Abstract
BALB/c mice sensitized to herpes simplex virus type 1 (HSV-1) develop a vigorous delayed-type hypersensitivity (DTH) response upon intradermal virus antigen challenge. Although CD4+ T cells are a key mediator of this response, neutrophils are the most abundant cells at the antigen challenge site both initially and at the peak of the reaction. We investigated what role, if any, neutrophils play in the DTH to a viral antigen. We show here that antibody-mediated depletion of neutrophils 1 day before antigen challenge significantly suppressed ear swelling and markedly reduced cellular influx. Additionally, neutrophil depletion was associated with decreased expression of macrophage inflammatory protein 2 (MIP-2) and MIP-1α, as well as with a >60-fold increase in HSV-1 replication. Neutralizing antibodies to neutrophil chemoattractants MIP-2 or MIP-1α but not KC significantly suppressed DTH and sharply reduced neutrophil accumulation in the ear pinna. Purified bone marrow-derived neutrophils exposed to interleukin-1α (IL-1α) produced chemokines in an 8-h assay. Administration of neutralizing antibody to IL-1α significantly reduced ear swelling and suppressed the levels of MIP-2, MIP-1α, MIP-1β, and RANTES. We conclude that neutrophils are a critical component of the DTH response to viral antigen. They are recruited to the DTH test site by MIP-2 and MIP-1α, where they can be activated by IL-1α. The infiltrating cells also help suppress virus replication in immunized mice.
The development of an inflammatory response in herpes simplex virus type 1 (HSV-1)-sensitized hosts upon reexposure to viral antigen has been known for decades (25, 32). However, the mechanism accounting for this immunologically specific response, delayed-type hypersensitivity (DTH), is quite complex, and identification of the cellular and molecular events inherent in this phenomenon remains incomplete. A clearer understanding of the DTH mechanism is pertinent because it represents a form of cell-mediated immunity and, as such, has the potential to provide protection against the virus or, alternatively, contribute to the pathogenesis of herpesvirus-induced disease (31).
DTH can be elicited by inoculation of virus antigen into the ear pinna (46) of the HSV-1-infected mouse. Optimal responsiveness occurs 6 to 9 days postinfection and has been shown via adoptive transfer studies and cell depletion experiments to be mediated by the CD4 T-cell subset (21, 38, 39). Although neutrophil influx after HSV-1 ocular infection is well documented (36, 37, 53), the accumulation of neutrophils in the skin, i.e., at the DTH test site, was not initially appreciated. Traditionally, neutrophils have been thought to function only as phagocytic effector cells. More recently, however, it has become evident that neutrophils can express a variety of immunoregulatory molecules (6, 34), and thus may participate more actively in DTH.
The identification of chemotactic factors involved in the recruitment of neutrophils to sites of DTH responsiveness also is incompletely defined. Chemokines are logical candidates (45, 60). They constitute a growing family of low-molecular-weight secreted proteins that can chemoattract leukocyte subpopulations from the blood to sites of inflammation, including DTH. Chemokines induce cell activation and migration by binding to specific G protein-coupled receptors expressed on the surface of leukocytes. In the mouse, two important neutrophil chemoattractants are macrophage inflammatory protein-2 (MIP-2) and KC (40, 55). These C-X-C chemokines are known to induce potent chemotaxis of the neutrophil in vitro, and injection of these chemokines either subcutaneously or intracorneally in mice results in a predominant neutrophil influx (15, 40, 49, 55, 58). Furthermore, these chemokines are found at the site of HSV-1 infection (58), and antibody neutralization of endogenous MIP-2 can alter the host response in models of both viral (58) and bacterial (18, 27, 47) infection. MIP-1α is a member of the C-C chemokine supergene family (45, 60). Like other members of this family, MIP-1α is chemotactic for mononuclear phagocytes and lymphocytes (44, 48). However, MIP-1α has also been reported to be chemotactic for neutrophils both in vivo and in vitro (1, 16). Experiments with mice carrying a disrupted MIP-1α gene (9, 51) or animals given antibody to neutralize endogenous MIP-1α (1, 22, 52) have revealed its importance for cellular recruitment in the expression phase of cell-mediated immunity, including that in HSV-1 infection (51, 52).
The focus of the present investigation was to evaluate the contribution of neutrophils in the DTH response to HSV-1 antigen and to identify mediators that affect the recruitment of these cells to the site of virus antigen deposition. We also investigated whether the recruited neutrophils helped to limit virus replication in immunized hosts.
MATERIALS AND METHODS
Animals.
Four-week-old female BALB/c mice were obtained from Charles River Laboratories (Wilmington, Mass.). Animals were cared for in compliance with federal, state, and local regulations.
Antibodies and reagents.
The rat hybridoma RB6-8C5, which produces anti-mouse granulocyte monoclonal antibody (MAb), was a gift from R. Coffman (DNAX Research Institute, Palo Alto, Calif.). This antibody was prepared as previously described (50). The rat hybridomas clones 2.43 and GK1.5, which produce anti-CD8 and anti-CD4 MAbs, respectively, were obtained from American Type Culture Collection (ATCC; Manassas, Va.). Hamster immunoglobulin G (IgG) MAb to mouse interleukin-1α (IL-1α) and polyclonal antibodies to MIP-1α (goat IgG) and KC (goat IgG) were all purchased from R&D Systems (Minneapolis, Minn.). A rabbit IgG polyclonal antibody to MIP-2 was a gift from S. Kunkel, University of Michigan. The recombinant mouse cytokines and chemokines IL-1α, MIP-2, MIP-1α, MIP-1β, and RANTES were purchased from R&D Systems, as was recombinant human IL-8.
Induction and elicitation of DTH reaction.
