Abstract
The bovine papillomavirus E5 protein activates the cellular platelet-derived growth factor β receptor (PDGFβR) tyrosine kinase in a ligand-independent manner. Evidence suggests that the small transmembrane E5 protein homodimerizes and physically interacts with the transmembrane domain of the PDGFβR, thereby inducing constitutive dimerization and activation of this receptor. Amino acids in the receptor previously found to be required for the PDGFβR-E5 interaction are a transmembrane Thr513 and a juxtamembrane Lys499. Here, we sought to determine if these are the only two receptor amino acids required for an interaction with the E5 protein. Substitution of large portions of the PDGFβR transmembrane domain indicated that additional amino acids in both the amino and carboxyl halves of the receptor transmembrane domain are required for a productive interaction with the E5 protein. Indeed, individual amino acid substitutions in the receptor transmembrane domain identified roles for the extracellular proximal transmembrane residues in the interaction. These data suggest that multiple amino acids within the transmembrane domain of the PDGFβR are required for a stable interaction with the E5 protein. These may be involved in direct protein-protein contacts or may support the proper transmembrane alpha-helical conformation for optimal positioning of the primary amino acid requirements.
The bovine papillomavirus E5 protein induces tumorigenic transformation of rodent fibroblasts and is the major transforming protein of this virus (1, 2, 7, 34). This small, 44-amino-acid transmembrane protein exists as a dimer with a subunit size of 7 kDa and localizes primarily to the endoplasmic reticulum and Golgi membranes of bovine papillomavirus-transformed cells (3, 35).
It has been demonstrated that the E5 protein forms a stable complex with and activates the platelet-derived growth factor beta receptor (PDGFβR) (29, 30). The PDGFβR is a cell surface receptor tyrosine kinase which stimulates cell proliferation (14) and has been associated with some cancers (9). The binding of the normal dimeric ligand, PDGF BB, to the extracellular domain of the receptor results in receptor dimerization, which in turn facilitates receptor activation and autophosphorylation (4, 14). Once phosphorylated, tyrosine residues in the receptor's cytoplasmic domain become docking sites for intracellular receptor substrates that initiate signaling cascades culminating in cell proliferation (14). Similarly, evidence suggests that binding of the bovine papillomavirus E5 dimer to the PDGFβR induces constitutive dimerization and hence sustained activation of the receptor (19), which in turn mediates the transforming effect of E5 (8, 26).
The E5 protein has been reported to interact with other transmembrane proteins such as the epidermal growth factor (EGF) receptor (6), α-adaptin (5), and the 16-kDa subunit of the vacuolar H+-ATPase (10-12). However, in these studies the E5 protein complexes were detected under conditions of transient overexpression and/or by utilizing an epitope or glutathione S-transferase-tagged E5 construct and therefore may not accurately represent naturally occurring phenomena.
Other studies showed that under conditions of more physiological protein expression levels, the E5 protein could not form a complex with the EGF receptor (31) or the closely related PDGFα receptor (13). Moreover, only the E5-PDGFβR interaction has been related to both a biochemical (receptor activation) and a biological (cellular transformation) response. Thus, the PDGFβR is likely to be the primary cellular target of the bovine papillomavirus E5 protein.
Several studies have explored the contributions of the receptor and those of the E5 protein necessary for the E5-PDGFβR interaction. Mutagenic dissection of the PDGFβR has elucidated that the transmembrane region, with minimal extracellular and intracellular sequences, is sufficient (V. M. Nappi, J. A. Schaefer, and L. M. Petti, unpublished data) and that specific amino acids are required for physical complex formation with the E5 protein (32). In particular, two amino acids of the receptor have been shown to be required for the interaction, Lys499 at the juxtamembrane position and Thr513 within the transmembrane domain (32). Similarly, mutagenesis analysis of the E5 protein revealed that the juxtamembrane-positioned Asp33 as well as Glu17, located in the transmembrane domain, are required for the transforming activity of E5 (15, 18) and its ability to interact with the receptor (27). In addition, the two extracellular cysteines of E5, demonstrated to be involved in E5 dimerization, and three amino acids within the transmembrane domain near the membrane interface with the extracellular space are also required for the transforming activity of this oncoprotein (15).
The nature of these amino acid requirements suggests that complex formation between the E5 protein and the PDGFβR involves direct protein-protein interactions. First, it was shown that maintenance of a negative charge at position 33 in the E5 protein (17) and a positive charge at residue 499 (32) of the receptor was required for complex formation. This suggested that the juxtamembrane Asp33 of the E5 protein participates in an electrostatic interaction with the analogously positioned Lys499 in the PDGFβR (17, 25, 32). Similarly, substitution of either Glu17 in E5 or Thr513 of the receptor with nonpolar residues was inhibitory for complex formation, suggesting that Gln17 of E5 and Thr513 of the receptor participate in hydrogen bonding (16, 32). Since both Lys499 and Thr513 of the receptor align precisely along the same face of the predicted transmembrane α-helix, it was proposed that complex formation between the PDGFβR and the E5 oncoprotein involves direct contacts along a single helical interface of the receptor transmembrane domain (32).
In this study, we set out to determine whether Lys499 and Thr513 of the PDGFβR are the minimal amino acids required for complete complex formation with the E5 protein. Using a variety of chimeric receptor constructs, we found that the presence of Lys499 and Thr513 alone was not completely sufficient for restoration of an interaction with the E5 protein and that additional amino acid requirements exist in both the amino and carboxyl halves of the receptor transmembrane domain. Consistent with these findings, we also demonstrated that specific extracellular proximal transmembrane amino acids of the PDGFβR play a role in an optimal functional interaction with the E5 protein. Taken together, our results suggest that indeed there are multiple amino acid requirements in the PDGFβR transmembrane domain for a stable interaction with the E5 protein. Whether these requirements are for direct protein-protein contacts or for helical stability within the membrane remains to be elucidated.
MATERIALS AND METHODS
Construction of mutant receptors.
Single, double, and triple amino acid changes were introduced into the transmembrane domain of the wild-type PDGFβR, ERTM, or NNTM by site-directed mutagenesis. ERTM and NNTM are chimeric receptor constructs that contain the EGF receptor and Neu transmembrane domain, respectively, in the context of the PDGFβR (32). In most cases, site-directed mutagenesis was performed with the QuikChange method (Stratagene) according to the manufacturer to introduce amino acid substitutions. In many cases, additional base pair mismatches were incorporated to introduce a silent mutation that created a SpeI or eliminated an ApaI restriction enzyme site as a means of selection for plasmids containing the desired mutations. The templates for mutagenesis were the cDNAs for the wild-type murine PDGFβR (PR), ERTM, and NNTM subcloned into the LXSN retroviral vector. The presence of the appropriate mutation was confirmed by DNA sequencing. Fig. 1 indicates the positions of the individual amino acid substitutions that were made.
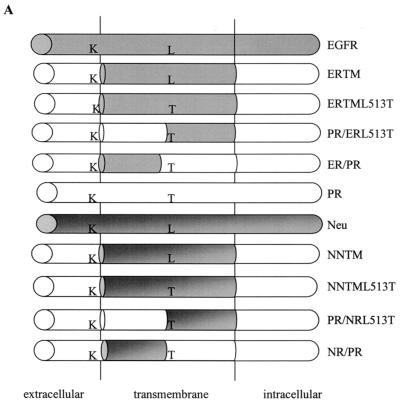
FIG. 1.
