Abstract
Many alphaherpesviruses establish a latent infection in the peripheral nervous systems of their hosts. This life cycle requires the virus to move long distances in axons toward the neuron's cell body during infection and away from the cell body during reactivation. While the events underlying entry of the virion into neurons during infection are understood in principle, no such consensus exists regarding viral egress from neurons after reactivation. In this study, we challenged two different models of viral egress from neurons by using pseudorabies virus (PRV) infection of the rat retina: does PRV egress solely from axon terminals, or can the virus egress from axon shafts as well as axon terminals? We took advantage of PRV gD mutants that are not infectious as extracellular particles but are capable of spreading by cell-cell contact. We observed that both wild-type virus and a PRV gD null mutant are capable of spreading from axons to closely apposed nonneuronal cells within the rat optic nerve after intravitreal infection. However, infection does not spread from these infected nonneuronal cells. We suggest that viral egress can occur sporadically along the length of infected axons and is not confined solely to axon terminals. Moreover, it is likely that extracellular particles are not involved in nonneuronal cell infections. Taking these together with previous data, we suggest a model of viral egress from neurons that unifies previous apparently contradictory data.
Many alphaherpesviruses have evolved a complex life cycle that requires establishment of a lifelong infection in the peripheral nervous systems of their hosts (reviewed in references 7, 18, and 34). In general, virions infect exposed epithelial tissue and replicate before entering the nervous system. Entry into the peripheral nervous system occurs when the viral envelope fuses with the plasma membrane of sensory and autonomic nerve endings innervating the infected tissue. After fusion, the capsid (and possibly part of the tegument) engages the microtubule-based motor system of neurons for transport to cell bodies (retrograde transport). The movement of capsids from peripheral epithelia to cell bodies of neurons is remarkable for several reasons. The journey of herpes simplex virus type 1 (HSV-1) from the human lip along sensory neuron axons to cell bodies in the trigeminal ganglia is around 10 cm, or approximately a million capsid diameters. Once viral genomes are deposited in nuclei of peripheral nervous system neurons, a latent infection is usually established in the natural host. During times of stress, the latent viral genome reactivates and virus is transported from cell bodies to axon terminals to reinfect peripheral epithelial cells (anterograde transport). After replication in peripheral epithelial cells, mature virions can spread to a new host to complete the viral life cycle.
While the events that occur during viral entry into neurons (e.g., retrograde transport) are understood in principle, no consensus exists regarding mechanisms of long-distance movement of newly replicated virions by anterograde transport to axon terminals and mechanisms of viral egress from axons. The traditional view is that mature virions are assembled in the cell bodies of neurons and are then transported in a vesicle in axons to the terminus. In this model, the transport vesicle fuses with the presynaptic membrane at the axon terminal, releasing mature virions in the extracellular space adjacent to the neuron. Data to support such a model are well documented. For example, enveloped virions within vesicles have been identified in axons by electron microscopy (3, 9, 10, 16). In addition, infectious virus can be isolated from infected nerves when the virus is moving in the anterograde but not the retrograde direction (13, 14, 15). Glial cells adjacent to infected neurons often are infected, suggesting that mature, infectious, enveloped virions are present in axons during virus egress (29). In contrast, glial cells in a nerve are not infected during virus entry, presumably because the capsid is separated from envelope proteins necessary for membrane fusion during retrograde transport (18).
In 1994, Cunningham and coworkers challenged the traditional view of virus egress by suggesting that in human primary sensory neurons, HSV-1 envelope proteins were transported separately from capsid and tegument components (11, 19, 25). The novel idea was that mature virions were not transported in axons but rather were assembled at the axon terminus. In this report, we refer to this model as the subvirion transport model. Other laboratories have provided supporting data for the subvirion transport model. Ohara et al. injected HSV-1 directly into rat trigeminal ganglia and examined transport of newly replicated virus to tissues of the eye (21, 22). They reported evidence for separate transport of virion components. Tomishima and Enquist reported that the PRV Us9 gene product is required for entry of all tested viral membrane proteins in axons but not for entry of capsids or tegument proteins (33). These data supporting the subvirion transport model stand in contrast to data supporting the traditional model of transport involving mature, infectious virions. If virion components indeed are transported separately within axons in a noninfectious form, how can infectious virus be isolated from nerves (13, 14), and how can glial cells within nerves become infected (29)?
At least two models have been proposed to explain the presence of infected glial cells in nerves. One proposes that mature viruses are present in axons and that these particles egress from axon shafts to infect neighboring glial cells (29). An alternative idea was developed by Ohara et al. using their model of direct injection of rat trigeminal ganglia (22). These workers suggested that a separate mode of infection occurs outside axons in the glial cells that are abundant in nerves. The idea was that infectious virions released from infected cells diffuse through the extracellular space outside of axons (but within the nerve, the endoneurium), infecting nonneuronal cells and thus propagating the infection. The extent of spread in a nerve by this mode would be limited by the rate of diffusion through the extracellular space in the nerve and the many rounds of replication necessary to propagate virions by diffusion, compared to fast-axonal transport inside axons. Ohara et al. suggested that this mode of spread would be important only at short distances from the infected cell body and would be distinct from fast-axonal transport within axons (22).
