Abstract
Adenoviruses (Ad) have a variety of immunoregulatory genes, many of which are clustered in a 3.5-kb segment of DNA known as early region 3 (E3). Ad E3 codes for proteins that downregulate surface expression of class I major histocompatibility antigens and also inhibit tumor necrosis factor alpha (TNF-α)- and Fas-induced cytolysis. We were interested in determining whether chemokine production or activity might also be inhibited by Ad E3 and we have studied this function in a human astrocytoma cell line, U373. Astrocytes constitute a part of the blood-brain barrier, and chemokines (IP-10, IL-8, MCP-1-4, and MIPs) expressed by them may contribute to leukocyte infiltration within the brain during inflammation. When U373 cells are activated by the proinflammatory molecule TNF-α, the increase in chemokine MCP-1, IL-8, and IP-10 transcripts is blocked by a recombinant Ad expressing the E3 genes under cytomegalovirus promoter control. Comparable Ads expressing green fluorescent protein in place of E3 have no effect on these chemokines. Ads also have been extensively studied as gene therapy vectors and most have a deletion of the E3 region to permit the insertion of larger fragments of foreign DNA. Our results suggest that construction of Ad vectors to include E3 expression cassettes will improve the efficacy and safety of such viral-based gene therapy protocols.
Adenovirus (Ad)-based gene therapy vectors have been useful for delivering therapeutic genes to a variety of nondividing cell types. However, studies with first-generation Ad gene therapy vectors have demonstrated that strong specific and innate immune responses limit the duration of transgene expression and cause significant dose-dependent tissue toxicity (8). Ad early region 3 (E3), a region not required for in vitro replication and commonly deleted from most gene therapy vectors to make room for the transgene, expresses a number of proteins which facilitate viral survival by antagonizing the host's efforts to eliminate infected cells (32, 46). Studies in our laboratory and others have suggested that Ad E3 genes can abrogate inflammatory cell infiltration in both nonviral models of immune-mediated disease and anti-Ad responses to gene therapy vectors (11, 12, 22, 44).
Of the six proteins in the Ad E3 region whose functions have been characterized, five are thought to be involved in immunoregulation (46). The E3 gp-19K protein (E3-19K) both retains the major histocompatibility complex (MHC) I in the endoplasmic reticulum and inhibits tapasin processing of peptides that bind to MHC I. These processes decrease the display of viral peptides on the cell surface and inhibit cytotoxic T lymphocyte killing of infected cells (3, 7). The complex of E3-10.4K and E3-14.5K, otherwise known as RID-α/β (receptor internalization and degradation alpha and beta), stimulates the internalization of proapoptotic receptors such as Fas and TRAIL R1 from the cell surface. RID-α/β also stimulates the internalization of the epidermal growth factor, insulin growth factor, and insulin-like growth factor receptors (14, 37, 42). E3-14.7K inhibits tumor necrosis factor alpha (TNF-α)-induced apoptosis without affecting TNF-α receptor number or distribution via mechanisms that are incompletely understood but may involve several downstream proteins in the NF-κB pathway. E3-14.7K also inhibits TNF-α-induced production of arachidonic acid (19, 20, 26, 29).
Chemokines have been studied extensively as important regulators of leukocyte trafficking to sites of immune challenge or tissue damage. This growing family of small, secreted proteins is grouped into two major (CC and CXC) and two minor (C and CX3C) structural families based on the location of several conserved cysteine residues (33). Viral disruption of the chemokine network has been described in the pathogenesis of several members of the poxvirus and herpesvirus families through the expression of either membrane-bound or secreted chemokine-binding proteins with the ability to scavenge chemokines from extracellular fluid (5, 27). Alternatively, transcriptional downregulation of monocyte chemoattractant protein-1 (MCP-1) expression by cytomegalovirus has been reported (21). The Ad genome does not contain a putative chemokine receptor or soluble chemokine-binding protein, but Ad's potential to modulate chemokine expression intracellularly has been reported in the early studies of MCP-1, then called JE (40, 41, 43). These previous studies described the downregulation of MCP-1 by the E1A region of Ad12 and Ad5.
Several studies have reported chemokine induction by first generation Ad vectors as a putative mechanism for early nonspecific inflammation responsible for Ad vector-induced toxicity (2, 34, 45). Treatment of mice with Ad from which E1 and E3 were deleted has been shown to induce the transcription of interferon-inducible protein-10 (IP-10), MCP-1, and other chemokine RNAs in the liver 1 to 6 h after infection, leading to a dose-dependent neutrophil-mediated toxicity (34). Similar vectors have been shown to induce interleukin-8 (IL-8) transcription and secretion into lung cell line supernatants. In the lung, Ad induces IL-8 in a serotype-specific manner (28). Wild-type Ad7 (group B) induces IL-8 in A549 cells and human fetal lung fibroblasts, while wild-type Ad5 (group C) does not. IL-8 was shown to be elevated in vivo in the bronchial alveolar lavage fluid of macaque monkeys 3 to 28 days after infection with a high dose (1010 PFU) of an Ad vector used to deliver the cystic fibrosis gene (45). Similarly, administration of an Ad vector into the cerebrospinal fluid of rhesus monkeys led to an elevated level of IL-8 and IL-6 (10). These studies and others like them show the importance of understanding the relationship between adenovirus infection and chemokine induction in the pathogenesis of both wild-type and gene therapy vector-virus infections. Both the cells and organs targeted as well as the dose and serotype of the adenovirus appear critical in the virus induction of chemokines.
Recent work in our laboratory has shown that incorporation of the Ad2 E3 transgenes into pancreatic islet β-cells not only prevents hyperglycemia in the lymphocytic choriomeningitis virus model of virus-induced autoimmune diabetes and prolongs the survival of pancreatic islet allografts, but also decreases the amount of inflammatory infiltrate present around the islets of these animals when compared to controls (11, 44). Other work using Ad with E3 deletions in a pneumonia pathogenesis model has suggested a role for E3 in affecting the composition and extent of lung inflammatory cell infiltrates (16, 17, 38).