To sensitize mice, the right cornea was scarified with three twists of a 2-mm trephine. Then, 1 × 104 to 2 × 104 PFU/2 μl of HSV-1 strain RE was massaged into the corneal surface by using the eyelids. DTH response was determined by using the ear swelling assay (31). Briefly, at 6 to 9 days postinfection a 10-μl volume of viral RE antigen, which had been UV-irradiated to reduce viral infectivity to <102 PFU/10 μl, in serum-free RPMI 1640 medium was injected into the right ear pinna, by using a 30-gauge needle and a Hamilton syringe (Hamilton, Reno, Nev.). The left control ear pinna received 10 μl of medium with 1% newborn calf serum. Ear swelling was measured 24 h later with a Mitutoyo 7326 Micrometer (Schlessinger Tools, New York, N.Y.), and the results were expressed as the mean swelling of antigen challenge ear minus the mean swelling of control ear (units, 10−4 inches). In experiments in which anti-chemokine antibodies were used, the antibodies were admixed with 10 μl of viral antigen to a final concentration of 2.5 μg/μl. Appropriate IgG controls were used at the same concentration. To neutralize endogenous IL-1α, specific antibody (2.5 μg/μl) was given mixed with DTH test antigen. A second 10-μl injection of antibody or control IgG was given 12 h postchallenge again at the DTH test site.
Cellular depletion.
Mice were depleted of specific leukocyte populations by intraperitoneal injection of 0.5 to 1.0 mg of MAb 1 day prior to DTH challenge (i.e., 5 days postsensitization). MAb RB6-8C5 was used to deplete mice of neutrophils and MAbs GK1.5 and 2.43 were administered to deplete CD4+ and CD8+ cells, respectively. Control mice received intraperitoneal injections of rat IgG antibody (Sigma, St. Louis, Mo.) or rat MAb specific for human HLA DR5 (clone SFR3-DR5; ATCC). Neutrophil depletion was confirmed to be at a level of >96% based on blood smears stained with Leukostat (Fisher Diagnostics, Orangeburg, N.Y.). After MAb treatment, cytofluorimetric analysis determined that CD4+ T cells and CD8+ T cells were reduced by >90 and >85%, respectively. No significant reductions in B cells, NK cells, or monocytes were observed after MAb treatment.
Cytokine-chemokine assay.
Sections (6 mm) of ear pinnas were excised at the indicated times after DTH challenge. Tissues from within each group were individually minced, homogenized, sonicated for 30 s, and clarified by centrifugation at 150 × g for 10 min. Samples were assayed for MIP-1α, MIP-1β, MIP-2, KC, and RANTES by using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems. The ELISA kit for IL-1α was obtained from Endogen, Inc. (Cambridge, Mass.).
Immunohistochemistry.
Staining was performed by using a modification of the method by Hendricks et al. (20). Ear sections were excised and imbedded in Tissue Tek O.C.T. compound (Sakura Finetek, Torrance, Calif.). Sections (6 μm) were cut at −20°C, fixed in ice-cold acetone for 10 min, and blocked with normal goat serum. Sections were stained for the presence of granulocytes (rat MAb RB6-8C5) or CD4+ T cells (rat MAb GK1.5) with 2 μg of the relevant primary antibody/ml for 1 h at room temperature. Control sections were treated with 2 ng of isotyped-matched rat MAb specific for human HLA DR5 (clone SFR3-DR5; ATCC)/ml. The sections were then treated with the streptavidin-biotin complex immunoperoxidase staining procedure according to the manufacturer's protocol (Zymed Laboratories, South San Francisco, Calif.). The slides were washed in distilled water and counter stained in Harris hematoxylin for 3 min. The stained neutrophil and CD4+ T cells were counted in a coded fashion, with the reader unaware of the treatment given.
MPO.
The assay for myeloperoxidase (MPO) assay, a marker for neutrophils, was performed as previously described (5). Individual ear pinna homogenates were tested for MPO 24, 48, and 72 h after DTH challenge. No MPO activity was detected in normal ear pinnas. The sensitivity of the assay was 0.005 IU.
Neutrophil isolation and stimulation.
Bone marrow (BM) was flushed from the femur and tibia of mice with media (RPMI 1640 plus 5% normal calf serum) with a syringe and 25G needle. The BM cells were centrifuged (1,200 rpm for 5 min), washed twice, layered over a gradient of Histopaque 1119 and 1077 (Sigma), and then centrifuged at 700 × g for 30 min. The enriched neutrophil layer was removed, washed twice in media, and treated with red blood cell lysis buffer (Sigma). Contaminating monocytes were depleted by adherence (30 min for 37°C) to a polystyrene tissue culture plate (Corning, New York, N.Y.). Neutrophil purity was consistently >99% as assessed by HEMA3 (Biochemical Sciences, Inc., Swedesboro, N.J.) staining of cytospin slides (Shandon, Pittsburgh, Pa.).
For stimulation, 106 neutrophils in 0.5 ml of media were plated in triplicate in 24-well tissue culture plates. The wells were precoated with newborn calf serum at 0.5 ml/well for 1 h at 37°C and then washed three times with phosphate-buffered saline. Stimulations were performed by the addition of recombinant mouse IL-1α at final concentrations of 0.1, 1.0, or 10 ng/ml. Phorbol myristate acetate (30 ng/ml) was used as a positive control, whereas negative controls were exposed to media alone. After 8 h of incubation at 37°C in 5% CO2, the supernatants were removed, clarified by centrifugation, and assayed for chemokine content by ELISA. In experiments in which neutrophils were stimulated with chemokines, MIP-1α and MIP-2 were used at 1, 10, and 100 ng/ml, whereas MIP-1β, RANTES, KC, and human IL-8 were used at stimulatory doses of 1 and 10 ng/ml.
Statistical analysis.
Student's t test was used to determine significant differences in the DTH responses and cytokine responses between control and test groups. The level of confidence at which experiments were judged significant was P < 0.05. All experiments were performed at least twice.
RESULTS
Neutrophil accumulation in the ear pinna after challenge with UV-inactived HSV-1.
Our initial experiments were aimed at characterizing the inflammatory response induced by subcutaneous inoculation of UV-HSV-1 into virus-sensitized mice. BALB/c mice were infected on the scarified cornea with the RE strain of HSV-1, and a DTH response was elicited 6 days postinfection. Sequential measurements of ear swelling over a 3-day period postchallenge established that the peak response occurred 24 h after antigen administration (data not shown). Immunohistochemical examination of ear pinnas with the anti-neutrophil MAb RB6-8C5 revealed that neutrophils were the predominant cell type present 24 h postchallenge (Table 1). Lower numbers of CD4+ T cells were also observed (Table 1), whereas CD8+ T cells were seen infrequently (not shown).