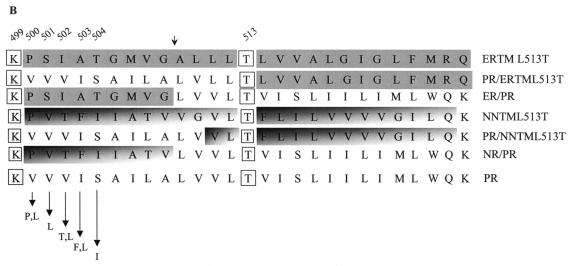
Structure of the mutant receptors. (A) Schematic representations of the chimeric mutant receptors generated and characterized in these studies. Chimeric receptor mutants were derived from ERTM or NNTM, which contain the transmembrane domain of the EGF receptor and Neu, respectively. Included are the ERTML513T and NNTML513T mutants, which contain a Thr at position 513 in ERTM and NNTM, respectively. Also included are the split transmembrane domain receptor mutants PR/ERL513T, ER/PR, PR/NRL513T, and NR/PR. The required Lys (K) located at position 499 and Thr (T) at position 513 are indicated. Shaded areas are derived from EGF receptor or Neu, while unshaded areas are derived from the PDGFβR. (B) Amino acid sequence of the transmembrane domain of each receptor examined in this study compared to the wild-type murine PDGFβR (PR). Fully shaded sequences were derived from the EGF receptor transmembrane domain, differentially shaded sequences were derived from the Neu receptor transmembrane domain, and unshaded sequences were derived from the wild-type PDGFβR transmembrane domain. The small arrow above the sequences denotes where a SpeI site was introduced into the PDGFβR, ERTML513T, and NNTML513T receptor constructs. Arrows at the bottom depict amino acid changes made in the wild-type PDGFβR at positions 500 to 504. The essential Lys (K) 499 and Thr (T) 513 of the PDGFβR required for an interaction with the E5 protein are boxed. Specific residue numbers are indicated above the sequences.
Construction of split transmembrane receptor chimeras.
Site-directed mutagenesis was first performed to introduce a SpeI restriction site at nucleotide position 1755 (which corresponds to transmembrane amino acids 509 to 510 [38]) of the murine PDGFβR as well as the ERTML513T and NNTML513T mutant receptor derivatives. In the case of the wild-type PDGFβR and the NNTML513T receptor mutant, introducing the SpeI site resulted in a silent mutation. In the case of the ERTML513T construct, introducing a SpeI site resulted in Ala509Leu and Leu510Val substitutions, which change the EGF receptor-derived amino acids to PDGFβR amino acids at these positions. The resulting PRSpeI, ERTML513TSpeI, and NNTML513TSpeI constructs were used to generate the split transmembrane mutants.
Since the LXSN vector also contains a SpeI site, SpeI digestion of these plasmids generated two fragments of approximately 6.7 and 2.5 kb, respectively. The 2.5-kb fragment of PRSpeI, which contains the amino half of the wild-type receptor transmembrane domain, was ligated to the 6.7-kb fragment of ERTML513TSpeI or NNTML513TSpeI, which contain the carboxyl half of the transmembrane domain of ERTML513T or NNTML513T, respectively, generating the PR/ERL513T and PR/NRL513T split transmembrane mutants, respectively. In addition, the 6.7-kb fragment from the PRSpeI plasmid, which contains the carboxyl half of the wild-type receptor transmembrane domain, was ligated to the 2.5-kb fragment of ERTML513TSpeI or NNTML513TSpeI, which contains the amino half of ERTM or NNTM, respectively, generating the split transmembrane chimeric receptors ER/PR and NR/PR, respectively. Fig. 1 shows a schematic representation and the amino acid sequences of the transmembrane domain of each split transmembrane receptor chimera. Note that the altered amino acid sequence of the ERTML513TSpeI intermediate resulted in a split transmembrane chimeric mutant with more sequence originating from the PDGFβR than from the EGF receptor transmembrane domain. Sequence analysis confirmed that the proper open reading frame was maintained during the ligation for each split transmembrane chimeric receptor mutant.
Cell culture.
The Phoenix ecotropic retrovirus producer cell line was obtained from the American Type Culture Collection with permission from Gary Nolan (Stanford University) and maintained in Dulbecco's modified Eagle's medium with high glucose supplemented with 10% fetal bovine serum (FBS) and antibiotics. Ba/F3 murine hematopoietic cells were maintained as previously described (8) in RPMI 1640 medium supplemented with 10% FBS, antibiotics, 50 μM β-mercaptoethanol, and 10% WEHI-conditioned medium as a source of interleukin-3 (RPMI/IL-3).
Production of retrovirus.
The various PDGFβR constructs, E5, and v-sis were introduced into Ba/F3 cells by retrovirus-mediated gene transfer. The recombinant retroviral vectors used were the receptor-LXSN constructs described above and constructs containing the E5 or v-sis open reading frames subcloned into the pBabepuro retroviral vector, which contains a puromycin resistance marker. High-titer ecotropic retrovirus was obtained from these retroviral vectors as described previously (33). Briefly, Phoenix ecotropic cells grown to 70 to 80% confluence in 60-mm dishes were transfected with 10 μg of plasmid DNA by the calcium phosphate method. The next day the medium was replaced, and 24 h later the virus-containing supernatant from each dish was collected and filtered through a 0.45-μm syringe filter.
Stable expression in Ba/F3 cells.
Retroviral infection of Ba/F3 cells was performed as described previously with some modifications (8). In general, approximately 5 × 106 cells were infected with approximately 1 × 105 to 2 × 105 CFU of ecotropic retrovirus in 10 ml of RPMI/IL-3 supplemented with 4 μg of Polybrene per ml. At 48 h postinfection, 2 ml of the infected cell culture was added to 8 ml of selective medium (RPMI/IL-3 containing 1 μg of puromycin [Sigma] per ml with or without 1 mg of G418 [Gemini] per ml). After reaching a density of approximately 106 cells/ml, cells were passaged again under selection conditions. Selection was continued through two to four additional passages until 100% of a mock-infected culture died, thus establishing stable cell lines. To establish Ba/F3 cells expressing a receptor construct with or without E5 or v-sis, cells were subjected to two sequential rounds of infection and selection, in which establishment of Ba/F3 cells expressing E5 or v-sis was followed by infection with each of the various LXSN-receptor constructs.
Assay for IL-3-independent growth.
Ba/F3 cells expressing a receptor construct with or without E5 or v-sis were grown to an approximate density of 106 cells/ml, washed twice with phosphate-buffered saline (PBS), and resuspended in an equal volume of RPMI lacking WEHI-conditioned medium and supplemented with only 1% FBS, antibiotics, and 50 μM β-mercaptoethanol (RPMI/−IL-3). Approximately 5 × 105 cells were seeded into 10 ml of RPMI/−IL-3, incubated at 37°C, and monitored for cell survival and proliferation. Total or viable cells were counted using a hemacytometer at various times after seeding. For each receptor analyzed, Ba/F3 cells that proliferated at least 20-fold during a 10-day period were considered IL-3-independent. Table 1 lists the number of IL-3-independent cell lines per total number of independently derived cell lines tested for each genotype.
TABLE 1.