Based on the importance of chemokines in orchestrating the movement of various leukocyte subsets and the significantly decreased inflammatory responses seen in our prior in vivo studies, we hypothesized that Ad E3 genes altered chemokine production within the infected cell as an additional mechanism to evade immune-mediated viral elimination. We utilized the astrocytoma cell line U373 for these studies because (i) U373 does not produce chemokines in response to the adenovirus E1/E3 deleted viral vectors alone at input multiplicities sufficient to infect all the cells; (ii) astrocytes play a central role during inflammation in the brain in response to various stimuli, including bacterial and viral infections; and (iii) astrocytes are highly responsive to TNF-α, which stimulates the production of large amounts of MCP-1 and IL-8 chemokines. The promoters of these two chemokines have consensus sites for NF-κB and AP-1, and it has been shown that these sequences are critical for their induction by TNF-α.
Our results demonstrate that Ad E3 gene expression downregulates the amount of chemokine mRNA and protein after induction of MCP-1, IL-8, and IP-10 by TNF-α in astrocytes and other cell lines. Similarities with results obtained using small molecule inhibitors that specifically block TNF-α signaling pathways at different levels of the NF-κB or related pathways suggest that there are multiple targets for Ad inhibition of chemokines. Finally, we propose that the ability of E3 to subvert the host cell's secretion of chemokines in response to Ad infection is an important and previously undescribed mechanism for Ad immune evasion during infection. This observation suggests that the inclusion of E3 into Ad vectors used for gene therapy would decrease the innate inflammatory response to these agents.
(The data in this paper will be submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (F.D.-L.) in the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University.)
MATERIALS AND METHODS
Cells and viruses.
U373 cells, a human astrocytoma cell line (American Type Culture Collection, Rockville, Md.) were grown in α-minimal essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin. HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and penicillin-streptomycin.
Adenoviral infections were performed in six-well plates with cells at 90% confluence in 2 ml of DMEM supplemented with antibiotics and 10% FBS. Virus was added directly to the plate to a concentration of 4,000 particles (p)/cell (40 to 200 PFU/cell) followed by gentle stirring. At this dose, all the cells are infected, as measured by using the Ad-green fluorescent protein (GFP) virus, and there is no cytotoxicity seen during the 24-h course of the experiment. TNF-α (R&D Systems) was added to a concentration of 10 ng/ml at various times postinfection. At each time point the medium was collected for enzyme-linked immunosorbent assay (ELISA) and the cells were lysed for total RNA or protein extraction. For the serum-starved cell experiments, the medium was changed to FBS-free medium 14 h prior to infection and the cells were maintained in serum-free conditions until the end of the experiment.
Ad7001, a virus with a deletion of E3 obtained from William Wold (19a), AdCMVGFP, a virus with a deletion of E1 and E3 constructed in the dl309 (24) background (pJM17) with GFP controlled by the cytomegalovirus (CMV) promoter inserted as a transgene in place of the deleted E1 genes, and AdCMVE3, a virus with a deletion of E1 and E3 with the E3 cassette driven by the CMV promoter reinserted as a transgene within the E1 deleted region in the pBGH11 (4) background were propagated in 293 cells and purified by Freon extraction and CsCl gradient centrifugation. Purified Ads were quantified by optical density at 260 nm (OD260), diluted 1:1 with sterile glycerol, and stored at −20°C.
Western blotting.
Cells were scraped into 1 ml of ice-cold phosphate-buffered saline (PBS) and centrifuged at 12,000 rpm in an Eppendorf centrifuge for 30 s. PBS was aspirated and the pellet was lysed with loading buffer (50 mM Tris-HCl, 100 mM dithiothreitol, 2% sodium dodecyl sulfate, 0.1% bromphenol blue, 10% glycerol) and boiled at 100°C for 3 min. Samples of the extracts were separated by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis and the proteins were transferred to nitrocellulose membrane for 3 h in 20 mM Tris (pH 8)-150 mM glycine-20% methanol at 4°C using the Bio-Rad transblot apparatus. The membrane was blocked for 1 h at room temperature in blocking buffer (1× PBS, 5% nonfat milk [Bio-Rad], 0.1% Tween 20), washed several times with wash buffer (1× PBS, 0.1% Tween 20), and incubated overnight at 4°C with polyclonal anti-gp19 protein antibody (1:2,000) (provided by William Wold) diluted in blocking buffer. After several washes, the blot was incubated for 1 h at room temperature with horseradish peroxidase-conjugated donkey anti-rabbit antibody diluted 1:5,000 in blocking buffer. Proteins were detected by enhanced chemiluminescence.
RNase protection assays.
Total RNA was extracted from U373 and HeLa cells using Trizol reagent (Life Technologies) per the company protocol. In brief, the cells were lysed with 1.0 ml of Trizol reagent on the plates and then mixed with 0.2 ml of chloroform in Eppendorf tubes, shaken by hand, allowed to stand at room temperature for 5 min, and centrifuged at 10,000 × g at 4°C for 15 min. The supernatants were collected and precipitated with isopropanol at −80°C for 15 min and centrifuged at 14,000 rpm at 4°C for 25 min. The pellet was washed with 70% ethanol at room temperature, dried, and resuspended in 10 μl of Ambion nuclease-free water. The yield and quality was measured by OD260/280 and by morpholinepropanesulfonic acid (MOPS)-formaldehyde agarose gel analysis.
RNase protection assays were performed using the RPA III kit (Ambion) with probe labeled using the Maxiscript T7 kit (Ambion). The multiprobe template set hCK-5 (Pharmingen) was transcribed using T7 RNA polymerase with a [32P]UTP (Amersham-Pharmacia) label and then was phenol-chloroform extracted and precipitated with ethanol and ammonium acetate; 32P incorporation was assessed by Cerenkov counting. Five micrograms of sample RNA was hybridized with 1.5 × 106 cpm of probe overnight at 44°C. Samples were then digested with RNase A and RNase T1 for 30 min at 37°C. RNase was inactivated and the samples were precipitated with proprietary buffer and ethanol. Protected samples were resuspended in 1× loading buffer and separated using 5% acrylamide-8 M urea gel electrophoresis. After drying, the gel was exposed overnight to Kodak film (MS).