TABLE 1.
CD4+ T-cell and neutrophil cell counts in ear pinnas of anti-chemokine-treated micea
| Leukocytesb | Mean cell count ± SE with:
|
||
|---|---|---|---|
| Rabbit IgGc | Anti-MIP-2 | Anti-MIP-1a | |
| CD4+ T cells | 40 ± 5 | 54 ± 9 | 32 ± 3 |
| Neutrophils | 216 ± 17 | 12 ± 3d | 44 ± 9d |
Ear pinnas (two per group) from sensitized mice given anti-chemokine antibody or rabbit IgG intradermally were obtained 24 h post challenge, and individual frozen sections were prepared.
CD4+ T cells and neutrophils were identified by immunohistochemical staining of frozen sections by using the MAbs GK1.5 and RB6-8C5, respectively. No positive leukocyte staining was observed in control sections stained with isotype-matched immunoglobulin.
The designated cell populations were counted in six different × 40 fields in each of two sections from each tissue (total of 24 fields).
The treatment group was significantly (P < 0.05) different from the control group as assessed by Student's t test.
Effect of neutrophil depletion on DTH responsiveness.
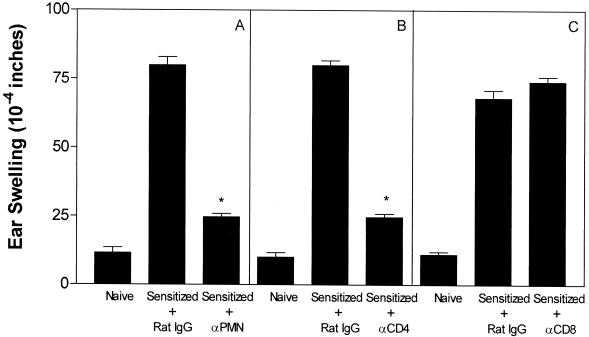
Depletion studies were carried out in a series of three independent experiments to assess whether neutrophils played an active as opposed to a passive role in DTH. Representative results are shown in Fig. 1A. Neutrophil depletion, achieved by giving RB6-8C5 MAb, reduced DTH responsiveness by 79%. For comparison purposes, T-cell subsets were also depleted (Fig. 1B and C). Depletion of CD4+ T cells with MAb GK1.5 suppressed ear swelling by 82%, whereas depletion of CD 8+ T cells via MAb 2.43 had little or no effect. Thus, neutrophils, as well as CD4+ T cells, are essential participants in the DTH response to HSV-1 antigen.
FIG. 1.
Effect of neutrophil or T-cell subset depletion on the DTH response to HSV-1. Mice (four per group) were sensitized via topical infection on the cornea with HSV-1 on day 0. On day 5 after infection each group received, intraperitoneally, 1.0 mg of the designated antibody (A, αPMN; B, αCD4; C, αCD8) or rat IgG. DTH testing was performed on day 6, and ear swelling was measured 24 h later. Nonsensitized (naive) controls were included for comparison purposes. An asterisk indicates that the antibody-treated group was significantly (P < 0.05) different from the rat IgG-treated control group.
Detection of neutrophil chemoattractants at the DTH test site.
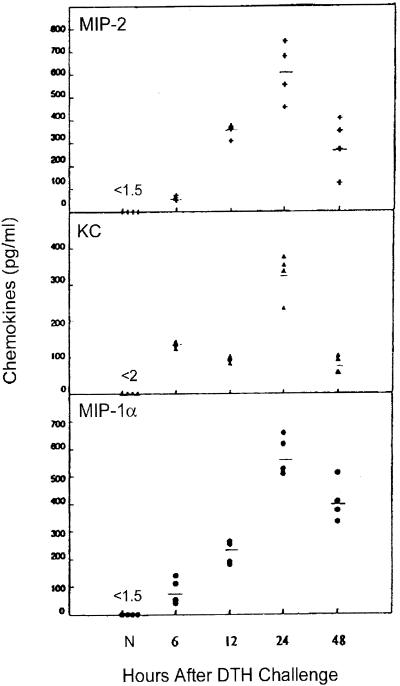
The DTH test site was examined for the expression of MIP-2, KC, and MIP-1α, chemokines known to attract murine neutrophils. All three chemokines were readily detected by ELISA 6 h after viral antigen challenge and reached peak levels at 24 h, the time of maximum ear swelling (Fig. 2). Peak chemokine levels correlated with peak neutrophil influx as monitored by MPO levels (data not shown). MIP-2 and MIP-1α levels were substantially greater (∼2-fold) than KC at 24 h. Chemokines were expressed only at background levels in the ear pinnas of naive hosts or of sensitized hosts that received medium in place of virus antigen.
FIG. 2.
Time course of chemokine protein expression in HSV-1-challenged ear pinnas. Virus-sensitized mice were given the DTH test antigen 7 days postinfection. Four ear pinnas were collected from sensitized hosts at the indicated times after challenge, and individual tissue lysates were prepared. The clarified lysates were assayed for the indicated chemokines via ELISA. Ear pinna lysates from nonsensitized (N) hosts were included for comparison.
Passive transfer of antibodies to MIP-2 and MIP-1α suppresses DTH and neutrophil accumulation.