In this study, we present a simple experimental paradigm to challenge the proposed modes of viral egress from neurons by using pseudorabies virus (PRV) infection of the rat retina: does PRV egress solely from axon terminals, or can the virus egress from axon shafts? We took advantage of PRV gD mutants that are not infectious as extracellular particles but are capable of spreading by cell-cell contact (1, 24, 27). We observed that both wild-type virus and a PRV gD null mutant are capable of spreading from axons to closely apposed nonneuronal cells within the rat optic nerve after intravitreal infection. However, infection does not spread from these infected nonneuronal cells. We also observed that both viruses efficiently infect second-order neurons in the brain, as noted previously by others (1, 24). We suggest that viral egress can occur sporadically along the lengths of infected axons and is not confined solely to axon terminals. Moreover, it is likely that extracellular particles are not involved in nonneuronal cell infections. We propose a modest revision of the subvirion transport model for viral egress from axons that reconciles previous apparently contradictory observations. We suggest that viral membrane proteins are transported separately from capsid and tegument proteins but that assembly and egress sites exist at scattered sites along the axon shaft as well as at terminals. These sites permit egress and infection of closely apposed glial cells in a gD-independent fashion. However, these glial cells do not spread infection to adjacent glial cells in the nerve.
MATERIALS AND METHODS
Virus strains and cells.
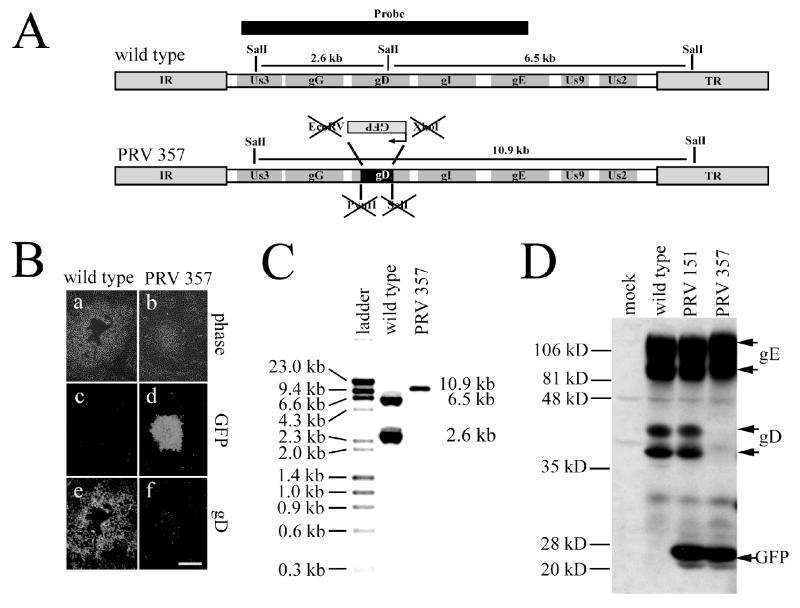
The wild-type strain PRV Becker (PRV Be) was used as the parental strain for the gD null mutant used in this report. PRV Be was propagated on PK15 (porcine kidney epithelial) cells in Dulbecco's modified Eagle's medium supplemented with 2% fetal bovine serum, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. The PRV Be stock used in the infections had a titer of 2 × 108 PFU/ml. The gD null virus (PRV 357) was created by constructing a transfer vector that contains a PstI-SphI fragment (from gG to gE) of PRV Be in pSP72 (Promega). This intermediate plasmid was digested with PvuII-SalI to remove part of the gD open reading frame. A cassette expressing green fluorescent protein (GFP) from the cytomegalovirus immediate-early promoter was excised from pEGFP-S1 with XhoI and EcoRV. The plasmid pEGFP-S1 is a derivative of the Clontech vector pEGFP-N1 that contains additional restriction enzyme sites in the multiple cloning site. The GFP cassette was ligated to the PvuII-SalI-digested transfer vector (see Fig. 2A), placing the direction of transcription of the cassette in the opposite orientation relative to the unique short region of the genome. This transfer vector is called pGS198.
FIG. 2.
(A) Diagram of the unique short region of the wild-type virus (top) and gD null virus PRV 357 (bottom). Most of the gD-coding sequence was removed by digesting a transfer vector with PvuII and SalI. A GFP expression cassette was excised with EcoRV and XhoI and was inserted into gD in the opposite orientation relative to transcription in the unique short region. Since the compatible ends of the SalI and XhoI sites and the blunt ends of the PvuII and EcoRV sites were ligated together, all sites were destroyed in the cloning process. (B) Indirect immunofluorescence of a wild-type plaque (a, c, and e) and a PRV 357 plaque (b, d, and f). PRV 357 exhibits GFP fluorescence (d) but not gD immunoreactivity (f), while the wild-type virus does not exhibit GFP fluorescence (c) and is gD immunoreactive (e). Bar, 0.5 mm. (C) Southern blot of the wild-type virus and PRV 357. Viral DNA was digested with SalI, and a probe (SphI fragment from wild-type virus as diagrammed in panel A) was prepared. As expected, two bands that corresponded to 6.5 and 2.6 kb were present in the wild-type lanes. The gD null virus, on the other hand, had a single band of approximately 10.9 kb, since the SalI site in gD was destroyed in the cloning process. (D) Western blot of PRV 357. Cell lysates were prepared from mock-infected PK15 cells or from PK15 cells infected with wild-type virus, PRV 151 (expresses GFP), or PRV 357. Antibodies against gE (as a loading control), gD, and GFP were used in this experiment. PRV 357 expresses GFP but not gD.
To make complemented stocks of PRV 357, PRV Be DNA and pGS198 were transfected into G5 cells (23). G5 cells are SK-6 (swine kidney) cells that constitutively express PRV gD (strain NIA-3). Plaques exhibiting GFP fluorescence were plaque purified three times on G5 cells before stock production.
