Abstract
Human herpesviruses utilize an impressive range of strategies to evade the immune system during their lytic replicative cycle, including reducing the expression of cell surface major histocompatibility complex (MHC) and immunostimulatory molecules required for recognition and lysis by virus-specific cytotoxic T cells. Study of possible immune evasion strategies by Epstein-Barr virus (EBV) in lytically infected cells has been hampered by the lack of an appropriate permissive culture model. Using two-color immunofluorescence staining of cell surface antigens and EBV-encoded lytic cycle antigens, we examined EBV-transformed B-cell lines in which a small subpopulation of cells had spontaneously entered the lytic cycle. Cells in the lytic cycle showed a four- to fivefold decrease in cell surface expression of MHC class I molecules relative to that in latently infected cells. Expression of MHC class II molecules, CD40, and CD54 was reduced by 40 to 50% on cells in the lytic cycle, while no decrease was observed in cell surface expression of CD19, CD80, and CD86. Downregulation of MHC class I expression was found to be an early-lytic-cycle event, since it was observed when progress through late lytic cycle was blocked by treatment with acyclovir. The immediate-early transactivator of the EBV lytic cycle, BZLF1, did not directly affect expression of MHC class I molecules. However, BZLF1 completely inhibited the upregulation of MHC class I expression mediated by the EBV cell-transforming protein, LMP1. This novel function of BZLF1 elucidates the paradox of how MHC class I expression can be downregulated when LMP1, which upregulates MHC class I expression in latent infection, remains expressed in the lytic cycle.
Epstein-Barr virus (EBV) is a ubiquitous gammaherpesvirus found in more than 90% of adults in all populations worldwide. Following primary infection in immunocompetent individuals, the virus generally establishes lifelong persistence in B lymphocytes. Most EBV-infected B cells in the healthy host show a resting phenotype with very limited expression of the viral genome (34, 41, 58). However, expression of a broader panel of 11 “latent” viral genes leads to growth transformation of B cells (26, 27). This growth transformation is thought to be a mechanism for expansion of the pool of EBV in the infected host. In addition, the lytic replicative cycle, which produces infectious virions, is likely to be important for periodic expansion of the pool of virus-infected cells within the host and for horizontal transmission of the virus. Latently infected, growth-transformed B-lymphoblastoid cells are good targets for EBV-specific cytotoxic T lymphocytes (CTL). This limits the potentially pathogenic consequences of uncontrolled proliferation of virus-infected cells (43). It is unclear whether lytically infected cells are subjected to the same immune controls.
CD8+ CTL are a major line of defense in the immune response to viral infection (23, 43, 44). The T-cell receptors on virus-specific CTL interact with major histocompatibility complex (MHC) class I molecules presenting viral peptides on the surface of the infected cell. Presentation of viral peptides involves several components of the endogenous antigen-processing pathway, including cleavage of antigen by proteasomes, translocation of peptides into the lumen of the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP), and loading onto heterodimers of newly synthesized MHC class I heavy chain and β2-microglobulin. This stabilized trimeric complex is then transported to the plasma membrane (33, 40). In EBV-transformed lymphoblastoid cells, the virally encoded latent membrane protein-1 (LMP1) enhances antigen-processing functions at several levels. LMP1 alters proteasome subunit expression and upregulates expression of TAP subunit proteins, MHC class I and class II molecules (51, 62), and cell surface adhesion molecules (20, 59). Conversely, the EBNA1 protein of EBV contains an unusual glycine-alanine repeat sequence which, while not interfering with antigen processing of other viral and cell genes, prevents processing of antigenic EBNA1 peptides and their presentation to CTL (29).
While it is crucial for the host-virus relationship that proliferation of growth-transformed EBV-infected B cells be controlled by virus-specific CTL immunosurveillance, efficient virus production in the lytic cycle would benefit from effective evasion of CTL responses. As reviewed elsewhere (5, 23, 40), the alpha- and betaherpesviruses utilize numerous immune evasion strategies in order to achieve persistence, and with rare exceptions, the viral genes demonstrated to interfere with immune recognition are expressed during the lytic cycle of these herpesviruses. Only recently have analogous immune evasion strategies been described for gammaherpesviruses. Thus, Kaposi's sarcoma-associated herpesvirus (also known as human herpesvirus 8) contains two open reading frames (ORFs) whose gene products, K3 and K5, have the ability to interfere with the cell surface expression of MHC class I molecules (22, 25, 37). A related K3 ORF in the murine gammaherpesvirus 68 has also been shown to downregulate cell surface MHC class I expression and to inhibit antigen presentation (56). To date, no such immune evasion strategy has been identified in the lytic program of EBV. Indeed, since the transformation-associated LMP1 gene is expressed throughout the EBV lytic cycle in B cells (52), it is possible that MHC expression and antigen presentation functions would remain enhanced.
Study of EBV lytic infection is difficult due to the lack of a fully permissive culture system for the virus (27). Normal B cells transformed in vitro with EBV give rise to lymphoblastoid cell lines (LCL) displaying a latency III type of infection, in which about 11 transformation-associated genes are expressed. Some EBV-positive Burkitt's lymphoma (BL) lines showing a more restricted, latency I pattern of EBV latent gene expression can be induced into the lytic cycle with variable efficiency (typically 5 to 40% of cells enter the lytic cycle) by activation with chemical inducers, such as phorbol esters or n-butyrate, or by ligation of surface immunoglobulin (12, 52, 57). However, these BL models are not helpful in the present context since, unlike LCL and BL lines with a latency III pattern of gene expression, latency I BL lines already have impaired antigen-processing functions (51). Therefore, we have taken advantage of the fact that some latency III BL lines and LCL consistently show a small proportion of cells, typically less than 5%, which have spontaneously entered the lytic cycle (27).
Isolation of the subpopulation of cells in the lytic cycle, using immunosorting strategies targeting a cell surface marker of the lytic cycle, would enable biochemical and functional studies. Unfortunately, this option is ruled out by the fact that lytic-cycle genes expressed at the cell surface (e.g., gp340) are also present on infectious viruses; the EBV released from lytic cells can bind neighboring latently infected cells expressing the CD21 receptor for the virus, thereby creating a large percentage of gp340+ cells which are not actually in the lytic cycle (14). We therefore developed a dichromatic flow cytometry assay to stain for surface cellular antigens on viable cells, followed by fixation, permeabilization, and intracellular staining of the EBV immediate-early lytic antigen, BZLF1, or the late viral capsid antigen (VCA). This allowed us to analyze cellular gene expression in lytic EBV infection compared to that in latent infection in the same culture. The results showed EBV lytic infection to be associated with a substantial reduction in cell surface MHC class I expression and a smaller, but significant, reduction in MHC class II expression. These observations were paradoxical, since expression of both MHC class I and MHC class II molecules can be upregulated by LMP1 (51, 62), which remains expressed during the lytic cycle (52). We therefore investigated whether lytic-cycle gene expression might interfere with the ability of LMP1 to upregulate MHC expression.
MATERIALS AND METHODS
Cells.
Eli-LCL is an EBV-positive LCL derived by infection of normal peripheral blood B cells with the B95.8 strain of EBV (53). Ag876 is an EBV-positive tumor B-cell line derived from a patient with BL (39). The Eli-LCL and Ag876 lines both display a predominantly latency III type of infection but contain up to 5% BZLF1-positive cells. The EBV-positive P3HR-1-c16 cell line is a strictly nonproducing subclone of the P3HR-1 mutant BL line (42), which can be induced into the lytic cycle by treatment with n-butyrate and phorbol esters. A deletion in the EBV genome of P3HR-1 removes the EBNA2 gene, and the loss of this transactivator results in downregulation of LMP1 expression in the latent infection state (1, 48). DG75 is an EBV-negative tumor B-cell line derived from a patient with BL (7). Cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, and antibiotics (200 U of penicillin/ml and 200 mg of streptomycin/ml) and were maintained at 37°C in a humidified atmosphere containing 5% CO2. Inhibition of viral DNA replication and late-lytic-cycle gene expression was achieved by culturing cells in a culture medium supplemented with 0.1 mM acyclovir (Sigma) for 2 weeks.