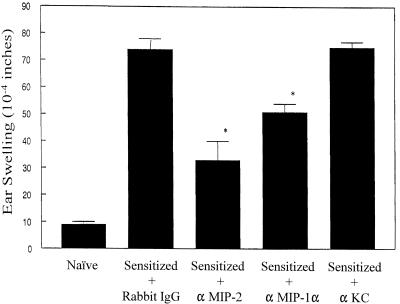
Neutralizing antibody to individual chemokines was administered locally admixed with virus challenge antigen in order to determine whether the chemokines detected at the DTH test site contributed to the inflammatory response. The results representative of two independent experiments are shown in Fig. 3. Local administration of antibody specific for MIP-2 and MIP-1α suppressed DTH responsiveness by 62 and 37%, respectively, relative to that seen in the IgG-treated controls. However, neutralizing antibody to KC given at a dose sufficient to neutralize at least 10 ng of KC in vivo failed to reduce the DTH response. Immunohistochemical staining of ear pinnas at 24 h postchallenge revealed that the neutrophil cell count was reduced by 94% in the anti-MIP-2 treated hosts and by 80% in the anti-MIP-1α recipients relative to that seen in the rabbit IgG-treated controls (Table 1). Anti-chemokine treatment, however, did not result in a significant reduction in the number of CD4+ T cells observed at the DTH site. In a independent followup study, the striking reduction in neutrophil content in the ear pinnas was supported via assays for the neutrophil marker enzyme MPO. Analysis performed on individual ear pinnas harvested 24 h postchallenge revealed that the mean MPO content was 0.32 IU/g (of tissue) in the rabbit IgG control group compared to 0.049 and 0.0084 IU/g in mice treated with anti-MIP-2 and anti-polymorphonuclear leukocyte (PMN) MAb, respectively.
FIG. 3.
Effect of anti-chemokine antibodies on DTH responsiveness in HSV-1-sensitized hosts. The indicated anti-chemokine antibodies (5 μg/μl) or rabbit IgG were admixed with virus antigen and inoculated into the ear pinnas of mice 7 days postinfection. Ear swelling was measured 24 h later. There were four to five mice per group. An asterisk indicates that the antibody-treated group was significantly (P < 0.05) different from the rabbit IgG-treated control group.
Effect of neutrophil depletion on mediator expression at the DTH site.
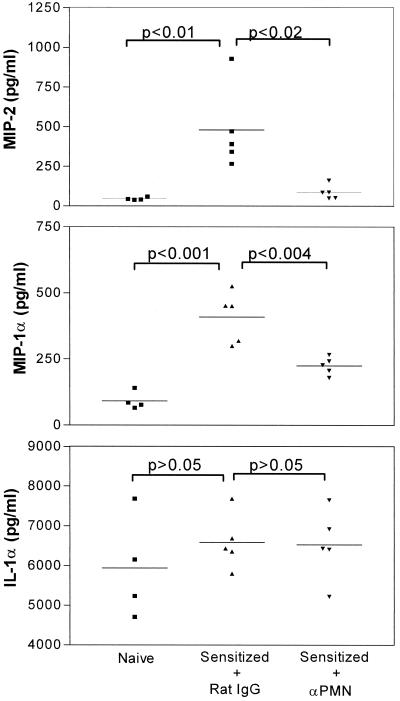
To evaluate the role of neutrophils, we tested whether these cells influenced the expression of mediators associated with DTH. Figure 4 shows that neutrophil depletion was accompanied by reduced chemokine expression. MIP-2 was decreased 82%, whereas MIP-1α levels fell by 45%. In contrast, the early warning cytokine IL-1α, expressed at high levels in ear pinnas from both naive and sensitized hosts, was not affected by neutrophil depletion. Tumor necrosis factor alpha (TNF-α) and granulocyte-macrophage colony-stimulating factor were not detected (<5 and <1 pg/ml, respectively) in any of the ear lysate preparations.
FIG. 4.
Effect of neutrophil depletion on the expression of MIP-2, MIP-1α, and IL-1α at the DTH test site. Mice were sensitized via HSV-1 corneal infection on day 0 and given RB6-8C5 MAb on day 5. DTH testing was carried out on day 6, and ear swelling was measured 24 h later. Individual ear pinna lysates were then prepared and assayed for the indicated mediators via ELISA. Ear lysates from nonsensitized controls were also analyzed.
IL-1α stimulates neutrophils to produce chemokines.
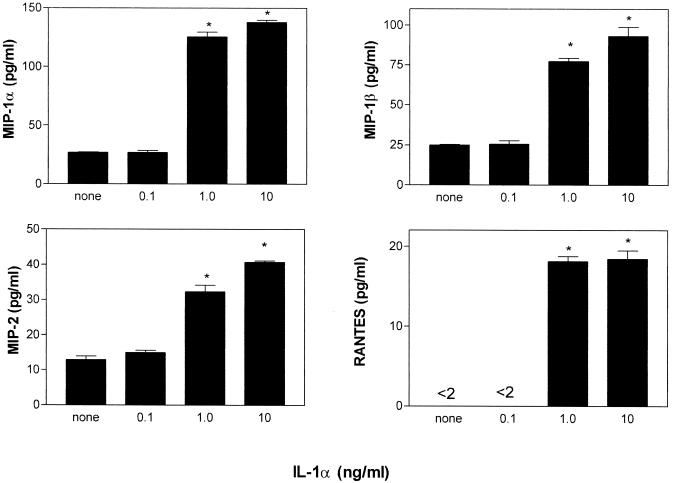
Since IL-1α was present in abundance at the DTH test site and is a potent inducer of proinflammatory mediators (13), we tested whether this cytokine could directly stimulate neutrophils to express chemokines. Figure 5 shows that purified neutrophils exposed to low-dose IL-1α secreted abundant MIP-1α, as well as MIP-1β, MIP-2, and RANTES (although at progressively lesser amounts).
FIG. 5.
Neutrophils exposed to IL-1α produce various chemokines. BM-derived neutrophils (106/well), purified as described in Materials and Methods, were stimulated in triplicate with concentrations of IL-1α ranging from 0.1 to 10 ng/ml for 8 h. Supernatants were then assayed for chemokines by ELISA. An asterisk indicates a significantly higher level (P < 0.005) than that of the unstimulated control.
We also investigated whether chemokines that recruited neutrophils could activate these cells to produce additional chemokines. It was found that addition of MIP-2 or MIP-1α at doses ranging from 1 to 100 ng/ml failed to induce chemokine production. Furthermore, stimulation of neutrophils with mediators known to bind the same receptors as MIP-2 (IL-8 and KC) and MIP-1α (RANTES) did not trigger a feedback response.
