Abstract
The third transmembrane domain (TM3) of serotonin transporter (SERT) contains two isoleucine residues previously proposed to be involved in binding and transport of serotonin. When Ile-172 was replaced with cysteine, SERT became sensitive to inactivation by externally added [2-(trimethylammonium)ethyl]methanethio-sulfonate (MTSET). The disulfide product of this inactivation was not sensitive to reduction by externally added sulfhydryl compounds, but apparently reacted with intracellular reducing agents to spontaneously regenerate active SERT. The apparent accessibility of this residue to both external and cytoplasmic reagents is consistent with its localization near a serotonin binding site that is alternately exposed to both internal and external media. In another SERT mutant, I179C, transport also was inactivated by MTSET but substrate binding was resistant. External substrate bound to the inactivated I179C and enhanced its reactivation by free thiols. In norepinephrine transporter (NET), cysteine replacement of Ile-155 (corresponding to SERT Ile-179) also rendered the transporter sensitive to MTSET inactivation. In NET I155C, cocaine enhanced this inactivation, and the substrate, dopamine, apparently protected against inactivation. The characteristics of this protection suggest that dopamine was transported, converting NET to a form in which Ile-155 was occluded. The results support the proposal that TM3 of SERT and NET constitute part of the substrate permeation pathway, and that Ile-172 in SERT resides close to the substrate binding site. They also suggest that Ile-179 in SERT (and Ile-155 in NET) is in a conformationally sensitive part of TM3, which may act as part of an external gate.
Transporters for the biogenic amines serotonin (5-hydroxytryptamine, 5-HT), norepinephrine (NE), and dopamine (DA) belong to a large family of Na+- and Cl−-coupled transporters that are responsible for taking up many neurotransmitters after their release during neurotransmission (1–4). They are important in vivo targets for drugs of abuse like cocaine and amphetamines (5–7). Serotonin transporter (SERT), in particular, is the site of action for antidepressants such as imipramine, fluoxetine (Prozac), and sertraline (Zoloft) (8).
Transport of 5-HT by SERT is coupled to influx of Na+ and Cl− and efflux of K+ or H+ (1, 9). The transport cycle consists of two parts. In the first part, 5-HT is transported into the cell together with Na+ and Cl+, and in the second part, K+ or H+ is transported out (9). In each reaction, the solutes are thought to bind to a site formed, at least in part, from the transmembrane domains (TMs) of the protein. This binding event may trigger a conformational change that alters the accessibility of the binding site.
SERT, like other ion-coupled transporters, is thought to catalyze transport by an “alternating access” or “mobile barrier” model (10) in which the binding site is exposed only to one side of the membrane at any given time, although other models for transport also exist (11). When the site is exposed to the external medium, a barrier, or gate, prevents access from the inside, and a similar external gate prevents access from the outside when the binding site is exposed to the internal medium. The conformational change that moves substrate from outside to inside is proposed to consist, therefore, of a concerted closing of the external gate and opening of the internal gate. For efficient coupling, this conformational change should occur only when 5-HT, Na+, and Cl− all are bound.
Identifying residues that form the binding sites and gates of transport proteins has become a pressing challenge. Yan and Maloney (12, 13) identified several residues in the permeation pathway of Escherichia coli glucose-6-phosphate transporter, UhpT. They classified these residues as being accessible from the inside, the outside, or both sides of the cell membrane. The substrate binding site is likely to be formed by a subset of those residues accessible from both sides. Because such a binding site must communicate with both sides of the membrane, it logically should be formed, at least in part, from the TMs of the transporter. We recently used site-directed chemical modification to verify the predicted transmembrane topology of SERT (14). By mutating the extracellular loop (EL) domains, we found that none of these hydrophilic regions, with the possible exception of EL4 or EL5, contributed to substrate and inhibitor binding selectivity (15). Therefore, the most likely location for substrate and inhibitor binding in SERT is in the TMs.
Residues forming the gates also may be located in transmembrane regions. However, they may have quite different properties from binding site residues. Binding site residues should be directly occluded by substrate binding and therefore protected from chemical modification. However, substrate binding should not protect a gate residue. During substrate translocation, residues forming the gate may be brought into close contact with other residues forming the permeation pathway, and thereby sequestered from the medium. The accessibility of a gate residue may, therefore, be extremely sensitive to conformational changes, especially those leading to substrate transport.
