Abstract
Cells infected with mammalian orthoreoviruses contain large cytoplasmic phase-dense inclusions believed to be the sites of viral replication and assembly, but the morphogenesis, structure, and specific functions of these “viral factories” are poorly understood. Using immunofluorescence microscopy, we found that reovirus nonstructural protein μNS expressed in transfected cells forms inclusions that resemble the globular viral factories formed in cells infected with reovirus strain type 3 Dearing from our laboratory (T3DN). In the transfected cells, the formation of μNS large globular perinuclear inclusions was dependent on the microtubule network, as demonstrated by the appearance of many smaller μNS globular inclusions dispersed throughout the cytoplasm after treatment with the microtubule-depolymerizing drug nocodazole. Coexpression of μNS and reovirus protein μ2 from a different strain, type 1 Lang (T1L), which forms filamentous viral factories, altered the distributions of both proteins. In cotransfected cells, the two proteins colocalized in thick filamentous structures. After nocodazole treatment, many small dispersed globular inclusions containing μNS and μ2 were seen, demonstrating that the microtubule network is required for the formation of the filamentous structures. When coexpressed, the μ2 protein from T3DN also colocalized with μNS, but in globular inclusions rather than filamentous structures. The morphology difference between the globular inclusions containing μNS and μ2 protein from T3DN and the filamentous structures containing μNS and μ2 protein from T1L in cotransfected cells mimicked the morphology difference between globular and filamentous factories in reovirus-infected cells, which is determined by the μ2-encoding M1 genome segment. We found that the first 40 amino acids of μNS are required for colocalization with μ2 but not for inclusion formation. Similarly, a fusion of μNS amino acids 1 to 41 to green fluorescent protein was sufficient for colocalization with the μ2 protein from T1L but not for inclusion formation. These observations suggest a functional difference between μNS and μNSC, a smaller form of the protein that is present in infected cells and that is missing amino acids from the amino terminus of μNS. The capacity of μNS to form inclusions and to colocalize with μ2 in transfected cells suggests a key role for μNS in forming viral factories in reovirus-infected cells.
The replication and assembly of viruses are often concentrated in specific locations within infected cells, such as on the actin cytoskeleton for human parainfluenza virus type 3 (13), on the outer mitochondrial membranes for flock house virus (24), in cytoplasmic inclusions for vaccinia virus (39), and in nuclear inclusions for herpes simplex virus (32). The nonfusogenic mammalian orthoreoviruses (reoviruses) are believed to replicate and assemble in cytoplasmic phase-dense inclusions in infected cells (31). These inclusions contain viral double-stranded RNA (34), viral proteins (9, 31), partially and fully assembled viral particles (31), and both microtubules (MTs) and thinner “kinky” filaments suggested to be intermediate filaments (8, 9, 35). It was reported that the intermediate filament vimentin is rearranged in reovirus-infected CV-1 cells such that it surrounds and is incorporated into the large inclusions at 48 h postinfection (p.i.) (33). The inclusions do not contain ribosomes (31, 33) and are not membrane bound (21). To differentiate these inclusions from the inclusion bodies formed by the aggregation of misfolded proteins (19), we refer to the reovirus phase-dense inclusions as “viral factories.” The viral and cellular factors responsible for the formation and morphology of viral factories and the exact functions and effects of these factories during reovirus infection are largely unknown.
A reovirus strain-dependent difference in the kinetics of viral factory formation was recently mapped to the M1 genome segment, which encodes the structurally minor core protein μ2, and secondarily to the S3 genome segment, which encodes the nonstructural protein σNS (22). Another recent study used temperature-sensitive reovirus mutants to define roles in viral factory formation for both μ2 and σNS (2). It was suggested that viral RNA-protein complexes containing σNS nucleate inclusion formation and that other viral proteins, including μ2, are recruited to these complexes. However, the expression of σNS in the absence of other viral proteins does not lead to factory formation; instead, σNS is diffusely distributed within the cytoplasm and nucleus (M. M. Becker, T. R. Peters, and T. S. Dermody, Abstr. 20th Meet. Am. Soc. Virol., abstr. W28-7, 2001; C. L. Miller, T. J. Broering, J. S. L. Parker, and M. L. Nibert, unpublished data). Thus, although σNS may be required, it is not sufficient for viral factory formation, and other viral components are needed for factory morphogenesis.
Parker et al. recently described two strain-dependent reovirus factory morphologies: filamentous, induced by the majority of strains tested, and globular, induced by only two strains (28). The filamentous viral factories are colinear with MTs, and MTs are stabilized and bundled in cells infected with strains that form filamentous factories, including reovirus strain type 1 Lang (T1L). In contrast, infection with reovirus strain type 3 Dearing from our laboratory (T3DN), a strain that forms globular factories, does not cause increased MT stability or bundling. Strain-dependent differences in both viral factory morphology and MT stabilization are determined by the μ2-encoding M1 genome segment (28). When μ2 derived from T1L [μ2(T1L)] is expressed in cells, it colocalizes with MTs and causes MT bundling and stabilization, whereas μ2 derived from T3DN [μ2(T3DN)] does not (28). These findings implicate protein μ2 as a major determinant of viral factory association with MTs. However, μ2 does not form structures that resemble factories when expressed alone, suggesting that one or more other viral proteins are involved in forming the matrix of the factories.
A reovirus nonstructural protein encoded by the M3 genome segment, μNS, can bind to a reovirus subviral particle (core) in vitro and form large amorphous complexes (5). Negative-stain electron microscopy (EM) revealed an extensive matrix surrounding the core particles; the morphology of this μNS and core complex resembled the morphology of factories in thin sections of reovirus-infected cells viewed by EM (33). By immunofluorescence (IF) microscopy, μNS was found concentrated within viral factories in infected cells (25, 28). Based on this evidence, we hypothesized as part of the current study that μNS plays a major role in viral factory structure and morphogenesis in infected cells.
μNS is an 80-kDa protein expressed at high levels in infected cells (41, 42). A second form of the protein, called μNSC, is also produced (20). Chemical cleavage of μNS and μNSC from infected cells demonstrated that μNSC lacks about 5 kDa of sequence from the amino (N) terminus of μNS (41). It was hypothesized that the synthesis of μNS results from ribosome initiation at the first AUG codon in the M3 plus-strand RNA (nucleotides 19 to 21) whereas the synthesis of μNSC results from initiation at the second, in-frame, AUG codon (nucleotides 139 to 141) (41). These first and second AUG codons are conserved in the three reovirus strains for which M3 sequences have been determined to date (23). The kinetics of μNSC formation in infected cells indicate that μNS and μNSC do not have a precursor-product relationship (41), supporting the hypothesis that μNSC arises by secondary initiation rather than by cleavage of full-length μNS. The relative functions of μNS and μNSC in infected cells are not known. To address the hypothesis that μNS and μNSC play roles in viral factory formation, we expressed these proteins in the absence of other reovirus proteins by transfection of DNA expression vectors containing the encoding genes. The results suggest a role for these proteins in factory formation. Moreover, colocalization and redistribution of μNS and μ2 when coexpressed suggest that specific associations between these two proteins may be key to forming viral factories and recruiting the factors necessary for replication and assembly.
MATERIALS AND METHODS
Cells, viruses, and reagents.
CHO, Mv1Lu, and CV-1 cells were maintained in Dulbecco's modified Eagle's medium (Life Technologies, Inc., Rockville, Md.) containing 10% fetal bovine serum (HyClone Laboratories, Logan, Utah) and 10 μg of gentamicin (Life Technologies) per ml. Reovirus strains T1L and T3DN were laboratory stocks. The designation T3DN differentiates our laboratory strain from another strain (T3DC) that differs in inclusion phenotype and M1 sequence as described previously (28). All enzymes were obtained from New England Biolabs, Inc. (Beverly, Mass.), unless otherwise stated.
Antibodies.
Monoclonal antibodies to Cy3-conjugated β-tubulin (TUB 2.1) were obtained from Sigma (St. Louis, Mo.). Goat anti-mouse immunoglobulin G (IgG) and goat anti-rabbit IgG conjugated to Alexa 488 or Alexa 594 were obtained from Molecular Probes (Eugene, Oreg.). Monoclonal antibodies to green fluorescent protein (GFP) (JL-8) were obtained from Clontech (Palo Alto, Calif.). We used rabbit polyclonal antisera against μNS (5) and μ2 (described below). μNS and μ2 polyclonal IgG antibodies purified with protein A-Sepharose were directly conjugated to Texas red and Oregon green by following the manufacturer's procedure (Molecular Probes). As determined by IF analysis, the μNS antiserum did not stain cells expressing μ2 or GFP, and the GFP antibodies did not stain cells expressing μNS or μ2. The μ2 antiserum showed a low level of background nuclear fluorescence with paraformaldehyde (PFA) fixation that was reduced with methanol fixation as previously described (28), but the antiserum did not stain μNS or GFP in transfected cells.