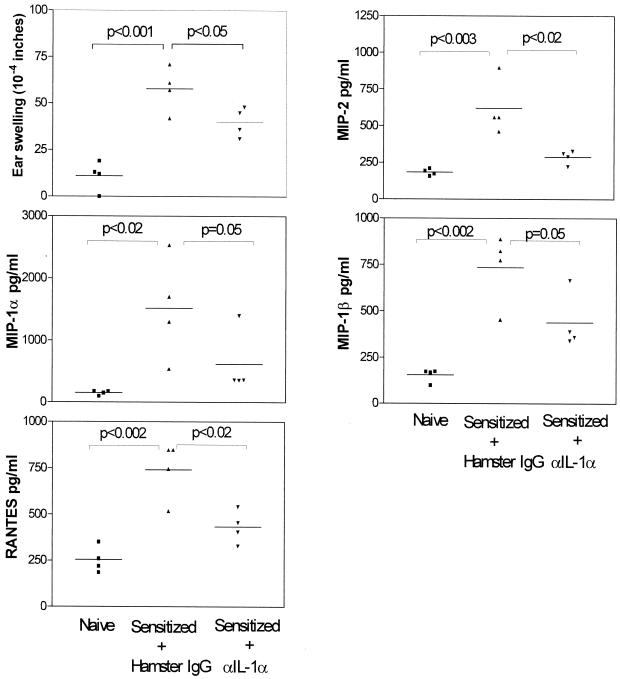
Antibody neutralization of IL-1α suppresses DTH and chemokine production.
If IL-1α is a potent activator of neutrophils in DTH responses as suggested by the foregoing in vitro results, then its neutralization in vivo should suppress this inflammatory response. To test this hypothesis, neutralizing antibody to IL-1α was administered together with the DTH viral antigen. A second antibody treatment was given locally 12 h later. Ear swelling was measured at 24 h. Figure 6 shows that antibody neutralization of endogenous IL-1α reduced ear swelling by 31%. In addition, the mean levels of MIP-1α, MIP-1β, MIP-2, and RANTES were reduced by 59, 44, 53, and 42%, respectively. These results support the view that IL-1α plays an important role in DTH and chemokine expression.
FIG. 6.
Effect of IL-1α neutralizing antibody treatment on DTH and mediator production. Antibodies to IL-1α (2.5 μg/μl) or hamster IgG were mixed with virus antigen and inoculated into the ear pinnas of mice 11 days postinfection. The antibody treatment was repeated 12 h later. At 24 h the degree of ear swelling was measured. Individual ear pinna lysates were then prepared and assayed for the indicated chemokines by ELISA. Ear pinna lysates from nonsensitized hosts were included for comparison.
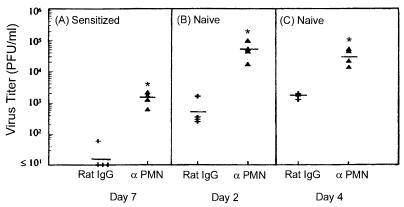
Effect of neutrophil depletion on virus replication at the DTH test site.
We speculated that the rapid and abundant neutrophil infiltrate may also contribute to host resistance to intradermal virus challenge. To test this hypothesis, 5 × 105 PFU viable HSV-1 was inoculated into the ear pinna of immunized mice 1 day after they were depleted of neutrophils. Figure 7A shows that the mean virus titer 24 h postinfection was 64-fold higher than in the sensitized controls. Neutrophil depletion also enhanced virus growth in the skin of naive mice. Figure 7B and C show that 2 and 4 days postinfection the mean titers were 84- and 20-fold higher, respectively, than in the IgG-treated controls. These results suggest that neutrophils help to limit HSV-1 replication in the skin of both sensitized and naive hosts.
FIG. 7.
Antiviral effect of neutrophils in acquired and innate immunity. (A) Mice were sensitized on the cornea with 2 × 104 PFU HSV-1 and treated with RB6-8C5 MAb or control rat IgG 5 days later. Both groups were challenged on day 6 with 5 × 105 PFU HSV-1, and ear pinnas were individually assayed for virus content 24 h later. ✽, P < 0.04 compared to the controls. (B and C) Naive mice were given RB6-8C5 MAb or control rat IgG at time 0, and inoculated in the right ear pinna with 5 × 105 PFU HSV-1 24 h later. On days 2 (B) and 4 (C) postinfection, ear pinnas were collected and individually assayed for virus content. ✽, P < 0.02 compared to the controls.
DISCUSSION
This study identifies a number of critical participants in DTH to viral antigen and probes how they contribute to this inflammatory response. First, we show that there is a rapid and extensive influx of neutrophils. The importance of these cells is demonstrated by the profound reduction (60 to 80%) in ear swelling in neutrophil depleted hosts. This decrease was only slightly less than that seen in mice depleted of CD4+ T cells (82 to 91% reduction), the initiating cells of DTH. Our results are compatible with reports of an essential role for the neutrophil in the DTH response to sheep red blood cells in the rat (29) and to tuberculin in the rabbit (30). Although macrophages play a prominent role in the prototypic DTH response to Mycobacterium tuberculosis (28), only a small number of these cells were observed at the DTH test site 24 h after HSV-1 antigen challenge. Recent studies in our laboratory have shown that depletion of macrophages from subconjunctival and draining lymph node tissue before but not after sensitization via the ocular route suppressed DTH responsiveness (7). This suggests that although macrophages participate in host sensitization they may not be dominant effectors of DTH responsiveness to HSV-1 in the mouse.
Chemokines active in the DTH response to viral antigen were also identified. Specifically, we found that antibody neutralization of MIP-2 resulted in marked reduction in ear swelling and neutrophil influx, whereas treatment with neutralizing antibody to KC was without effect. Additionally, local administration of neutralizing antibody to MIP-1α reduced DTH and neutrophil accumulation. However, the reduction was more modest than that achieved with antibody to MIP-2. Since murine neutrophils express receptors for MIP-2 (CXCR2 [33]) and MIP-1α (CCR1 [59]) and are chemoattracted by these chemokines in vitro, it is likely that both are directly chemotactic for these cells in vivo. MIP-2 (4) and IL-8 (30), the functional analogue of MIP-2, have been previously linked to neutrophil recruitment in nonviral antigen models of immune response in skin, as have MIP-1α (11) and TCA-3 (12). KC has been linked to neutrophil recruitment in the elicitation of contact hypersensitivity (10). Why this chemokine is a chemoattractant in a response to a hapten but not viral antigen will require further study.