Summary of data obtained from IL-3 independence growth assays
| Ba/F3 cell genotype | No. of positive cell lines/total no. of independently derived cell lines tested | Growth phenotypea | Ba/F3 cell genotype | No. of positive cell lines/total no. of independently derived cell lines tested | Growth phenotype | |
|---|---|---|---|---|---|---|
| LXSN | 0/21 | − | PTF | 0/2 | − | |
| E5 | 0/21 | − | PTF + E5 | 0/2 | − | |
| v-sis | 0/20 | − | PTF + v-sis | 2/2 | + | |
| PR | 1b/23 | − | ERTM | 5b/13 | − | |
| PR + E5 | 23/23 | + | ERTM + E5 | 3b/13 | − | |
| PR + v-sis | 22/22 | + | ERTM + v-sis | 12/12 | + | |
| PRSpeI | 0/3 | − | ERTML513T | 2b/12 | − | |
| PRSpeI + E5 | 3/3 | + | ERTML513T + E5 | 5c/12; 2b/12 | +/− | |
| PRSpeI + v-sis | 3/3 | + | ERTML513T + v-sis | 11/11 | + | |
| V500P | 0/3 | − | ERTML513TSpeI | 0/3 | − | |
| V500P + E5 | 2/3 | + | ERTML513TSpeI + E5 | 2c/3 | +/− | |
| V500P + v-sis | 3/3 | + | ERTML513TSpeI + v-sis | 3/3 | + | |
| V500L | 0/2 | − | PR/ERL513T | 1b/3 | − | |
| V500L + E5 | 2/2 | + | PR/ERL513T + E5 | 1b/3 | − | |
| V500L + v-sis | 2/2 | + | PR/ERL513T + v-sis | 2/2 | + | |
| V501L | 0/2 | − | ER/PR | 0/2 | − | |
| V501L + E5 | 2/2 | + | ER/PR + E5 | 0/2 | − | |
| V501L + v-sis | 2/2 | + | ER/PR + v-sis | 2/2 | + | |
| V502T | 0/3 | − | NNTM | 1b/3 | − | |
| V502T + E5 | 3c/3 | +/− | NNTM + E5 | 1b/3 | − | |
| V502T + v-sis | 3/3 | + | NNTM + v-sis | 3/3 | + | |
| V502L | 0/2 | − | NNTML513T | 1b/3 | − | |
| V502L + E5 | 2/2 | + | NNTML513T + E5 | 1b/3 | − | |
| V502L + v-sis | 2/2 | + | NNTML513T + v-sis | 3/3 | + | |
| I503F | 0/3 | − | NNTML513TSpeI | 0/2 | − | |
| I503F + E5 | 3c/3 | +/− | NNTML513TSpeI + E5 | 0/2 | − | |
| I503F + v-sis | 3/3 | + | NNTML513TSpeI + v-sis | 2/2 | + | |
| I503L | 0/2 | − | PR/NRL513T | 0/2 | − | |
| I503L + E5 | 2/2 | + | PR/NRL513T + E5 | 0/2 | − | |
| I503L + v-sis | 2/2 | + | PR/NRL513T + v-sis | 2/2 | + | |
| S504I | 0/2 | − | NR/PR | 0/2 | − | |
| S504I + E5 | 2c/2 | +/− | NR/PR + E5 | 0/2 | − | |
| S504I + v-sis | 2/2 | + | NR/PR + v-sis | 2/2 | + |
+ and − indicate ability or inability, respectively, to grow in the absence of IL-3. An IL-3-independent (+) growth phenotype was assigned to cells that could consistently proliferate in the absence of IL-3 20-fold over a period of 10 days. +/− was assigned to cells that inconsistently displayed an intermediate IL-3-independent growth phenotype, as described below.
Considered background growth if growth was slowed, the saturation density attained was suboptimal, and cells not expressing E5 or v-sis established in parallel grew in a similar manner.
Considered intermediate growth because proliferation was significantly delayed and the saturation density attained was 8 to 20 times the seeded density.
Immunoprecipitation and immunoblotting.
Ba/F3 cells were lysed by incubation in cold EBC buffer (50 mM Tris-HCl [pH 8.0], 120 mM NaCl, 0.5% NP-40) supplemented with 1 mM phenylmethylsulfonyl fluoride, 2 mM sodium orthovanadate, 20 μg of leupeptin per ml, and 20 μg of aprotinin per ml on ice for 15 min. Lysates were cleared of nuclei and cellular debris by microcentrifugation, and the supernatant extracts were used for immunoprecipitation. To immunoprecipitate the E5 protein and any associated protein, 1,000 μg of extracted protein was incubated overnight at 4°C with approximately 10 μl of a rabbit polyclonal antibody directed against the 16 C-terminal amino acids of the E5 protein (gift from D. DiMaio). To immunoprecipitate the wild-type and mutant forms of the PDGFβR, 500 to 1,000 μg of extracted protein was incubated overnight at 4°C with approximately 5 μl of a rabbit polyclonal antibody directed against the C-terminal 13 amino acids of the human PDGFβR (gift from D. DiMaio). Following incubation with primary antibody, extracts were incubated with 60 μl of a 1:1 slurry of protein A-Sepharose CL-4B beads (Pharmacia) for 60 min at 4°C. After washing the beads, protein complexes were dissociated from beads by boiling in 2× Laemmli protein sample buffer.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis were performed as described previously (32). Briefly, immunoprecipitates were either electrophoresed on an SDS-6.5% (or 7.5% in experiments represented in Fig. 4B) polyacrylamide gel and transferred to nitrocellulose (for PDGF receptor or phosphotyrosine immunoblotting) or electrophoresed on an SDS-15% polyacrylamide gel and transferred to Immobilon (Millipore) (for E5 immunoblotting). Blots were blocked in milk buffer (5% nonfat dry milk in TBST [10 mM Tris-HCl, pH 7.4; 154 mM NaCl; 0.1% Tween-20]) and then incubated with primary antibody for 3 h or overnight using a 1:2,000 dilution of monoclonal antiphosphotyrosine antibody P-Tyr-100 (Cell Signaling), a 1:500 to 1:1,000 dilution of the anti-PDGFβ receptor antiserum, or a 1:500 to 1:750 dilution of the anti-E5 antiserum. Following incubation with primary antibody, immunoblots were washed in TBST and then incubated for 1 h with a 1:5,000 dilution of either horseradish peroxidase (HRP)-conjugated protein A (Pierce) (for PDGFβR or E5 blots) or HRP-conjugated goat anti-mouse immunoglobulin G (IgG) (Pierce) (for P-Tyr-100 antiphosphotyrosine blots). Blots were then washed again and subjected to enhanced chemiluminescence (ECL) detection (Amersham) as described by the manufacturer.
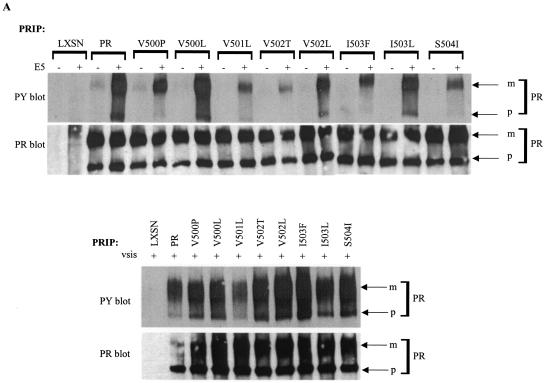
FIG. 4.
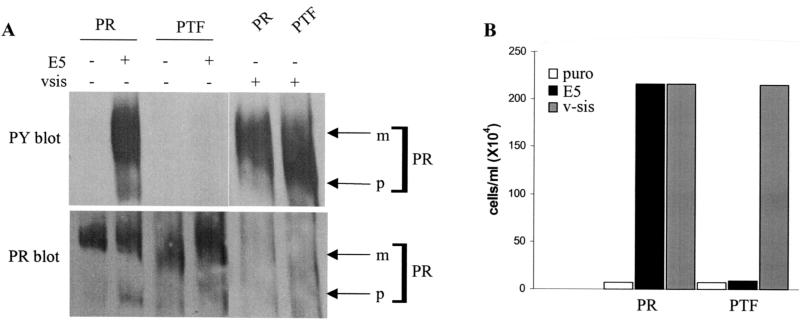
Biochemical and functional analysis of PDGFβR mutants containing extracellular proximal transmembrane amino acid substitutions. Each of the PDGFβR mutants containing an extracellular proximal transmembrane amino acid substitution, the wild-type PDGFβR (PR), or no exogenous receptor (LXSN) was expressed in Ba/F3 cells with (+) or without (−) E5 or v-sis as described in Materials and Methods. (A) PDGFβR was immunoprecipitated (PRIP) from cell extracts and subjected to antiphosphotyrosine (PY) or anti-PDGFβR (PR) immunoblotting to detect receptor activation and total receptor levels, respectively. (B) The E5 protein with any associated proteins was immunoprecipitated (E5IP) from cell extracts and subjected to anti-PDGFβR (PR) or antiphosphotyrosine (PY) immunoblotting to assess physical complex formation between the E5 protein and the PDGFβR. Anti-E5 (E5) immunoblotting was performed to detect E5 protein expression levels. In these experiments, the presence of the 165-kDa precursor form of the PDGFβR in E5 immunoprecipitates is indicative of complex formation between the E5 protein and the PDGFβR. Arrows to the right mark the mature (m) and precursor (p) isoforms of each receptor as well as the E5 protein. Each lane represents approximately 585 μg (A) or 675 μg (B) of extracted protein.