We analyzed the third TM (TM3) of SERT by using the substituted-cysteine accessibility method (16). This analysis identified two isoleucine residues, Ile-172 and Ile-179, separated by two turns of a putative transmembrane α-helix, which were accessible to extracellular reagents (17). When either residue was replaced with cysteine, the resultant mutant catalyzed 5-HT transport normally, but the substitution rendered SERT sensitive to inactivation by the cysteine reagent [2-(trimethylammonium)ethyl]methanethiosulfonate (MTSET). Protection experiments demonstrated that mutant I172C was protected from inactivation by 5-HT and cocaine but inactivation of I179C was unaffected by these ligands. Furthermore, MTSET prevented binding of the high affinity cocaine analog 2β-carbomethoxy-3β-(4-[125I]-iodophenyl)tropane (β-CIT) to I172C but not to I179C. We suggested that Ile-172 was located close to the common binding site for 5-HT and cocaine, and that Ile-179 did not directly interact with this site. We also proposed that Ile-179 might form part of an external gate that was prevented from closing when a cysteine at that position was modified by MTSET. Recent evidence suggests that residues in TM1 also contribute to substrate binding and selectivity (18).
In the work reported here, we have extended these studies by investigating further the inactivation and reactivation of cysteine replacement mutants I172C and I179C [and the corresponding norepinephrine transporter (NET) mutant I155C]. We have examined the interaction of Na+, substrate, MTSET, and free thiols with these residues. The results support the proposal that TM3 constitutes part of the substrate translocation pathway in biogenic amine transporters, that Ile-172 is alternately exposed to internal and external media, and that the accessibility of Ile-179 is sensitive to conformational changes resulting from substrate binding and translocation.
Methods
Mutation and Expression.
SERT mutants I172C, I179C, and L564C used in this study were generated previously by site-directed mutagenesis (14, 17). In each of these mutants, Cys-109 was mutated to alanine to reduce background MTSET reactivity. NET mutant I155C was created by using the QuickChange kit (Stratagene). Each of these mutants was inserted downstream of the T7 promoter in pBluescript. The transporters were expressed transiently in intestine 407 cells (CCL-6, American Type Culture Collection) by using the vaccinia-T7 expression system (19) and prepared for transport and binding as described (20).
Treatment with MTSET and Free Thiols, Binding and Transport Assays.
Transfected cells in 24-well plates were washed with PBS (21) containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS/CM) and then incubated with MTSET as described (20). Transport of 20.5 nM [3H]5-HT (3,500 cpm/pmol, NEN Life Science Products) or 50 nM [3H]DA (2,821 cpm/pmol) in 250 μl PBS/CM was measured as described (17, 20). When Na+ was absent, it was replaced with N-methyl-d-glucamine (NMDG). β-CIT was used to measure the equilibrium binding affinity of 5-HT or DA, as described (20, 22). Membranes were incubated with MTSET, and [125I] β-CIT binding was measured for 90 min as described (17). Where indicated, membranes were treated with MTSET and DTT before measuring [125I] β-CIT binding.
Data Analysis.
Nonlinear regression fits of experimental and calculated data were performed with origin (Microcal Software, Northampton, MA). Each figure shows a representative experiment that was performed at least twice. The statistical analysis given in the text was from multiple experiments. Data with error bars represented the mean ± SD for duplicate or triplicate samples.
Results
Accessibility of SERT Cysteine Residues.
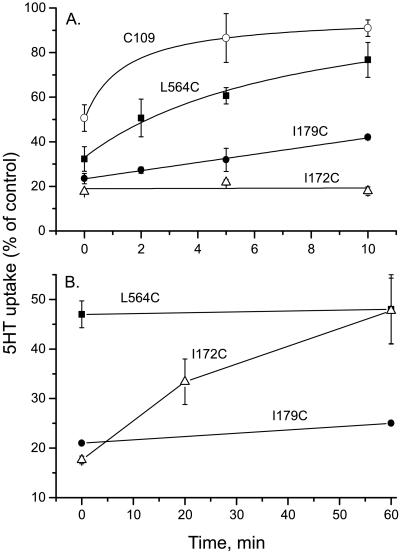
To evaluate the relative accessibility of various parts of SERT, we used the ability of external reagents to reactivate SERT and SERT mutants after modification by MTSET at identified cysteine residues. We showed previously that SERT contains a reactive cysteine residue, Cys-109, in the first EL (EL1) (20). In addition, the third TM of SERT (TM3) contains residues, including Ile-172 and Ile-179, where cysteine substitution rendered the transporter sensitive to inactivation by MTSET (17). Yet another cysteine replacement, at Leu-564 in EL6, also caused the transporter to become sensitive to MTSET inactivation (14). Because the product formed when cysteine reacts with MTSET is a disulfide between the cysteine sulfhydryl and 2-trimethylammonium ethanethiol, we tested the ability of free cysteine to reduce this disulfide to the active thiol form. The results, shown in Fig. 1A, demonstrate that the MTSET-modified EL cysteine residues, Cys-109 and L564C, were more rapidly reduced by 12 mM free cysteine than were the two cysteine substitutions in TM3. Of the two TM3 cysteines, I179C was slowly reactivated by free cysteine, but I172C was not affected by 10-min treatment with 12 mM free cysteine (Fig. 1A) or 12 mM DTT (data not shown). These results are consistent with the topological model for SERT (14) in which EL1 and EL6 are exposed to the external medium, and the N-terminal end of TM3 is closer to the cytoplasmic face than the C-terminal end.