ELISA.
Quantikine kits (R&D) for IL-8 and MCP-1 were used to measure the chemokines secreted by U373 cells. Briefly, the plates were incubated with 200 μl of supernatant for MCP-1 determinations and 50 μl of supernatant for IL-8 (both diluted 1:100 in DMEM) or the respective standards (0 to 2,000 ng/ml) for 2 h (MCP-1) or 3 h (IL-8) at room temperature. After four washes with wash buffer (1× PBS, 0.1% Tween 20), 200 μl of horseradish peroxidase-conjugated secondary antibody was added per well and incubated at room temperature for two more hours. After four washes, 200 μl of substrate was added per well and incubated for 20 min, and then 50 μl of 2 N sulfuric acid was added to stop the reaction. The plates were read at 450 nm.
RESULTS
Ad E3 inhibits TNF-α-induced MCP-1 and IL-8 mRNA in U373 cells.
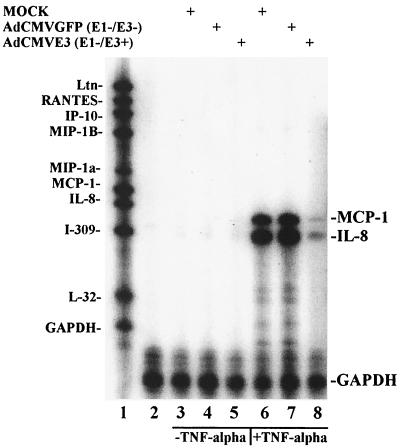
The effects of Ad E3 proteins on chemokine induction were measured in U373 cells infected with 4,000 p/cell of AdCMVE3, a replication-deficient recombinant virus with a deletion of all E1 and endogenous E3 genes and into which the E3 genes were inserted behind the CMV promoter. The same amount of recombinant AdCMVGFP, a virus that also has a deletion of E1 and E3, was used as a control for the effects of viral infection and to monitor infection efficiency by the expression of GFP. After 12 h of infection, the cells were treated with 10 ng of TNF-α per ml for an additional 4 h. RNA was harvested from 5 × 105 cells and analyzed by RNase protection using a multiprobe set from Pharmingen (hck-5) with representative chemokines from the CXC, CC, and C families. Although it has been reported that adenovirus vectors induced expression of MCP-1, IL-8, IP-10, and other chemokines in some cell lines (6, 34), we did not observe Ad induction of chemokines in U373 cells until the addition of TNF-α (Fig. 1). MCP-1 and IL-8 transcripts were the dominant chemokine genes induced by TNF-α in approximately equal amounts, and a small amount of IP-10 was noted when the gel was overexposed (data not shown). Infection with AdCMVE3 resulted in an almost complete inhibition of TNF-α-induced MCP-1 and IL-8 expression, whereas AdCMVGFP virus infection had essentially no inhibitory effects. Parallel Western blot analyses of whole-cell extracts using a rabbit polyclonal anti-AdE3gp19K revealed strong gp19K expression, as expected, only in the AdCMVE3-infected U373 cells (data not shown). Thus, the U373 cell line is a good system for the isolated study of chemokine inhibition by Ad vectors.
FIG. 1.
Ad E3 inhibition of TNF-α-induced chemokines in U373. U373 cells (106) were grown in α-MEM medium supplemented with 10% FBS and infected with 4,000 particles of virus per cell; TNF-α was added at 12 hpi and the samples were harvested as described in the text at 16 hpi. RNase protection was performed with the hck-5 probe set using 5 μg of sample RNA. Lane 1, α-32P-labeled, undigested hck-5 probe; lane 2, probes from lane 1 digested with RNase but without an RNA sample; lanes 3 to 5, U373 cells infected with mock, AdCMVGFP, and AdCMVE3 virus, respectively, in the absence of TNF-α treatment; lanes 6 to 8, U373 cells infected with mock, AdCMVGFP, and AdCMVE3 virus, respectively, and treated with 10 ng of TNF-α per ml. The protected bands, indicated by the labels on the right (MCP-1, IL-8, and GAPDH), migrate faster than undigested probes, as expected.
Ad E1 and E3 independently downregulate TNF-α-induced chemokine RNA expression differentially in serum-treated versus serum-depleted HeLa and U373 cells.
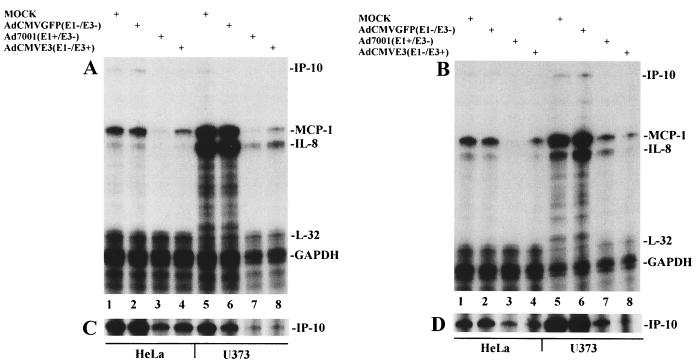
The Ad5 E1A is known to suppress the transcription of MCP-1 (40, 41, 43). In order to evaluate the effects of Ad E1 verus E3 on chemokine expression, we used Ad7001, an E1-positive but E3 deleted virus, to infect U373 or HeLa cells. HeLa cells were used in identical experiments to determine whether the observations about chemokine control could be extended to another human cell line. AdCMVGFP, a non-E1- or -E3-expressing virus, was again used to control for effects of viral infection and as a marker for successful cell infection. Induction of MCP-1 and IL-8 by TNF-α is more dramatic in U373 cells than in HeLa cells. Whereas the induction of both chemokines in U373 is approximately equal, in HeLa cell lines, the induction of MCP-1 is about fivefold stronger than that of IL-8 (Fig. 2A). A distinct induction of IP-10 was detected in both U373 and HeLa cells upon TNF-α stimulation in mock- or AdCMVGFP-infected HeLa cells (Fig. 2C). In both HeLa and U373 cells, MCP-1 and IL-8 induction by TNF-α was not affected by AdCMVGFP infection, but the amounts of MCP-1 and IL-8 mRNA were profoundly decreased by Ad7001 (E1 positive and E3 negative). Although AdCMVE3 infection inhibited TNF-α-induced transcription of MCP-1 and IL-8 in both U373 and HeLa cells, there was more inhibition seen in U373 cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and L32 RNA levels remained unchanged in each cell line and were used to normalize data. Thus, TNF-α induced transcription of MCP-1, IL-8, and IP-10, each of which can be downregulated either by Ad E1 or Ad E3 gene products.