Antibodies.
The EE reagent is a human serum from a chronic infectious-mononucleosis patient with unusually high titers of antibodies to lytic-cycle antigens (52). BZ.1 is a murine monoclonal antibody (MAb) to the immediate-early protein encoded by the BZLF1 ORF (60). MAb L2 recognizes a 125-kDa late-lytic-cycle antigen which is a major component of the VCA complex (28) and was a kind gift from G. Pearson (Georgetown University, Washington, D.C.). CS.1-4 is a pool of MAbs to LMP1 (49). OX34, a MAb specific for rat CD2, and W6/32, a MAb specific for MHC class I (HLA-A, -B, and -C alleles), were produced from hybridomas purchased from the American Tissue Culture Collection. MAb Tü149, recognizing MHC class I (HLA-B and -C, and some HLA-A alleles) was kindly provided by B. Uchanska-Ziegler (Berlin, Germany). MAb BU.12, specific for CD19, was a kind gift from D. Hardie (University of Birmingham, Birmingham, United Kingdom). The murine MAb WR18, specific for MHC class II (DP, DQ, and DR antigens) and the red phycoerythrin (RPE)-conjugated MAbs specific for CD54 and CD40 were purchased from Serotec, Oxford, United Kingdom. MAbs L307.4 (anti-CD80) and FUN-1 (anti-CD86) were purchased from BD Biosciences Pharmingen.
Biotinylation of antibody BZ.1.
Antibody BZ.1 (60) was purified from hybridoma culture supernatant by affinity chromatography with Sepharose-protein A. The purified antibody was biotinylated by using the Immunoprobe Biotinylation kit (Sigma) according to the manufacturer's instructions.
Flow cytometry analysis of BZLF1 and VCA expression.
Cells were fixed and permeabilized to allow for detection of the intracellular markers of the EBV lytic cycle by indirect immunofluorescence. This was achieved by incubating the cells with a 2% paraformaldehyde solution in phosphate-buffered saline (PBS) for 30 min on ice, followed by incubation with 0.2% Triton X-100 for 30 min on ice. After extensive washes in PBS, cells were incubated either with 1 μg of MAb BZ.1 (anti-BZLF1)/ml or with 1/1,000-diluted L2 ascites fluids (anti-VCA) for 1 h at 37°C. Cells were washed twice in PBS and incubated with 1:50-diluted fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG; Sigma) for 1 h at 37°C. After further washes, cells were resuspended in 2% paraformaldehyde and analyzed on a FACScalibur flow cytometer (Becton Dickinson Co., San Jose, Calif.).
Flow cytometry analysis of cell surface antigen expression in BZLF1- and VCA-positive cells.
Cell surface antigen and intracellular EBV lytic-cycle antigens were detected simultaneously by first staining viable cells with an antibody specific for cell surface protein detected with RPE-conjugated antibodies, then fixing and permeabilizing the cells (see above), and staining for intracellular antigen detected with FITC-conjugated antibodies. Optimal two-color staining of surface MHC class I molecules and intracellular BZLF1 or VCA was achieved by staining viable cells with 5 μg of W6/32 (IgG2a MAb)/ml for 30 min at 4°C, fixing and permeabilizing, and then staining with 1 μg of BZ.1 (IgG1 MAb to BZLF1)/ml or 1:1,000-diluted L2 (IgG1 MAb to VCA) for 1 h at 37°C. The two primary antibodies were subsequently detected by incubation for 1 h at 37°C with a mixture of RPE-conjugated goat anti-mouse IgG2a antibodies (diluted 1:50; Serotec) and FITC-conjugated goat anti-mouse IgG1 antibodies (diluted 1:50; Serotec).
For MAbs used in cell surface staining which, like antibody BZ.1 against BZLF1, belonged to the IgG1 isotype, the procedure was modified to allow specific simultaneous detection of cell surface and intracellular antibody staining. Prior to fixation and permeabilization, the MAb bound to the cell surface was first detected with RPE-conjugated rabbit anti-mouse IgG (diluted 1:50; Serotec) and then free binding sites of the secondary antibody were blocked by incubation for 20 min at room temperature with 20% whole mouse serum (The Binding Site, Birmingham, United Kingdom) in PBS. Following fixation and permeabilization, cells were incubated with 1 μg of biotinylated MAb BZ.1/ml followed by incubation with a 1/20 dilution of streptavidin-FITC (Southern Biotechnology Associates Inc., Birmingham, Ala.) in PBS for 25 min at room temperature. In some experiments, primary antibodies directly conjugated with RPE were used (e.g., anti-CD40-RPE and anti-CD54-RPE); in these cases, the incubation steps with secondary RPE-conjugated anti-mouse IgG1 and with blocking mouse serum were omitted.
Plasmids.
Plasmid pSG5-LMP1 expresses wild-type LMP1 cDNA derived from the B95.8 strain of EBV (24). The pCMV-IκBα ΔN plasmid expresses a phosphorylation-defective mutant IκBα and was kindly provided by Dean W. Ballard (Howard Hughes Medical Institute, Nashville, Tenn.). The pSG5-trCD2 plasmid expresses a C terminus-truncated rat CD2 and has been described previously (19). The green fluorescent protein (GFP) expression vector pEGFP-N1 was purchased from Clontech. The NF-κB luciferase reporter 3Enh.κB-ConALuc (3Enh-Luc) contains three tandem repeats of the NF-κB binding sites from the Ig κ promoter upstream of a minimal conalbumin promoter and has been described previously (6). Plasmid p509, a pCMV-1-based plasmid that expresses the BZLF1 transactivator of the EBV lytic cycle (46), was obtained from A. Rickinson (Birmingham University).
Gene transfection and luciferase reporter assay.
Electroporation of 107 cells in 0.5 ml of RPMI medium was performed by using a Bio-Rad Genepulser II electroporator set to 270 V and 950 μF for DG75 cells, or 240 V and 950 μF for P3HR1-c16 cells. The cells were then seeded in 4 ml of fresh growth medium and cultured under normal conditions. Transfection efficiency was typically 30 to 40% for the DG75 cell line and 10 to 15% for the P3HR1-c16 cell line, as determined by transfection with pEGFP-N1 and flow cytometry analysis of GFP expression. Luciferase activity from the 3Enh-Luc reporter plasmid was measured 24 h after transfection, exactly as described previously (32).
Immunomagnetic separation.
Following transfection with pSG5-rCD2, cells expressing rat CD2 were labeled by staining with the rat CD2-specific MAb OX34 followed by FITC-conjugated anti-mouse IgG2a antibodies. These cells were positively selected by using magnetic cell sorting anti-FITC microbeads and MS columns (Miltenyi Biotech) according to the manufacturer's guidelines. More than 90% sort purity was achieved for transfected P3HR-1-c16 cells.