Figure 1.
Reactivation of wild-type SERT and cysteine replacement mutants. (A) Cysteine-dependent reactivation. Confluent INT-407 cells in 24-well plates were infected with T7 vaccinia virus and transfected with cDNA encoding wild-type SERT and mutants I172C, I179C, and L564C. After incubation for 16–20 h at 37°C cells were treated with MTSET for 10 min, rinsed with PBS, treated with 12 mM cysteine for the indicated time, and assayed for transport activity. (B) Spontaneous reactivation. Cells expressing I172C, I179C, and L564C were inactivated with MTSET as described above and incubated in the absence of cysteine for the indicated time before assaying transport.
After inactivation of I172C by MTSET, external thiols had no effect on activity (Fig. 1A), but further incubation led to spontaneous recovery of activity even in the absence of added thiol. Fig. 1B shows the time course of this recovery and, by comparison, the lack of recovery of MTSET-inactivated I179C and L564C mutants. In other experiments (not shown) the activity recovered spontaneously was completely sensitive to a second treatment with MTSET. The recovery of activity was slow, requiring over 60 min, but no significant recovery was observed with the other mutants tested in the absence of added thiol. Each of these mutants has similar activity, expression, and cell surface localization (14, 17), so it is unlikely that newly synthesized transporter is responsible for the recovery. Because I172 is predicted to be close to the cytoplasmic end of TM3, we considered the possibility that reduction by cytoplasmic thiols was responsible for reactivation of I172C. We therefore tested the ability of reducing agents to reactivate I172C in membrane preparations, where the cytoplasmic face of the membrane is expected to be more accessible.
Using β-CIT binding as an assay for activity, we treated membranes prepared from cells expressing SERT I172C with 0.1 mM MTSET. Binding of β-CIT then was measured with or without a 10-min pretreatment with 12 mM DTT. The results show, in Table 1, that DTT markedly increased binding activity relative to the MTSET-treated control, whereas the same treatment of MTSET-inactivated cells did not reactivate transport activity. Control membranes from C109A expressing cells were inactivated much less by MTSET and only slightly reactivated by DTT (Table 1), in contrast to the inactivation of binding to dopamine transporter in membrane preparations (at 1–30 mM MTSET) (23). In the absence of added thiols, MTSET-treated membranes did not spontaneously reactivate during a 90-min incubation (data not shown). To minimize MTSET modification of internal cysteines, cells expressing I172C or C109A were treated with 0.1 mM MTSET. Membranes prepared from cells expressing I172C were reactivated by 20 ± 5% by using DTT, whereas C109A, as previously shown (20), was resistant to MTSET treatment in intact cells. These results support the conclusion that Cys-172 is susceptible to reduction from the cytoplasmic side of the plasma membrane.
Table 1.
Inactivation and recovery of I172C in cells and membranes
| Activity, percent of untreated control
|
|||
|---|---|---|---|
| Transport in cells
|
Binding in membranes
|
||
| I172C | I172C | C109A | |
| MTSET 0.1 mM, 10 min | 26 ± 1 | 29 ± 3 | 82 ± 2 |
| DTT 12 mM, 10 min | 28 ± 1 | 43 ± 1 | 86 ± 2 |
Cells expressing SERT mutant I172C (second column) or membranes prepared from those cells (third column) or cells expressing SERT C109A (fourth column) were treated with MTSET for 10 min. Samples of the treated cells or membranes were further incubated with DTT for 10 min before measuring 5-HT uptake or β-CIT binding. Results are expressed as the percentage of control transport in cells or binding in membranes that had not been treated with any of these reagents. All incubations were in PBS containing 0.1 mM CaCl2 and 1 mM MgCl2.
Protection of I172C.