For the experiment presented in Fig. 2 and 6A, after ECL detection of the phosphotyrosine immunoblot, primary and secondary antibodies were removed according to the stripping protocol provided by the manufacturer. In brief, membranes were incubated in stripping buffer (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) for 30 min at 60°C. The stripped blot was then washed and subjected to ECL detection to ensure that the stripping process was effective. The membrane was then washed in TBST several times, blocked in milk buffer, and subjected to anti-PDGFβR immunoblotting as described above.
FIG. 2.
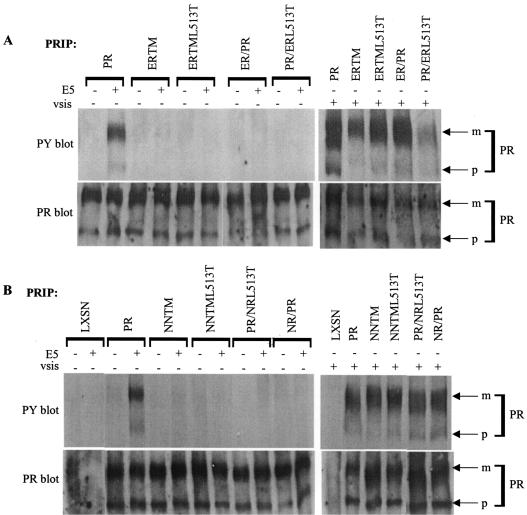
Biochemical and functional analysis of the split transmembrane PDGFβR chimeras. Ba/F3 cell lines expressing the wild-type PDGFβR (PR), the ERTM, ERTML513T, NNTM, or NNTML513T chimeric receptors, or the PR/ERL513T, ER/PR, PR/NRL513T, or NR/PR split transmembrane receptor chimeras with (+) or without (−) E5 or v-sis were generated as described in Materials and Methods. (A and B) PDGFβ receptor (PR) was immunoprecipitated (IP) from cell extracts and immunoblotted with an antiphosphotyrosine antibody (PY) for detection of activated receptor (upper panels). The blots then were stripped (Materials and Methods) and incubated with anti-PDGFβR antiserum (PR) to assess receptor expression levels (lower panels). Each lane represents approximately 300 μg of extracted protein. Arrows on the right point to the mature (m) and precursor (p) forms of the receptor.
FIG. 6.
Biochemical and functional analysis of the triple substitution PTF mutant. Ba/F3 cells expressing the wild-type PDGFβR (PR) or the PTF mutant receptor with (+) or without (−) E5 or v-sis were established as described in Materials and Methods. (A) PDGFβR immunoprecipitates from cell extracts were immunoblotted with an antiphosphotyrosine antibody to assess receptor activation levels (top panel). The phosphotyrosine immunoblot was then stripped (see Materials and Methods) and reprobed with the PDGFβR antiserum to detect receptor expression levels (bottom panel). Each lane represents approximately 300 μg of extracted protein. Arrows on the right point to the mature (m) and precursor (p) forms of the receptors. (B) IL-3 independence assay of cells expressing the PTF mutant receptor. Ba/F3 cells expressing the wild-type PDGFβR (PR) or the PTF mutant receptor without (puro) or with E5 or v-sis were incubated in the absence of IL-3 for 11 days and then counted. The data are representative of multiple experiments listed in Table 1.
RESULTS
In this study, to further assess the PDGFβR requirements for a complete functional interaction with the E5 protein, we constructed a variety of additional receptor chimeras and mutants, which are depicted in Fig. 1. Each receptor mutant was assessed for its ability to physically and functionally interact with the E5 protein after stable coexpression with E5 in Ba/F3 cells, a murine hematopoietic cell line that ordinarily does not express the PDGFβR. Ba/F3 cells depend on the cytokine IL-3 for survival and proliferation (28). Since this dependence on IL-3 can be alleviated by coexpression of the PDGFβR and E5 or v-sis, which encodes the viral homologue of PDGF B (37), Ba/F3 cells afford a functional assay to demonstrate cooperativity between a particular PDGFβR mutant and E5 (8). Therefore, Ba/F3 cells allowed us to determine the functionality of the E5/PDGFβR complex in terms of its ability to produce a proliferative signal. Each receptor mutant was also coexpressed with v-sis to ensure that the receptor could be appropriately activated by ligand. Stable Ba/F3 cell lines in which each receptor mutant was expressed alone without E5 or v-sis also were established to determine if any amino acid substitutions conferred constitutive receptor activation.
Requirement for both the amino and carboxyl portions of the PDGFβR transmembrane domain for productive interaction with the E5 protein.
Evidence reported previously (32; V. M. Nappi, J. A. Schaefer, and L. M. Petti, unpublished data) suggests that specific amino acids located in the transmembrane region of the PDGFβR are required for an interaction with the E5 protein. To examine the global contribution of amino acids within the amino and carboxyl halves of the receptor transmembrane domain toward a productive interaction with the E5 protein, we constructed four PDGFβR mutants having chimeric transmembrane domains (Fig. 1). These chimeric receptors all retained the essential Lys499 and Thr513 but had either the amino or carboxyl half of the transmembrane domain replaced with that of a different receptor tyrosine kinase. In the PR/ERL513T and PR/NRL513T “split” transmembrane mutants, the first half of the transmembrane domain was derived from the PDGFβR, while the remaining half was derived from the EGF receptor or Neu receptor, respectively, with the exception of a Thr at position 513. In the ER/PR and NR/PR mutants, the first portion of the transmembrane domain was derived from the EGF receptor or Neu, respectively, while the remainder maintained the PDGFβR sequence. The ability of these mutant receptors to productively interact with the E5 protein was assessed after establishing stable expression in Ba/F3 cells. The chimeric receptors ERTML513T and NNTML513T, which contained a Thr at position 513 in the context of the EGF receptor or Neu transmembrane domain, respectively, served as precursors to the split transmembrane mutants and were examined in parallel.
First, we examined the status of these receptor mutants stably expressed in Ba/F3 cells. PDGF receptor immunoblotting was performed to detect total receptor expression levels and revealed that abundant amounts of both the mature and a faster-migrating, incompletely processed precursor form of the receptor were expressed in the cell lines tested (Fig. 2, lower panels of A and B). The activity of the split transmembrane receptor mutants and their precursors was assessed by PDGFβR immunoprecipitation followed by phosphotyrosine immunoblot analysis, as shown in Fig. 2 (upper panels). Increased receptor tyrosine phosphorylation over background typically implies receptor autophosphorylation, which requires the kinase activity of the receptor (14). None of the receptor mutants displayed detectable levels of receptor tyrosine phosphorylation when expressed alone. However, each receptor was abundantly tyrosine phosphorylated when coexpressed with v-sis, illustrating that each receptor mutant could be activated by ligand and thus was a functional receptor tyrosine kinase.