Rabbit polyclonal antiserum specific for μ2 was produced by using Escherichia coli-expressed protein. The T1L M1 gene was excised from pBluescript II KS(+) (Stratagene, La Jolla, Calif.) (28) with SmaI and XhoI and ligated to pET-21b (Novagen, Madison, Wis.) cut with HindIII and XhoI, with the HindIII overhang converted to a blunt end with the Klenow fragment of DNA polymerase I; this procedure generated pET-M1(T1L). μ2 was expressed in BL21-DE3 cells (Novagen) by following the procedure in the pET system manual (Novagen). In brief, expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside. The cells were incubated at 37°C for 3 h, pelleted, resuspended, and lysed by sonication. Approximately 50% of the insoluble fraction was μ2. To further purify μ2 from the insoluble fraction, it was subjected to preparative sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and electroeluted (14). The eluent was concentrated in dialysis tubing by buffer absorption with polyethylene glycol. The antiserum was generated in a rabbit by the Polyclonal Antibody Service at the Animal Care Unit of the University of Wisconsin Medical School.
Rat antiserum specific for the N-terminal 41 amino acids of μNS was produced by using a fusion protein of glutathione S-transferase (GST) and amino acids 1 to 41 of the T1L μNS protein [GST-μNS(T1L)(1-41)]. To express the protein, pGEM4Z-M3(T1L) (5) was cut with AflIII and SalI, and the piece of DNA containing M3 nucleotides 1 to 142 was isolated. The Klenow fragment was used to convert the AflIII site to a blunt end. The small piece of DNA was ligated to pGEX-4T-3 (Amersham Biosciences, Piscataway, N.J.) cut with NotI and SalI, with the NotI site converted to a blunt end with the Klenow fragment; this procedure generated pGEX-M3(T1L)(1-41). The fusion protein, GST-μNS(T1L)(1-41), was expressed in BL21-DE3 cells and purified by using glutathione-agarose beads (Pierce, Rockford, Ill.) according to the instructions in the pGEX manual (Amersham Biosciences). The antiserum was generated in a rat by the Polyclonal Antibody Service at the Animal Care Unit of the University of Wisconsin Medical School.
Generation of μNS mammalian expression constructs.
The DNA clone of the T3D M3 gene used in this study was originally generated by Cashdollar et al. (7). As a result, it was likely derived from a viral plaque isolate most closely related to T3DC (28). We have chosen not to include the Cashdollar (C) or Nibert (N) designation on the M3 gene (28) because there is as yet no demonstrated sequence or functional difference between the M3 genes of the T3DC and T3DN viruses. The T3D M3 gene was cut from pGEM4Z-M3(T3D) (5) by using the KpnI and SalI sites. T4 DNA polymerase was used to convert the KpnI site to a blunt end, which was ligated to pCI-neo (Promega, Madison, Wis.) that had been cut with SmaI and XhoI; this procedure generated pCI-M3(T3D). The T1L M3 gene was cut from pGEM4Z-M3(T1L) by using the BamHI and NheI sites. The Klenow fragment was used to convert the BamHI site to a blunt end, which was ligated to pCI-neo that had been cut with SmaI and NheI; this procedure generated pCI-M3(T1L).
Generation of a μNS(41-721) expression construct.
To generate a vector to direct the expression of a μNS protein that lacks N-terminal amino acids, we exploited the second in-frame AUG codon in the T1L M3 gene (nucleotides 139 to 141) and a unique StyI restriction site in the T1L M3 gene (nucleotides 62 to 67). We used the StyI restriction site to introduce a frameshift into the μNS open reading frame between the first two AUG codons. pGEM4Z-M3(T1L) was cut with StyI, and the ends were made blunt with the Klenow fragment. The plasmid was religated, thereby adding an insertion of 4 bp after nucleotide 62. The mutated M3 gene was cloned into pCI-neo as described above for the T1L M3 gene, generating pCI-M3(41-721). The insertion shifts the reading frame from the first AUG, associating it with a stop codon at nucleotides 79 to 81. Ribosome initiation at the first AUG codon (nucleotides 19 to 21) should therefore produce a protein containing only amino acids 1 to 16 of μNS and an additional 4 amino acids. We have not been able to detect this product in immunoblots with μNS antisera (data not shown). Ribosome initiation at the second AUG codon should produce a protein containing amino acids 41 to 721 of μNS, with a predicted molecular mass of 76 kDa. We have detected this product (see Fig. 7).
FIG. 7.
Distribution of μNS(41-721) in transfected cells with and without μ2 coexpression. (A) CV-1 cells were transfected with 1 μg of pCI-M3(T1L) or pCI-M3(41-721) and 1 μg of pCI-neo as a carrier plasmid per well. CV-1 cells were infected (inf) with T1L at a multiplicity of infection of 5. Lysates were collected at 18 h p.t. or p.i. and analyzed by SDS-PAGE and immunoblotting with polyclonal antisera specific for full-length μNS (α-μNS) and the N-terminal 41 amino acids of μNS (α-1-41 μNS). A band appearing in untransfected CV-1 cells is indicated on the right with an asterisk. The position of full-length μNS is indicated on the right. (B) Phase-contrast microscopy (left) and IF microscopy (right) of CV-1 cells transfected with 2 μg of pCI-M3(41-721) per well. Cells were fixed at 18 h p.t. and immunostained with Texas red-conjugated anti-μNS rabbit IgG. (C) CV-1 cells were cotransfected with 1 μg of pCI-M3(41-721) and 1 μg of pCI-M1(T1L) (upper panels) or 1 μg of pCI-M3(41-721) and 1 μg of pCI-M1(T3DN) (lower panels) per well and fixed at 18 h p.t. Cells were immunostained for μNS (red) and μ2 (green) as described in the legend to Fig. 3A. Bars, 10 μm.
Generation of GFP fusion vectors.
To generate a fusion of μNS amino acids 1 to 41 to the N terminus of GFP, pGEM4Z-M3(T1L) was cut with AflIII and NheI, and the piece of DNA containing M3 nucleotides 1 to 142 was isolated. The Klenow fragment was used to convert the AflIII site to a blunt end, which was ligated to pEGFP-N1 (Clontech) that had been cut with BamHI and NheI, with the BamHI site converted to a blunt end with the Klenow fragment; this procedure generated pEGFP-M3(1-41).
To generate a full-length μNS fusion with GFP, the stop codon of the M3 gene was removed by using PCR with a primer complementary to the 5′ end of M3 containing no stop codon and a new BamHI site. Nucleotides 1842 to 2181 of M3 were amplified with a forward primer complementary to nucleotides 1842 to 1859, a reverse primer (5′-GTGTATCCGCCAGCTCATCAGTTGGAAC-3′), pGEM4Z-M3(T1L) as a template, and Vent polymerase. The PCR product was cut with SalI and BamHI and ligated to pGEM4Z-M3(T1L) that had been cut with SalI and BamHI to remove nucleotides 1842 to 2241; this procedure generated pGEM4Z-M3(T1L)(no stop). The intended mutation was verified by sequencing. The M3 gene was cut from pGEM4Z-M3(T1L)(no stop) with NheI and BamHI and ligated to pEGFP-N1 that had been cut with NheI and BamHI; this procedure generated pEGFP-M3.
IF microscopy.
CV-1 cells were seeded on the day before transfection or infection at a density of 1.0 × 104 per cm2 in six-well plates (9.6 cm2 per well) containing glass coverslips. Transfections were performed with 2 μg of DNA (total) and 6 μl of Lipofectamine (Life Technologies) per well according to the manufacturer's directions. Infections were done for 1 h at room temperature with 200 μl of phosphate-buffered saline (PBS) (137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1 mM KH2PO4 [pH 7.5]) supplemented to contain 2 mM MgCl2. Cells were further incubated at 37°C before being processed for IF microscopy. Cells were fixed for 10 min at room temperature in 2% PFA or for 3 min at −20°C in 100% methanol. No significant differences in morphology were seen between PFA fixation and methanol fixation of inclusions or filamentous structures in infected or transfected cells. PFA fixation was insufficient to fix GFP and the GFP fusion to μNS amino acids 1 to 41. Instead, methanol fixation was used, and GFP was recognized with the specific monoclonal antibody. Cells were permeabilized, blocked, incubated with antibodies and 4′,6-diamidino-2-phenylindole (DAPI), and mounted as described previously (28). Samples were examined by using a Nikon TE-300 inverted microscope equipped with phase and fluorescence optics, and images were collected digitally as described previously (28). Images were processed and prepared for presentation by using Photoshop and Illustrator software (Adobe Systems, San Jose, Calif.).
Immunoblot analysis.
To compare viral proteins expressed in infected and transfected cells, we collected cell lysates at 18 to 24 h p.i. or posttransfection (p.t.). CV-1 cells (1.2 × 106) were washed briefly in PBS, scraped into 2 ml of PBS, and pelleted. The pelleted cells were resuspended in 60 μl of PBS containing protease inhibitors (Roche, Indianapolis, Ind.), lysed in sample buffer, boiled for 10 min, and subjected to SDS-PAGE. Proteins were electroblotted from the gels to nitrocellulose in 25 mM Tris-192 mM glycine (pH 8.3). The binding of antibodies was detected with alkaline phosphatase-coupled goat anti-mouse or anti-rabbit (Bio-Rad Laboratories, Hercules, Calif.) or anti-rat (Pierce) IgG and the colorimetric reagents p-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt (Bio-Rad).
RESULTS
Globular inclusions are formed in cells that express μNS.