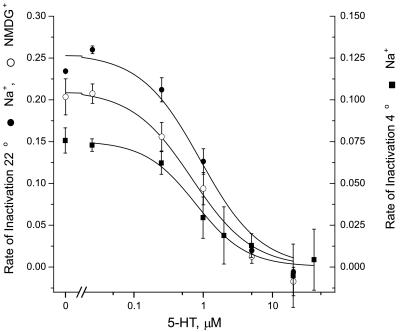
Protection of I172C by 5-HT and cocaine (17) suggested that this residue is close to the 5-HT binding site. To evaluate the alternative explanation that 5-HT binding induced a conformational change that occluded I172, we measured the Na+ and temperature dependence of the protection process. Binding of 5-HT to SERT is not Na+ dependent (22), but Na+ is required for transport, and Na+-dependent changes have been observed by electrophysiological methods for other Na+-coupled transporters (24–27). The results shown in Fig. 2 indicate that Na+ does not influence the ability of 5-HT to protect I172C from MTSET inactivation. Na+ also did not significantly change the rate at which MTSET inactivated I172C (Fig. 2). Therefore, Na+-dependent conformational changes do not seem to play a role in the accessibility of Ile-172. At low temperature (4°C) the rate constant for inactivation decreased to 10% of the value at 22°C (Fig. 2), consistent with a Q10 of approximately 3. However, the ability of 5-HT to protect against MTSET inactivation was unchanged. Fig. 2 shows that neither the extent of protection nor the potency of 5-HT was affected by the decrease in temperature. These results are consistent with the proposal that 5-HT directly protects position 172 and does not induce this effect through Na+- and temperature-dependent conformational changes.
Figure 2.
Na+and temperature dependence of 5-HT protection of I172C. Cells expressing SERT I172C were incubated in PBS (●) or Na+-free (NMDG) PBS solution (○) containing 0.1 mM MTSET for 10 min at 22°C (left axis), or 0.3 mM MTSET at 2–4°C (■, right axis) in the presence of the indicated concentrations of 5-HT. Cells were rinsed with PBS and assayed for transport in PBS at 22°C. The rate of inactivation was expressed as a pseudo first-order rate constant, assuming a simple exponential decay of the transport activity.
Evidence for Conformational Changes in Inactivation and Reactivation of I179C.
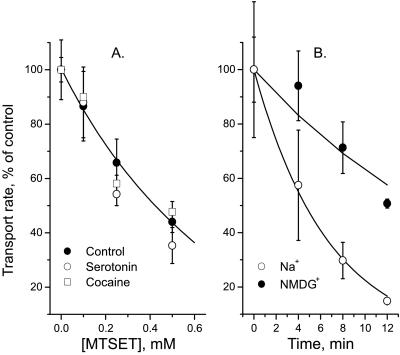
Previous results showed that for I179C, neither 5-HT nor cocaine protected against inactivation by 1 mM MTSET (17). Fig. 3A shows that, even at lower MTSET concentrations (0.1–0.5 mM), 5-HT and cocaine also failed to affect the MTSET inactivation. Na+, however, enhanced the inactivation of I179C (Fig. 3B), in contrast with its lack of effect on I172C. The maximal effect of Na+ was a 3.3-fold increase in rate at 160 mM Na+ with the half-maximal increase at approximately 12 mM (data not shown).
Figure 3.
Effect of ligands and Na+on inactivation of I179C. (A) Cells expressing SERT I179C were incubated for 10 min with the indicated concentration of MTSET in PBS with no addition (●), 16 μM 5-HT (○), or 16 μM cocaine (□). Cells were washed free of MTSET and ligand and assayed for 5-HT transport in PBS. (B) Cells expressing SERT I179C were incubated for the indicated time with 1 mM MTSET in PBS (○) or Na+-free (NMDG) PBS (●). After rinsing with PBS, cells were assayed for transport in PBS.
Although MTSET inactivated transport by I179C, the transporter retained its ability to bind β-CIT (17). Moreover, the equilibrium binding affinity for 5-HT, as measured by displacement of β-CIT, was unchanged by MTSET inactivation of this mutant. The inactivated transporter had a binding affinity of 1.7 ± 0.23 μM, similar to the value of 2.1 ± 0.25 μM for the unmodified SERT I179C (data not shown). This finding suggests that the 5-HT binding site in the inactivated form of I179C is similar to that in the native form of the transporter.