High-titer stocks were made by infecting 10-cm-diameter dishes with virus obtained from an isolated green plaque. As foci of infection formed, the monolayer was trypsinized, trypsin was removed by centrifugation, and cells were resuspended in 2% fetal calf serum with 50 U of penicillin per ml and 50 μg of streptomycin per ml (Gibco BRL) in Dulbecco's modified Eagle's medium. After resuspension, cells were reseeded into a fresh dish. This allowed the dispersal of infected cells, permitting viral spread throughout the monolayer. This was repeated until the entire monolayer was infected, typically after two rounds of trypsinization. The titer of PRV 357 used in this study was 107 PFU/ml. The PRV 357 stock used in these studies was tested for revertants in three ways. First, we examined 196 isolated plaques from the stock for green fluorescence, and all were positive. Second, we infected PK15 cells to make infected-cell lysates and Western blotted with antibodies against gD, and we verified that no gD could be detected in these lysates. Third, we performed Southern blotting on PRV 357 stocks and found bands of the expected sizes for the gD null allele and not for the wild-type gD allele.
Antisera.
The rabbit polyclonal antiserum (Rb133) against acetone-inactivated PRV and the rabbit polyclonal antiserum (Rb601) against gE have been described previously (3, 29). The monoclonal antibody against the glial fibrillary acidic protein (GFAP) was purchased from Sigma-Aldrich (clone G-A-5). The polyclonal antiserum against GFP and Alexa 488- and 568-conjugated secondary antibodies were purchased from Molecular Probes. A polyclonal antiserum against PRV gD was kindly provided by Krystyna Bienkowska-Szewczyk (University of Gdansk).
Southern and Western blots.
NEBlot and Phototope kits (New England Biolabs) were used according to the manufacturer's instructions for the Southern blotting. Western blotting was performed by infecting PK15 cells for 16 h to make cell lysates. Cell lysates were made from mock-, wild-type-, PRV 357-, and PRV 151-infected cells. PRV 151 is a virus that expresses GFP in the gG locus, and it was included as a positive control for GFP expression (30). Infected cells were rinsed once with phosphate-buffered saline (PBS) and incubated with Western lysis buffer (10 mM Tris [pH 7.5], 1% NP-40, 1% sodium deoxycholate, and 150 mM NaCl) on ice (10 min). After the incubation, cells were pipetted off the tissue culture dish and vortexed briefly. Cell lysates were centrifuged at full speed (16,000 × g) in a microcentrifuge (4°C, 15 min) to eliminate insoluble aggregates. Ten microliters of supernatant in reducing sample buffer was boiled (5 min) before being loaded on a sodium dodecyl sulfate-polyacrylamide gel to separate proteins in the lysate, and protein was transferred to a Hybond membrane with a semidry transfer apparatus. After transfer, the membrane was rinsed extensively with PBS and then blocked overnight in PBS containing 5% nonfat milk with 0.1% Tween 20. The membrane was rinsed four times (5 min each) with wash buffer (1% nonfat milk and 0.1% Tween 20 in PBS). Primary antibodies against gE (1:1,000), GFP (1:1,000), and gD (1:1,000) were diluted in wash buffer and incubated with the membrane for 1 h. The membrane was rinsed four times (5 min each) in wash buffer before a 1-h incubation with secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit antibody [Jackson Labs] diluted 1:10,000 in wash buffer). The membrane was rinsed four times (5 min each) with wash buffer, incubated in chemiluminescent substrate for 10 min, and then exposed to film.
Animals.
Adult male Sprague-Dawley albino rats weighing at least 230 g were used in this study. Animals were housed in a biosafety level 2/3 facility with a controlled temperature (22°C) and a constant photoperiod. Rats were maintained in individual cages with food and water freely available throughout the experiment. All experimental protocols were approved by the Princeton University Animal Welfare Committee and were consistent with the regulations stipulated by the American Association for Accreditation of Laboratory Animal Care and the Animal Welfare Act (Public Law 99-198).
Intraocular injections.
Animals were anesthetized with intramuscular xylazine (13 mg/kg) and ketamine (86 mg/kg). The animals were inoculated intravitreally with 2.5 μl of virus at a rate of approximately 100 nl/min. Total PFU per injection were 500,000 for the wild-type virus and 25,000 PFU for the gD null virus. The syringe was left in situ for 5 min after injection to reduce expulsion of inoculum into the orbit. Prior to injections, cell debris was removed from viral stocks by centrifugation (5,000 × g for 5 min) after sonication.
Perfusion and tissue preparation.
Animals were deeply anesthetized with xylazine and ketamine and exsanguinated by transcardiac perfusion of isotonic saline (0.9%) followed by 4% paraformaldehyde containing lysine and sodium metaperiodate at 4°C. Both ocular orbits were enucleated, and optic nerves and brains were removed. Tissue was postfixed in 4% paraformaldehyde for 12 to 24 h at 4°C and cryoprotected in phosphate-buffered 30% sucrose at 4°C for at least 72 h. Sagittal sections of optic nerve (35 μm thick) were cut with a freezing rotary microtome and stored in a cryopreservative (35) at −20°C prior to indirect immunofluorescence.
Indirect immunofluorescence on infected optic nerves.