Detection of lytic-cycle antigens and LMP1 by immunoblotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and immunoblotting were performed as described previously (50). Briefly, cells were solubilized by sonication in reducing gel sample buffer, and solubilized proteins equivalent to 2 × 105 cells were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Amersham Life Sciences) for immunoblotting. Lytic-cycle antigens were detected by incubating the membranes for 1 h with a 1/10,000 dilution of EE human serum followed by incubation for 1 h with a 1/10,000 dilution of alkaline phosphatase-conjugated goat anti-human IgG (Bio-Rad). LMP1 was detected by using 1 μg of MAbs CS.1-4/ml followed by a 1/10,000 dilution of alkaline phosphatase-conjugated goat anti-mouse IgG (Bio-Rad). Bound alkaline phosphatase was developed by using the CDP-Star (Tropix Inc.) chemiluminescent reagent.
RESULTS
Cell surface MHC class I expression is decreased in the lytic cycle.
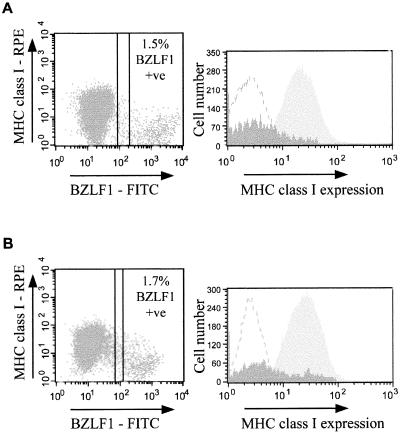
In order to analyze the cell surface phenotype of EBV lytic cells, we performed two-color flow cytometry assays to detect cell surface antigens simultaneously with the immediate-early lytic-cycle nuclear antigen BZLF1. Figure 1A shows results of a representative experiment with the latency III BL cell line Ag876, in which 1.5% of cells stained positive with MAb BZ.1 against BZLF1, detected with FITC-conjugated anti-IgG1 secondary antibodies. Simultaneous detection of cell surface MHC class I molecules with antibody W6/32 to HLA-A, -B, and -C, followed by RPE-conjugated anti-IgG2a secondary antibodies, revealed a 4.8-fold reduction in the fluorescence intensity of MHC class I molecules staining on BZLF1+ cells (mean fluorescence intensity [MFI], 5) relative to that on latently infected, BZLF1− cells (MFI, 24). Similar results were obtained with a normal EBV-transformed LCL, Eli-LCL. Thus, Fig. 1B shows results of one representative experiment with Eli-LCL where 1.7% of cells were BZLF1+, and simultaneous staining with antibody W6/32 revealed a 3.5-fold reduction in the level of MHC class I molecules on the surfaces of BZLF1+ cells (MFI, 8) relative to the level on latently infected, BZLF1− cells (MFI, 28).
FIG. 1.
Downregulation of MHC class I expression in EBV lytic infection. (A) Surface expression of MHC class I molecules on the latency III and lytic EBV-positive BL cell line Ag876. Viable cells were stained with antibody W6/32 (IgG2a isotype, anti-HLA-A, -B, and-C) and then fixed and permeabilized to allow staining with antibody BZ.1 (IgG1 isotype, anti-BZLF1 immediate-early EBV antigen), followed by detection with a pool of FITC-conjugated anti-IgG1 and RPE-conjugated anti-IgG2a secondary antibodies. The left-hand panel is a dot plot of the flow cytometry results of RPE staining (surface MHC class I molecules) (y axis) and FITC staining (nuclear BZLF1) (x axis). The right-hand panel is a histogram of the RPE staining (surface MHC class I molecules) of the latent, BZLF1− population (light shading) and the lytic, BZLF1+ population (dark shading). The MFI of surface MHC class I expression on the latent, BZLF1− cells is 24, whereas the corresponding MFI for the lytic, BZLF1+ cell population is 5. Results shown are representative of four independent experiments. (B) Surface expression of MHC class I molecules on the normal EBV-transformed LCL Eli-LCL. Immunofluorescence staining and analysis were performed exactly as for the Ag876 cells in panel A. In this representative experiment, the MFI of surface MHC class I expression obtained for the latent, BZLF1− cells was 28, and the corresponding MFI for the lytic, BZLF1+ cells was 8.
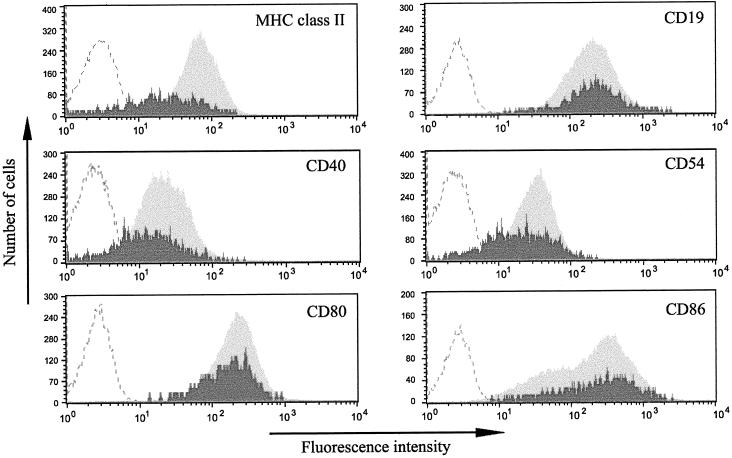
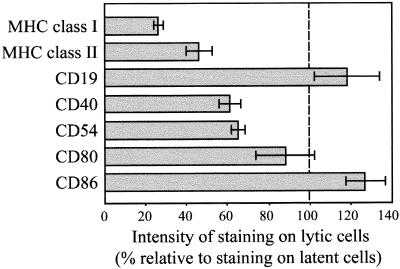
Next, we examined whether expression of other cellular proteins was similarly modulated in the lytic cycle. Figure 2 shows representative results obtained with the Ag876 BL cell line simultaneously stained for nuclear BZLF1 and each of the indicated cell surface antigens. The pooled results of at least three experiments are summarized in Fig. 3. Although not as severe as for MHC class I molecules, BZLF1+ cells expressed reduced levels of surface MHC class II molecules, ranging from 40 to 50% of levels in latent cells. Furthermore, CD40 and ICAM-1 (CD54) expression was reduced by as much as 40% on cells in the lytic cycle. In contrast, cell surface CD19 expression showed a small increase (18%) on BZLF1+ cells, but this did not reach statistical significance (P = 0.2132 by a paired Student t test). Small differences were also observed in the levels of CD80 and CD86, which are the B7-1 and B7-2 ligands for the CD28 costimulatory receptor on T cells. The 10% decrease in CD80 levels on BZLF1+ cells was not statistically significant (P = 0.2274), while the 25% increase in CD86 expression did reach statistical significance (P = 0.0298). As shown in Fig. 2, in contrast to all other surface antigens analyzed, which gave a single, defined population of cells, CD86 staining gave two overlapping populations.
FIG. 2.
Analysis of cell surface protein expression in the lytic cycle. Ag876 cells were stained with antibodies specific for a range of cell surface antigens (for MHC class II, MAb WR18; for CD19, MAb BU.12; for CD40, MAb LOB7/6; for CD54, MAb 15.2; for CD80, MAb L307.4; and for CD86, MAb FUN-1) and labeled with RPE, together with MAb BZ.1 against the BZLF1 immediate-early EBV antigen, labeled with FITC. A representative flow cytometry analysis is shown for each combination of antibodies. Dark shading represents cell surface antigen staining on lytic, BZLF1+ cells; light shading represents cell surface antigen staining on latent, BZLF1− cells; curves defined by dashed lines represent the background RPE fluorescence obtained on cells stained with control antibodies.
FIG. 3.
Summary of the relative levels of cell surface antigen expression on lytic, BZLF1+ cells in Ag876 BL cultures. Each result represents the mean relative intensity from at least three separate experiments and is shown as a percentage of the MFI of RPE staining of latently infected cells in the same experiment. Error bars represent the standard errors of the means from at least three separate experiments.