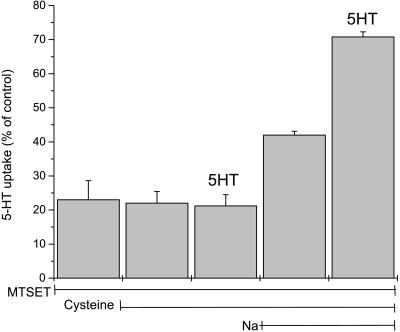
SERT I179C inactivated by MTSET was blocked in a step subsequent to substrate binding. This phenomenon provided a way to study the effect of substrate binding in the absence of translocation. In the experiment shown in Fig. 4, MTSET-treated I179C was treated subsequently with 10 mM free cysteine under the conditions shown. When NMDG+ replaced Na+, no reactivation occurred, and 5-HT addition also had no effect. In the presence of Na+, however, cysteine reactivated a significant portion of the activity, and this reactivation was enhanced in the presence of 5-HT. Therefore, like its stimulation of inactivation, Na+ enhanced I179C reactivation by cysteine. Although Na+ had little effect on equilibrium 5-HT binding (22) it was required for 5-HT to stimulate reactivation (Fig. 4).
Figure 4.
Reactivation of I179C. Cells expressing I179C were incubated with 1 mM MTSET for 10 min. After washing with PBS, some of the cells were further incubated with PBS or Na+-free (NMDG) PBS containing 12 mM cysteine for 10 min, in the presence or absence of 16 μM 5-HT. After rinsing with PBS, cells were assayed for transport in PBS.
NET Ile-155 Reactivity.
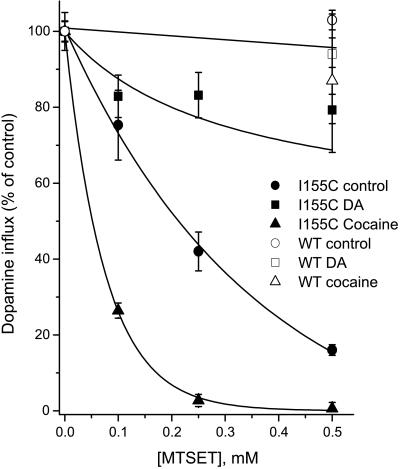
NET is a member of the same NaCl-coupled transporter family as SERT and shares with SERT the sensitivity to cocaine and tricyclic antidepressants. Ile-155 in NET corresponds to Ile-179 in SERT. Although wild-type NET is resistant to MTSET inactivation, mutant I155C is readily inactivated (Fig. 5). We attempted to measure reactivation of NET I155C by cysteine, but the reaction was so slow that we could not reliably determine its rate or extent (data not shown). In contrast to MTSET inactivation of SERT I179C, cocaine and DA (a NET transport substrate) dramatically affect inactivation of NET I155C (but not wild-type NET) by MTSET. As shown in Fig. 5, inactivation is enhanced by cocaine and dramatically slowed by DA. One possible explanation for this behavior is that cocaine and DA both bind to the outward-facing form of NET, but that DA, as it is transported, converts NET to the inward-facing form, in which Ile-155 is occluded.
Figure 5.
Effect of ligands on inactivation of NET I155C. Cells expressing wild-type (WT) NET (●) or NET I155C were incubated for 10 min with the indicated concentration of MTSET in PBS with no addition (○), 12.5 μM DA (□), or 5 μM cocaine (■). Cells were washed free of MTSET and ligand and assayed for DA transport in PBS.
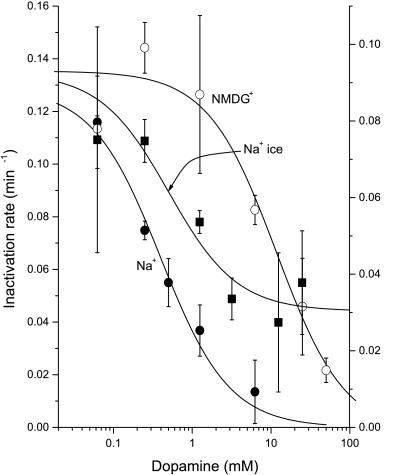
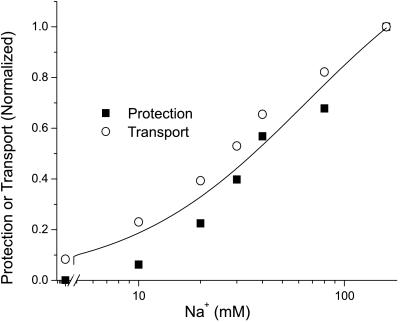
To test the possibility that DA translocation was responsible for the protection of I155C, we examined the effect of Na+ and temperature, both of which profoundly affect transport. Na+ markedly enhanced the potency of DA in blocking inactivation (Fig. 6). In the presence of Na+, the DA concentration required for half maximal protection was approximately 0.39 μM, which is close to the KM for transport (28). When Na+ was replaced with NMDG, 11.1 μM DA was required for half maximal protection (Fig. 6). The Na+ dependence for DA protection and DA transport were identical (Fig. 7). In contrast to the effect of Na+ on DA protection from MTSET, Na+ had little effect on DA binding. As assessed by equilibrium displacement of [125I]β-CIT binding, the KD for DA in NET I155C was 3.7 ± 0.4 μM in Na+ and 2.0 ± 1.1 in NMDG (data not shown). The ability of DA to protect I155C is also temperature dependent. Fig. 6 shows that DA can completely protect against inactivation at 22°C, but at 4°C, higher concentrations of DA are required for protection, and saturating concentrations do not provide complete protection.