In addition to neutrophils and chemokines, the third important participant in DTH identified in this study was IL-1α. We found that antibody neutralization of endogenous IL-1α locally resulted in a significant reduction in ear swelling and diminished expression of MIP-1α, MIP-1β, MIP-2, and RANTES. Furthermore, in vitro exposure of purified mouse neutrophils to low-dose IL-1α resulted in the secretion of all four chemokines. Since neutrophil depletion also reduced chemokine levels, collectively, the results support the view that IL-1α interaction with neutrophils is a means of recruiting additional inflammatory cells to the DTH site. Previous investigators noted that IL-1 was required for initiation of primary immune responses in skin (14), that it was found in abundance in tuberculin-induced DTH in humans (8), and that IL-1 played a role in preventing antigen-specific suppression of systemic DTH (3). However, to our knowledge IL-1α involvement in effecting DTH has not been previously reported. It is important to point out that TNF-α and granulocyte-macrophage colony-stimulating factor, other cytokines with the potential to stimulate PMN to produce chemokines were not detected at the DTH site (S. Molesworth-Kenyon and R. N. Lausch, unpublished results). Also there was no evidence that MIP-1α or MIP-2 could act in an autocrine fashion to induce neutrophils to produce more chemokines. Thus, IL-1α, in addition to microbial components such as lipopolysaccharide (6, 26, 56) and certain other cytokines (17), can stimulated neutrophils to produce chemokines. This cytokine-neutrophil interaction may be an important means of regulating leukocyte migration into the DTH site.
Neutrophils are among the first inflammatory cells to arrive at the site of virus infection and thus have the potential to contain virus replication and spread. Indeed, there are reports of a high incidence of HSV infection in patients rendered neutropenic due to chemotherapy (2, 24). In the present study we found that HSV-1 titers were more than an order of magnitude greater in the ears of neutrophil-depleted mice, relative to the controls, 2 and 4 days after primary cutaneous infection. These results conform with those of earlier studies in which more severe HSV-1 infections were observed in corneas and footpads of naive mice depleted of mouse neutrophils (50, 54). Of particular interest, we found that when immunized mice were depleted of neutrophils, a striking increase (64-fold) in the virus titer relative to the controls were observed. This strongly implies that neutrophils play an important antiviral role in the immune as well as in the naive host.
Precisely how neutrophils exert their antiviral effect is unclear. The cells may act directly by releasing interferons or TNF-α, both of which possess antiviral activity (43, 57). Additionally, in the presence of specific antibody (19) or complement (23) neutrophils can kill virus-infected cells via cellular cytotoxicity reactions. The cells also have the capacity to release reactive oxygen metabolites (35, 42). Neutrophils may also act indirectly by producing chemokines and/or cytokines which recruit or activate other cell types to participate in nonspecific or specific antiviral defense mechanisms (6).
Collectively, our data suggest the following scenario. Sensitized CD4+ T cells recognize HSV-1 antigen and respond by releasing proinflammatory mediators, some of which are presumed to initiate neutrophil recruitment. Infiltrating neutrophils are induced by IL-1α (possibly made by keratinocytes) to produce and secrete a number of chemokines, including MIP-2 and MIP-1α. These chemokines in turn recruit additional neutrophils and probably other white blood cells. The accumulated neutrophils participate directly or indirectly in promoting DTH and limiting HSV-1 growth. In an analogous fashion, Doyle and Murphy (11) observed that the suppression of neutrophil recruitment was associated with decreased DTH and increased levels of Cryptococcus neoformans in the lungs and brains of infected mice. Although the relationship between DTH and specific resistance to pathogens requires further clarification (41), the importance of neutrophils in both the former and the latter is apparent.
Acknowledgments
This work was supported by NIH grant EY07564.
We thank Patricia Couling for careful preparation of the manuscript.
REFERENCES
- 1.Appelberg, R. 1992. Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell-mediated neutrophil recruitment. J. Leukoc. Biol. 52:303-306. [DOI] [PubMed] [Google Scholar]
- 2.Beattie, G., J. Whelan, J. Cassidy, L. Milne, S. Burns, and R. Leonard. 1989. Herpes simplex virus, Candida albicans, and mouth ulcers in neutropenic patients with non-haematological malignancy. Cancer Chemother. Pharmacol. 25:75-76. [DOI] [PubMed] [Google Scholar]
- 3.Benson, J. L., and J. Y. Niederkorn. 1990. Interleukin-1 abrogates anterior chamber-associated immune deviation. Investig. Ophthalmol. Vis. Sci. 31:2123-2128. [PubMed] [Google Scholar]
- 4.Biedermann, T., M. Kneilling, R. Mailhammer, K. Maier, C. A. Sander, G. Kollias, S. L. Kunkel, L. Hultner, and M. Rocken. 2000. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 192:1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley, P., R. Christensen, and G. Rothstein. 1982. Cellular and extracelluar myeloperoxidase in pyogenic inflammation. Blood 60:618-625. [PubMed] [Google Scholar]
- 6.Cassatella, M. A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16:21-26. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, H., T. M. Tumpey, H. F. Staats, N. van Rooijen, J. E. Oakes, and R. N. Lausch. 2000. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Investig. Ophthalmol. Vis. Sci. 41:1402-1409. [PubMed] [Google Scholar]