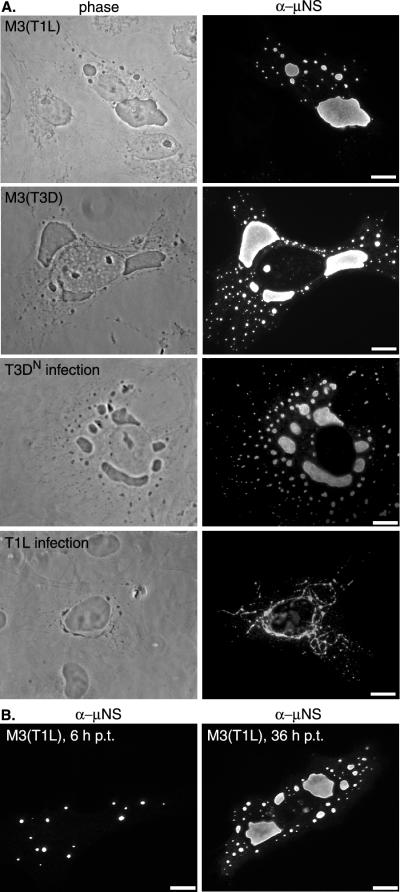
To test whether μNS can induce structures that resemble viral factories when expressed in the absence of other reovirus proteins, we transfected CV-1 cells with expression vectors containing the M3 gene from either reovirus T1L [pCI-M3(T1L)] or reovirus T3D [pCI-M3(T3D)] and examined the subcellular distribution of μNS by IF microscopy at 18 h p.t. Large and small globular phase-dense inclusions, with smooth edges, were observed in the cytoplasm of cells transfected with either M3 gene (Fig. 1A). These structures contained μNS and closely resembled the μNS-containing globular phase-dense viral factories in reovirus T3DN-infected cells (28) (Fig. 1A). The untransfected cells in the samples (Fig. 1A, top panels) and separate untransfected cell controls (data not shown) did not react with the μNS-specific antiserum and did not contain globular phase-dense structures. We observed a similar distribution of the μNS protein from T1L [μNS(T1L)] in transfected CHO and Mv1Lu cells (supplemental data can be found at http://micro.med.harvard.edu/nibert/suppl/broering02a/fig1.html). Unlike the filamentous viral factories in T1L-infected cells (28), the inclusions in cells expressing μNS(T1L) were globular (Fig. 1A).
FIG. 1.
Distribution of μNS in transfected and reovirus-infected cells. (A) Phase-contrast microscopy (left column) and IF microscopy (right column) of CV-1 cells transfected (upper four panels) with 2 μg of pCI-M3(T1L) or pCI-M3(T3D) per well or infected (lower four panels) with T3DN or T1L at a multiplicity of infection of 5. The cells were fixed at 18 h p.t. or p.i. and immunostained with rabbit anti-μNS IgG directly conjugated to Texas red (T3DN and T1L infection) or Oregon green [pCI-M3(T3D) transfection] or immunostained with rabbit anti-μNS serum followed by goat anti-rabbit IgG conjugated to Alexa 488 [pCI-M3(T1L) transfection]. (B) CV-1 cells transfected with 2 μg of pCI-M3(T1L) per well were fixed at 6 h p.t. (left) or 36 h p.t. (right) and immunostained with Oregon green-conjugated anti-μNS rabbit IgG. Bars, 10 μm.
Analysis of cells at different times revealed that at 6 h p.t., μNS inclusions were uniformly small and spread throughout the cytoplasm (Fig. 1B), whereas at 18 h p.t. (Fig. 1A) and 36 h p.t. (Fig. 1B), large perinuclear inclusions were present along with smaller inclusions. These changes in the size and distribution of μNS inclusions over time p.t. resemble the time-dependent changes in the viral factories in T3DN-infected cells (2, 28). The striking similarity between the viral factories in T3DN-infected cells and the inclusions in cells transfected with either the T1L or the T3D M3 gene is consistent with our hypothesis that μNS plays a role in the formation and structure of the viral factories in infected cells.
Because misfolded proteins can accumulate in globular phase-dense structures called aggresomes (18), which appear similar to some viral inclusions (15, 28), we examined samples for two hallmarks of aggresome formation in association with the μNS inclusions: polyubiquitination and collapse of the vimentin intermediate filament network around the inclusions (18). For CV-1 cells transfected with pCI-M3(T1L), we found no staining of the μNS inclusions with an antibody to polyubiquitin and no evidence for redistribution of vimentin intermediate filaments to surround the inclusions (supplemental data can be found at http://micro.med.harvard.edu/nibert/suppl/broering02a/fig2.html), suggesting that the μNS inclusions are not aggresomes.
Size and distribution of μNS(T1L) globular inclusions are dependent on MTs.
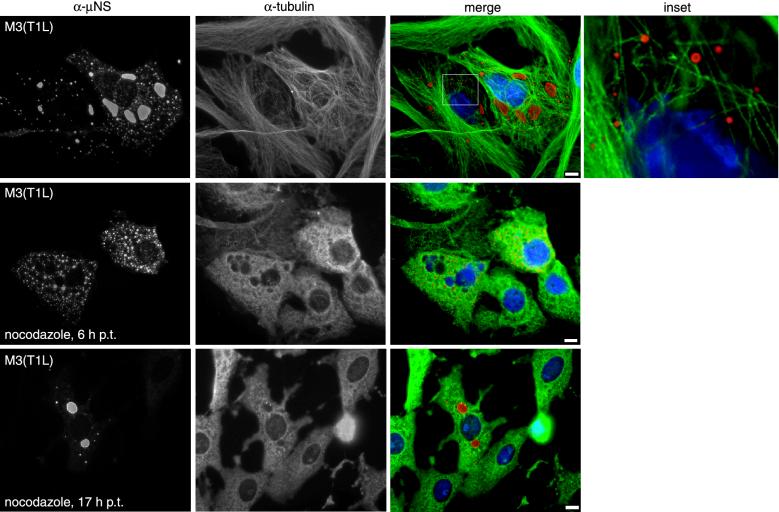
Based on the previous observations that MTs are embedded in reovirus factories (8, 12, 35) and that μNS may associate with MTs (25), we examined samples for the colocalization of μNS(T1L) with MTs by staining pCI-M3(T1L)-transfected cells for μNS and β-tubulin. μNS globular inclusions did not colocalize with or reorganize MTs (Fig. 2), but small isolated inclusions on the periphery of the cells were found adjacent to MTs (Fig. 2, inset). These findings are similar to those for the viral factories in T3DN-infected cells (28).
FIG. 2.
Distribution of μNS(T1L) and β-tubulin in transfected cells with and without nocodazole treatment. CV-1 cells transfected with 2 μg of pCI-M3(T1L) per well were left untreated (upper panels), treated with 10 μM nocodazole added at 6 h p.t. (middle panels), or treated with 10 μM nocodazole added at 17 h p.t. (lower panels). The cells were fixed at 18 h p.t. and immunostained with Oregon green-conjugated anti-μNS rabbit IgG (red) (first column) and Cy3-conjugated mouse monoclonal antibody to β-tubulin (green) (second column). Nuclei were counterstained with DAPI (blue). The boxed area in the merged image (third column) is enlarged to show detail (inset). Bars, 10 μm.
To determine whether the morphology of μNS globular inclusions is altered by the depolymerization of MTs, we treated transfected cells with 10 μM nocodazole for 12 h beginning at 6 h p.t. to depolymerize MTs as described previously (28). With nocodazole treatment at 6 h p.t., MTs were completely depolymerized, and μNS(T1L) was located in numerous small globular inclusions dispersed throughout the cytoplasm (Fig. 2). When transfected cells were treated with 10 μM nocodazole for only 1 h beginning at 17 h p.t., MTs were completely depolymerized, but the large perinuclear μNS inclusions were not disrupted (Fig. 2). Similar observations were made for the viral factories in T3DN-infected cells (28). We conclude that the MT network is required for the formation of large perinuclear μNS inclusions but is not required for the maintenance of the large inclusions once they are formed.
μNS and μ2 colocalize when coexpressed in cells.
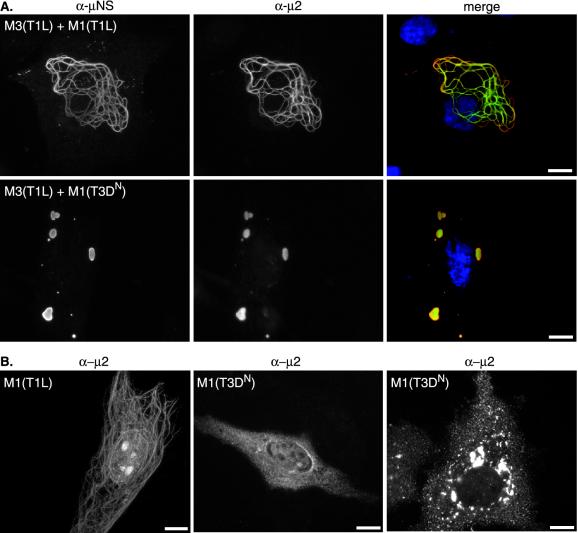
The μNS globular inclusions in transfected cells expressing μNS(T1L) contrast with the filamentous distribution of μNS in T1L-infected cells (28) (Fig. 1A, compare top and bottom panels). Because the filamentous appearance of viral factories in T1L-infected cells is determined by the capacity of μ2(T1L) to associate with MTs (28), we hypothesized that μ2 associates with μNS and redistributes it to MTs. To test this hypothesis, we transfected CV-1 cells with pCI-M3(T1L) and pCI-M1(T1L) at a 1:1 (wt/wt) ratio and examined the distribution of μNS and μ2 by using protein-specific polyclonal antisera. The μNS(T1L) and μ2(T1L) proteins colocalized in thick filamentous structures that surrounded the nucleus (Fig. 3A). The distribution of μNS(T1L) and μ2(T1L) in cells coexpressing the two proteins was clearly different from the distribution of each protein expressed separately (compare Fig. 3A with Fig. 1A and 3B). The results suggest that μNS(T1L) and μ2(T1L) associate and that μ2(T1L) likely mediates the redistribution of μNS(T1L) to MTs.