As expected, the wild-type receptor expressed with E5 was abundantly tyrosine phosphorylated. In contrast, both the NNTML513T and ERTML513T mutants were reproducibly defective for being tyrosine phosphorylated in response to E5. It is important to note that tyrosine phosphorylation of the ERTML513T mutant was occasionally observed (in three of eight independently derived cell lines tested; data not shown), suggesting that this mutant may not have been completely defective for receptor activation by E5. Nonetheless, it is reasonable to conclude that the required Lys499 and Thr513 alone are insufficient for complete receptor activation by E5.
Surprisingly, none of the split transmembrane mutants exhibited detectable levels of tyrosine phosphorylation when expressed with E5. The lack of receptor activation observed in response to E5 was not due to decreased receptor levels, since all receptors were expressed at comparable levels in the cell lines tested (Fig. 2A and B, bottom panels). These results suggest that there exist additional amino acid requirements in both the amino and carboxyl halves of the PDGFβR transmembrane domain for activation of this receptor by the E5 protein.
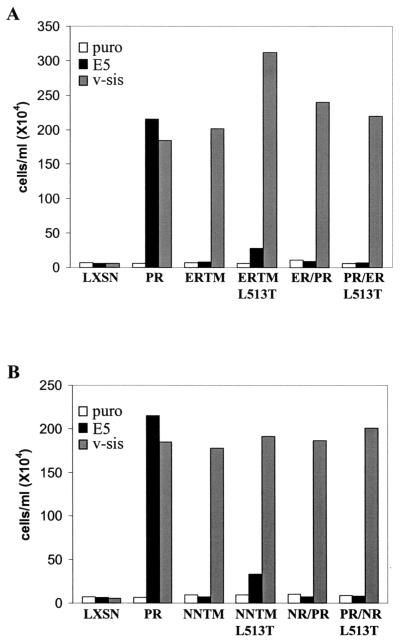
To assess the ability of the split transmembrane domain constructs to biologically respond to the E5 protein, an IL-3 independence assay was performed. Ba/F3 cells expressing each receptor in the presence or absence of E5 or v-sis were incubated in medium lacking IL-3, monitored for proliferation, and counted. The graphs in Fig. 3 are representative of data summarized in Table 1 and depict cell density after a 10-day incubation in the absence of IL-3. Ba/F3 cells expressing E5 or v-sis alone or each receptor alone did not exhibit an IL-3-independent growth phenotype. For all receptor constructs tested, coexpression of receptor with v-sis allowed IL-3-independent growth, confirming that they were biologically functional receptors. As expected, proliferation in the absence of IL-3 was observed for Ba/F3 cells coexpressing the wild-type receptor with E5. However, neither the NNTML513T chimera nor any of the split transmembrane chimera mutants were able to efficiently cooperate with E5 to induce IL-3-independent growth. Approximately half of the independently established cell lines expressing the ERTML513T chimera with E5 were unable to proliferate in the absence of IL-3 (Fig. 3), while the other half exhibited a delayed proliferative response (Table 1). This inconsistent intermediate mitogenic response may indicate a weak interaction between this mutant and the E5 protein. Nevertheless, taken together, our results suggest that besides Lys499 and Thr513, other amino acids in both the amino- and carboxyl-terminal halves of the PDGFβR transmembrane domain are required for a biologically functional interaction with the E5 protein.
FIG. 3.
IL-3 independence assay of Ba/F3 cells expressing the split transmembrane chimeric receptors. Cells expressing the split transmembrane chimeric receptors with or without E5 or v-sis were seeded in medium lacking IL-3 and counted 10 days later. The densities of the cell lines expressing the ERTM-derived chimeras are shown in panel A, and the densities of the cell lines expressing the NNTM-derived chimeras are shown in panel B. These data are representative of experiments listed in Table 1.
Role for the extracellular proximal portion of the PDGFβR transmembrane domain in the interaction with the E5 protein.
The data obtained from the above experiments suggested that additional amino acids within the transmembrane region of the PDGFβR may be required for a stable and productive interaction with the E5 protein. Three of the eight amino acids in the E5 protein that were found to be required for its transformation activity are located inside the membrane near the lipid interface with the extracellular milieu (15). Here, we determined if amino acids 500 to 504 within the corresponding “extracellular proximal” region of the transmembrane domain of the receptor also played a role in a productive interaction with the E5 protein. Conservative as well as nonconservative amino acid substitutions were made within this region of the PDGFβR transmembrane domain (Fig. 1B), and the resulting mutant receptors were analyzed for the ability to productively interact with the E5 protein in Ba/F3 cells.
To determine the tyrosine phosphorylation status of these receptor mutants, phosphotyrosine immunoblot analysis of PDGFβR immunoprecipitates was performed and is shown in Fig. 4A. None of the receptor constructs when expressed alone was tyrosine phosphorylated, indicating that none of the amino acid substitutions created a condition of constitutive receptor activation. All receptor constructs exhibited substantial tyrosine phosphorylation when coexpressed with v-sis (Fig. 4A, lower panel), suggesting that none of the amino acid substitutions affected receptor activation in response to ligand stimulation.
Receptor mutants containing single amino acid substitutions at positions 500 to 504 also displayed increased tyrosine phosphorylation when expressed with E5, although the level of receptor tyrosine phosphorylation varied depending on the particular mutation present. Like the wild-type receptor, mutant receptors V500L, V502L, and I503L, containing a Leu substitution at position Val500, Val502, or Ile503, respectively, displayed abundant tyrosine phosphorylation when expressed with E5. This indicates that conservative substitutions at these positions did not inhibit receptor activation in response to E5. Although the conservative Val501Leu substitution appeared to significantly inhibit receptor activation in the experiment shown in Fig. 4A, tyrosine phosphorylation of this mutant in response to E5 appeared only slightly less than that of the wild-type receptor for a different set of independently derived cell lines (data not shown). When expressed with E5, receptor mutants V500P, V502T, I503F, and S504I, containing a Val500Pro, Val502Thr, Ile503Phe, and Ser504Ile substitution, respectively, were significantly less tyrosine phosphorylated than the wild-type receptor. Tyrosine phosphorylation of the precursor form of these receptor mutants, unlike that of the wild-type receptor, was nearly undetectable with E5.
The differences observed in tyrosine phosphorylation of these receptor mutants were not due to significant variability in the amount of receptor expressed in the different cell lines (Fig. 4A, lower part of top panel). Thus, more drastic amino acid changes at these extracellular proximal transmembrane positions of the PDGFβR appeared to inhibit but not abolish activation of this receptor by E5. The V502T mutant appeared the most reproducibly defective for being activated by E5. Therefore, the amino acids within this region of the PDGFβR, particularly Val502, may play a role in receptor activation by the E5 protein.
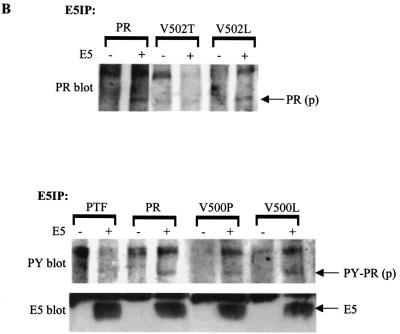
The ability of some of these receptor mutants to physically interact with the E5 protein was assessed by coimmunoprecipitation experiments (Fig. 4B). Since nonspecific precipitation of the mature but not the precursor form of the receptor could be detected in the absence of E5 expression, coimmunoprecipitation of the precursor form was considered an indication of complex formation with the E5 protein. Coimmunoprecipitation of the precursor form of the V500L, V500P, and V502L mutants with the E5 protein was comparable to that of the wild-type receptor. In contrast, coimmunoprecipitation of the V502T precursor was undetectable. Thus, the mutation that inhibited receptor activation most severely, Val502Thr, also appeared to be the most inhibitory for complex formation with the E5 protein, further suggesting that Val502 plays an important role in the interaction.