Tissue sections were rinsed four times (15 min each) in 0.01 M PBS (pH 7.5). After washing, optic nerve sections were incubated for 72 h in primary antibody Rb133 (1:5,000) or anti-GFAP (1:400) diluted in 0.01 M PBS containing 2% normal goat serum and 0.3% Triton X-100. Sections were washed again in 0.01 M PBS (pH 7.5) four times (15 min each) before a 90-min incubation with secondary antibodies. Alexa 488- or 568-conjugated secondary antibodies (Molecular Probes) were used at 1:400, diluted in 0.01 M PBS (pH 7.5) containing 2% normal goat serum and 0.3% Triton X-100. After the removal of secondary antibodies, sections were washed in 0.01 M PBS (pH 7.5) four times (15 min each). Sections were mounted onto gelatin-coated slides after the last rinse, and a drop of Vectashield mounting medium (H 1000; Vector Laboratories, Inc.) was placed on the nerves. A coverslip was placed on the Vectashield and sealed with nail polish. Single optical sections were captured using a Nikon Optiphot-2 microscope equipped with a Bio-Rad Laboratories MRC600 scan head. Images were processed with Adobe Photoshop (version 5.0).
Indirect immunofluorescence on viral plaques.
PK15 cells were cultured on coverslips, and wild-type or PRV 357 plaques were grown for 3 days. Coverslips were rinsed three times in PBS and then fixed in 3.2% paraformaldehyde in PBS before permeabilization in PBS containing 0.5% saponin and 3% bovine serum albumin. All subsequent manipulations were done in PBS containing 0.5% saponin and 3% bovine serum albumin. Coverslips were incubated with polyclonal antibodies against gD (1:200) at room temperature. After incubation for 60 min, coverslips were washed three times and then incubated with goat anti-rabbit or anti-mouse Alexa 568-conjugated secondary antibodies (1:400; Molecular Probes). Coverslips were washed three times, and a final rinse in water was performed before the coverslips were mounted on glass slides with Vectashield mounting medium (H 1000; Vector Laboratories). Images were captured on a Nikon Eclipse TE300 inverted microscope with a 10× phase-contrast objective fitted with a Spot RT color camera (Diagnostic Instruments, Inc.). Images were processed on Adobe Photoshop (version 5.0).
RESULTS
Infection of the rodent visual system.
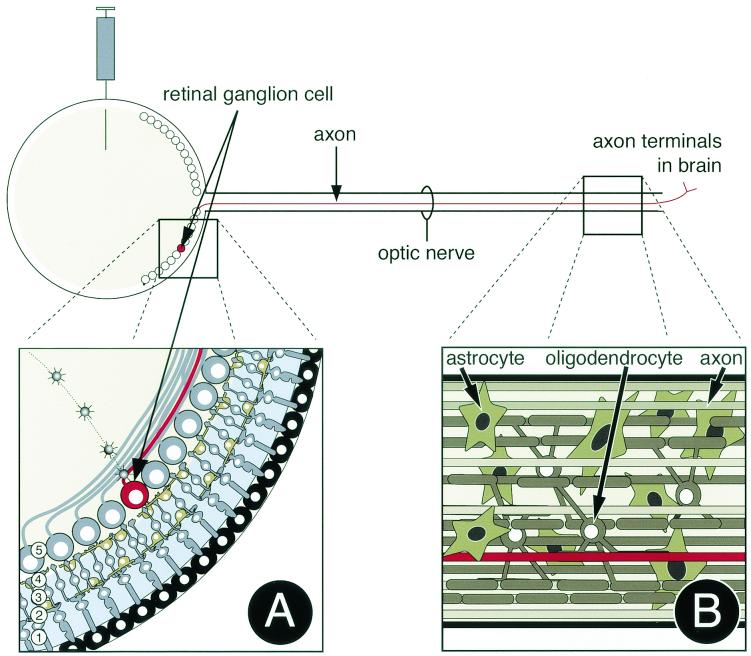
We used in vivo infection of the rodent visual system to determine whether viral egress occurs from the sides of axons in a nerve. Infection of the retina via intravitreal injections provides many advantages for assaying directional viral spread in neurons and circuits within an infected animal (Fig. 1) (4-6, 8, 12, 36). Retinal ganglion cells (Fig. 1A) are the neurons of the retina that convey visual information and virus in the assay, to the brain. Axons of the retinal ganglion cells leave the retina and project long distances through the optic nerve (Fig. 1B). Retinal ganglion cell axon terminals are found in distinct regions of the brain (5, 6).
FIG. 1.
Visual neuronal circuitry and experimental design. (A) Light enters the retina and penetrates through the layers of cells before exciting the rods and cones (1). Changes in membrane potential are transmitted first to bipolar cells (3) and then to retinal ganglion cells (5). Aside from this linear signaling pathway, both horizontal and amacrine cells (2 and 4) signal laterally to help process visual information before retinal ganglion cells send information to the brain through the optic nerve. Virus injected into the vitreous humor of the eye has direct contact with retinal ganglion cells (5). (B) After replication in retinal ganglion cells, the virus travels through retinal ganglion cell axons in the optic nerve. In this study, we sectioned the optic nerve longitudinally so that infected axons could be visualized for long distances. We focused our analysis on the posterior region of the nerve far from the retina yet before the axon terminals in the brain. Nonneuronal cells adjacent to retinal ganglion cell axons in the optic nerve include astrocytes and oligodendrocytes.
Virus is injected into the vitreous humor of the eye, where it infects and replicates in cell bodies of retinal ganglion cells (4, 5, 9). After replication in the retinal ganglion cell bodies in the retina, virus is transported long distances in axons of the optic nerve to retinorecipient regions of the brain. Infection of second-order neurons in the brain requires viral transport across a synapse. We determined if nonneuronal cells in the nerve are infected as virus is transported toward the brain but has not yet reached synapses in the brain. Nonneuronal cells in the optic nerve include oligodendrocytes that form the myelin sheath around axons and astrocytes that are thought to perform a variety of functions in the nervous system.