FIG. 3.
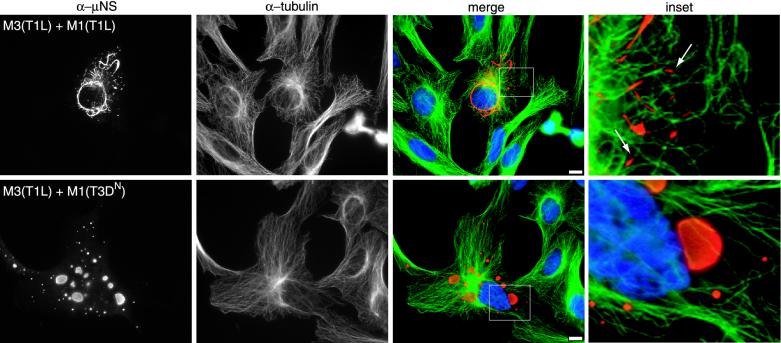
Colocalization of μNS(T1L) and μ2 in cotransfected cells. (A) CV-1 cells were cotransfected with 1 μg of pCI-M3(T1L) and 1 μg of pCI-M1(T1L) (upper panels) or 1 μg of pCI-M3(T1L) and 1 μg of pCI-M1(T3DN) (lower panels) per well and fixed at 18 h p.t. Cells were immunostained with Texas red-conjugated anti-μNS rabbit IgG (red) (first column) and Oregon green-conjugated μ2 rabbit IgG (green) (second column). Nuclei were counterstained with DAPI (blue). (B) CV-1 cells were transfected with 2 μg of pCI-M1(T1L) (left) or pCI-M1(T3DN) (middle and right) per well, fixed at 18 h p.t., and stained with rabbit anti-μ2 polyclonal serum and goat anti-rabbit IgG conjugated to Alexa 488. Bars, 10 μm.
When expressed in transfected cells, the μ2(T3DN) protein does not have a filamentous distribution and does not colocalize with MTs (28). Instead, μ2(T3DN) is diffusely distributed throughout the cytoplasm and nucleus or, in cells that express more of the protein, is concentrated in rough-edged structures that differ in appearance from μNS inclusions and may be aggregates of misfolded protein (28) (Fig. 3B). To determine whether the lack of MT association would affect the capacity of μ2(T3DN) to colocalize with μNS(T1L), CV-1 cells were cotransfected with pCI-M3(T1L) and pCI-M1(T3DN) at a 1:1 (wt/wt) ratio. We found that the distribution of the μ2(T3DN) protein changed when it was coexpressed with μNS(T1L) such that the two proteins were colocalized in large globular inclusions with smooth edges (Fig. 3A), very similar to those seen when μNS(T1L) was expressed in the absence of μ2 (Fig. 1A). Results essentially the same as those described for μNS(T1L) were observed when the μNS protein from T3D [μNS(T3D)] was coexpressed with either μ2(T1L) or μ2(T3DN) (data not shown). These results indicate that the colocalization of μNS and μ2 is independent of the strain derivations of these proteins but that the morphology of μNS/μ2 structures is determined by the strain-dependent difference in the association of μ2 with MTs.
As described previously, some of the μ2 protein—especially μ2(T1L)—appears to be located in the nuclei of many transfected cells (28) and may be concentrated in as-yet-unidentified nuclear structures (Fig. 3B). When we examined the distribution of μ2(T1L) or μ2(T3DN) in cells coexpressing μNS(T1L), we found that the nuclear association of μ2 was much less evident (Fig. 3A). This result suggests the existence of an equilibrium between μ2 proteins in different subcellular compartments (nucleus and cytoplasm) that is altered by the association of μ2 with μNS.
Varying the relative levels of μNS and μ2 expression alters the morphology of μNS/μ2 structures.
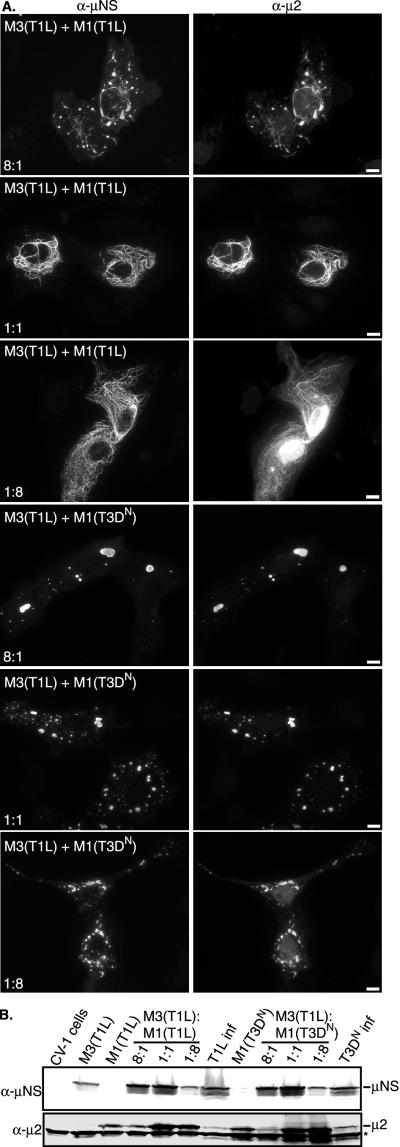
Although the globular inclusions containing μNS(T1L) and μ2(T3DN) closely resemble the globular factories in T3DN-infected cells, the filamentous structures containing μNS(T1L) and μ2(T1L) lack the globular areas connected by thin filaments found in T1L-infected cells (28) (compare Fig. 3A with Fig. 1A). To investigate the effects of changes in the levels of protein expression on the morphology of μNS/μ2 structures, we cotransfected cells with pCI-M3(T1L) and either pCI-M1(T1L) or pCI-M1(T3DN) at a ratio of 8:1, 1:1, or 1:8 (wt/wt). We found that in all instances, μNS(T1L) colocalized with μ2 (Fig. 4A ); however, the relative amounts of transfected DNA affected the morphology of μNS/μ2 structures. When more of the μNS expression plasmid was transfected relative to the μ2 expression plasmid (8:1), globular inclusions connected by thin filaments were predominantly seen with μNS(T1L) and μ2(T1L) and large globular perinuclear inclusions were predominantly seen with μNS(T1L) and μ2(T3DN) (Fig. 4A). Alternatively, when less of the μNS expression plasmid was transfected relative to the μ2 expression plasmid (1:8), the morphology of μNS/μ2 structures was closer to that of μ2 alone: distributed filaments for μ2(T1L) and small perinuclear structures, with rough edges, for μ2(T3DN) (Fig. 4A). In addition, the association of μ2 with the nucleus was more evident in these cells (Fig. 4A). Structures containing μNS and μ2 in cells transfected with M3 and M1 at a ratio of 8:1 most closely resembled the viral factories in reovirus-infected cells (compare Fig. 4A with Fig. 1A), suggesting that this relative level of protein expression most closely mimics that in infected cells.
FIG.4.
Morphologies of μNS/μ2 structures with different relative levels of expression of μNS(T1L) and μ2. (A) CV-1 cells were cotransfected with 2 μg of DNA (total) of pCI-M3(T1L) and pCI-M1(T1L) (upper six panels) or pCI-M3(T1L) and pCI-M1(T3DN) (lower six panels) with different ratios of M3 DNA to M1 DNA (8:1, 1:1, and 1:8) per well. Cells were fixed at 18 h p.t. and immunostained for μNS (left column) and μ2 (right column) as described in the legend to Fig. 3A. Bars, 10 μm. (B) CV-1 cells were transfected with 1 μg of pCI-M3(T1L), pCI-M1(T1L), or pCI-M1(T3DN) and 1 μg of pCI-neo as a carrier plasmid per well and cotransfected as described above. CV-1 cells were infected (inf) with T1L or T3DN at a multiplicity of infection of 5. Lysates were collected at 18 h p.t. or p.i. and analyzed by SDS-PAGE and immunoblotting with polyclonal μNS and μ2 antisera (α-μNS and α-μ2, respectively). A band appearing in untransfected CV-1 cells is indicated on the right with an asterisk. The positions of μNS and μ2 are indicated on the right.