In order to determine whether each of these amino acid substitutions affected the ability of the receptor to cooperate with E5 to induce IL-3-independent growth of Ba/F3 cells, an IL-3 independence assay was performed. Neither the wild-type receptor nor any of the mutant receptors when expressed alone was able to confer IL-3-independent growth (Fig. 5 and data not shown). Each receptor construct when expressed with v-sis allowed IL-3-independent growth, in which a saturation density of approximately 2 × 106 cells/ml was attained by 9 days (Table 1 and data not shown). This indicates that each mutant was fully competent for the transmission of proliferative signals stimulated by ligand.
FIG. 5.
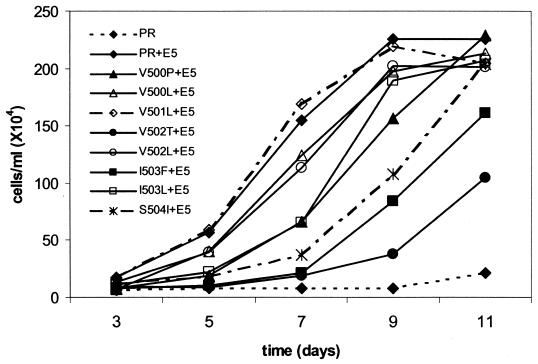
IL-3 independence assay of Ba/F3 cells expressing PDGFβR mutants with the extracellular proximal transmembrane substitutions. The Ba/F3 cell lines described in the Fig. 4 legend were seeded into medium lacking IL-3 and counted at the indicated intervals. These growth curves are representative of multiple experiments listed in Table 1.
The IL-3-independent growth kinetics of each Ba/F3 cell line expressing a particular receptor construct with E5 are depicted in Fig. 5 and are representative of data summarized in Table 1. As expected, Ba/F3 cells coexpressing the wild-type PDGFβR with E5 grew in the absence of IL-3, achieving a saturation density of 2.3 × 106 cells/ml by 9 days. Similar growth kinetics was observed for cells coexpressing E5 with the conservative amino acid substitution mutants (V500L, V501L, and V502L). Cells expressing the I503L mutant with E5 displayed a longer initial lag period prior to exponential growth but still were able to attain a density of approximately 2 × 106 cells/ml after 9 days without IL-3. Thus, conservative amino acid changes in the extracellular proximal transmembrane region did not significantly inhibit the ability of the receptor to functionally interact with the E5 protein. Cells expressing E5 with any of the more drastic amino acid substitution mutants, V500P, V502T, I503F, or S504I, exhibited various degrees of delayed growth in the absence of IL-3 compared to cells expressing the wild-type receptor with E5. Cells expressing the V500P or the S504I receptor with E5 experienced an extended lag period and a 2-day delay in reaching their saturation density. The IL-3-independent growth observed for Ba/F3 cells coexpressing the I503F or V502T receptor mutant with E5 was significantly slower, requiring 6 or 7 additional days, respectively, to reach a saturation density of 2 × 106 cells/ml (Fig. 5 and data not shown). The V502T mutant was reproducibly the most defective for cooperating with E5 to induce a mitogenic response in these cells. Therefore, more drastic amino acid substitutions within the extracellular proximal transmembrane region of the PDGFβR inhibit but do not completely abolish functional cooperativity with the E5 protein. This is perhaps indicative of a less stable receptor-E5 interaction.
In summary, the data in Fig. 4 and 5 suggest that amino acids in the extracellular proximal region of the PDGFβR transmembrane domain may play a role in the ability of the receptor to interact with the E5 protein. Conservative substitutions at Val500, Val501, Val502, and Ile503 in the receptor do not appear to affect an interaction with E5. However, more drastic amino acid substitutions at these positions affect a range of intermediate responses to E5 and therefore probably weaken the interaction. The Val500Pro and Ser504Ile mutations appear to have the least effect on the interaction, whereas the Val502Thr and Ile503Phe mutations have the most severe effect. This suggests that perhaps Val502 and Ile503 are the most important amino acids in this region of the PDGFβR for an interaction with the E5 protein.
In order to test the combined effect of the Val500Pro, Val502Thr, and Ile503Phe mutations, a triple substitution mutant receptor, PTF, which contains all three amino acid substitutions together was constructed. The ability of this mutant receptor to interact with the E5 protein in Ba/F3 cells was determined. First, the tyrosine phosphorylation level of the PTF mutant was assessed (Fig. 6A, upper panel). Neither the wild-type receptor nor the PTF mutant was tyrosine phosphorylated when expressed alone. Like the wild-type receptor, the PTF receptor exhibited abundant tyrosine phosphorylation when coexpressed with v-sis, indicating that the three amino acid substitutions in this mutant did not affect receptor activation in response to ligand stimulation. However, unlike the wild-type receptor, no tyrosine phosphorylation of the PTF receptor was observed after coexpression with E5. Thus, the combined substitutions of Val500Pro, Val502Thr, and Ile503Phe completely inhibited the ability of the receptor to be activated by E5. Coimmunoprecipitation data suggest that these three mutations together also completely inhibited the ability of the receptor to physically complex with the E5 protein (Fig. 4B).
An IL-3 independence assay was also performed to determine if the PTF receptor mutant could functionally cooperate with the E5 protein (Fig. 6B). Ba/F3 cells expressing either the wild-type receptor or the PTF mutant alone remained IL-3 dependent for growth. When expressed with v-sis, the PTF receptor, like the wild-type receptor, was able to induce IL-3-independent proliferation of Ba/F3 cells, illustrating that the triple amino acid substitution does not inhibit the ability of this receptor to induce proliferation in response to ligand stimulation. However, unlike the wild-type receptor, expression of the PTF receptor with E5 did not permit IL-3-independent proliferation. This suggests that the combined Val500Pro, Val502Thr, and Ile503Phe amino acid substitutions abolished a functional interaction between the receptor and the E5 protein. Thus, individually the Val500Pro, Val502Thr, and Ile503Phe mutations in the receptor partially inhibited an interaction with E5, while the three mutations together completely abolished the interaction. This suggests that at least two of these amino acids may function cooperatively to stabilize an interaction with the E5 protein.
DISCUSSION
Evidence from previous studies suggests that the E5-PDGFβR complex is formed by direct intermolecular contacts between specific amino acids within the transmembrane domains of the two proteins (16, 17, 27, 32). One contact is thought to involve an electrostatic interaction between Lys499 at the extracellular juxtamembrane position of the receptor and the analogously positioned Asp33 of the E5 protein. A second contact is thought to occur at a central transmembrane position, involving hydrogen bonding between Thr513 in the receptor and Gln17 in the E5 protein. The data presented in this paper indicate that additional transmembrane amino acids of the receptor are required for an optimal interaction with the E5 protein and that perhaps there exist more than two sites of contact.
First, we generated split transmembrane domain receptor mutants to explore the global contribution of the amino or carboxyl portion of the transmembrane domain for an interaction with the E5 protein. These mutants contain a hybrid transmembrane domain, with one half retaining PDGFβR sequence and the other half derived from an analogous portion of the EGF receptor or Neu transmembrane domain. Although all of these split transmembrane domain chimeras retained the required Lys499 and Thr513, none appeared capable of functionally interacting with the E5 protein. Thus, additional amino acids in both the amino and carboxyl halves of the transmembrane domain must be required for the interaction.