Construction of a gD mutant virus.
We designed and constructed a gD null virus (PRV 357) for use in these studies (Fig. 2A). gD interacts with a variety of cell surface receptors, leading to the fusion of the virion envelope with the plasma membrane of a cell (for a review, see reference 32). Therefore, gD null viruses must be grown on a complementing cell line to produce infectious virions. Such complemented stocks allow the virion to penetrate the first cell it encounters, but any virions produced are not infectious. Although released virions are not infectious, infection can spread directly from cell to cell after the first round of replication of gD null mutants (23, 27). One concern in the propagation of gD null stocks is that the gD null allele can recombine with the gD gene encoded in the complementing cell line, giving rise to gD+ revertants in the stock. We designed PRV 357 so that a GFP expression cassette was placed within a large deletion in gD. If recombination between the viral genome and the complementing cell line occurred, the virus would no longer express GFP. Viral stocks were prepared as outlined in Materials and Methods. We screened 196 plaques of the PRV 357 stock used in this study and found that all expressed GFP, indicating that fewer than 0.5% revertants were present in the stock (for example, see Fig. 2B). Plaques of PRV 357 expressed GFP but were not gD immunopositive (Fig. 2B, panel f), unlike the wild-type virus, which expressed gD (Fig. 2B, panel e) but not GFP (Fig. 2B, panel c). In addition, Southern blots demonstrated that the gD null allele had recombined as expected into gD, and no contaminating wild-type genomes were detected in PRV 357 stocks (Fig. 2C). Western blotting was performed on cell lysates from PRV 357-infected cells (Fig. 2D). No gD was detected, even when the blot was overexposed. PRV 357 formed plaques in media without methocellulose, unlike the wild-type virus, which requires methocellulose to inhibit the diffusion of released virus for plaque formation (data not shown). Finally, PRV 357 plaques did not yield infectious virus, unlike wild-type virus (data not shown). These data are consistent with that obtained from PRV gD null mutants (23, 27).
Wild-type infection.
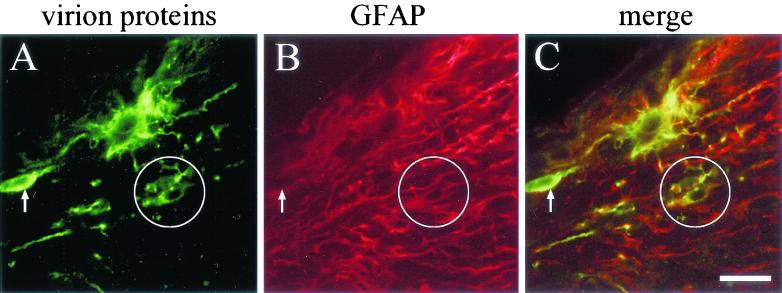
We first performed intravitreal injections with a wild-type strain of PRV (PRV Be; n = 7 animals). Infected optic nerves were dissected and sectioned longitudinally on a freezing microtome. Antibodies that recognize viral structural proteins (Rb133) or a glial marker (anti-GFAP, a marker of astrocytes) were used in indirect-immunofluorescence experiments to examine the distribution of immunoreactivity within optic nerves. Many nonneuronal cells were infected deep within the nerve, often up to 5 mm away from the retina (Fig. 3). Some of these cells reacted with anti-GFAP antibodies and thus are likely glial cells (the circled cell in Fig. 3), and a few of the infected cells had the morphology of oligodendrocytes (data not shown). Infected nonneuronal cells were often adjacent to infected axons (Fig. 4).
FIG. 3.
Confocal micrograph of wild-type-infected optic nerves. The rat visual system was infected with wild-type virus, and 35-μm-diameter sections were prepared and incubated with antibody against PRV virion proteins (in green) (A) and an antibody against the astroglial cell marker GFAP (in red) (B). Secondary antibodies were then used to visualize where primary antibody had bound, and confocal microscopy was used to capture images. The merged image is shown in panel C. The circled nonneuronal cell is immunopositive for both virion proteins and GFAP, whereas the arrow points to a nonneuronal cell that is not GFAP positive. Bar, 25 μm.
FIG. 4.
Infected nonneuronal cells are often next to infected axons. (A) A representative wild-type-infected nerve has infected nonneuronal cells adjacent to immunopositive axons. (B) The same section as in panel A, except that infected axons are shown as black lines. White circles were placed around infected nonneuronal cells that are near axons, and black circles were placed around infected nonneuronal cells that are not near an obviously infected axon. Bar, 150 μm. (C) High-magnification image of a nonneuronal cell adjacent to an infected axon. Bar, 25 μm.
Infected nonneuronal cells are scattered throughout the nerve.
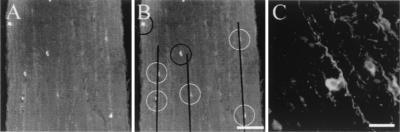
In one experiment, we analyzed sections of wild-type-infected optic nerves from four animals. Low-magnification confocal images were obtained (Fig. 5), and seven fields were analyzed. A 150-μm-diameter circle was placed around infected nonneuronal cells, and cells were scored as to whether another infected nonneuronal cell was within a 75-μm radius. Based on an average cell diameter of approximately 20 μm for a nonneuronal cell (Fig. 5), 75 μm was judged to provide a conservative estimate for spread to adjacent cells. We found that 50 of 72 (69%) of the infected cells analyzed did not have another infected nonneuronal cell within 75 μm of the infected cell.