Results similar to those shown in Fig. 2 and 3 were also obtained with Eli-LCL. In three separate experiments, the mean MHC class I expression in lytic cells was 29.5% ± 2.93% of expression in latent cells (P = 0.0321 by a paired Student t test). In single experiments, performed in parallel with MHC class I staining, the expression of MHC class II molecules, CD40, and CD54 on lytic cells was reduced to 45 to 50% of that on latent cells, while CD80 and CD86 showed a 15% reduction and a 15% increase, respectively, on lytic cells. Together, these data obtained with Ag876 cells and Eli-LCL suggest that the marked reduction in MHC class I expression observed during EBV lytic infection is unlikely to be the result of a global shutdown in protein synthesis.
Downregulation of MHC class I expression is an early event in the lytic program.
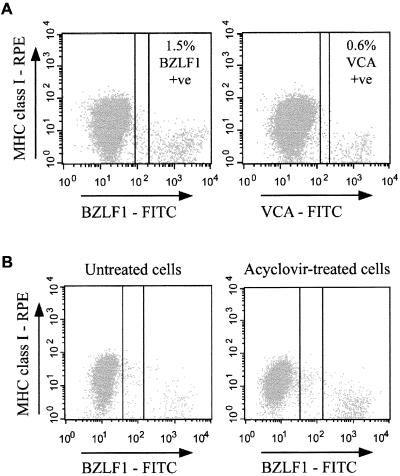
Because the downregulation of MHC class I expression may be important for immune escape from CTL responses, it is of interest to know at what stage in the lytic cycle the expression of MHC class I molecules is affected. BZLF1 is expressed by cells at both early and late stages of the lytic cycle. Therefore, if downregulation of MHC class I expression were a late-lytic-cycle event, it is possible that a greater degree of downregulation would be observed by analyzing only cells expressing a late-lytic-cycle marker such as VCA. The next set of experiments addressed this question. Figure 4A shows results of a representative two-color immunofluorescence experiment with Ag876 cells stained either for MHC class I molecules and BZLF1 (left panel) or for MHC class I molecules and VCA (right panel). In this experiment, 1.5% of cells stained positive for BZLF1 while 0.6% of cells stained positive for VCA. MHC class I expression was reduced to similar degrees on BZLF1+ cells (4.8-fold reduction) and VCA+ cells (5-fold reduction) relative to expression in the latently infected population.
FIG. 4.
Downregulation of MHC class I expression is an early-lytic-cycle event. (A) Surface expression of MHC class I molecules on BZLF1+ and VCA+ cells in the lytic cycle. Ag876 BL cells were simultaneously stained to detect surface MHC class I expression and intracellular lytic-cycle antigen expression exactly as for Fig. 1. The left-hand flow cytometry dot plot shows staining with MAbs W6/32 (anti-MHC class I; RPE conjugated) (y axis) and BZ.1 (anti-BZLF1; FITC conjugated) (x axis). The right-hand dot plot shows parallel staining of cells with MAb W6/32 (y axis) and L2 (anti-VCA; FITC conjugated) (x axis). (B) Surface expression of MHC class I molecules on acyclovir-treated and untreated Ag876 BL cells. Cells cultured in acyclovir for 2 weeks and replicate untreated cells were simultaneously stained with MAbs W6/32 and BZ.1 exactly as for Fig. 1. Results shown are dot plot flow cytometry profiles of W6/32 staining (RPE conjugated) (y axis) versus BZ.1 staining (FITC conjugated) (x axis) obtained for untreated control cells (left panel) and acyclovir-treated cells (right panel).
The results in Fig. 4A indicate that downregulation of MHC class I expression is an early event in the lytic cascade. To confirm this, Ag876 cells were cultured in the presence or absence of acyclovir for 2 weeks. Acyclovir is phosphorylated by EBV-encoded thymidine kinase to generate acyclovir monophosphate and then by cellular kinases to generate the di- and triphosphate forms. Acyclovir triphospate, an analogue of the nucleoside dGTP, is preferentially incorporated into growing chains of viral DNA, and it terminates extension of the DNA template. Culturing cells in acyclovir inhibits lytic viral DNA replication and, hence, expression of late-lytic-cycle antigens (30). Immunofluorescence staining with an anti-VCA MAb demonstrated that while VCA was detected on a subpopulation of control Ag876 cultures, it was undetectable in cells cultured with acyclovir for 2 weeks (data not shown). Figure 4B shows the results of two-color flow cytometry analysis of nuclear BZLF1 and surface MHC class I expression in control Ag876 cells and acyclovir-treated Ag876 cells. MHC class I expression was reduced to similar degrees on BZLF1+ cells in the two cultures. Thus, in the experiment shown, BZLF1+ cells in control cultures showed a mean intensity of MHC class I staining that was 28.6% of that observed in BZLF1− cells (a 3.5-fold reduction), while BZLF1+ cells in acyclovir-treated cultures showed a mean intensity of MHC class I staining that was 29.4% of that observed in BZLF1− cells (a 3.4-fold reduction). Therefore, downregulation of cell surface MHC class I expression is initiated during the immediate-early or early phase of the lytic cycle and persists through the late lytic cycle.
LMP1 is expressed in the lytic cycle.
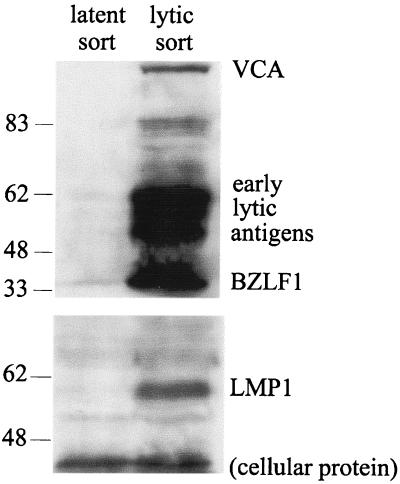
In latent infection, LMP1 has been shown to upregulate expression of MHC class I molecules (51). Investigation of the mechanism of downregulation of MHC class I expression in the lytic cycle needs to take into consideration the fact that the latent gene product, LMP1, is also expressed in the lytic cycle (52). Thus, as illustrated by the results in Fig. 5, when cells with a latency I type of infection are induced into the lytic cycle, LMP1 expression is also induced. In this experiment, EBV+ P3HR1-c16 cells were electroporated with a BZLF1 expression plasmid to initiate the lytic-cycle cascade and with a GFP-rCD2 marker gene to allow immunosorting of transfected cells expressing cell surface rCD2. At 24 h after transfection, immunoblot analysis was performed on the negative and positive sorted cells with a human serum reactive with several early- and late-lytic-cycle antigens (Fig. 5, upper blot). The results confirmed that lytic-cycle antigen expression was undetectable in untransfected, latently infected cells, while high levels of expression of early- and late-lytic-cycle antigens were seen in BZLF1-transfected cells. Reprobing the immunoblot with MAbs CS.1-4 (Fig. 5, lower blot) showed that LMP1 was undetectable in the latently infected population but was clearly expressed in the lytic population. We have also shown by two-color immunofluorescence that LMP1 remains expressed in cells which spontaneously enter the lytic cycle from a latency III type of infection, as in the Ag876 and Eli-LCL lines (M. Rowe, unpublished data). These observations raise the question: why is LMP1 unable to induce or maintain high levels of MHC class I expression in the lytic cycle? Recently, Dreyfus et al. (13) reported that BZLF1 inactivated the transcription factor NF-κB in human T cells. Since activation of NF-κB is a major signaling function of LMP1, we examined whether coexpression of BZLF1 in the lytic cycle might interfere with the ability of LMP1 to upregulate MHC class I expression.