FIG. 2.
Ad E1 and E3 independently inhibit chemokines in U373 and HeLa cells. (A) Cells (106) grown in DMEM supplemented with 10% FBS were infected with 2,000 particles of virus per cell, treated with 10 ng of TNF-α per ml at 12 hpi, and harvested as described in the text at 16 hpi. (B) Cells (106) were first serum starved for 14 h and then treated as in panel A and harvested at 16 hpi. For panels A and B, lanes 1 to 4, HeLa cells; and lanes 5 to 8, U373 cells; lanes 1 and 5, mock-infected cells; lanes 2 and 6, AdCMVGFP-infected cells; lanes 3 and 7, Ad7001-infected cells; lanes 4 and 8, AdCMVE3-infected cells. MCP-1, IL-8, and to a lesser degree IP-10 were the predominant chemokines induced with TNF-α; L32 and GAPDH were used for normalization of data points. Each lane represents RNase protection of the hck-5 labeling probe set by 5 μg of RNA. (C) The same experiment as for panel A is shown; however, the gel was exposed for 72 h instead of 3 h to visualize the IP-10 results. (D) The gel shown in panel B was reexposed for 72 h. The amounts of extracts loaded per lane were equivalent as measured by the intensity of GAPDH; however, in panels A and C, the amounts loaded in lanes 7 and 8 were 54% of the controls in lanes 5 and 6 as analyzed by densitometry.
In certain cell lines such as primary astrocytes, the presence of serum is known to induce MCP-1 transcription, presumably due to the presence of platelet-derived growth factor. Although basal chemokine production was minimal in both U373 and HeLa cells and no detectable differences were observed in the presence or absence of serum, we employed serum starvation to examine how serum factors affected the observed inhibitory effects of the E1 and E3 genes on TNF-α-induced chemokine transcription (Fig. 2B). Viral modulation of TNF-α-induced MCP-1, IL-8, or IP-10 (Fig. 2D) in serum-depleted HeLa cells was similar to that in serum-treated HeLa cells. In contrast, in U373 cells in the absence of serum, IL-8 induction by TNF-α was slightly decreased relative to the levels seen in the presence of serum, while IP-10 induction was increased relative to levels seen in the presence of serum (Fig. 2D). Although patterns of inhibition of MCP-1 and IL-8 in HeLa cells differed little in the presence or absence of serum, the same measurements in U373 cells produced differential inhibition as a function of the addition of serum. For example, the residual amounts of expression of MCP-1 compared to IL-8 in U373 cells were reversed between serum-depleted and serum-containing cultures. This differential inhibition might indicate that E3 does not inhibit MCP-1 and IL-8 by identical interactions with signaling molecules or transcription regulatory regions for each of these chemokines. In addition, the different patterns of chemokine downregulation between the Ad7001 and AdCMVE3 lanes in U373 cells in the absence of serum suggest that different intracellular mechanisms may be responsible for chemokine modulation by the E1 and E3 regions.
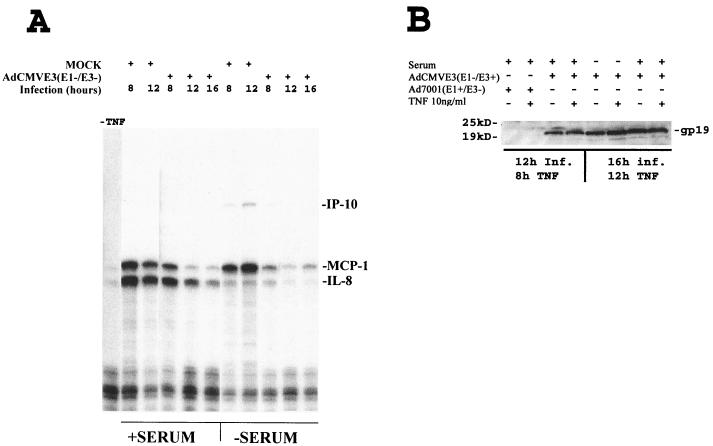
Kinetics of chemokine inhibition correlate with Ad E3 protein expression.
In wild-type Ad infection, E1 proteins are made first and transactivate expression of proteins from the other early regions, including E3. In the AdCMVE3 vector, E3 is expressed constitutively and is expected to accumulate progressively after infection. In order to better characterize how the levels of E3 proteins correlate with reduction of chemokine transcription, we infected U373 cells with 4,000 p of AdCMVE3 per cell for 4, 8, and 12 h prior to a 4-h treatment with 10 ng of TNF-α per ml in the presence and absence of serum. As expected, the amounts of both IL-8 and MCP-1 mRNA decreased with longer infection times prior to TNF-α treatment (Fig. 3A) and this correlated with an increase of E3-19K protein expression as measured by Western blot analysis (Fig. 3B). Inhibition of TNF-α-induced IL-8 and MCP-1 transcription was first noted with the addition of TNF-α at 12 h postinfection (hpi) in the presence of serum and 8 hpi in the absence of serum (Fig. 3A). Almost complete inhibition of TNF-α-induced MCP-1 and IL-8 messages in the presence of serum was seen between 12 and 16 hpi. In the absence of serum, almost complete inhibition of MCP-1 was achieved by 8 to 12 hpi. In addition, under serum starvation conditions the message for IL-8 was induced weakly (probably with different kinetics) when compared with the level of message for MCP-1 (Fig. 3A). Thus, increased E3 protein expression, as measured by E3-19K expression, correlated with increasing inhibition of TNF-α-induced MCP-1 and IL-8 in both the presence and absence of serum.