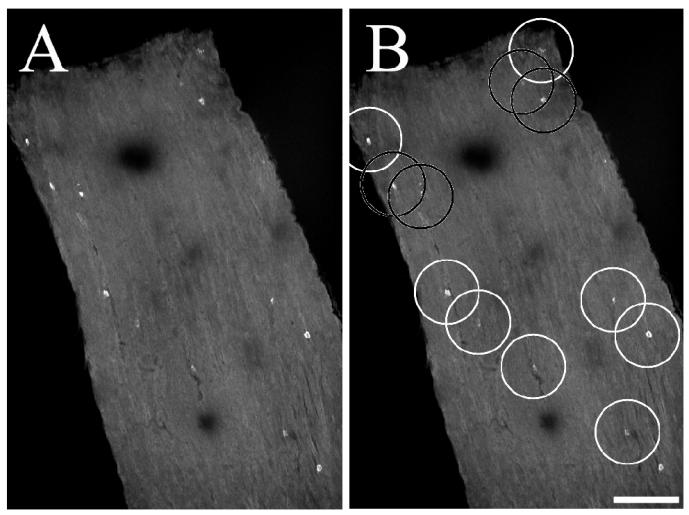
FIG. 5.
Representative example of infected nonneuronal cells that are often separated large distances from other infected nonneuronal cells. (A) Infected optic nerve before analysis; (B) infected optic nerve after scoring. White circles have been placed around infected nonneuronal cells that are separated from other infected cells by at least 75 μm, and black circles have been placed around cells that are closer than 75 μm to other infected nonneuronal cells. Each circle is 150 μm in diameter.
gD null infections.
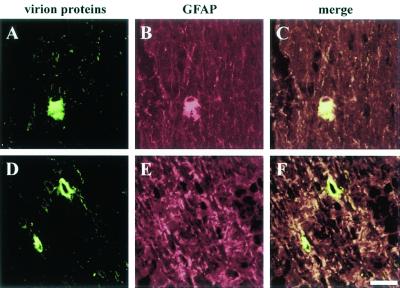
Consistent with previous reports, the gD null virus (PRV 357) was less virulent than the wild-type virus yet spread through neural circuits to infect second-order neurons like the wild-type virus (references 1, 20, and 24 and data not shown). The distribution of infected nonneuronal cells in PRV 357-infected optic nerves was similar to that for wild-type infections, although the infection was not as extensive as found in wild-type infections (n = 7 animals). This observation may reflect the reduced titer obtained for gD null virus stocks compared to the wild-type virus. However, previous studies with swine reported that gD null mutants spread more slowly in neural circuits relative to the wild type even when similar infectious doses were used (20). Despite the less extensive infection, we found many infected nonneuronal cells adjacent to infected axons up to 5 mm away from the retina (the nerve enters the brain after about 5 mm and cannot be easily dissected for analysis). Some of these infected nonneuronal cells were positive for the glial cell marker GFAP (Fig. 6A to C), while others were not (Fig. 6D to F). Similar to the case for wild-type infections, infected nonneuronal cells were sparsely distributed along the nerve and rarely were found adjacent to each other.
FIG. 6.
Confocal micrograph of gD null virus (PRV 357)-infected optic nerves. The rat visual system was infected with PRV 357, and 35-μm-diameter sections were prepared and incubated with antibody against PRV structural proteins (in green) (A, C, D, and F) and the glial cell marker GFAP (in red) (B, E, C, and F). The gD null virus-infected nonneuronal cells that were positive (A to C) and negative (D to F) for GFAP are shown. Bar, 25 μm.
DISCUSSION
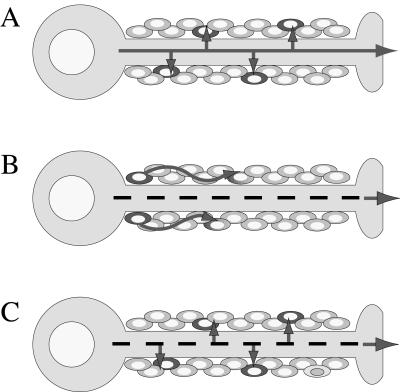
Our data suggest that PRV can egress not only from axon terminals, but also at sites along the axon shaft during anterograde spread of infection along the rat optic nerve. Such spread does not require gD and thus may not involve infection of released, mature virions. This finding directly affects how we think about viral spread and assembly during anterograde viral transport. If nonneuronal cells are infected because viral egress occurs from axon shafts, viral assembly may occur along the length of axons as well as at axon terminals. Alternatively, as suggested by Ohara et al. (22), viral assembly in axons might be confined to the axon terminal, but glial cell infections reflect a secondary wave of infection outside axons (Fig. 7B). Here, we present data that suggest that such extra-axonal infection is unlikely, and the data are consistent with the idea that PRV can exit from the axon shaft and not just at axon terminals (Fig. 7A and C).
FIG. 7.
Models of axonal transport of virus and infection of nonneuronal cells within nerves. Solid lines represent axonal transport of fully assembled virus, whereas dashed lines represent transport of subvirion assemblies. (A) Virus is assembled in the cell body and is transported to the axon terminal in an infectious form. Since it is preassembled, virus can bud out of the side of the axon during transit to the axon terminal. (B) Fast-axonal transport of virus is mediated by subvirion structures for later assembly at the axon terminal. Glial cells are infected by a second wave of extracellular virus that occurs within nerves but outside axons. (C) Fast-axonal transport of virus is mediated by subvirion structures, but virus can assemble at points along the axon shaft as well as at the axon terminal.
