takeda, J. Asayama, H. Fliss, and M. Nakagawa. 2000. Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. J. Am. Coll. Cardiol. 35:1338-1346. [DOI] [PubMed] [Google Scholar]
- 70.Taylor, B. S., and D. A. Geller. 2000. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock 13:413-424. [DOI] [PubMed] [Google Scholar]
- 71.Terajima, M., J. D. Hendershot, I. I. I., H. Kariwa, F. T. Koster, B. Hjelle, D. Goade, M. C. DeFronzo, and F. A. Ennis. 1999. High levels of viremia in patients with the hantavirus pulmonary syndrome. J. Infect. Dis. 180:2030-2034. [DOI] [PubMed] [Google Scholar]
- 72.Tracey, W. R., C. Xue, V. Klinghofer, J. Barlow, J. S. Pollock, U. Förstermann, and R. A. Johns. 1994. Immunochemical detection of inducible NO synthase in human lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 266:L722-L727. [DOI] [PubMed]
- 73.van der Vliet, A., J. P. Eiserich, M. K. Shigenaga, and C. E. Cross. 1999. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? Am. J. Respir. Crit. Care Med. 160:1-9. [DOI] [PubMed] [Google Scholar]
- 74.Werdan, K. 2001. Pathophysiology of septic shock and multiple organ dysfunction syndrome and various therapeutic approaches with special emphasis on immunoglobulins. Ther. Apher. 5:115-122. [DOI] [PubMed] [Google Scholar]
- 75.Zaki, S. R., P. W. Greer, L. M. Coffield, C. S. Goldsmith, K. B. Nolte, K. Foucar, R. M. Feddersen, R. E. Zumwalt, G. L. Miller, and A. S. Khan. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552-579. [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu, S., I. Y. Haddad, and S. Matalon. 1996. Nitration of surfactant protein A (SP-A) tyrosine residues results in decreased mannose binding ability. Arch. Biochem. Biophys. 333:282-290. [DOI] [PubMed] [Google Scholar]
- 77.Zhu, S., L. B. Ware, T. Geiser, M. A. Matthay, and S. Matalon. 2001. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am. J. Respir. Crit. Care Med. 163:166-172. [DOI] [PubMed] [Google Scholar]