FIG. 3.
Kinetics of MCP-1 and IL-8 inhibition by Ad E3. U373 cells (106) were grown in α-MEM medium supplemented with 10% FBS or depleted of serum for 14 h prior to infection. Cells were subsequently infected with 4,000 particles of virus per cell; 10 ng of TNF-α per ml was added 4 h prior to harvesting each sample at the time points indicated. (A) RNase protection assay. The first lane is a negative control (no virus or TNF). The time of harvest of the mock- or AdCMVE3 (E1 and E3 positive)-infected cells and the presence or absence of serum are indicated for each lane. (B) Western blot. U373 cells were infected with Ad7001 and AdCMVE3 and some samples were treated with TNF-α 4 h prior to harvesting at 12 or 16 hpi for protein and analysis as described in Materials and Methods. The infecting adenovirus and the presence of serum or TNF stimulation is indicated for each lane. The Ad E3-gp19K band, detected by rabbit polyclonal antibodies, is labeled
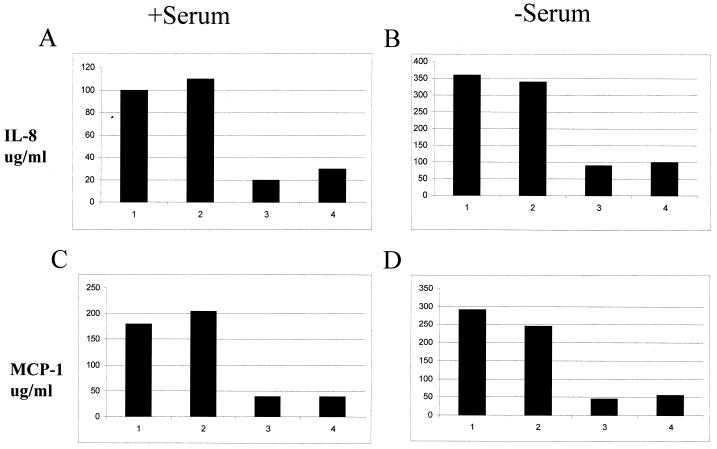
Ad E1 or Ad E3 decreases the amount of MCP-1 and IL-8 protein that is secreted into TNF-α-treated U373 supernatants.
U373 cells were infected with Ad7001, AdCMVE3, or AdCMVGFP for 12 h and treated with 10 ng of TNF-α per ml for an additional 12 h. Supernatants were collected at 12 h after TNF-α treatment and analyzed by sandwich ELISA using the method described in Materials and Methods to determine how Ad E1 or Ad E3 expression changed the extracellular concentrations of MCP-1 or IL-8. Presence of either the E1 or E3 genes resulted in a three- to fourfold decrease of MCP-1 and IL-8 proteins in the medium (Fig. 4). The overall amounts of chemokines secreted were higher from the cells grown in the absence of serum.
FIG. 4.
E1 and E3 genes impair secretion of MCP-1 and IL-8. U373 cells (5 × 105 per well) were mock infected or infected with AdCMVGFP, Ad7001, or AdCMVE3 for 12 h with 6,000 particles/cell. At that time, 10 ng of TNF-α per ml was added per well. After 12 h of stimulation with TNF-α, the supernatants were collected and analyzed by ELISA for IL-8 (A and B) and MCP-1 (C and D) as described in Materials and Methods. One group of cells was maintained with normal FBS in the medium (A and C), while the others were treated for 12 h with serum-depleted medium before infection began (B and D). The numbers at the bottom of each bar represent different treatments as follows: 1, mock; 2, AdCMVGFP (E1 and E3 negative); 3, Ad7001 (E1 positive and E3 negative); and 4, AdCMVE3 (E1 negative and E3 positive).
Differential inhibition of TNF-α-induced MCP-1 and IL-8 levels by inhibitors of NF-κB signal transduction pathway.
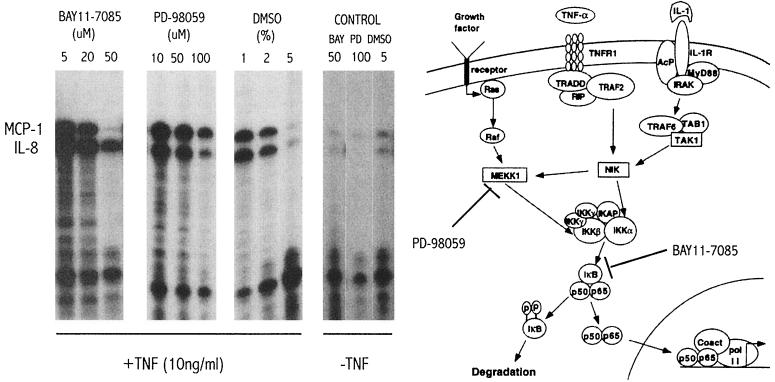
As discussed above, the promoters for the IL-8 and MCP-1 chemokines contain consensus sequences for AP-1 and NF-κB transcription factors, and it has been shown in other systems that both chemokines can be upregulated by NF-κB. However, preliminary experiments did not observe an equal upregulation of both MCP-1 and IL-8 chemokines using different proinflammatory molecules. For example, IL-1β showed a similar pattern of upregulation compared to TNF-α, the phorbol ester phorbol myristate acetate upregulated the IL-8 message but not the MCP-1 message, and CD-40L had no effect (data not shown). There is evidence for the role of NF-κB in the regulation of IL-8 in astrocytes (23, 39), but corresponding data for MCP-1 are unavailable. In the case of the astrocytoma cell line U373, it is not known if TNF-α induces these chemokines through the NF-κB signaling pathway. We attempted to approach the question of what pathways may be involved by using specific small molecule chemical inhibitors to learn some of the mechanisms involved in chemokine upregulation by TNF-α in our system. One specific inhibitor of MEKK1, PD-98059 (1) showed a preferential inhibition of IL-8 transcription over MCP-1, implying that the mitogen-activated protein kinase pathway can affect the NF-κB pathway to upregulate IL-8, possibly through IKKβ phosphorylation (25, 31). Bay11-7085, a specific and irreversible inhibitor of IκB-α phosphorylation (36), showed a more pronounced inhibition of MCP-1 expression than of IL-8 expression. It has been reported that the antioxidant solvent dimethyl sulfoxide (DMSO) inhibits NF-κB (36). We found that high concentrations of DMSO (above 2%) inhibited the expression of both chemokines (Fig. 5).