To analyze the relative levels of μNS and μ2 expression in transfected cells, samples identical to those used for the IF microscopy experiments were analyzed by SDS-PAGE and immunoblotting (Fig. 4B). In both T1L- and T3DN-infected cells, μNS was expressed at a higher level than μ2 (Fig. 4B). In transfected cells, the ratio of M3 DNA to M1 DNA changed the relative levels of μNS and μ2 expression, and as the IF results suggested, an M3/M1 ratio of 8:1 provided relative levels of μNS and μ2 expression that were most similar to those in infected cells (Fig. 4B). It is thus clear that the relative levels of μNS and μ2 expression are important for determining particular aspects of inclusion morphology.
μNS(T1L) is colinear with MTs in the presence of μ2(T1L) but not in the presence of μ2(T3DN).
It was previously shown that μ2(T1L) colocalizes with MTs in transfected cells and that the filamentous inclusions in T1L-infected cells are colinear with MTs (28). Hence, we examined samples for the colocalization of μNS/μ2 inclusions with MTs by costaining transfected cells for μNS and β-tubulin (Fig. 5). Similar to previous findings for T1L-infected cells (28), we did not find significant colocalization of μNS(T1L) with MTs, but we did find that the finer filamentous structures in cells coexpressing μNS(T1L) and μ2(T1L) were colinear with MTs (Fig. 5, upper inset). In cells expressing high levels of recombinant proteins, we detected decreased amounts of MTs stained with the tubulin-specific antibody (supplemental data can be found at http://micro.med.harvard.edu/nibert/suppl/broering02a/fig3.html), suggesting that the μNS(T1L)/μ2(T1L) inclusions mask the underlying MTs and prevent access of the tubulin-specific antibody (28). We also cotransfected cells with pCI-M3(T1L) and pCI-M1(T3DN) and costained samples for μNS and β-tubulin (Fig. 5). μNS globular inclusions did not colocalize with or reorganize MTs and were not colinear with MTs (Fig. 5, lower inset), like the viral factories in T3DN-infected cells (28) and inclusions formed by μNS(T1L) expressed in the absence of other viral proteins (Fig. 2). We obtained similar results when cells were cotransfected with μNS and μ2 expression plasmids at a 7:1 ratio (Fig. 5) or a 1:1 M3/M1 antibody ratio (Fig. 5 and supplemental data that can be found at http://micro.med.harvard.edu/nibert/suppl/broering02a/fig3.html). These results suggest that μNS(T1L)/μ2(T1L) inclusions associate with MTs.
FIG. 5.
Distribution of μNS(T1L) and β-tubulin in cells coexpressing μNS(T1L) and μ2. CV-1 cells were transfected with 1.75 μg of pCI-M3(T1L) and 0.25 μg of pCI-M1(T1L) (upper panels) or 1.75 μg of pCI-M3(T1L) and 0.25 μg of pCI-M1(T3DN) (lower panels) per well. Cells were fixed at 18 h p.t. and immunostained for μNS (red) and β-tubulin (green) as described in the legend to Fig. 2. The boxed areas in the merged images are enlarged to show detail (insets). Colinearity of μNS and tubulin is indicated by arrows. Bars, 10 μm.
The morphology of μNS/μ2 inclusions depends on an intact MT network.
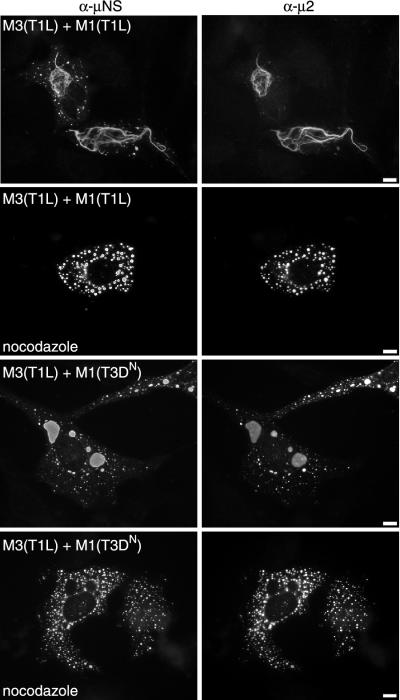
To test whether μNS(T1L)/μ2(T1L) filamentous structures are altered by the depolymerization of MTs, we treated cotransfected cells with 10 μM nocodazole for 12 h beginning at 6 h p.t. and analyzed the distribution of μNS and μ2 by IF microscopy. Simultaneous control experiments confirmed that MTs were depolymerized by this treatment. In cells coexpressing μNS(T1L) and μ2(T1L), nocodazole treatment at 6 h p.t. prevented the formation of filamentous structures. Instead, μNS(T1L) and μ2(T1L) colocalized in many small globular structures that were dispersed throughout the cytoplasm (Fig. 6). The appearance of small dispersed globular structures after MT depolymerization was also noted for both T3D- and T1L-infected cells treated with nocodazole at 6 h p.i. (28) and for pCI-M3(T1L)-transfected cells treated with nocodazole at 6 h p.t. (Fig. 2). To determine whether MT depolymerization would also affect the morphology of μNS(T1L)/μ2(T3DN) inclusions, we treated cotransfected cells with 10 μM nocodazole for 12 h beginning at 6 h p.t. Cells coexpressing μNS(T1L) and μ2(T3DN) showed a dispersed distribution of small globular structures in which μNS(T1L) and μ2(T3DN) colocalized (Fig. 6). We obtained similar results when cells were cotransfected with μNS and μ2 expression plasmids at a 7:1 or a 1:1 M3/M1 ratio (Fig. 6 and data not shown). These results show that intact MTs are required for the formation of filamentous structures in cells expressing μNS(T1L) and μ2(T1L) and for the formation of large perinuclear inclusions in cells expressing μNS(T1L) and μ2(T3DN).
FIG. 6.
Colocalization of μNS(T1L) and μ2 in cotransfected cells treated with nocodazole. CV-1 cells were cotransfected with 1.75 μg of pCI-M3(T1L) and 0.25 μg of pCI-M1(T1L) (upper four panels) or 0.25 μg of pCI-M1(T3DN) (lower four panels) per well. Cells were left untreated or treated with 10 μM nocodazole added at 6 h p.t. (nocodazole), fixed at 18 h p.t., and immunostained for μNS (left column) and μ2 (right column) as described in the legend to Fig. 3A. Bars, 10 μm.
When cells coexpressing μNS(T1L) and μ2(T1L) were treated with 10 μM nocodazole for 1 h beginning at 17 h p.t. and costained for μNS and β-tubulin, the μNS(T1L)/μ2(T1L) filamentous structures were not disrupted (supplemental data can be found at http://micro.med.harvard.edu/nibert/suppl/broering02a/fig4.html). MTs in untransfected cells were depolymerized by treatment with nocodazole for 1 h, but MTs in cells that contained μNS(T1L) and μ2(T1L) remained intact (supplemental data can be found at http://micro.med.harvard.edu/nibert/suppl/broering02a/fig4.html). MTs in T1L-infected cells were also resistant to depolymerization by 1 h of nocodazole treatment beginning at 17 h p.t. (28), but MTs in transfected cells expressing only μNS(T1L) were not resistant (Fig. 2). Parker et al. previously identified the M1 genome segment as the determinant of the viral strain-dependent difference in MT stabilization and demonstrated that MTs are stabilized in transfected cells expressing μ2(T1L) but not μ2(T3DN) (28). The current results suggest that μ2(T1L) also stabilizes MTs when coexpressed with μNS.
The μNS N terminus is required for colocalization with μ2.
A second form of μNS, called μNSC, is found in reovirus-infected cells and is missing amino acids from the N terminus of μNS (41). To determine whether the N terminus of μNS is required for inclusion formation and colocalization with μ2, we engineered a construct [pCI-M3(41-721)] to allow initiation at the second AUG codon in the M3 gene, producing a protein containing amino acids 41 to 721 of μNS(T1L) [μNS(41-721)]. The protein expressed in cells upon transfection of pCI-M3(41-721) reacted with the full-length μNS polyclonal antiserum but was not recognized by a polyclonal antiserum generated to the first 41 amino acids of μNS (Fig. 7A); these results confirm that the N terminus of μNS is absent from μNS(41-721). Full-length μNS expressed in transfected cells and in reovirus-infected cells was recognized by both full-length and N-terminal μNS polyclonal antisera (Fig. 7A). Phase-contrast microscopy of CV-1 cells expressing μNS(41-721) revealed globular phase-dense structures that contained μNS(41-721), as determined by IF microscopy (Fig. 7B). Therefore, amino acids 1 to 40 of μNS are not required for the formation of globular inclusions in transfected cells.
When μNS(41-721) was coexpressed with μ2(T1L), the two proteins did not colocalize in filamentous structures (Fig. 7C). Rather, when coexpressed with μ2(T1L), μNS(41-721) was seen in globular inclusions similar to those seen when the protein was expressed without μ2 (Fig. 7B), and μ2(T1L) had a filamentous and nuclear distribution similar to that seen when it was expressed without μNS (28) (Fig. 3B). μNS(41-721) and μ2(T3DN) also failed to colocalize (Fig. 7C). Smooth-edged globular structures containing μNS(41-721), similar to those found without μ2 coexpression (Fig. 7B), were surrounded by μ2(T3DN)-containing rough-edged structures; this appearance was similar to the pattern of μ2(T3DN) expressed in the absence of other reovirus proteins (28) (Fig. 3B). We conclude that amino acids 1 to 40 of μNS(T1L) are required for colocalization with μ2 but not for inclusion formation.