Figure 6.
Na+and temperature dependence of DA protection of NET I155C. Cells expressing NET I155C were incubated in PBS (●) or Na+-free (NMDG) PBS solution (○) containing 0.25 mM MTSET for 10 min at 22°C or 1.5 mM MTSET at 4°C (■, left axis) in the presence of the indicated concentrations of DA. Cells were rinsed with PBS and assayed for DA transport in PBS at 22°C. The rate of inactivation was expressed as a pseudo first-order rate constant, assuming a simple exponential decay of the transport activity. In the absence of DA, the inactivation rate was 520 min−1⋅M−1 at 22°C and 61.7 min−1⋅M−1 at 4°C, for a Q10 of 3.27. The DA concentration required for half protection increased from 0.39 μM in the presence of Na+ to 11.1 μM when Na+ was absent.
Figure 7.
Sodium dependence of DA transport and DA protection from MTSET. For protection (■), cells were treated with 0.25 mM MTSET for 10 min in the presence of 1 μM DA and the indicated concentration of Na+. The extent of protection is expressed as (AM−DA−AM)/(AC−AM), where AC is the control activity, AM is the activity after MTSET treatment, and AM-DA is the activity after MTSET treatment in the presence of DA. For transport (○), uptake of 1 μM DA was measured as described in Methods.
Discussion
The results presented here illustrate the striking difference in behavior for two residues found in TM3 of biogenic amine transporters. In SERT, two isoleucine residues, Ile-172 and Ile-179, are predicted to lie on the same face of this helical TM, separated by two helical turns. Despite their similar structure, location and orientation, these two positions were affected very differently by Na+ and substrate binding. Ile-172 apparently is located in the permeation pathway close to the substrate binding site where it is accessible to both external and intracellular reagents. In contrast, Ile-179 appears to be located in a conformationally sensitive part of TM3, closer to the external medium, where its accessibility is enhanced by ligand binding and blocked by the substrate translocation step.
Previous work had shown that replacement of Ile-172 with cysteine rendered SERT sensitive to inactivation by MTSET, and that the inactivation could be completely blocked by 5-HT or cocaine binding (17). We proposed that this protection represented direct occlusion of I172C by bound ligand. However, an alternate interpretation was that ligand binding led to a conformational change that blocked the reactivity of I172C toward MTSET. Two of the observations reported here argue against the latter interpretation and in favor of Ile-172 being in close proximity to the substrate binding pocket.
Residue 172 is accessible to external reagents such as MTSET, which inactivate transport when a cysteine is in this position (17). It is also apparently accessible to internal reducing agents, which reactivate the MTSET-modified transporter in the absence of external thiols (Fig. 1B). We expect the substrate binding site to be accessible from both faces of the membrane because 5-HT binds to that site from the external medium and dissociates from it to the cytoplasm, although these binding and dissociation steps are likely to be separated temporally by a conformational change. Therefore, residues associated with the substrate site are expected to react with both external and internal reagents. Although we cannot exclude the possibility that other residues have this property, the finding that Cys-172 reacts both with external MTSET and an internal reductant supports the proposal that position 172 is in the 5-HT permeation pathway, close to the binding site.
Additional support for this conclusion comes from the observation that 5-HT protected against MTSET inactivation of I172C and that this protection was independent of Na+ and temperature (Fig. 2), in contrast to results with NET I155C (see below). Na+ independence is expected if the protection is a result of 5-HT binding, an event that does not require Na+ (22). The lack of temperature dependence further suggests that the reaction was a direct occlusion of Cys-172 by 5-HT because a secondary effect resulting from a 5-HT induced conformational change would likely be associated with a large temperature dependence (29). In glutamate transporter EAAT1, Seal and Amara (30) reported that substrate protected against inactivation of A395C at both 22°C and 2–4°C and also concluded that the protection did not require a conformational change.