FIG. 5.
MCP-1 and IL-8 chemokine upregulation induced by TNF-α is not coordinately affected by inhibitors of the NF-κB pathway. Confluent wells with 5 × 105 U373 cells were pretreated for 1 h with different chemical compounds before TNF-α stimulation for an additional 4 h before harvest and total RNA isolation for RNase protection assay analysis. The inhibitors were tested at different concentrations. BAY11-7085 (BIOMOL), a specific inhibitor of IκB phosphorylation, PD-98059 (BIOMOL), a specific inhibitor of MEKK1 kinase activity, and DMSO, a solvent with antioxidant properties that has been shown to inhibit NF-κB in a nonspecific manner, were tested. In the control panel, the chemicals were tested at the maximal concentration and under the same conditions but without TNF-α stimulation. A diagram of the NF-κB signal transduction pathway (reproduced with permission from reference 9) is shown together with the steps inhibited by BAY11-7085 and PD-98059.
DISCUSSION
Our initial hypothesis that Ad E3 modulates cellular chemokine secretion was based on indirect evidence of E3's ability to reduce inflammatory cell infiltration in the context of either viral infection or immune-mediated disease (22). However, here we provide the first direct evidence that Ad E3 gene expression can independently downregulate the expression of chemokine RNAs, thus extending the immunoregulatory targets controlled by Ad E3 genes. Inhibition of the amounts of chemokine mRNAs by E3 is also reflected in decreased secretion of MCP-1 and IL-8 proteins as shown by ELISA. Although the levels of IP-10 induction following TNF induction were lower than those for IL-8 and MCP-1, it also appears that Ad E3 decreases IP-10 transcripts. We chose to do our experiments in cell lines and at input multiplicities at which there was no induction of chemokines by adenovirus infection alone. This allowed us to quantify the reduction of chemokines by E3 genes more accurately than using conditions during which the viral vector itself would be inducing changes. For example, in human umbilical vein endothelial cells MCP-1 and IL-8 RNA levels were both moderately elevated in response to AdCMVGFP infection relative to mock infection and did not require the presence of TNF-α induction (data not shown); however, even human umbilical vein endothelial cell infection with AdCMVE3 also decreased MCP-1 and IL-8 levels below the basal levels seen in mock-infected U373 cells (data not shown).
Induction of chemokine RNA has been reported in response to Ad infection in several cell lines and in vivo, leading to dose-dependent tissue toxicity (2, 34, 45). Besides the particular cell type infected in other studies, the almost constant feature in the experimental design was the use of large amounts of virus necessary to achieve chemokine upregulation (up to 5,000 p/cell [34] or ∼105 particles/cell [6]). These previous reports increase the significance of our data describing that Ad E3 can control chemokine transcription in response to inflammatory stimuli.
Our experimental results were limited to the chemokines induced by TNF-α in the U373 and HeLa cell lines. It appears that TNF-α induction of chemokines is a valid representation of what occurs in vivo in response to Ad infection. This is based on observations that infection with both wild-type Ads and Ad gene therapy vectors have been shown to induce the secretion of TNF-α in vivo (18, 30). However, administration of TNF-α induces chemokine secretion differentially in specific tissue such as stromal fibroblasts, microvascular endothelium, and mononuclear phagocytes (35). In support of the importance of the TNF response in vivo, TNF-α knockout mice have reduced early leukocyte infiltrate in the liver, prolonged transgene expression, and a significantly decreased anti-Ad immunoglobulin G antibody response to infection compared to wild-type animals infected with first-generation Ad vectors (13). Thus, the TNF-α functions in both the initial innate immunity and later acquired immunity phases of Ad vector-induced responses. Also, the presence of four genes (E1B-19K, E3-14.7K, and the complex of E3-10.4K/14.7K) in the early regions of the Ad genome, which counteract the antiviral functions of TNF-α, further supports the importance of this cytokine in adenoviral biology.
Serum-depleted conditions for some experiments were utilized because normal astrocytes as components of the central nervous system are not normally in direct contact with serum proteins unless there is inflammation triggered by infectious agents or by injury. The different patterns of inhibition of MCP-1 and IL-8 by Ad E1 versus Ad E3 and the differential effects in the presence or absence of serum suggest multiple intracellular targets for the modulation of chemokines by Ad early proteins. Multiple transcription factors including NF-κB have been implicated in the control of MCP-1 and IL-8 transcription. The mechanisms by which Ad E3 proteins interact with the TNF-α receptor and TNF-α downstream signaling pathways are an area of active research (22, 46). Experiments using specific inhibitors of different molecules on the NFκB pathway have demonstrated differential inhibition of MCP-1 or IL-8 induction by TNF-α (Fig. 5). Although E3-14.7K interacts with a component of the IKK complex, important in the NF-κB signaling pathway (29, 31), and E3-RID has been shown to inhibit TNF-α activation of NF-κB (15), it is unclear which E3 protein interactions are important in the inhibition of MCP-1 and IL-8. Experiments are under way in order to make additional vectors expressing single E3 proteins, but with a deletion of the E1 region, to further dissect the E3 genes involved in chemokine inhibition and to better understand the molecular mechanisms of this phenomenon. Based on the information provided in previous publications regarding the promoters of these two chemokines, data showing a direct involvement of NF-κB in the regulation of MCP-1 and IL-8, and our observation that both chemokines are being upregulated, we anticipated that in astrocytes the TNF-α stimulation would affect a common pathway. However, the results with the cell-permeable inhibitors (Fig. 5) provide us with some examples of differences in the pathways that are being activated upon TNF-α stimulation or inhibited by Ad E3 protein expression.