μNS(T1L) amino acids 1 to 41 are sufficient for μ2(T1L) colocalization.
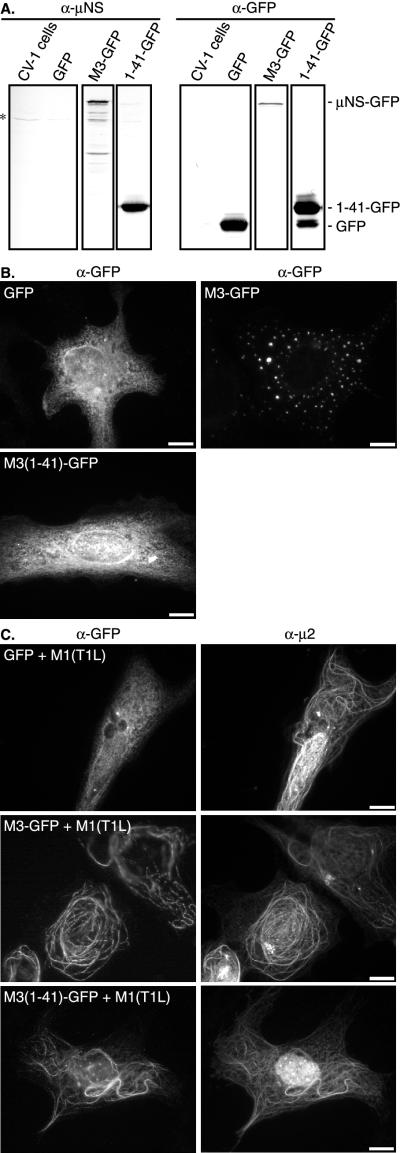
To determine whether the N-terminal amino acids of μNS that are required for μ2 colocalization are sufficient for colocalization, we constructed a plasmid [pEGFP-M3(1-41)] that expresses amino acids 1 to 41 of μNS(T1L) fused to the N terminus of GFP [μNS(1-41)-GFP]. To control for any effect of GFP on μNS/μ2 colocalization or inclusion formation, a plasmid (pEGFP-M3) was constructed that expresses full-length μNS(T1L) fused to the N terminus of GFP (μNS-GFP), and a plasmid expressing GFP alone (pEGFP) was also used. Cells transfected with pEGFP-M3(1-41) and pEGFP-M3 produced proteins of the expected sizes that were recognized by immunoblotting with both full-length μNS polyclonal antiserum and GFP antibodies, whereas cells expressing GFP alone produced a protein that was recognized by only GFP antibodies (Fig. 8A ). The N-terminal antiserum generated against amino acids 1 to 41 of μNS recognized μNS(1-41)-GFP and μNS-GFP but not GFP (data not shown). By immunoblotting, a protein of about the same size as native GFP was detected with the μNS(1-41)-GFP fusion protein (Fig. 8A), but the fusion protein was the predominant product (Fig. 8A). The production of this GFP-sized protein could have resulted from removal of the fused, μNS(1-41) portion by cleavage or from ribosomes initiating at the AUG codon at the beginning of the GFP-encoding gene (141 nucleotides downstream of the M3 AUG codon in the expression construct).
FIG.8.
Distribution of GFP, μNS-GFP, and μNS(1-41)-GFP in transfected cells with and without the coexpression of μ2(T1L). (A) CV-1 cells were transfected with 1 μg of pEGFP, pEGFP-M3, orpEGFP-M3(1-41) and 1 μg of pCI-neo as a carrier plasmid per well. Lysates were collected at 18 h p.i. and analyzed by SDS-PAGE and immunoblotting with polyclonal anti-μNS serum (α-μNS) or monoclonal anti-GFP IgG (α-GFP). A band appearing in untransfected CV-1 cells is indicated on the left with an asterisk. The positions of μNS-GFP, μNS(1-41)-GFP, and GFP are indicated on the right. (B) CV-1 cells were transfected with 2 μg of pEGFP (upper left panel), pEGFP-M3 (upper right panel), or pEGFP-M3(1-41) (lower left panel), fixed at 18 h p.t., and immunostained with mouse monoclonal antibody to GFP followed by goat anti-mouse IgG conjugated to Alexa 488. (C) CV-1 cells were cotransfected with 0.25 μg of pEGFP (upper panels), pEGFP-M3 (middle panels), or pEGFP-M3(1-41) (lower panels) and 1.75 μg of pCI-M1(T1L) per well. Cells were fixed at 18 h p.t. and stained for GFP (left column) as described for panel B and μ2 (right column) with rabbit anti-μ2 serum followed by goat anti-rabbit IgG conjugated to Alexa 594. Bars, 10 μm.
By IF microscopy, we found that GFP was distributed diffusely throughout pEGFP-transfected cells (Fig. 8B). Although fusion of GFP to the carboxyl terminus of μNS reduced the efficiency of μNS inclusion formation, globular inclusions were detected in approximately 80% of cells expressing μNS-GFP (Fig. 8B). The other μNS-GFP-positive cells displayed a diffuse distribution for μNS-GFP that was similar to that for GFP alone (data not shown). In cells transfected with pEGFP-M3(1-41), μNS(1-41)-GFP was diffusely distributed, demonstrating that the first 41 amino acids of μNS(T1L) are not sufficient for inclusion formation (Fig. 8B). When GFP was coexpressed with μ2(T1L), GFP remained diffusely distributed in cells and did not colocalize with μ2(T1L) (Fig. 8C). When μNS-GFP was coexpressed with μ2(T1L), on the other hand, μNS-GFP reorganized into filamentous structures that colocalized with μ2(T1L) (Fig. 8C). These structures were similar to those found when μNS(T1L) and μ2(T1L) were coexpressed (Fig. 4A), demonstrating that the fusion of GFP to the carboxyl terminus of μNS(T1L) did not alter μNS colocalization with μ2(T1L). When μNS (1-41)-GFP was coexpressed with μ2(T1L), μNS(1-41)-GFP also colocalized with μ2(T1L) in filamentous structures and in concentrated areas of the nucleus (Fig. 8C). These results provide strong evidence that amino acids 1 to 41 of μNS are sufficient to support colocalization with μ2.
DISCUSSION
Formation of globular phase-dense structures by μNS.
Reovirus-infected cells contain phase-dense inclusions that are believed to be the sites of virus replication and assembly and are therefore referred to as viral factories. Little is known about the viral and cellular factors that are needed to form these structures. We found that transfected cells expressing μNS(T1L) or μNS(T3D) contain μNS globular inclusions that appear very similar to the globular viral factories in T3DN-infected cells (Fig. 1A). This finding supports our hypothesis (see introductory paragraphs) that μNS plays a major role in the formation and structure of viral factories in infected cells. We recognize, however, that these similarities do not formally prove that the structures are functionally equivalent. The inclusions formed in transfected cells after the overexpression of μNS may be the result of aggregation that coincidently forms structures with an appearance similar to that of globular viral factories. Misfolded proteins can accumulate in inclusions called aggresomes (18). These phase-dense structures are often ubiquitinated, surrounded by a collapsed vimentin intermediate filament network, and located in the perinuclear region (18). They require MTs to form but not to be maintained (18). The structures formed by μNS resemble aggresomes because they are phase dense (Fig. 1A), accumulate in the perinuclear region (Fig. 1A), and appear to use MTs to travel to this region; once formed, however, they are not disrupted by MT depolymerization (Fig. 2). We do not believe, however, that the μNS in globular inclusions is misfolded, because it can be relocalized upon μ2(T1L) coexpression (Fig. 3A and 4A). Also, μNS inclusions are neither polyubiquitinated nor surrounded by a collapsed vimentin filament network (supplemental data can be found at http://micro.med.harvard.edu/nibert/suppl/broering02a/fig2.html). Future experiments, such as negative-stain EM of thin sections from M3-transfected and T3DN-infected cells, will allow further comparison of μNS globular inclusions and viral factories.
The suggestion that viral nonstructural proteins form the structure of viral factories has been made for the Reoviridae family members rotavirus and bluetongue virus. IF microscopy was used to identify spherical inclusions that formed when rotavirus nonstructural proteins NSP5 and NSP2 were coexpressed in MA104 cells after transfection but not when the proteins were expressed individually (11). Negative-stain EM identified inclusions, similar in morphology to those in bluetongue virus-infected cells, in insect cells infected with a recombinant baculovirus that expresses bluetongue virus nonstructural protein NS2 (38). Rotavirus NSP5, rotavirus NSP2, and bluetongue virus NS2 can all be phosphorylated (10, 17, 36, 40) and are all proposed to hydrolyze nucleoside triphosphates (NTPs) (3, 16, 29, 36, 37). The phosphorylation status and NTP-hydrolyzing activities of μNS are uncharacterized. However, the M1 genome segment that encodes μ2 was previously shown to determine differences in the NTPase activities of reovirus cores, and the μ2 sequence includes regions with some similarity to the A and B motifs of NTPases (27). Investigating the similarities among these proteins may provide a better understanding of their capacities to form inclusions in the absence of other viral proteins.
μNS and μ2 association.