FIG. 5.
LMP1 expression during the lytic cycle. Western blot analysis of LMP1 expression in EBV lytic cycle-positive P3HR1-c16 cells was performed. Cells were cotransfected with 8 μg of a BZLF1 expression plasmid to induce the EBV lytic cycle and with 3 μg of a rat CD2 surface antigen expression plasmid to allow transfected cells to be positively selected by immunomagnetic separation. At 24 h following transfection, viable cells were labeled with OX34 (an anti-rat CD2 MAb) followed by FITC-conjugated anti-IgG; then they were immunomagnetically sorted with anti-FITC MACS microbeads. BZLF1-transfected cells were positively sorted on the basis of rat CD2 expression, while the unbound flowthrough cells were taken to be untransfected, latent cells. Cell samples were analyzed by Western immunoblotting. When probed with EE human serum (top panel), the blot showed staining of BZLF1, early antigens, and late antigens in the positively sorted cells and not in the untransfected, flowthrough cells. Reprobing the blot for LMP1 with MAbs CS.1-4 (lower panel) demonstrated induced expression of LMP1 in the positively sorted, lytically infected cells.
BZLF1 inhibits the ability of LMP1 to upregulate MHC class I expression.
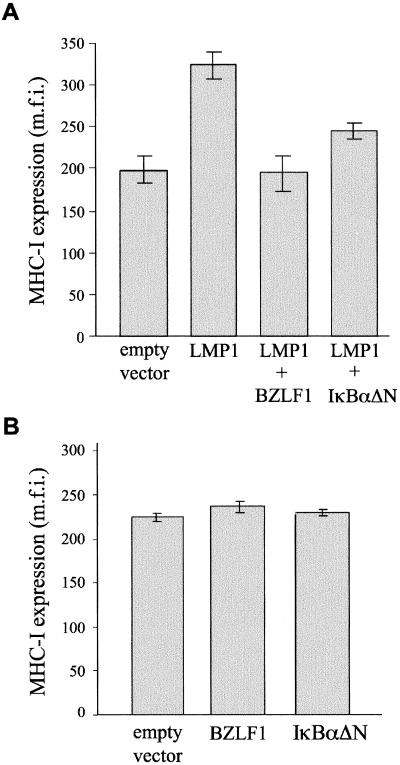
The effect of BZLF1 on LMP1-mediated upregulation of MHC class I expression was investigated by cotransfection experiments with the EBV− BL line DG75. Replicate cultures of DG75 cells were transfected either with empty vector DNA or with an LMP1 expression plasmid, together with a GFP expression plasmid to serve as a marker of the transfected subpopulation of cells. At 48 h after transfection, MHC class I expression was detected with RPE-conjugated antibodies and the intensity of MHC class I expression in the GFP-gated population was analyzed by flow cytometry. The results in Fig. 6A show that expression of LMP1 causes a 70% increase in the intensity of MHC class I staining relative to that for control cells transfected with empty vector DNA. Coexpression of BZLF1 with LMP1 completely abolished the ability of LMP1 to upregulate MHC class I expression. Thus, Fig. 6A shows that when BZLF1 was cotransfected with LMP1, MHC class I expression remained at the constitutive level observed in vector control-transfected cells. Coexpression of the NF-κB inhibitory molecule IκBαΔN with LMP1 also had an inhibitory effect, although it was less effective than BZLF1 at inhibiting LMP1-mediated upregulation of MHC class I expression (Fig. 6A). As shown in Fig. 6B, expression of BZLF1 or IκBαΔN alone did not affect the level of MHC class I expression relative to that for control cells transfected with empty vector DNA.
FIG. 6.
BZLF1 inhibits LMP1-mediated upregulation of MHC class I expression. (A) Flow cytometric analysis of surface MHC class I expression in LMP1-transfected EBV-negative DG75 cells. Each transfection included 1 μg of the GFP expression vector pEGFP-N1, cotransfected with either 4 μg of pSG5 empty vector DNA, 4 μg of an LMP1 expression plasmid, 4 μg each of an LMP1 expression plasmid and a BZLF1 expression plasmid, or 4 μg each of an LMP1 expression plasmid and an IκBαΔN expression plasmid. The total amount of DNA per transfection was kept constant by addition of an appropriate amount of pSG5 empty vector DNA. MHC class I expression was detected 48 h following transfection by staining cells with MAb Tü149 followed by RPE-conjugated anti-IgG antibodies and analyzing by flow cytometry. The MFI of RPE staining (cell surface MHC class I molecules) on the GFP-positive (transfected) viable cell population was quantified. Results shown are means and standard errors from triplicate experiments. (B) Effect of BZLF1 and IκBαΔN on constitutive surface MHC class I expression in EBV-negative cells. DG75 cells were cotransfected with 1 μg of a marker plasmid (the GFP expression vector pEGFP-N1) together with 4 μg of either pSG5 empty vector DNA, a BZLF1 expression plasmid, or an IκBαΔN expression plasmid. At 48 h following transfection, the cells were stained for MHC class I expression exactly as for Fig. 6A, and the MFI of RPE staining (cell surface MHC class I molecules) on the GFP-positive (transfected) viable cell population was quantified. Results shown are means and standard errors from triplicate experiments.
The inhibitory effect of BZLF1 involves mechanisms in addition to NF-κB.
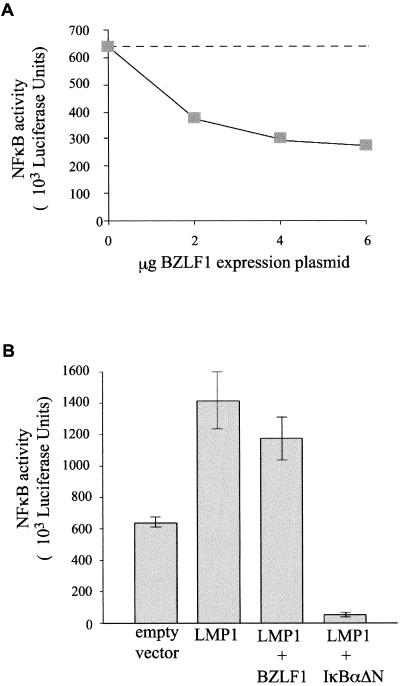
The results shown in Fig. 6 raise the question of whether BZLF1 is in fact mediating its inhibitory effects on LMP1 function by interfering with activation of NF-κB. To confirm that BZLF1 does inhibit NF-κB in B cells, we cotransfected DG75 cells with increasing amounts of a BZLF1 expression vector together with a constant amount of NF-κB-luciferase reporter DNA. Figure 7A shows results of a representative experiment in which the high basal level of NF-κB activity was inhibited by BZLF1 in a dose-responsive manner, with a maximum inhibition of 42% at 4 to 6 μg of the BZLF1 expression plasmid. In repeated experiments, inhibition of 40 to 50% was typically obtained at a dose of 4 μg of transfected BZLF1 plasmid. The inhibition of NF-κB activity by BZLF1 was never as efficient as the inhibition achieved when the NF-κB reporter plasmid was cotransfected with the IκBαΔN expression plasmid, which typically produced greater than 95% inhibition of basal NF-κB activity in DG75 cells (data not shown).
FIG. 7.