In conclusion, we have demonstrated that Ad E3 overexpression can inhibit chemokine mRNA expression and subsequent secretion in response to inflammatory stimuli such as TNF-α. Chemokines represent a novel target for immunoregulation by the E3 region as well as an additional area of interest in the study of adenovirus-host interactions. These findings represent a possible mechanism for the decreased inflammatory infiltration seen in response to E3 expression in prior studies. Finally, we suggest that the overexpression of the E3 region in Ad gene therapy vectors will improve vector safety by reducing chemokine induction in response to infection.
Acknowledgments
A.M.L. and F.D.-L. contributed equally to the results.
This work was supported by grants from the National Institutes of Health (NIH) (NCI RO1 CA72963) (M.S.H.), NIAID (RO1 AI-42295) (M.S.H. and F.D.-L.), Cancer Center Core (grant CA13330) (to M.S.H.), the Forchheimer Foundation (M.S.H.), and a Howard Hughes Research Training Fellowship (A.M.L.).
REFERENCES
- 1.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 2.Amin, R., R. Wilmott, Y. Schwarz, B. Trapnell, and J. Stark. 1995. Replication-deficient adenovirus induces expression of interleukin-8 by airway epithelial cells in vitro. Hum. Gene Ther. 6:145-153. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, E. M., J. R. Bennink, J. W. Yewdell, and F. M. Brodsky. 1999. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J. Immunol. 162:5049-5052. [PubMed] [Google Scholar]
- 4.Bett, A. J., W. Haddara, L. Prevec, and F. L. Graham. 1994. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc. Natl. Acad. Sci. USA 91:8802-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodaghi, B., T. R. Jones, D. Zipeto, C. Vita, L. Sun, L. Laurent, F. Arenzana-Seisdedos, J. L. Virelizier, and S. Michelson. 1998. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J. Exp. Med. 188:855-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgland, S. L., G. P. Bowen, N. C. Wong, T. A. Libermann, and D. A. Muruve. 2000. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-kappaB. J. Virol. 74:3941-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgert, H. G., and S. Kvist. 1985. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell 41:987-997. [DOI] [PubMed] [Google Scholar]
- 8.Dai, Y., E. M. Schwarz, D. Gu, W. W. Zhang, N. Sarvetnick, and I. M. Verma. 1995. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc. Natl. Acad. Sci. USA 92:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delfino, F., and W. H. Walker. 1999. Hormonal regulation of the NF-kappaB signaling pathway. Mol. Cell. Endocrinol. 157:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Driesse, M. J., M. C. Esandi, J. M. Kros, C. J. Avezaat, C. Vecht, C. Zurcher, I. van der Velde, D. Valerio, A. Bout, and P. A. Sillevis Smitt. 2000. Intra-CSF administered recombinant adenovirus causes an immune response-mediated toxicity. Gene Ther. 7:1401-1409. [DOI] [PubMed] [Google Scholar]
- 11.Efrat, S., G. Fejer, M. Brownlee, and M. S. Horwitz. 1995. Prolonged survival of pancreatic islet allografts mediated by adenovirus immunoregulatory transgenes. Proc. Natl. Acad. Sci. USA 92:6947-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efrat, S., D. V. Serreze, A. Svetlanov, C. M. Post, E. A. Johnson, K. Herold, and M. S. Horwitz. 2001. Adenovirus early region 3 (E3) immunomodulatory genes decrease the incidence of autoimmune diabetes in nonobese diabetic (NOD) mice. Diabetes 50:980-984. [DOI] [PubMed] [Google Scholar]
- 13.Elkon, K. B., C.-C. Liu, J. G. Gall, J. Trevejo, M. W. Marino, K. A. Abrahamsen, X. Song, J. L. Zhou, L. J. Old, R. G. Crystal, and E. Falck-Pedersen. 1997. Tumor necrosis factor α plays a central role in immune-mediated clearance of adenoviral vectors. Proc. Natl. Acad. Sci. USA 94:9814-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsing, A., and H. G. Burgert. 1998. The adenovirus E3/10.4K-14.5K proteins down-modulate the apoptosis receptor Fas/Apo-1 by inducing its internalization. Proc. Natl. Acad. Sci. USA 95:10072-10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman, J., and M. S. Horwitz. 2002. Inhibition of tumor necrosis factor alpha-induced NF-κB activation by the adenovirus E3-10.4/14.5K complex. J.Virol. 76:5515-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg, H. S., R. L. Horswood, R. M. Chanock, and G. A. Prince. 1990. Role of early genes in pathogenesis of adenovirus pneumonia. Proc. Natl. Acad. Sci. USA 87:6191-6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg, H. S., U. Lundholm-Beauchamp, R. L. Horswood, B. Pernis, W. S. M. Wold, R. M. Chanock, and G. A. Prince. 1989. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc. Natl. Acad. Sci. USA 86:3823-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginsberg, H. S., L. L. Moldawer, P. B. Sehgal, M. Redington, P. L. Kilian, R. M. Chanock, and G. A. Prince. 1991. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc. Natl. Acad. Sci. USA 88:1651-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooding, L. R., L. W. Elmore, A. E. Tollefson, H. A. Brady, and W. S. M. Wold. 1988. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell 53:341-346. [DOI] [PubMed] [Google Scholar]
- 19a.Gooding, L. R., T. S. Ranheim, A. E. Tollefson, L. Aquino, P. Duerksen-Hughes, T. M. Horton, and W. S. M. Wold. 1991. The 10,400- and 14,500-dalton proteins encoded by region E3 adenovirus function together to protect many but not all mouse cell lines against lysis by tumor necrosis factor. J. Virol. 65:4114-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooding, L. R., I. O. Sofola, A. E. Tollefson, P. J. Duerksen-Hughes, and W. S. M. Wold. 1990. The adenovirus E3-14.7K protein is a general inhibitor of tumor necrosis factor-mediated cytolysis. J. Immunol. 145:3080-3086. [PubMed] [Google Scholar]