The precursors of these split transmembrane chimeric receptors, ERTML513T and NNTML513T, actually provided a means for assessing the contribution of Lys499 and Thr513 alone in the absence of any other PDGFβR transmembrane amino acids. Both chimeric receptors appeared completely defective for a functional interaction with the E5 protein in the experiments presented in Fig. 2 and 3, as well as in several other independent experiments. However, the ERTML513T chimera was occasionally activated by E5 and inconsistently cooperated with E5 to induce a proliferative response that was delayed (Table 1 and data not shown). These results could be explained by an ability of this chimeric receptor to weakly interact with the E5 protein. Nonetheless, our results indicate that provision of Lys499 and Thr513 in the context of the EGF receptor transmembrane domain is still insufficient for a stable association with the E5 protein, further substantiating the requirement for additional receptor transmembrane amino acids.
This led us to examine whether receptor amino acids 500 to 504, located just inside the transmembrane domain near the interface with the extracellular space, play a role in the interaction with the E5 protein. We thought that this extracellular proximal region of the transmembrane domain might be important for the interaction because the analogous region of the E5 protein plays a role in its transforming activity (15). We found that individual, as well as combined, amino acid replacements of Val500, Val502, Ile503, and Ser504 of the receptor negatively affected receptor activation and cell proliferation in response to E5, suggesting that these residues may play a role in maintaining a stable E5-PDGFβR complex.
Disparate but not conservative substitutions of these amino acid residues inhibited but did not abolish an interaction with the E5 protein. The Val500Pro substitution may be partially defective due to the presence of a proline, which could introduce a kink in the transmembrane α-helix, rather than absence of the valine at this position. For this reason, and because this substitution appeared only moderately inhibitory for the interaction, the role of Val500 remains unclear. However, the Val502Thr and Ile503Phe substitution mutants, which appeared the most defective for the interaction, implicate an important role for two hydrophobic amino acids having nonpolar and nonaromatic side chains at positions 502 and 503, respectively. While the less conservative amino acid changes Val500Pro, Val502Thr, and Ile503Phe individually only partially inhibited the interaction, the combined mutations in the PTF receptor mutant appeared to completely abolish the interaction. This suggests that at least two of these extracellular proximal amino acids, possibly Val502 and Ile503, participate in the interaction cooperatively.
If this is the case, it is more likely that these amino acids play a supportive role in stabilizing the complex rather than making specific contacts with the E5 protein. For example, as the receptor enters the hydrophobic transmembrane environment, these amino acids, given their location close to the membrane interface, may allow optimal positioning of the receptor transmembrane α-helix for the interaction. Moreover, they may also play a role in maintaining the proper receptor conformation required for optimal receptor activation and signaling. The analogously positioned amino acids in the E5 protein may function in this manner. Mutation of these amino acids does not inhibit the ability of E5 to bind to or induce autophosphorylation of the receptor but severely inhibits its focus-forming activity (15, 27). The conformation of the E5-PDGFβR complex in this case is likely to be altered in such a way as to disrupt receptor signaling. Finally, since Val502 and Ile503 are predicted to be spatially adjacent to Lys499 and Thr513 within the transmembrane α-helix (32), these amino acids could play a supportive role by assisting Lys499 and Thr513 in making contacts with the E5 protein.
Experiments are under way to explore the contribution of other transmembrane amino acids of the receptor towards forming a stable and functional complex with the E5 protein. In glycophorin A, amino acids spaced at seven amino-acid intervals in the transmembrane domain play a role in homodimerization of this protein (20-23, 36, 39). We recently showed that Ile506, which is located in the amino half of the receptor transmembrane domain, seven amino acids between Lys499 and Thr513, is required for a complete interaction with the E5 protein (V. M. Nappi, J. A. Schaefer, and L. M. Petti, unpublished data). We speculated that perhaps Ile506 is involved in hydrophobic interactions with a similarly located Leu residue in the transmembrane domain of the E5 protein. We are currently testing the requirement for Leu520, which is located in the carboxyl half of the transmembrane, seven amino acids away from Thr513. Ile506 and Leu520, along with Lys499 and Thr513, may be involved in direct protein-protein contacts along a single alpha helical interface of the receptor. Other amino acids in the carboxyl half of the receptor transmembrane domain required for the interaction might be those located near the membrane interface with the cytoplasm (i.e., residues 523 to 525). Like the extracellular proximal amino acids, these intracellular proximal residues may be required for anchoring the transmembrane α-helix in an appropriate position and/or conformation for an interaction with the E5 protein.
Given the evidence for multiple requirements within the transmembrane domain of the PDGFβR for formation of a stable functional complex with the E5 protein, we propose that this protein-protein interaction is highly specific. Previous reports have suggested that other transmembrane proteins such as the EGF receptor may be cellular targets for the E5 protein (5, 6, 10, 11, 24). However, in many of these studies the interaction was observed in systems of overexpression or in vitro, conditions favorable for promoting nonspecific interactions. In fact, when both the EGF receptor and the PDGFβR are coexpressed with E5 under conditions of overexpression, the E5 protein preferentially binds to and activates the PDGFβR and not the EGF receptor (31), suggesting that the interaction with the PDGFβR is more specific. Moreover, unlike the E5-PDGFβR interaction, the ability of E5 to interact with any of these other proteins has never been causally related to a biochemical or biological activity. Therefore, we propose that the PDGFβR may be the only specific target of the E5 protein in fibroblasts.
In conclusion, the E5-PDGFβR interaction provides a unique model for structure-function studies of a protein-protein complex formed within the lipid bilayer. Cumulative evidence indicates that the nature of this interaction is probably quite specific and complex, involving several amino acids that either participate in direct protein-protein contacts or play a role in conformational stabilization. E5 and PDGFβR mutants displaying partially defective phenotypes may relate the phenotypic consequences of an altered interaction to the presence or absence of a particular signaling pathway.
Acknowledgments
We thank Paul Black, Jeff Banas, Tom Friedrich, and Jim McSharry for enthusiastic discussions. We also thank Dan DiMaio for E5 and PDGFβR antisera.
This work was supported by a grant from the National Institutes of Health (CA73682).