- 8.Chu, C. Q., M. Field, E. Andrew, D. Haskard, M. Feldmann, and R. N. Maini. 1992. Detection of cytokines at the site of tuberculin-induced delayed-type hypersensitivity in man. Clin. Exp. Immunol. 90:522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, D. N., M. A. Beck, T. M. Coffman, S. L. Kirby, J. F. Sheridan, I. B. Pragnell, and O. Smithies. 1995. Requirement of MIP-1α for an inflammatory response to viral infection. Science 269:1583-1585. [DOI] [PubMed] [Google Scholar]
- 10.Dilulio, N. A., T. Engeman, D. Armstrong, C. Tannenbaum, T. A. Hamilton, and R. L. Fairchild. 1999. Groα-mediated recruitment of neutrophils is required for elicitation of contact hypersensitivity. Eur. J. Immunol. 29:3485-3495. [DOI] [PubMed] [Google Scholar]
- 11.Doyle, H. A., and J. W. Murphy. 1997. MIP-1α contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans. J. Leukoc. Biol. 61:147-155. [DOI] [PubMed] [Google Scholar]
- 12.Doyle, H. A., and J. W. Murphy. 1999. Role of the C-C chemokine, TCA3, in the protective anticryptococcal cell-mediated immune response. J. Immunol. 162:4824-4833. [PubMed] [Google Scholar]
- 13.Durum, S. K., J. A. Schmidt, and J. J. Oppenheim. 1985. Interleukin 1: an immunological perspective. Annu. Rev. Immunol. 3:263-287. [DOI] [PubMed] [Google Scholar]
- 14.Enk, A. H., V. L. Angeloni, M. C. Udey, and S. I. Katz. 1993. An essential role for Langerhans cell-derived IL-1β in the initiation of primary immune response in skin. J. Immunol. 150:3698-3704. [PubMed] [Google Scholar]
- 15.Frevert, C. W., S. Huang, H. Danaee, J. D. Paulauskis, and L. Kobzik. 1995. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J. Immunol. 154:335-344. [PubMed] [Google Scholar]
- 16.Gao, J.-L., T. A. Wynn, Y. Chang, E. J. Lee, H. E. Broxmeyer, S. Cooper, H. L. Tiffany, H. Westphal, J. Kwon-Chung, and P. M. Murphy. 1997. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J. Exp. Med. 185:1959-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasperini, S., M. Marchi, F. Calzetti, C. Laudanna, L. Vicentini, H. Olsen, M. Murphy, F. Liao, J. Farber, and M. A. Cassatella. 1999. Gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T-cell alpha chemoattractant (I-TAC), and IFN-γ-inducible protein-10 (IP-10) chemokines by human neutrophils. J. Immunol. 162:4928-4937. [PubMed] [Google Scholar]
- 18.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, L. L. Laichalk, D. C. McGillicuddy, and T. J. Standiford. 1996. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumoniae. J. Infect. Dis. 173:159-165. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto, G., P. Wright, and D. Karzon. 1983. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J. Infect. Dis. 148:785-794. [DOI] [PubMed] [Google Scholar]
- 20.Hendricks, R. L., R. J. Epstein, and T. Tumpey. 1989. The effect of cellular immune tolerance to HSV-1 antigens on the immunopathology of HSV-1 keratitis. Investig. Ophthalmol. Vis. Sci. 30:105-115. [PubMed] [Google Scholar]
- 21.Hendricks, R. L., and T. Tumpey. 1990. Contribution of virus and immune factors to herpes simplex virus type 1-induced corneal pathology. Investig. Ophthalmol. Vis. Sci. 31:1929-1939. [PubMed] [Google Scholar]
- 22.Huffnagle, G. B., R. M. Strieter, L. K. McNeil, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1997. Macrophage inflammatory protein-1α (MIP-1α) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J. Immunol. 159:318-327. [PubMed] [Google Scholar]
- 23.Ihara, T., S. Starr, M. Ito, S. Douglas, and A. Arbeter. 1984. Human polymorphonuclear leukocyte-mediated cytotoxicity against varicellar-zoster virus-infected fibroblasts. J. Virol. 51:110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janmohamed, R., J. E. Morton, D. W. Milligan, M. J. Leyland, and B. Coupland. 1990. Herpes simplex in oral ulcers in neutropenic patients. Br. J. Cancer 61:469-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jawetz, E., V. Coleman, and M. F. Allende. 1951. Studies on herpes simplex virus. II A soluble antigen of herpesvirus possessing skin-reactive properties. J. Immunol. 67:197-205. [PubMed] [Google Scholar]
- 26.Kasama, T., R. M. Strieter, N. W. Lukacs, M. D. Burdick, and S. L. Kunkel. 1994. Regulation of neutrophil-derived chemokine expression by IL-10. J. Immunol. 152:3559-3569. [PubMed] [Google Scholar]
- 27.Kernacki, K. A., R. P. Barrett, J. A. Hobden, and L. D. Hazlett. 2000. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J. Immunol. 164:1037-1045. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi, K., K. Kaneda, and T. Kasama. 2001. Immunopathogenesis of delayed-type hypersensitivity. Microsc. Res. Tech. 53:241-245. [DOI] [PubMed] [Google Scholar]
- 29.Kudo, C., T. Yamashita, A. Araki, M. Terashita, T. Watanabe, M. Atsumi, M. Tamura, and F. Sendo. 1993. Modulation of in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3. I. Inhibition by RP-3 treatment of the priming and effector phases of delayed type hypersensitivity to sheep red blood cells in rats. J. Immunol. 150:3728-3738. [PubMed] [Google Scholar]
- 30.Larsen, C. G., M. K. Thomsen, B. Gesser, P. D. Thomsen, B. W. Deleuran, J. Nowak, V. Skodt, H. K. Thomsen, M. Deleuran, K. Thestrup-Pedersen, et al. 1995. The delayed-type hypersensitivity reaction is dependent on IL-8: inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J. Immunol. 155:2151-2157. [PubMed] [Google Scholar]
- 31.Lausch, R. N., C. Monterio, W. R. Kleinschrodt, and J. E. Oakes. 1987. Superiority of antibody versus delayed hypersensitivity in clearance of HSV-2 from eye. Investig. Ophthalmol. Vis. Sci. 28:565-570. [PubMed] [Google Scholar]
- 32.Lausch, R. N., J. S. Swyers, and H. E. Kaufman. 1966. Delayed hypersensitivity to herpes simplex virus in the guinea pig. J. Immunol. 96:981-987. [PubMed] [Google Scholar]
- 33.Lee, J., G. Cacalano, T. Camerato, K. Toy, M. Moore, and W. Wood. 1995. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 155:2158-2164. [PubMed] [Google Scholar]