- 21.Hirsch, A. J., and T. Shenk. 1999. Human cytomegalovirus inhibits transcription of the CC chemokine MCP-1 gene. J. Virol. 73:404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz, M. S. 2001. Adenovirus immunoregulatory genes and their cellular targets. Virology 279:1-8. [DOI] [PubMed] [Google Scholar]
- 23.John, G. R., J. E. Simpson, M. N. Woodroofe, S. C. Lee, and C. F. Brosnan. 2001. Extracellular nucleotides differentially regulate interleukin-1beta signaling in primary human astrocytes: implications for inflammatory gene expression. J. Neurosci. 21:4134-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, N., and T. Shenk. 1979. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell 17:683-689. [DOI] [PubMed] [Google Scholar]
- 25.Kim, T., T. Y. Kim, W. G. Lee, J. Yim, and T. K. Kim. 2000. Signaling pathways to the assembly of an interferon-beta enhanceosome. Chemical genetic studies with a small molecule. J. Biol. Chem. 275:16910-16917. [DOI] [PubMed] [Google Scholar]
- 26.Krajcsi, P., T. Dimitrov, T. W. Hermiston, A. E. Tollefson, T. S. Ranheim, S. B. Vande Pol, A. H. Stephenson, and W. S. Wold. 1996. The adenovirus E3-14.7K protein and the E3-10.4K/14.5K complex of proteins, which independently inhibit tumor necrosis factor (TNF)-induced apoptosis, also independently inhibit TNF-induced release of arachidonic acid. J. Virol. 70:4904-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalani, A. S., and G. McFadden. 1999. Evasion and exploitation of chemokines by viruses. Cytokine Growth Factor Rev. 10:219-233. [DOI] [PubMed] [Google Scholar]
- 28.Leland, B. J., and J. P. Metcalf. 1999. Type-specific induction of interleukin-8 by adenovirus. Am. J. Respir. Cell Mol. Biol. 21:521-527. [DOI] [PubMed] [Google Scholar]
- 29.Li, Y., J. Kang, J. Friedman, L. Tarassishin, J. Ye, A. Kovalenko, D. Wallach, and M. S. Horwitz. 1999. Identification of a cell protein (FIP-3) as a modulator of NF-kappaB activity and as a target of an adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis. Proc. Natl. Acad. Sci. USA 96:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieber, A., C.-Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol. Cell. Biol. 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahr, J. A., and L. R. Gooding. 1999. Immune evasion by adenoviruses. Immunol. Rev. 168:121-130. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani, A. 1999. Chemokines. Introduction and overview. Chem. Immunol. 72:1-6. [PubMed] [Google Scholar]
- 34.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 35.Ohmori, Y., L. Wyner, S. Narumi, D. Armstrong, M. Stoler, and T. A. Hamilton. 1993. Tumor necrosis factor-alpha induces cell type and tissue-specific expression of chemoattractant cytokines in vivo. Am. J. Pathol. 142:861-870. [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce, J. W., R. Schoenleber, G. Jesmok, J. Best, S. A. Moore, T. Collins, and M. E. Gerritsen. 1997. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272:21096-21103. [DOI] [PubMed] [Google Scholar]
- 37.Shisler, J., C. Yang, B. Walter, C. F. Ware, and L. R. Gooding. 1997. The adenovirus E3-10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J. Virol. 71:8299-8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparer, T., R. A. Tripp, D. L. Dilleha, T. W. Hermiston, W. S. Wold, and L. R. Gooding. 1996. The role of human adenovirus early region 3 proteins (gp19K, 10.4K, 14.5K, and 14.7K) in a murine pneumonia model. J. Virol. 70:2431-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanimirovic, D., W. Zhang, C. Howlett, P. Lemieux, and C. Smith. 2001. Inflammatory gene transcription in human astrocytes exposed to hypoxia: roles of the nuclear factor-kappaB and autocrine stimulation. J. Neuroimmunol. 119:365-376. [DOI] [PubMed] [Google Scholar]
- 40.Timmers, H. T., D. De Wit, J. L. Bos, and A. J. van der Eb. 1988. E1A products of adenoviruses reduce the expression of cellular proliferation-associated genes. Oncogene Res. 3:67-76. [PubMed] [Google Scholar]
- 41.Timmers, H. T., H. van Dam, G. J. Pronk, J. L. Bos, and A. J. van der Eb. 1989. Adenovirus E1A represses transcription of the cellular JE gene. J. Virol. 63:1470-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tollefson, A. E., T. W. Hermiston, D. L. Lichtenstein, C. F. Colle, R. A. Tripp, T. Dimitrov, K. Toth, C. E. Wells, P. C. Doherty, and W. S. Wold. 1998. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature 392:726-730. [DOI] [PubMed] [Google Scholar]
- 43.van Dam, H., R. Offringa, A. M. Smits, J. L. Bos, N. C. Jones, and A. J. van der Eb. 1989. The repression of the growth factor-inducible genes JE, c-myc and stromelysin by adenovirus E1A is mediated by conserved region 1. Oncogene 4:1207-1212. [PubMed] [Google Scholar]
- 44.von Herrath, M., S. Efrat, M. B. A. Oldstone, and M. S. Horwitz. 1997. Expression of adenoviral E3 transgenes in β cells prevents autoimmune diabetes. Proc. Natl. Acad. Sci. USA 94:9808-9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilmott, R. W., R. S. Amin, C. R. Perez, S. E. Wert, G. Keller, G. P. Boivin, R. Hirsch, J. De Inocencio, P. Lu, S. F. Reising, S. Yei, J. A. Whitsett, and B. C. Trapnell. 1996. Safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lungs of nonhuman primates. Hum. Gene Ther. 7:301-318. [DOI] [PubMed] [Google Scholar]
- 46.Wold, W. S., K. Doronin, K. Toth, M. Kuppuswamy, D. L. Lichtenstein, and A. E. Tollefson. 1999. Immune responses to adenoviruses: viral evasion mechanisms and their implications for the clinic. Curr. Opin. Immunol. 11:380-386. [DOI] [PubMed] [Google Scholar]