PRV virions lacking gD are not infectious, but gD null mutants can spread by direct cell-cell contact. gD null mutants provide a direct test of the idea that virus can spread by diffusion of particles inside nerves. gD null virions were grown on a complementing cell line to produce infectious particles, but after the first round of replication in retinal cells, the virus can spread only from cell to cell. Spread of PRV virions inside the extraneural space should be very slow, given that the average size of nonneuronal cells is roughly 20 μm, a replication cycle for PRV is approximately 8 h, and the infections progressed in vivo from 3 to 7 days. Based on these numbers, extraneural spread of virus should vary from ∼180 μm (3 days) to ∼420 μm (7 days). We observed infected glial cells several millimeters away from the retina. Moreover, infected nonneuronal cells were almost always isolated infected cells. Our data are inconsistent with the idea that the infection was spreading from nonneuronal cell to nonneuronal cell in the nerve.
Two other observations during wild-type infections support the hypothesis that PRV can egress from axon shafts. We noticed that infected nonneuronal cells were almost always isolated infected cells (Fig. 5) in direct contact with the neural shaft (Fig. 4). When clusters of infected nonneuronal cells were observed, they invariably were confined to the most heavily infected regions near the retina in wild-type infections. The most likely explanation for the frequent occurrence of isolated nonneuronal cells is that these cells are susceptible to infection from the infected axons but are incapable of producing infectious virus. Previous investigators have suggested that nonneuronal cells undergo an abortive infection due to a defect in viral assembly (2-4, 9, 28). Our results with both wild-type and gD null infections are consistent with these earlier suggestions that glial cells and other nonneuronal cells likely respond to infection and limit the extraneural spread of virus (3, 17, 28).
This work and our previous data (33), together with the data of Cunningham's group (11, 19, 25) and LaVail's group (21, 22) suggest a modest revision of the subvirion transport model and alphaherpesvirus egress from axons (Fig. 7C). In the revised model, capsid and tegument proteins are transported into axons separately from vesicles containing viral membrane proteins. These noninfectious structures undergo fast-axonal transport towards the axon terminal. However, viral egress and assembly can occur at sites within the axon and not solely at the terminal of the axon. It is likely that assembly occurs as capsid and tegument structures bud into a membranous structure containing viral membrane proteins that forms along the axon shaft. This assembly step would constitute the events of secondary envelopment at the trans-Golgi network or endosomes, as proposed for nonneuronal cells (7, 18, 34). Once assembled, mature virus is within a vesicle capable of fusing with the surface of the axon, releasing an enveloped virus adjacent to axons. The revised model predicts that subvirion structures undergo fast-axonal transport but that assembling virus in a membranous vesicle could also be present in the axons. The key distinction is that these assembling virions will no longer be engaged in fast-axonal transport, a possibility suggested by the work of Smith et al. (31). Some data support this model. We have observed that Us9 null mutants do not infect nonneuronal cells in the optic nerve, although axons contain immunoreactive PRV proteins. We believe that the axons are immunoreactive because they contain capsid and some tegument proteins, but nonneuronal cells in the optic nerve are not infected because the viral membrane proteins necessary for egress from axons are not present (M. J. Tomishima and L. W. Enquist, unpublished results).
Although the revised model is attractive because it unifies apparently contradictory evidence on viral egress from axons, it is important to view it with some caution. This study has addressed where viral egress occurs in vivo, but it has not demonstrated actual assembly of virus in axons. While the model hypothesizes that both cell-to-cell spread and particle infection occur from axons, it is not yet obvious that assembly of mature enveloped virions occurs in axons. Since we do not understand the mechanics of cell-to-cell spread in any detail, it is possible that egress from axon shafts occurs without full assembly of a virion (a possibility discussed in reference 34). For example, it is possible that viral membrane proteins promote formation of a transient pore to facilitate the transfer of capsids from neuron to nonneuronal cell. In fact, when examining the cellular architecture of the nervous system, it is hard to imagine how a fully enveloped particle could exist in the extracellular space between many types of cells in the nervous system. The distance between the axon and the myelin sheaths of oligodendrocytes in the central nervous system varies from approximately 2 to 12 nm, which is insufficient space for a 200-nm virion (26). Other cell contacts, such as the extracellular space between axons and astrocytes, have been estimated to be 20 nm, approximately the size of chemical synapses in the central nervous system (27). It is possible that the membranes of these opposing cells are plastic enough to allow a virion to reside in this extracellular space, despite the large size of the virion. Alternatively, viral assembly might not occur in axons; perhaps these viruses rely exclusively on cell-to-cell spread in the nervous system, a strong possibility given that PRV gD null mutants spread like wild-type virus. Another possibility is that fully enveloped virions emerge only from nonmyelinated axons in a nerve.
Our data suggest that PRV can egress from axon shafts as well as axon terminals and can use gD-independent cell-to-cell spread mechanisms to infect nonneuronal cells in nerves and synaptically connected neurons. The biological significance of spread from axonal shafts to nonneuronal cells is unclear. It might reflect as-yet-unknown aspects of axonal architecture and contacts with supporting cells. Clearly, more work is needed to clarify how alphaherpesviruses move, assemble, and spread in the nervous system.
Acknowledgments
We thank Greg Smith (Northwestern University) for supplying reagents necessary to make the gD null virus and for many constructive discussions on the manuscript. We also thank Leontine Galante for her work that served as a proof of principle necessary to begin this project.
This work was supported by NIH grant NS33506.