In NET, Ile-155 corresponds to SERT Ile-179. Replacing this residue with cysteine renders NET sensitive to MTSET inactivation, which is protected by the substrate DA (Fig. 5). In contrast to SERT I172C, however, substrate protection of I155C depended on both Na+ and temperature (Fig. 6). Although binding of DA to I155C was similar in the presence or absence of Na+, DA protection was enhanced by Na+ with the same Na+ dependence as the transport process (Fig. 6). This finding suggests that it is the conformational change that occurs with substrate translocation, rather than just substrate binding, that is responsible for DA protection of I155C. The temperature dependence of protection is also consistent with involvement of a conformational change in the protection.
Remarkably, cocaine and DA had opposite effects on the inactivation process. The situation is similar to inactivation of glutamate transporter GLT-1 in which Tyr-403 was replaced with cysteine (31). In both cases, substrate protected against inactivation but a nontransported inhibitor potentiated. We interpret the NET I155C data, as did Zarbiv et al. (31), to mean that cocaine, the inhibitor, trapped the transporter in an external conformation that exposed the reactive cysteine residue. Independent evidence from the kinetic analysis of cocaine inhibition supports the proposal that cocaine binds to the outward facing conformation of NET (32). By comparison, DA binding was followed by translocation, a process that converted the transporter to an inward-facing form in which residue 155 was sequestered from the external medium (Fig. 5). It is possible that NET Ile-155 (SERT Ile-179) is part of the external gate that closes when substrate is transported inward.
In contrast to I172C, SERT I179C inactivated by MTSET was blocked in a step subsequent to substrate binding. External Na+ bound to the inactivated transporter and increased the accessibility Cys-179 (Fig. 4), just as in the unmodified transporter (Fig. 3). Moreover, external 5-HT also can bind to the inactivated I179C, suggesting that the transporter was locked in an outward facing form. Furthermore, binding of Na+ and 5-HT together produce a cooperative conformational change. In the unmodified transporter, we expect that a similar conformational change (Fig. 4), triggered by formation of a complex containing Na+, Cl−, and substrate, leads to translocation. In the inactivated SERT I179C this conformational change is manifested by its effect on reactivation, but it does not lead to translocation, possibly because modification of Cys-179 prevents the external gate from closing. Ile-179 may become buried in a hydrophobic environment during the conversion of SERT from the outward facing form to the inward facing form. Attaching the bulky and charged 2-trimethylammonium ethanethiol group to a cysteine at this position would block the conformational change required for the transport process and trap the transporter in an outward facing form.
It is noteworthy that 5-HT enhances reactivation of I179C but has no effect on inactivation. An important difference between these two reactions is that in MTSET-inactivated I179C, 5-HT could bind, but was not translocated (17). This event would stabilize the outward facing conformation in which Na+ and 5-HT were bound. In contrast, this conformation in the unmodified form of I179C may exist for only a brief time before translocation occurs. This may be the reason that we could not detect the effect of 5-HT and Na+ binding on the MTSET reactivity of unmodified I179C.
We observed a striking difference between SERT I179C and NET I155C in the response to cocaine and substrate (Figs. 3A and 5). These agents had opposite effects on MTSET inactivation of NET I155C but had no effect on the corresponding SERT mutant I179C, even if DA was used instead of 5-HT (data not shown). The difference may be because of the equilibrium between inward and outward facing forms of the two transporters. If NET favors the inward facing form, only a minor fraction of the transporter will be available for reaction with MTSET unless an inhibitor such as cocaine traps the protein in the outward form. The substrate, DA, apparently protects by being translocated and converting the transporter to its inward form, from which it only slowly or incompletely returns. In SERT, the predominant form may be outward facing, and cocaine therefore would have little effect. The lack of a protective effect from 5-HT transport may simply be a result of more rapid return of SERT than NET to the outward form. The turnover number for SERT is about 110 min−1, far greater than for NET (6.5 min−1) (28).
Overall, the results support the idea that TM3 of biogenic amine transporters is associated with the substrate permeation pathway and intimately involved with the conformational changes that accompany transport. SERT Ile-172 appears to be located near the substrate binding site where it may be alternately exposed to the external medium and the cytoplasm as the transporter progresses through the transport cycle. Ile-179 of SERT, in contrast, is in a part of TM3 that is sensitive to conformational changes induced by the binding of Na+ and 5-HT, and may be maximally accessible in the form of the transporter that is poised to translocate substrate. We speculate that this translocation event normally closes the gate and sequesters Ile-179 from the medium, but modification of a cysteine in this position prevents the gate from closing and thereby blocks translocation.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse.