REFERENCES
- 1.Bergman, P., M. Ustav, J. Sedman, J. Moreno-Lopez, B. Vennstrom, and U. Pettersson. 1988. The E5 gene of bovine papillomavirus type 1 is sufficient for complete oncogenic transformation of mouse fibroblasts. Oncogene 2:453-459. [PubMed] [Google Scholar]
- 2.Burkhardt, A., D. DiMaio, and R. Schlegel. 1987. Genetic and biochemical definition of the bovine papillomavirus E5 transforming protein. EMBO J. 6:2381-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhardt, A., M. Willingham, C. Gay, K. T. Jeang, and R. Schlegel. 1989. The E5 oncoprotein of bovine papillomavirus is oriented asymmetrically in Golgi and plasma membranes. Virology 170:334-339. [DOI] [PubMed] [Google Scholar]
- 4.Claesson-Welsh, L. 1994. Platelet-derived growth factor receptor signals. J. Biol. Chem. 269:32023-32026. [PubMed] [Google Scholar]
- 5.Cohen, B. D., D. R. Lowy, and J. T. Schiller. 1993. The conserved C-terminal domain of the bovine papillomavirus E5 oncoprotein can associate with an alpha-adaptin-like molecule: a possible link between growth factor receptors and viral transformation. Mol. Cell. Biol. 13:6462-6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, B. D., D. J. Goldstein, L. Rutledge, W. C. Vass, D. R. Lowy, R. Schlegel, and J. T. Schiller. 1993. Transformation-specific interaction of the bovine papillomavirus E5 oncoprotein with the platelet-derived growth factor receptor transmembrane domain and the epidermal growth factor receptor cytoplasmic domain. J. Virol. 67:5303-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMaio, D., D. Guralski, and J. T. Schiller. 1986. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc. Natl. Acad. Sci. USA 83:1797-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond-Barbosa, D. A., R. R. Vaillancourt, A. Kazlauskas, and D. DiMaio. 1995. Ligand-independent activation of the platelet-derived growth factor beta receptor: requirements for bovine papillomavirus E5-induced mitogenic signaling. Mol. Cell. Biol. 15:2570-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming, T. P., A. Saxena, W. C. Clark, J. T. Robertson, E. H. Oldfield, S. A. Aaronson, and I. U. Ali. 1992. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 52:4550-4553. [PubMed] [Google Scholar]
- 10.Goldstein, D. J., and R. Schlegel. 1990. The E5 oncoprotein of bovine papillomavirus binds to a 16 kDa cellular protein. EMBO J. 9:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein, D. J., M. E. Finbow, T. Andresson, P. McLean, K. Smith, V. Bubb, and R. Schlegel. 1991. Bovine papillomavirus E5 oncoprotein binds to the 16K component of vacuolar H+-ATPases. Nature 352:347-349. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein, D. J., T. Andresson, J. J. Sparkowski, and R. Schlegel. 1992. The bovine papillomavirus-1 E5 protein, the 16 kDa membrane pore-forming protein and the PDGF receptor exist in a complex that is dependent on hydrophobic transmembrane interactions. EMBO J. 11:4851-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein, D. J., W. Li, L. M. Wang, M. A. Heidaran, S. Aaronson, R. Shinn, R. Schlegel, and J. H. Pierce. 1994. The bovine papillomavirus type 1 E5 transforming protein specifically binds and activates the beta-type receptor for the platelet-derived growth factor but not other related tyrosine kinase-containing receptors to induce cellular transformation. J. Virol. 68:4432-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldin, C. H. 1995. Dimerization of cell surface receptors in signal transduction. Cell 80:213-223. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz, B. H., A. L. Burkhardt, R. Schlegel, and D. DiMaio. 1988. 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol. Cell. Biol. 8:4071-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein, O., G. W. Polack, T. Surti, D. Kegler-Ebo, S. O. Smith, and D. DiMaio. 1998. Role of glutamine 17 of the bovine papillomavirus E5 protein in platelet-derived growth factor beta receptor activation and cell transformation. J. Virol. 72:8921-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein, O., D. Kegler-Ebo, J. Su, S. Smith, and D. DiMaio. 1999. The bovine papillomavirus E5 protein requires a juxtamembrane negative charge for activation of the platelet-derived growth factor beta receptor and transformation of C127 cells. J. Virol. 73:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulke, R., B. H. Horwitz, T. Zibello, and D. DiMaio. 1992. The central hydrophobic domain of the bovine papillomavirus E5 transforming protein can be functionally replaced by many hydrophobic amino acid sequences containing a glutamine. J. Virol. 66:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai, C. C., C. Henningson, and D. DiMaio. 1998. Bovine papillomavirus E5 protein induces oligomerization and trans-phosphorylation of the platelet-derived growth factor beta receptor. Proc. Natl. Acad. Sci. USA 95:15241-15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmon, M. A., J. M. Flanagan, H. R. Treutlein, J. Zhang, and D. M. Engelman. 1992. Sequence specificity in the dimerization of transmembrane alpha-helices. Biochemistry 31:12719-12725. [DOI] [PubMed] [Google Scholar]
- 21.Lemmon, M. A., J. M. Flanagan, J. F. Hunt, B. D. Adair, B. J. Bormann, C. E. Dempsey, and D. M. Engelman. 1992. Glycophorin A dimerization is driven by specific interactions between transmembrane alpha-helices. J. Biol. Chem. 267:7683-7689. [PubMed] [Google Scholar]
- 22.Lemmon, M. A., and D. M. Engelman. 1994. Specificity and promiscuity in membrane helix interactions. Q. Rev. Biophys. 27:157-218. [DOI] [PubMed] [Google Scholar]
- 23.Lemmon, M. A., H. R. Treutlein, P. D. Adams, A. T. Brunger, and D. M. Engelman. 1994. A dimerization motif for transmembrane alpha-helices. Nat. Struct. Biol. 1:157-163. [DOI] [PubMed] [Google Scholar]
- 24.Martin, P., W. C. Vass, J. T. Schiller, D. R. Lowy, and T. J. Velu. 1989. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell 59:21-32. [DOI] [PubMed] [Google Scholar]
- 25.Meyer, A. N., Y. F. Xu, M. K. Webster, A. E. Smith, and D. J. Donoghue. 1994. Cellular transformation by a transmembrane peptide: structural requirements for the bovine papillomavirus E5 oncoprotein. Proc. Natl. Acad. Sci. USA 91:4634-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilson, L. A., and D. DiMaio. 1993. Platelet-derived growth factor receptor can mediate tumorigenic transformation by the bovine papillomavirus E5 protein. Mol. Cell. Biol. 13:4137-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilson, L. A., R. L. Gottlieb, G. W. Polack, and D. DiMaio. 1995. Mutational analysis of the interaction between the bovine papillomavirus E5 transforming protein and the endogenous beta receptor for platelet-derived growth factor in mouse C127 cells. J. Virol. 69:5869-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacios, R., and M. Steinmetz. 1985. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell 41:727-734. [DOI] [PubMed] [Google Scholar]
- 29.Petti, L., L. A. Nilson, and D. DiMaio. 1991. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 10:845-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petti, L., and D. DiMaio. 1992. Stable association between the bovine papillomavirus E5 transforming protein and activated platelet-derived growth factor receptor in transformed mouse cells. Proc. Natl. Acad. Sci. USA 89:6736-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petti, L., and D. DiMaio. 1994. Specific interaction between the bovine papillomavirus E5 transforming protein and the beta receptor for platelet-derived growth factor in stably transformed and acutely transfected cells. J. Virol. 68:3582-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petti, L. M., V. Reddy, S. O. Smith, and D. DiMaio. 1997. Identification of amino acids in the transmembrane and juxtamembrane domains of the platelet-derived growth factor receptor required for productive interaction with the bovine papillomavirus E5 protein. J. Virol. 71:7318-7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petti, L. M., and F. A. Ray. 2000. Transformation of mortal human fibroblasts and activation of a growth inhibitory pathway by the bovine papillomavirus E5 oncoprotein. Cell Growth Differ. 11:395-408. [PubMed] [Google Scholar]
- 34.Schiller, J. T., W. C. Vass, K. H. Vousden, and D. R. Lowy. 1986. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J. Virol. 57:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlegel, R., M. Wade-Glass, M. S. Rabson, and Y. C. Yang. 1986. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science 233:464-467. [DOI] [PubMed] [Google Scholar]
- 36.Treutlein, H. R., M. A. Lemmon, D. M. Engelman, and A. T. Brunger. 1992. The glycophorin A transmembrane domain dimer: sequence-specific propensity for a right-handed supercoil of helices. Biochemistry 31:12726-12732. [DOI] [PubMed] [Google Scholar]
- 37.Waterfield, M. D., G. T. Scrace, N. Whittle, P. Stroobant, A. Johnsson, A. Wasteson, B. Westermark, C. H. Heldin, J. S. Huang, and T. F. Deuel. 1983. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature 304:35-39. [DOI] [PubMed] [Google Scholar]
- 38.Yarden, Y., J. A. Escobedo, W. J. Kuang, T. L. Yang-Feng, T. O. Daniel, P. M. Tremble, E. Y. Chen, M. E. Ando, R. N. Harkins, U. Francke, et al. 1986. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature 323:226-232. [DOI] [PubMed] [Google Scholar]
- 39.Zhou, F. X., M. J. Cocco, W. P. Russ, A. T. Brunger, and D. M. Engelman. 2000. Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nat. Struct. Biol. 7:154-160. [DOI] [PubMed] [Google Scholar]