Effect of BZLF1 on LMP1-mediated activation of NF-κB. (A) Dose-dependent inhibition of NF-κB activation by BZLF1. As much as 6 μg of a BZLF1 expression plasmid was cotransfected with 3 μg of the NF-κB reporter 3Enh-Luc in DG75 cells. The total amount of DNA per transfection was kept to a constant 9 μg by addition of an appropriate amount of empty vector DNA. Luciferase activity was measured after 24 h. Results shown are means of results from duplicate experiments. (B) Effect of BZLF1 on LMP1-mediated NF-κB activation. DG75 cells were cotransfected with 3 μg of the NF-κB reporter 3Enh-Luc together with either 4 μg of pSG5 empty vector DNA, 4 μg of an LMP1 expression plasmid, 4 μg each of an LMP1 expression plasmid and a BZLF1 expression plasmid, or 4 μg each of an LMP1 expression plasmid and an IκBαΔN expression plasmid, exactly as for Fig. 6A. The total amount of DNA per transfection was kept constant by addition of an appropriate amount of empty vector DNA. Luciferase activity was measured after 24 h. Results shown are means and standard errors from triplicate experiments.
The effect of BZLF1 on NF-κB activation in LMP1-transfected cells was less pronounced. Figure 7B shows results of a representative NF-κB reporter experiment that was set up in parallel with the transfection experiment for which results are shown in Fig. 6A. Because of the high basal NF-κB activity of DG75 cells, expression of LMP1 results in a modest 2.5-fold increase in reporter activity. However, while coexpression of IκBαΔN with LMP1 inhibited NF-κB reporter activity by 96%, coexpression of BZLF1 with LMP1 inhibited NF-κB reporter activity by only 18%. Taken together, the results in Fig. 6 and 7 indicate that the ability of BZLF1 to inhibit LMP1-induced MHC class I expression is predominantly mediated by mechanisms other than the effects of BZLF1 on NF-κB activation.
DISCUSSION
These experiments show that the cell surface phenotype of cells spontaneously entering into lytic cycle differs in important respects from the surface phenotype of latency III cells in the same BL or LCL cultures. Cell surface MHC class I expression was reduced four- to fivefold from the levels seen on latency III cells, while MHC class II expression was reduced twofold (Fig. 3). It has not been possible to test the functional significance of this reduced MHC expression for recognition by EBV-specific CTL experimentally. Nevertheless, the reduced MHC expression in the lytic cycle is comparable to the differences between latency I and latency III subclones of EBV-positive BL lines (18, 31, 51), which are sufficient to impair the sensitivity of the BL cells to CTL lysis and to impair their T-cell-stimulatory capacity in mixed lymphocyte reactions (11, 47, 51). In BL lines displaying a latency I pattern of EBV infection, expression of MHC class I molecules is only one of several components of the antigen-processing pathway that appears to contribute to impaired antigen presentation. It is not known whether the same is also true of cells in the lytic cycle. However, even a relatively modest downregulation of MHC class I expression could conceivably have a significant effect on antigen presentation if de novo MHC class I synthesis (i.e., those MHC molecules synthesized after EBV replication begins) were predominantly inhibited. The 40% reduction seen in CD54 and CD40 expression in the lytic cycle (Fig. 3) could also affect the T-cell-stimulatory capacity of these cells, although it is worth noting that the CD80 and CD86 costimulatory molecules were not substantially affected by the entry of cells into lytic cycle.
Little is known about presentation of EBV target peptides to CTL during the lytic cycle (44). While CTL responses to several peptides of early-lytic-cycle antigens have been demonstrated (9, 38, 55), there have been no reports of recognition and lysis of cells in the lytic cycle by these CTL. In this context, it is of interest that CTL specific for EBNA1 peptides can similarly be reactivated from the peripheral T cells of infected individuals, even though these EBNA1-specific CTL cannot lyse EBV-transformed B cells expressing EBNA1 (8, 29). Therefore, demonstration of CTL specific for lytic-cycle antigens is not by itself sufficient to ascertain whether the cells in the lytic cycle are sensitive to CTL. Addressing this issue directly has been hampered by the practical problems associated with the lack of a fully permissive culture model for the EBV lytic cycle (27). Nevertheless, our present study has provided indirect evidence for impaired antigen presentation in the lytic cycle.
There is one previous report, suggesting that MHC class I and class II expression may be upregulated in the lytic cycle (12), which contradicts our observations. However, this earlier study investigated the expression of cell surface MHC molecules on cell lines which were induced into lytic cycle by treatment with a number of different chemical activators, such as n-butyrate and phorbol esters. Such chemicals have broad effects on cellular gene expression; hence it is difficult to attribute such changes specifically to induction of the lytic cycle. Indeed, closer inspection of the data published by Di Renzo et al. (12) shows that MHC expression was elevated not only in the subpopulation of cells in the lytic cycle but also in latently infected cells in the same culture. In our present study, we avoided the complications of chemical induction by examining lines which showed a predominant latency III type infection but which also contained a subpopulation that had spontaneously entered into lytic cycle.
Downregulation of surface MHC class I expression is maintained throughout the lytic cycle of EBV and can be seen on cells which have progressed into late lytic cycle and express VCA (Fig. 4A). Because treatment with acyclovir had no effect on the downregulation of MHC class I expression, we concluded that this modulation of MHC expression is an early-lytic-cycle event, occurring in the immediate-early or early stage of lytic infection, and is not dependent on the expression of a late-lytic-cycle gene (Fig. 4B). It is possible that reduced expression of MHC molecules is not caused by lytic-cycle genes but that the lytic cycle is preferentially induced in a population of latently infected cells with a phenotype that includes low expression of MHC molecules. This scenario would not diminish the potential importance of the low-MHC-expression phenotype in the lytic cycle with regard to the effect on CTL recognition. Analogy with other herpesviruses, however, favors a scenario where EBV lytic-cycle genes cause reduced MHC expression and perhaps also interfere with other components of antigen presentation. Our investigation of the possible mechanisms of the downregulation of MHC expression in the lytic cycle focused on two EBV-encoded proteins: (i) BZLF1, since recent evidence points to immunomodulatory functions for this immediate-early transactivator protein (see below), and (ii) LMP1, which is expressed as an early-lytic-cycle antigen (52). Since LMP1 is known to upregulate expression of various components of the antigen-processing pathway (51), we reasoned that some signaling functions of LMP1 must be impaired in the lytic cycle
The BZLF1 immediate-early transactivator plays an essential role in induction of the lytic cycle from latent infection (17, 57). BZLF1 is required for expression of many of the early lytic genes and is also important in mediating viral DNA replication through the lytic origin of replication (15, 16, 54). There is increasing evidence suggesting that BZLF1 also interacts with a number of cellular proteins to provide favorable conditions for replication. BZLF1 brings about cell cycle arrest through induction of cyclin-dependent kinase inhibitors (10, 45). It can also alter CREB-binding protein (3, 61) and p53 functions (63), interact with the transcription factor NF-κB (13, 21), and increase activation of the ATF2 transcription factor (2). While this report was in preparation, BZLF1 was reported to inhibit expression of the gamma interferon receptor (35). Recently, it was also shown that BZLF1 can cause dispersal of nuclear PML bodies and is SUMO-1 modified (4). PML protein appears to play a role in the regulation of MHC class I presentation and apoptosis (64). Together, these data indicate that BZLF1, in addition to its essential role in activating the lytic cycle, may have important immunomodulatory functions.