Abbreviations
- β-CIT
2β-carbomethoxy-3β-(4-[125I]-iodophenyl)tropane
- MTSET
[2-(trimethylammonium)ethyl]methanethiosulfonate
- SERT
serotonin transporter
- NET
norepinephrine transporter
- 5-HT
serotonin (5-hydroxytryptamine)
- DA
dopamine
- NE
norepinephrine
- NMDG
N-methyl-d-glucamine
- EL
external loop, TM, transmembrane domain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rudnick G, Clark J. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- 2.Blakely R D, De Felice L J, Hartzell H C. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- 3.Borowsky B, Hoffman B J. Int Rev Neurobiol. 1995;38:139–199. doi: 10.1016/s0074-7742(08)60526-7. [DOI] [PubMed] [Google Scholar]
- 4.Nelson N. J Neurochem. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- 5.Amara S. Nature (London) 1992;360:420–421. doi: 10.1038/360420d0. [DOI] [PubMed] [Google Scholar]
- 6.Amara S, Kuhar M. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 7.Uhl G, Johnson P. J Exp Biol. 1994;196:229–236. doi: 10.1242/jeb.196.1.229. [DOI] [PubMed] [Google Scholar]
- 8.Schloss P, Williams D C. J Psychopharmacol. 1998;12:115–121. doi: 10.1177/026988119801200201. [DOI] [PubMed] [Google Scholar]
- 9.Nelson P J, Rudnick G. J Biol Chem. 1979;254:10084–10089. [PubMed] [Google Scholar]
- 10.Mitchell P. Res Microbiol. 1990;141:286–289. doi: 10.1016/0923-2508(90)90002-8. [DOI] [PubMed] [Google Scholar]
- 11.Su A, Mager S, Mayo S L, Lester H A. Biophys J. 1996;70:762–777. doi: 10.1016/S0006-3495(96)79616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan R, Maloney P. Cell. 1993;75:37–44. [PubMed] [Google Scholar]
- 13.Yan R T, Maloney P C. Proc Natl Acad Sci USA. 1995;92:5973–5976. doi: 10.1073/pnas.92.13.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J G, Liu-Chen S, Rudnick G. J Biol Chem. 1998;273:12675–12681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- 15.Smicun Y, Campbell S D, Chen M A, Gu H H, Rudnick G. J Biol Chem. 1999;274:36058–36064. doi: 10.1074/jbc.274.51.36058. [DOI] [PubMed] [Google Scholar]
- 16.Karlin A, Akabas M H. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen J G, Sachpatzidis A, Rudnick G. J Biol Chem. 1997;272:28321–28327. doi: 10.1074/jbc.272.45.28321. [DOI] [PubMed] [Google Scholar]
- 18.Barker E L, Moore K R, Rakhshan F, Blakely R D. J Neurosci. 1999;19:4705–4717. doi: 10.1523/JNEUROSCI.19-12-04705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blakely R D, Clark J A, Rudnick G, Amara S G. Anal Biochem. 1991;194:302–308. doi: 10.1016/0003-2697(91)90233-j. [DOI] [PubMed] [Google Scholar]
- 20.Chen J G, Liu-Chen S, Rudnick G. Biochemistry. 1997;36:1479–1486. doi: 10.1021/bi962256g. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 22.Humphreys C J, Wall S C, Rudnick G. Biochemistry. 1994;33:9118–9125. doi: 10.1021/bi00197a014. [DOI] [PubMed] [Google Scholar]
- 23.Ferrer J V, Javitch J A. Proc Natl Acad Sci USA. 1998;95:9238–9243. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parent L, Supplisson S, Loo D, Wright E. J Membr Biol. 1992;125:49–62. doi: 10.1007/BF00235797. [DOI] [PubMed] [Google Scholar]
- 25.Mager S, Naeve J, Quick M, Labarca C, Davidson N, Lester H A. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- 26.Mager S, Min C, Henry D J, Chavkin C, Hoffman B J, Davidson N, Lester H A. Neuron. 1994;12:845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 27.Sonders M, Zhu S, Zahniser N, Kavanaugh M, Amara S. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu H H, Wall S C, Rudnick G. J Biol Chem. 1994;269:7124–7130. [PubMed] [Google Scholar]
- 29.Tanford C. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- 30.Seal R P, Amara S G. Neuron. 1998;21:1487–1498. doi: 10.1016/s0896-6273(00)80666-2. [DOI] [PubMed] [Google Scholar]
- 31.Zarbiv R, Grunewald M, Kavanaugh M P, Kanner B I. J Biol Chem. 1998;273:14231–14237. doi: 10.1074/jbc.273.23.14231. [DOI] [PubMed] [Google Scholar]
- 32.Chen N, Justice J B J. J Neurosci. 1998;18:10257–10268. doi: 10.1523/JNEUROSCI.18-24-10257.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]