The reovirus μ2 protein was recently shown to play a role in determining the morphology of filamentous viral factories by associating with and stabilizing MTs, but μ2 expression after transfection did not produce structures resembling viral factories (28). Similarly, when μNS was expressed in transfected cells, we found only globular inclusions (Fig. 1A). Filamentous structures resembling those in T1L-infected cells formed only upon coexpression of μ2(T1L) and μNS (Fig. 4A). These results suggest that μNS and μ2 cooperate in T1L-infected cells to determine the distribution and morphology of viral factories. The colocalization and redistribution of μNS and μ2 in cotransfected cells strongly suggest an interaction between these proteins in vivo, either direct binding of the two proteins or indirect interaction through a cellular intermediate. The association of μNS and μ2 is not dependent on the localization of μ2 to MTs, because μNS and μ2(T3DN) colocalized in transfected cells (Fig. 3A and 4A). The μNS/μ2 association is also not dependent on the capacity of μNS to form inclusions, since μNS(1-41)-GFP colocalized with μ2(T1L) but did not form inclusions (Fig. 8). Only the N-terminal 41 amino acids of μNS(T1L) are needed to mediate the association with μ2(T1L). Further evidence for an association between μNS and μ2 was the increase in μ2 expression in transfected cells when μNS(T1L) was coexpressed (Fig. 4B). This increased expression of μ2 could be due to increased translation or increased stability of this protein, and studies to address these possibilities are under way. Previous genetic data also linked the M1 and M3 segments: in strains that accumulated deletions upon high passage, the capacity to accumulate deletions in M1 was mapped to M3 (6).
In both this study and a previous one (28), μ2 staining in the nucleus of M1-transfected cells was observed. The size of the μ2 protein (83 kDa) exceeds the 60-kDa limit for passive diffusion into the nucleus (30). However, μ2 contains predicted nuclear import and export signals (J. S. L. Parker, J. Kim, and M. L. Nibert, unpublished data) that may explain its distribution in the nucleus and the cytoplasm of transfected cells. Significant μ2 staining in the nucleus of infected cells has not been reported (28). In this study, we found that coexpression of μNS reduced μ2 staining in the nucleus of M3- and M1-cotransfected cells (Fig. 3). As μNS (80 kDa) does not localize to the nucleus and does not have a known nuclear import signal, a reasonable explanation is that μNS sequesters μ2 within cytoplasmic inclusions, thus reducing the amount of cytoplasmic μ2 that is free to enter the nucleus. μNS(1-41)-GFP, on the other hand, colocalized with μ2 in the nucleus of cotransfected cells. As the μNS(1-41)-GFP fusion protein does not form inclusions (Fig. 8) and is smaller than the size limit for nuclear entry by passive diffusion, we hypothesize that μNS(1-41)-GFP enters the nucleus passively and is then retained there through its association with μ2. These results suggest that μ2 may enter the nucleus of infected cells, but further studies are needed to confirm that prediction and to assess any functional role that μ2 nuclear localization may play in reovirus replication.
In reoviruses with filamentous viral factories (22 of 24 strains tested in the study of Parker et al. [28]), the μNS/μ2 association may specifically function to recruit μNS to MTs and may be the first of multiple associations that bring together both viral and cellular factors to form viral factories. An association also occurs between μNS and μ2(T3DN), which does not associate with MTs, suggesting that there may be other functions of the μNS/μ2 association. For example, μNS association with μ2 could regulate the proposed NTPase (27) and RNA-binding (4) activities of μ2. Similarly, activities of μNS such as core binding (5) or proposed RNA binding (1) could be affected by μNS association with μ2. Much work remains to be done to determine the functions of μNS and μ2 and how they alter each other's activities in infected cells.
Possible μNSC function.
We found that μNS(41-721) forms inclusions but does not colocalize with μ2 (Fig. 7), whereas μNS(1-41)-GFP does not form inclusions but colocalizes with μ2(T1L) (Fig. 8). These observations identify a small region of μNS(T1L) (amino acids 1 to 41) that is necessary and sufficient (in terms of μNS regions) for μ2 colocalization and a much larger region of μNS(T1L) (amino acids 41 to 721) that is necessary and sufficient (in terms of μNS regions) for inclusion formation. We believe that the μNS(41-721) protein is properly folded because it retains the capacity to form globular inclusions in transfected cells (Fig. 7B) and the capacity to bind to cores in vitro (T. J. Broering, P. L. Joyce, and M. L. Nibert, unpublished data). The results obtained with μNS(41-721) may be relevant to reovirus infection because the μNSC protein present in reovirus-infected L cells (20) is missing sequences from the N terminus of μNS and is postulated to comprise amino acids 41 to 721 of μNS (23, 41). Immunoblot analysis of reovirus-infected L cells with the N-terminal antiserum generated against amino acids 1 to 41 of μNS confirmed the absence of N-terminal amino acids in μNSC (T. J. Broering and M. L. Nibert, unpublished data). However, the analysis of μNSC in reovirus-infected CV-1 cells and recombinant baculovirus-infected insect cells has been complicated by the presence of additional protein bands, between the μNS and μNSC bands, which react with the N-terminal μNS antiserum (Fig. 7A) (Broering and Nibert, unpublished). The compositions of these additional μNS bands are under investigation. If μNSC does indeed lack as much as 5 kDa of sequence from the N terminus of μNS, our data suggest a difference in μNS and μNSC activities in reovirus-infected cells. For example, if μNSC does not associate with μ2(T1L), as the results obtained with μNS(41-721) suggest, it may alter the amount of inclusion material associated with MTs and may also be free to interact with other components. The relative levels of expression of μNS and μNSC may be regulated during infection to coordinate their different activities.
Potential role for μNS in the formation of viral factories in infected cells: a current model.
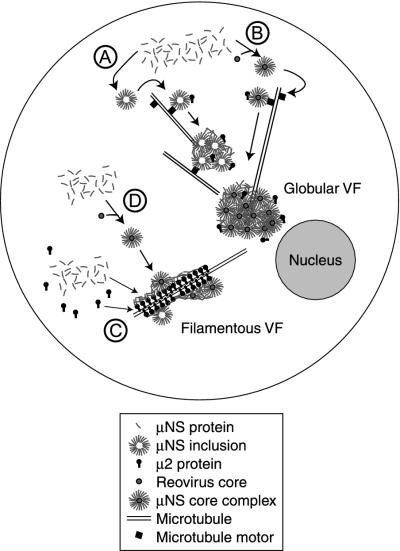
When expressed by transfection, μNS forms globular inclusions that can recruit coexpressed μ2(T3DN) (Fig. 9A) (see Fig. 1A and 3A for data). These initially small inclusions may travel along MTs and coalesce to form large perinuclear inclusions (Fig. 9A) (see Fig. 1B and 2 for data). In cotransfected cells, μNS is recruited to MTs by an association with μ2(T1L) (see Fig. 3A and 5 for data). We hypothesize that these two proteins form the coat around MTs previously identified in infected cells (8) and that μ2 mediates the previously identified association of μNS with the cytoskeletal fractions of infected cells (25) (Fig. 9C). Based on the capacity of μNS to bind to cores in vitro (5), the observation that cores are embedded within factories in infected cells (12, 31), and the isolation of core-like particles with μNS from infected cells (26), we also hypothesize that μNS may retain cores in viral factories as well as recruit unassembled core proteins. A viral factory may begin as a single transcribing core bound by μNS and grow as more μNS is added and new cores are assembled (Fig. 9B and D). We propose that the morphology and location of the viral factories are controlled through the μNS association with μ2, which determines whether the factory is globular or filamentous (28) (Fig. 9). Based on our findings, we hypothesize that μNS initiates the formation and provides the structure for viral factories in infected cells. Reovirus particles, proteins, and RNA are then recruited to the sites of replication and particle assembly. By functioning as a scaffold, μNS may increase the local concentrations of reovirus proteins and RNA, organize the double-stranded RNA synthesis or particle assembly process, recruit specific cellular factors to contribute to these processes, and/or exclude other cellular factors from the area of reovirus assembly. The unique distributions of μNS and μ2(T1L) when expressed individually and together are useful tools for identifying in vivo associations with other reovirus proteins and will allow us to test our hypothesis that μNS recruits other reovirus proteins and perhaps cellular proteins as well to inclusions.
FIG. 9.
Model for viral factory formation. (A) When expressed in the absence of other viral proteins, μNS forms globular phase-dense inclusions that travel along MTs toward the nucleus. When μ2(T3DN) is coexpressed with μNS, it is recruited to μNS globular inclusions. (B) In a T3DN-infected cell, μNS binds to cores and incorporates them into inclusions to generate a globular viral factory (VF). (C) μNS coats MTs when coexpressed with μ2(T1L), forming filamentous structures. (D) In a T1L-infected cell, cores bound by μNS are recruited to MTs by the μNS/μ2 association, forming a filamentous VF.
Acknowledgments
Many thanks are due to Laura Breun and Elaine Freimont for technical assistance, Aimee McCutcheon for assistance with plasmid construction, and Caroline Piggott for antibody titration. We are grateful to Darren Higgins and Angelika Gründling for essential maintenance and advice regarding microscope facilities.
This work was supported by NIH grants R29 AI-39533 and R01 AI-47904 (to M.L.N.) and by a USDA Hatch grant through the University of Wisconsin Extension (to M.L.N.). T.J.B. acknowledges previous support from predoctoral fellowships from the Wisconsin Alumni Research Foundation and NIH research training grant T32 GM0712 to the Molecular Biosciences Program at the University of Wisconsin—Madison. J.S.L.P. is the recipient of individual NRSA fellowship F32 AI-10134.