In the present study, we demonstrated that BZLF1 completely inhibits upregulation of surface MHC class I expression induced by LMP1 in EBV-negative B cells (Fig. 6A). NF-κB activation is thought to play a major role in LMP1-mediated upregulation of surface MHC class I expression (36), and because there is evidence to suggest that BZLF1 may interfere with the activation of this cellular transcription factor, it was reasonable to hypothesize that BZLF1 may block upregulation of MHC class I expression by LMP1 through a mechanism involving NF-κB. Following up on the work of Dreyfus et al. (13), we demonstrated that BZLF1 can directly inhibit the constitutive activation of NF-κB in EBV-negative B cells by as much as 50% (Fig. 7A). Interestingly, this negative effect of BZLF1 on NF-κB activation is not sufficient to explain the inhibition of LMP1-mediated upregulation of MHC class I expression by BZLF1. Investigation of the effect of BZLF1 on the activation of NF-κB by LMP1 demonstrated that BZLF1 could not substantially block LMP1-mediated NF-κB activation (Fig. 7B). On the other hand, expression of IκBαΔN blocked not only LMP1-mediated but also constitutive NF-κB activation. Thus, IκBαΔN has a partial inhibitory effect on LMP1-mediated upregulation of MHC class I expression and a dramatic inhibitory effect on LMP1-mediated NF-κB activation, whereas BZLF1 can totally block LMP1-mediated MHC class I upregulation while having little inhibitory effect on LMP1-mediated NF-κB activation. While these observations do not contradict evidence implicating NF-κB activation in LMP1-mediated induction of MHC class I expression (36), they do suggest that BZLF1 mediates its inhibitory effects on LMP1 predominantly via another pathway.
This study has identified a novel role for BZLF1 in completely inhibiting the ability of LMP1 to upregulate expression of cell surface MHC class I molecules. Since LMP1 is also known to induce expression of MHC class II molecules, CD40, and CD54 in B cells (51, 59, 62), this function of BZLF1 may be sufficient to account for the reduced expression of all these cellular proteins following the switch from latency III to the lytic cycle (Fig. 3). The more severe reduction in MHC class I expression relative to the other LMP1-regulated genes may be a consequence of interference with the ability of LMP1 to alter proteasome composition and to upregulate expression of TAP-1 and TAP-2 (51). Alternatively, as has been observed with other herpesviruses, it is possible that additional lytic-cycle genes specifically interfere with the antigen presentation pathway to augment the inhibitory effect of BZLF1 on LMP1 function.
Acknowledgments
We gratefully acknowledge the financial support of the Medical Research Council and the Wellcome Trust, which funded this work.
We thank Paul Brennan, Gavin Wilkinson, Anja Mehl, Ceri Fielding, and Eddie Wang for practical assistance and for critical review of the manuscript.
REFERENCES
- 1.Abbot, S. D., M. Rowe, K. Cadwallader, A. Ricksten, J. Gordon, F. Wang, L. Rymo, and A. B. Rickinson. 1990. Epstein-Barr virus nuclear antigen 2 (EBNA2) induces expression of the virus-coded latent membrane protein (LMP). J. Virol. 64:2126-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson, A. L., and S. Kenney. 1999. The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase CREB-binding protein. J. Virol. 73:6551-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamson, A. L., and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J. Virol. 75:2388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Immunol. Today 21:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arenzana-Seisdedos, F., B. Fernandez, I. Dominguez, J. M. Jacque, D. Thomas, M. T. Diaz-Meco, J. Moscat, and J. L. Virelizier. 1993. Phosphatidylcholine hydrolysis activates NF-κB and increases human immunodeficiency virus replication in human monocytes and T lymphocytes. J. Virol. 67:6596-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Bassat, H., N. Goldblum, S. Mitrani, T. Goldblum, J. M. Yoffey, M. M. Cohen, Z. Bentwich, B. Ramot, E. Klein, and G. Klein. 1977. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt like” malignant lymphoma (line D.G.-75). Int. J. Cancer 19:27-33. [DOI] [PubMed] [Google Scholar]
- 8.Blake, N., S. Lee, I. Redchenko, W. Thomas, N. Steven, A. Leese, P. Steigerwald-Mullen, M. Kurilla, L. Frappier, and A. Rickinson. 1997. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity 7:791-802. [DOI] [PubMed] [Google Scholar]
- 9.Bogedain, C., H. Wolf, S. Modrow, G. Stuber, and W. Jilg. 1995. Specific cytotoxic T lymphocytes recognize the immediate-early transactivator Zta of Epstein-Barr virus. J. Virol. 69:4872-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cayrol, C., and E. K. Flemington. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 11.de Campos-Lima, P. O., S. Torsteinsdottir, L. Cuomo, G. Klein, D. Sulitzeanu, and M. G. Masucci. 1993. Antigen processing and presentation by EBV-carrying cell lines: cell-phenotype dependence and influence of the EBV-encoded LMP1. Int. J. Cancer 53:856-862. [DOI] [PubMed] [Google Scholar]
- 12.Di Renzo, L., J. Avila-Carino, and E. Klein. 1993. Induction of the lytic viral cycle in Epstein Barr virus carrying Burkitt lymphoma lines is accompanied by increased expression of major histocompatibility complex molecules. Immunol. Lett. 38:207-214. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfus, D. H., M. Nagasawa, J. C. Pratt, C. A. Kelleher, and E. W. Gelfand. 1999. Inactivation of NF-κB by EBV BZLF-1-encoded ZEBRA protein in human T cells. J. Immunol. 163:6261-6268. [PubMed] [Google Scholar]
- 14.Fais, F., G. Cutrona, M. Ulivi, S. Roncella, M. C. Gagliardi, P. Cornaglia-Ferraris, M. Rowe, V. Barnaba, and M. Ferrarini. 1996. Lymphoblastoid cells transfected with c-myc: downregulation of EBV-lytic antigens and impaired response of autologous CD4+ T cells in vitro. Int. J. Cancer 68:810-816. [DOI] [PubMed] [Google Scholar]
- 15.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1995. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J. Virol. 69:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1992. trans-acting requirements for replication of Epstein-Barr virus oriLyt. J. Virol. 66:5030-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flemington, E., and S. H. Speck. 1990. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floettmann, E., K. Ward, A. B. Rickinson, and M. Rowe. 1996. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 (LMP1) analysed using tetracycline-regulated expression. Virology 223:29-40. [DOI] [PubMed] [Google Scholar]
- 19.Floettmann, J. E., and M. Rowe. 1997. Epstein-Barr virus latent membrane protein-1 (LMP1) C-terminus activation region 2 (CTAR2) maps to the far C-terminus and requires oligomerisation for NF-κB activation. Oncogene 15:1851-1858. [DOI] [PubMed] [Google Scholar]
- 20.Gregory, C. D., R. J. Murray, C. F. Edwards, and A. B. Rickinson. 1988. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J. Exp. Med. 167:1811-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutsch, D. E., E. A. Holley-Guthrie, Q. Zhang, B. Stein, M. A. Blanar, A. S. Baldwin, and S. C. Kenney. 1994. The bZIP transactivator of Epstein-Barr virus, BZLF1, functionally and physically interacts with the p65 subunit of NF-κB. Mol. Cell. Biol. 14:1939-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haque, M., K. Ueda, K. Nakano, Y. Hirata, C. Parravicini, M. Corbellino, and K. Yamanishi. 2001. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. J. Gen. Virol. 82:1175-1180. [DOI] [PubMed] [Google Scholar]
- 23.Hengel, H., and U. H. Koszinowski. 1997. Interference with antigen processing by viruses. Curr. Opin. Immunol. 9:470-476. [DOI] [PubMed] [Google Scholar]
- 24.Huen, D. S., S. A. Henderson, D. Croom-Carter, and M. Rowe. 1995. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene 10:549-560. [PubMed] [Google Scholar]