REFERENCES
- 1.Babic, N., T. C. Mettenleiter, A. Flamand, and G. Ugolini. 1993. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J. Virol. 67:4421-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bak, I. J., C. H. Markham, M. L. Cook, and J. G. Stevens. 1977. Intra-axonal transport of herpes simplex virus in the rat nervous system. Brain Res. 136:415-429. [DOI] [PubMed] [Google Scholar]
- 3.Card, J. P., L. Rinaman, R. B. Lynn, B. H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Card, J. P., L. Rinaman, J. S. Schwaber, R. R. Miselis, M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1990. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J. Neurosci. 10:1974-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card, J. P., M. E. Whealy, A. K. Robbins, R. Y. Moore, and L. W. Enquist. 1991. Two alpha-herpesviruses are transported differentially in the rodent visual system. Neuron 6:957-969. [DOI] [PubMed] [Google Scholar]
- 7.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 8.Enquist, L. W., J. Dubin, M. E. Whealy, and J. P. Card. 1994. Complementation analysis of pseudorabies virus gE and gI mutants in retinal ganglion cell neurotropism. J. Virol. 68:5275-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field, H. J., and T. J. Hill. 1974. The pathogenesis of pseudorabies virus in mice following peripheral inoculation. J. Gen. Virol. 23:145-157. [DOI] [PubMed] [Google Scholar]
- 10.Hill, T. J., H. J. Field, and A. P. C. Roome. 1972. Intra-axonal localization of herpes simplex virus particles. J. Gen. Virol. 15:253-255. [DOI] [PubMed] [Google Scholar]
- 11.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husak, P. J., T. Kuo, and L. W. Enquist. 2000. Pseudorabies virus membrane proteins gI and gE facilitate anterograde spread of infection in projection-specific neurons in the rat. J. Virol. 74:10975-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kritas, S. K., H. J. Nauwynck, and M. B. Pensaert. 1995. Dissemination of wild-type and gC-, gE- and gI-deleted mutants of Aujeszky's disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J. Gen. Virol. 76:2063-2066. [DOI] [PubMed] [Google Scholar]
- 14.Kritas, S. K., M. B. Pensaert, and T. C. Mettenleiter. 1994. Invasion of spread of single glycoprotein deleted mutants of Aujeszky's disease virus (ADV) in the trigeminal pathway of pigs after intranasal inoculation. Vet. Microbiol. 40:323-334. [DOI] [PubMed] [Google Scholar]
- 15.Kritas, S. K., M. B. Pensaert, and T. C. Mettenleiter. 1994. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky's disease virus in the olfactory nervous pathway of the pig. J. Gen. Virol. 75:2319-2327. [DOI] [PubMed] [Google Scholar]
- 16.LaVail, J. H., K. S. Topp, P. A. Giblin, and J. A. Garner. 1997. Factors that contribute to the transneuronal spread of herpes simplex virus. J. Neurosci. Res. 49:485-496. [PubMed] [Google Scholar]
- 17.Levine, J. M., L. W. Enquist, and J. P. Card. 1998. Reactions of oligodendrocyte precursor cells to alpha herpesvirus infection of the central nervous system. Glia 23:316-328. [PubMed] [Google Scholar]
- 18.Lycke, E., K. Kristensson, B. Svennerholm, A. Vahlne, and R. Ziegler. 1984. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J. Gen. Virol. 65:55-64. [DOI] [PubMed] [Google Scholar]
- 18a.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder, W., J. Pol, T. Kimman, G. Kok, J. Priem, and B. Peeters. 1996. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J. Virol. 70:2191-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohara, P. T., M. S. Chin, and J. H. LaVail. 2000. The spread of herpes simplex virus type 1 from trigeminal neurons to the murine cornea: an immunoelectron microscopy study. J. Virol. 74:4776-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohara, P. T., A. N. Tauscher, and J. H. LaVail. 2001. Two paths for dissemination of herpes simplex virus from infected trigeminal ganglion to the murine cornea. Brain Res. 899:260-263. [DOI] [PubMed] [Google Scholar]
- 23.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moorman. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeters, B., J. Pol, A. Gielkens, and R. Moormann. 1993. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J. Virol. 67:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penfold, M. E. T., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters, A., S. L. Palay, and H. D. Webster. 1991. The fine structure of the nervous system, 3rd ed., p. 147, 252, 290. Oxford University Press, New York, N.Y.
- 27.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J. Virol. 65:5348-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinaman, L., J. P. Card, and L. W. Enquist. 1993. Spatiotemporal response of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J. Neurosci. 13:685-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimeld, C., S. Efstathiou, and T. Hill. 2001. Tracking the spread of a lacZ-tagged herpes simplex virus type 1 between the eye and the nervous system of the mouse: comparison of primary and recurrent infection. J. Virol. 75:5252-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, B. N., B. W. Banfield, C. A. Smeraski, C. L. Wilcox, F. E. Dudek, L. W. Enquist, and G. E. Pickard. 2000. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc. Natl. Acad. Sci. USA 97:9264-9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bi-directional fast axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 98:3466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spear, P. G., R. J. Eisenberg., and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virol. 275:1-8. [DOI] [PubMed] [Google Scholar]
- 33.Tomishima, M. J., and L. W. Enquist. 2001. A conserved α-herpesvirus protein necessary for the axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 35.Watson, R. E., S. T. Wiegand, R. W. Clough, and G. E. Hoffman. 1986. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155-159. [DOI] [PubMed] [Google Scholar]
- 36.Whealy, M. E., J. P. Card, A. K. Robbins, J. R. Dubin, H.-J. Rziha, and L. W. Enquist. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J. Virol. 67:3786-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]