REFERENCES
- 1.Antczak, J. B., and W. K. Joklik. 1992. Reovirus genome segment assortment into progeny genomes studied by the use of monoclonal antibodies directed against reovirus proteins. Virology 187:760-776. [DOI] [PubMed] [Google Scholar]
- 2.Becker, M. M., M. I. Goral, P. R. Hazelton, G. S. Baer, S. E. Rodgers, E. G. Brown, K. M. Coombs, and T. S. Dermody. 2001. Reovirus σNS protein is required for nucleation of viral assembly complexes and formation of viral inclusions. J. Virol. 75:1459-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackhall, J., A. Fuentes, K. Hansen, and G. Magnusson. 1997. Serine protein kinase activity associated with rotavirus phosphoprotein NSP5. J. Virol. 71:138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brentano, L., D. L. Noah, E. G. Brown, and B. Sherry. 1998. The reovirus protein μ2, encoded by the M1 gene, is an RNA-binding protein. J. Virol. 72:8354-8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broering, T. J., A. M. McCutcheon, V. E. Centonze, and M. L. Nibert. 2000. Reovirus nonstructural protein μNS binds to core particles but does not inhibit their transcription and capping activities. J. Virol. 74:5516-5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, E. G., M. L. Nibert, and B. N. Fields. 1983. The L2 gene of reovirus serotype 3 controls the capacity to interfere, accumulate deletions and establish persistent infection, p. 275-288. In R. W. Compans and D. H. L. Bishop (ed.), Double-stranded RNA viruses. Elsevier Science Publishing Co., Inc., New York, N.Y.
- 7.Cashdollar, L. W., R. Chmelo, J. Esparza, G. R. Hudson, and W. K. Joklik. 1984. Molecular cloning of the complete genome of reovirus serotype 3. Virology 133:191-196. [DOI] [PubMed] [Google Scholar]
- 8.Dales, S. 1963. Association between the spindle apparatus and reovirus. Proc. Natl. Acad. Sci. USA 50:268-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dales, S., P. Gomatos, and K. C. Hsu. 1965. The uptake and development of reovirus in strain L cells followed with labelled viral ribonucleic acid and ferritin-antibody complexes. Virology 25:193-211. [DOI] [PubMed] [Google Scholar]
- 10.Devaney, M. A., J. Kendall, and M. J. Grubman. 1988. Characterization of a nonstructural phosphoprotein of two orbiviruses. Virus Res. 11:151-164. [DOI] [PubMed] [Google Scholar]
- 11.Fabbretti, E., I. Afrikanova, F. Vascotto, and O. R. Burrone. 1999. Two non-structural rotavirus proteins, NSP2 and NSP5, form viroplasm-like structures in vivo. J. Gen. Virol. 80:333-339. [DOI] [PubMed] [Google Scholar]
- 12.Fields, B. N., C. S. Raine, and S. G. Baum. 1971. Temperature-sensitive mutants of reovirus type 3: defects in viral maturation as studied by immunofluorescence and electron microscopy. Virology 43:569-578. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, S., B. P. De, J. A. Drazba, and A. K. Banerjee. 1998. Involvement of actin microfilaments in the replication of human parainfluenza virus type 3. J. Virol. 72:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hager, D. A., and R. R. Burgess. 1980. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal. Biochem. 109:76-86. [DOI] [PubMed] [Google Scholar]
- 15.Heath, C. M., M. Windsor, and T. Wileman. 2001. Aggresomes resemble sites specialized for virus assembly. J. Cell Biol. 153:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horscroft, N. J., and P. Roy. 2000. NTP binding and phosphohydrolase activity associated with purified bluetongue virus non-structural protein NS2. J. Gen. Virol. 81:1961-1965. [DOI] [PubMed] [Google Scholar]
- 17.Huismans, H., A. A. van Dijk, and A. R. Bauskin. 1987. In vitro phosphorylation and purification of a nonstructural protein of bluetongue virus with affinity for single-stranded RNA. J. Virol. 61:3589-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston, J. A., C. L. Ward, and R. R. Kopito. 1998. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143:1883-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopito, R. R. 2000. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 10:524-530. [DOI] [PubMed] [Google Scholar]
- 20.Lee, P. W., E. C. Hayes, and W. K. Joklik. 1981. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology 108:134-146. [DOI] [PubMed] [Google Scholar]
- 21.Mayor, H. D. 1965. Studies on reovirus 3. A labile, single-stranded ribonucleic acid associated with the late stages of infection. J. Natl. Cancer Inst. 35:919-925. [PubMed] [Google Scholar]
- 22.Mbisa, J. L., M. M. Becker, S. Zou, T. S. Dermody, and E. G. Brown. 2000. Reovirus μ2 protein determines strain-specific differences in the rate of viral inclusion formation in L929 cells. Virology 272:16-26. [DOI] [PubMed] [Google Scholar]
- 23.McCutcheon, A. M., T. J. Broering, and M. L. Nibert. 1999. Mammalian reovirus M3 gene sequences and conservation of coiled-coil motifs near the carboxyl terminus of the μNS protein. Virology 264:16-24. [DOI] [PubMed] [Google Scholar]
- 24.Miller, D. J., M. D. Schwartz, and P. Ahlquist. 2001. Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells. J. Virol. 75:11664-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora, M., K. Partin, M. Bhatia, J. Partin, and C. Carter. 1987. Association of reovirus proteins with the structural matrix of infected cells. Virology 159:265-277. [DOI] [PubMed] [Google Scholar]
- 26.Morgan, E. M., and H. J. Zweerink. 1975. Characterization of transcriptase and replicase particles isolated from reovirus-infected cells. Virology 68:455-466. [DOI] [PubMed] [Google Scholar]
- 27.Noble, S., and M. L. Nibert. 1997. Core protein μ2 is a second determinant of nucleoside triphosphatase activities by reovirus cores. J. Virol. 71:7728-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker, J. S. L., T. J. Broering, J. Kim, D. E. Higgins, and M. L. Nibert. 2002. Reovirus core protein μ2 determines the filamentous morphology of viral inclusion bodies by interacting with and stabilizing microtubules. J. Virol. 76:4483-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poncet, D., P. Lindenbaum, R. l'Haridon, and J. Cohen. 1997. In vivo and in vitro phosphorylation of rotavirus NSP5 correlates with its localization in viroplasms. J. Virol. 71:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quimby, B. B., and A. H. Corbett. 2001. Nuclear transport mechanisms. Cell. Mol. Life Sci. 58:1766-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhim, J. S., L. E. Jordan, and H. D. Mayor. 1962. Cytochemical, fluorescent-antibody and electron microscopic studies on the growth of reovirus (ECHO 10) in tissue culture. Virology 17:342-355. [DOI] [PubMed] [Google Scholar]
- 32.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 33.Sharpe, A. H., L. B. Chen, and B. N. Fields. 1982. The interaction of mammalian reoviruses with the cytoskeleton of monkey kidney CV-1 cells. Virology 120:399-411. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein, S. C., and P. H. Schur. 1970. Immunofluorescent localization of double-stranded RNA in reovirus-infected cells. Virology 41:564-566. [DOI] [PubMed] [Google Scholar]
- 35.Spendlove, R. S., E. H. Lennette, J. N. Chin, and C. O. Knight. 1964. Effect of antimitotic agents on intracellular reovirus antigen. Cancer Res. 24:1826-1833. [PubMed] [Google Scholar]
- 36.Taraporewala, Z. F., D. Chen, and J. T. Patton. 1999. Multimers formed by the rotavirus nonstructural protein NSP2 bind to RNA and have nucleoside triphosphatase activity. J. Virol. 73:9934-9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taraporewala, Z. F., D. Chen, and J. T. Patton. 2001. Multimers of the bluetongue virus nonstructural protein, NS2, possess nucleotidyl phosphatase activity: similarities between NS2 and rotavirus NSP2. Virology 280:221-231. [DOI] [PubMed] [Google Scholar]
- 38.Thomas, C. P., T. F. Booth, and P. Roy. 1990. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the singe-stranded RNA species. J. Gen. Virol. 71:2073-2083. [DOI] [PubMed] [Google Scholar]
- 39.Tolonen, N., L. Doglio, S. Schleich, and J. Krijnse Locker. 2001. Vaccinia virus DNA replication occurs in endoplasmic reticulum-enclosed cytoplasmic mini-nuclei. Mol. Biol. Cell 12:2031-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welch, S. K., S. E. Crawford, and M. K. Estes. 1989. Rotavirus SA11 genome segment 11 protein is a nonstructural phosphoprotein. J. Virol. 63:3974-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiener, J. R., J. A. Bartlett, and W. K. Joklik. 1989. The sequences of reovirus serotype 3 genome segments M1 and M3 encoding the minor protein μ2 and the major nonstructural protein μNS, respectively. Virology 169:293-304. [DOI] [PubMed] [Google Scholar]
- 42.Zweerink, H. J., M. J. McDowell, and W. K. Joklik. 1971. Essential and nonessential noncapsid reovirus proteins. Virology 45:716-723. [DOI] [PubMed] [Google Scholar]