- 34.Lloyd, A. R., and J. J. Oppenheim. 1992. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol. Today 13:169-172. [DOI] [PubMed] [Google Scholar]
- 35.Malech, H., and J. Gallin. 1987. Neutrophils in human disease. N. Engl. J. Med. 317:687-694. [DOI] [PubMed] [Google Scholar]
- 36.Metcalf, J. F., and R. W. Reichert. 1979. Histological and electron microscopic studies of experimental herpetic keratitis in the rabbit. Investig. Ophthalmol. Vis. Sci. 18:1123-1138. [PubMed] [Google Scholar]
- 37.Meyers-Elliott, R. H., and P. A. Chitjian. 1981. Immunopathogenesis of corneal inflammation in herpes simplex virus stromal keratitis: role of the polymorphonuclear leukocyte. Investig. Ophthalmol. Vis. Sci. 20:784-798. [PubMed] [Google Scholar]
- 38.Nash, A. A., and P. G. Gell. 1983. Membrane phenotype of murine effector and suppressor T cells involved in delayed hypersensitivity and protective immunity to herpes simplex virus. Cell. Immunol. 75:348-355. [DOI] [PubMed] [Google Scholar]
- 39.Newell, C. K., S. Martin, D. Sendele, C. M. Mercadal, and B. T. Rouse. 1989. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J. Virol. 63:769-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oquendo, P., J. Alberta, D. Z. Wen, J. L. Graycar, R. Derynck, and C. D. Stiles. 1989. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J. Biol. Chem. 264:4133-4137. [PubMed] [Google Scholar]
- 41.Orme, I. M., and A. M. Cooper. 1999. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol. Today 20:307-312. [DOI] [PubMed] [Google Scholar]
- 42.Roberts, R. L., B. J. Ank, and E. R. Stiehm. 1994. Antiviral properties of neonatal and adult human neutrophils. Pediatr. Res. 36:792-798. [DOI] [PubMed] [Google Scholar]
- 43.Rouse, B., L. Babiuk, and P. Henson. 1980. Neutrophils in antiviral immunity: inhibition of virus replication by a mediator produced by bovine neutrophils. J. Infect. Dis. 141:223-232. [DOI] [PubMed] [Google Scholar]
- 44.Schall, T. J., K. Bacon, R. D. Camp, J. W. Kaspari, and D. V. Goeddel. 1993. Human macrophage inflammatory protein α (MIP-1α) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J. Exp. Med. 177:1821-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schall, T. J., and K. B. Bacon. 1994. Chemokines, leukocyte trafficking, and inflammation. Curr. Opin. Immunol. 6:865-873. [DOI] [PubMed] [Google Scholar]
- 46.Schrier, R. D., L. I. Pizer, and J. W. Moorhead. 1982. Delayed hypersensitivity to herpes simplex virus: murine model. Infect. Immun. 35:566-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seebach, J., D. Bartholdi, K. Frei, K.-S. Spanaus, E. Ferrero, U. Widmer, S. Isenmann, R. M. Strieter, M. Schwab, H.-W. Pfister, and A. Fontana. 1995. Experimental Listeria meningoencephalitis: macrophage inflammatory protein-1α and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J. Immunol. 155:4367-4375. [PubMed] [Google Scholar]
- 48.Taub, D. D., K. Conlon, A. R. Lloyd, J. J. Oppenheim, and D. J. Kelvin. 1993. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science 260:355-358. [DOI] [PubMed] [Google Scholar]
- 49.Tessier, P., P. Naccache, I. Clark-Lewis, R. Gladue, K. Neote, and S. McColl. 1997. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-α. J. Immunol. 159:3595-3602. [PubMed] [Google Scholar]
- 50.Tumpey, T. M., S. H. Chen, J. E. Oakes, and R. N. Lausch. 1996. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 70:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tumpey, T. M., H. Cheng, D. N. Cook, O. Smithies, J. E. Oakes, and R. N. Lausch. 1998. Absence of macrophage inflammatory protein-1α prevents the development of blinding herpes stromal keratitis. J. Virol. 72:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tumpey, T. M., H. Cheng, X. T. Yan, J. E. Oakes, and R. N. Lausch. 1998. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J. Leukoc. Biol. 63:486-492. [DOI] [PubMed] [Google Scholar]
- 53.Wang, H.-M., M. M. Sheu, R. D. Stulting, and H. J. Kaplan. 1989. Immunohistochemical evaluation of murine HSV-1 keratouveitis. Curr. Eye Res. 8:37-46. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe, D., A. Adachi, Y. Tomita, M. Yamamoto, M. Kobayashi, and Y. Nishiyama. 1999. The role of polymorphonuclear leukocyte infiltration in herpes simplex virus infection of murine skin. Arch. Dermatol. Res. 291:28-36. [DOI] [PubMed] [Google Scholar]
- 55.Wolpe, S. D., B. Sherry, D. Juers, G. Davatelis, R. W. Yurt, and A. Cerami. 1989. Identification and characterization of macrophage inflammatory protein 2. Proc. Natl. Acad. Sci. USA 86:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xing, Z., M. Jordana, H. Kirpalani, K. E. Driscoll, T. J. Schall, and J. Gauldie. 1994. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-α, macrophage inflammatory protein-2, interleukin-1β, and interleukin-6 but not RANTES or transforming growth factor-β1 mRNA expression in acute lung inflammation. Am. J. Respir. Cell. Mol. Biol. 10:148-153. [DOI] [PubMed] [Google Scholar]
- 57.Xing, Z., H. Kirpalani, D. Torry, M. Jordana, and J. Gauldie. 1993. Polymorphonuclear leukocytes as a significant source of tumor necrosis factor-alpha in endotoxin-challenged lung tissue. Am. J. Pathol. 143:1009-1015. [PMC free article] [PubMed] [Google Scholar]
- 58.Yan, X. T., T. M. Tumpey, S. L. Kunkel, J. E. Oakes, and R. N. Lausch. 1998. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Investig. Ophthalmol. Vis. Sci. 39:1854-1862. [PubMed] [Google Scholar]
- 59.Zhang, S., B. S. Youn, J. L. Gao, P. M. Murphy, and B. S. Kwon. 1999. Differential effects of leukotactin-1 and macrophage inflammatory protein-1α on neutrophils mediated by CCR1. J. Immunol. 162:4938-4942. [PubMed] [Google Scholar]
- 60.Zlotnik, A., J. Morales, and J. A. Hedrick. 1999. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 19:1-47. [PubMed] [Google Scholar]