- 25.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph, A. M., G. J. Babcock, and D. A. Thorley-Lawson. 2000. Cells expressing the Epstein-Barr virus growth program are present in and restricted to the naive B-cell subset of healthy tonsils. J. Virol. 74:9964-9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 28.Kishishita, M., J. Luka, B. Vroman, J. F. Poduslo, and G. R. Pearson. 1984. Production of monoclonal antibody to a late intracellular Epstein-Barr virus-induced antigen. Virology 133:363-375. [DOI] [PubMed] [Google Scholar]
- 29.Levitskaya, J., M. Coram, V. Levitsky, S. Imreh, P. M. Steigerwald-Mullen, G. Klein, M. G. Kurilla, and M. G. Masucci. 1995. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature 375:685-688. [DOI] [PubMed] [Google Scholar]
- 30.Lin, J.-C., M. C. Smith, and J. S. Pagano. 1984. Prolonged inhibitory effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine against replication of Epstein-Barr virus. J. Virol. 50:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masucci, M. G., S. Torsteinsdottir, J. Colombani, C. Brautbar, E. Klein, and G. Klein. 1987. Down-regulation of class-I HLA antigens and of the Epstein-Barr virus (EBV)-encoded latent membrane protein (LMP) in Burkitt's lymphoma lines. Proc. Natl. Acad. Sci. USA 84:4567-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehl, A. M., J. E. Floettmann, M. Jones, P. Brennan, and M. Rowe. 2001. Characterization of intercellular adhesion molecule-1 regulation by Epstein-Barr virus-encoded latent membrane protein-1 identifies pathways that cooperate with nuclear factor κB to activate transcription. J. Biol. Chem. 276:984-992. [DOI] [PubMed] [Google Scholar]
- 33.Miller, D. M., and D. D. Sedmak. 1999. Viral effects on antigen processing. Curr. Opin. Immunol. 11:94-99. [DOI] [PubMed] [Google Scholar]
- 34.Miyashita, E., B. Yang, G. Babcock, and D. Thorley-Lawson. 1997. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J. Virol. 71:4882-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, T. E., A. Mauser, A. Wong, J. P. Ting, and S. C. Kenney. 2001. Inhibition of IFN-γ signaling by an Epstein-Barr virus immediate-early protein. Immunity 15:787-799. [DOI] [PubMed] [Google Scholar]
- 36.Pai, S., B. J. O'Sullivan, L. Cooper, R. Thomas, and R. Khanna. 2002. RelB nuclear translocation mediated by C-terminal activator regions of Epstein-Barr virus-encoded latent membrane protein 1 and its effect on antigen-presenting function in B cells. J. Virol. 76:1914-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulson, E., C. Tran, K. Collins, and K. Fruh. 2001. KSHV-K5 inhibits phosphorylation of the major histocompatibility complex class I cytoplasmic tail. Virology 288:369-378. [DOI] [PubMed] [Google Scholar]
- 38.Pepperl, S., G. Benninger-Doring, S. Modrow, H. Wolf, and W. Jilg. 1998. Immediate-early transactivator Rta of Epstein-Barr virus (EBV) shows multiple epitopes recognized by EBV-specific cytotoxic T lymphocytes. J. Virol. 72:8644-8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzo, P., I. Magrath, R. Chattopadhyady, R. Biggar, and P. Gerber. 1978. A new tumour-derived transforming strain of Epstein-Barr virus. Nature 272:629-631. [DOI] [PubMed] [Google Scholar]
- 40.Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280:248-253. [DOI] [PubMed] [Google Scholar]
- 41.Qu, L., and D. T. Rowe. 1992. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J. Virol. 66:3715-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabson, M., L. Heston, and G. Miller. 1983. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc. Natl. Acad. Sci. USA 80:2762-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 44.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez, A., E. J. Jung, Q. Yin, C. Cayrol, and E. K. Flemington. 2001. Role of c-myc regulation in Zta-mediated induction of the cyclin-dependent kinase inhibitors p21 and p27 and cell growth arrest. Virology 284:159-169. [DOI] [PubMed] [Google Scholar]
- 46.Rooney, C. M., D. T. Rowe, T. Ragot, and P. J. Farrell. 1989. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J. Virol. 63:3109-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rooney, C. M., M. Rowe, L. E. Wallace, and A. B. Rickinson. 1985. Epstein-Barr virus-positive Burkitt's lymphoma cells not recognized by virus-specific T-cell surveillance. Nature 317:629-631. [DOI] [PubMed] [Google Scholar]
- 48.Rowe, D., L. Heston, J. Metlay, and G. Miller. 1985. Identification and expression of a nuclear antigen from the genomic region of the Jijoye strain of Epstein-Barr virus that is missing in its non-immortalizing deletion mutant, P3HR-1. Proc. Natl. Acad. Sci. USA 82:7429-7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe, M., H. S. Evans, L. S. Young, K. Hennessy, E. Kieff, and A. B. Rickinson. 1987. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J. Gen. Virol. 68:1575-1586. [DOI] [PubMed] [Google Scholar]
- 50.Rowe, M., and M. Jones. 2001. Detection of EBV latent proteins by Western blotting. Methods Mol. Biol. 174:229-242. [DOI] [PubMed] [Google Scholar]
- 51.Rowe, M., R. Khanna, C. A. Jacob, V. Argaet, A. Kelly, S. Powis, M. Belich, D. Croom-Carter, S. Lee, S. R. Burrows, et al. 1995. Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein-Barr virus latent membrane protein-1: coordinate up-regulation of peptide transporters and HLA-class I antigen expression. Eur. J. Immunol. 25:1374-1384. [DOI] [PubMed] [Google Scholar]
- 52.Rowe, M., A. L. Lear, D. Croom-Carter, A. H. Davies, and A. B. Rickinson. 1992. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J. Virol. 66:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowe, M., D. T. Rowe, C. D. Gregory, L. S. Young, P. J. Farrell, H. Rupani, and A. B. Rickinson. 1987. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 6:2743-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schepers, A., D. Pich, and W. Hammerschmidt. 1993. A transcription factor with homology to the AP-1 family links RNA transcription and DNA replication in the lytic cycle of Epstein-Barr virus. EMBO J. 12:3921-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steven, N. M., N. E. Annels, A. Kumar, A. M. Leese, M. G. Kurilla, and A. B. Rickinson. 1997. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J. Exp. Med. 185:1605-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevenson, P. G., S. Efstathiou, P. C. Doherty, and P. J. Lehner. 2000. Inhibition of MHC class I-restricted antigen presentation by gamma-2 herpesviruses. Proc. Natl. Acad. Sci. USA 97:8455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takada, K., and Y. Ono. 1989. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J. Virol. 63:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tierney, R. J., N. Steven, L. S. Young, and A. B. Rickinson. 1994. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J. Virol. 68:7374-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young, L. S., R. Lau, M. Rowe, G. Niedobitek, G. Packham, F. Shanahan, D. T. Rowe, D. Greenspan, J. S. Greenspan, A. B. Rickinson, et al. 1991. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J. Virol. 65:2868-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zerby, D., C. J. Chen, E. Poon, D. Lee, R. Shiekhattar, and P. M. Lieberman. 1999. The amino-terminal C/H1 domain of CREB binding protein mediates Zta transcriptional activation of latent Epstein-Barr virus. Mol. Cell. Biol. 19:1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, Q., L. Brooks, P. Busson, F. Wang, D. Charron, E. Kieff, A. B. Rickinson, and T. Tursz. 1994. Epstein-Barr virus (EBV) latent membrane-protein-1 increases HLA class-II expression in an EBV-negative B-cell line. Eur. J. Immunol. 24:1467-1470. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, Q., D. Gutsch, and S. Kenney. 1994. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol. Cell. Biol. 14:1929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng, P., Y. Guo, Q. Niu, D. E. Levy, J. A. Dyck, S. Lu, L. A. Sheiman, and Y. Liu. 1998. Proto-oncogene PML controls genes devoted to MHC class I antigen presentation. Nature 396:373-376. [DOI] [PubMed] [Google Scholar]