Abstract
Varicella-zoster virus (VZV) glycoprotein I (gI) is dispensable in cell culture; the SCIDhu model of VZV pathogenesis was used to determine whether gI is necessary in vivo. The parental and repaired viruses grew in human skin and thymus/liver implants, but the gI deletion mutant was not infectious. Thus, gI is essential for VZV infectivity in skin and T cells.
Varicella-zoster virus (VZV) causes varicella, or chicken pox, during primary infection and establishes latency in sensory ganglia; VZV reactivation produces herpes zoster, or shingles. In hosts with primary VZV infection, VZV is detected by in situ hybridization in mononuclear cells that have the morphology of T cells (6). Primary and recurrent VZV infections are associated with cutaneous vesicles. Therefore, viral genes that are involved in T-cell viremia and cutaneous infectivity are critical for VZV pathogenesis.
The SCIDhu mouse model provides a unique opportunity to examine genes that mediate the tropism of VZV for skin and T cells (8-10). Low-passage clinical VZV isolates induced lesions in skin implants, with histopathologic changes characteristic of varicella or herpes zoster, and the live attenuated VZV vaccine was infectious but replicated more slowly (8, 9). Blocking the open reading frame 47 (ORF47) kinase prevented replication in skin (10), whereas the naturally occurring glycoprotein E (gE) mutation in the VZV MSP strain was associated with accelerated infectivity (13).
Experiments using VZV-infected cells and transient expression systems demonstrate that gI is likely to play an important role in VZV infection (4). VZV gI forms a heterodimer with gE and shuttles gE between the trans-Golgi network (TGN) and the plasma membrane (11). Using recombinant Oka (rOka) cosmids to make VZV gI deletion mutants, Mallory et al. showed that rOkaΔgI replicated but produced small plaques and less infectious virus in melanoma cells and that characteristic localization of gE to the plasma membrane was disrupted (7). Envelopment of tegument-coated capsids at the cytosolic side of the TGN was grossly abnormal in fibroblasts infected with rOkaΔgI (15). When gI was deleted from a different set of VZV cosmids, designated ROka cosmids, the mutant virus was distinctive for its inability to grow in Vero cells (2). These abnormal phenotypes in cell culture were corrected when repaired viruses were made in which ORF67 was restored at a nonnative site in rOka-gI@Avr or at the native site in ROkaΔ67R (2, 7).
The purpose of these experiments was to assess the role of gI in VZV infectivity in vivo. The approach was to evaluate infectivity in SCIDhu mice with thymus/liver implants that contain human T cells and skin implants that contain all of the differentiated cell types found in the dermal and epidermal layers of human skin (8). Our prediction was that VZV mutants lacking gI would replicate, although more slowly than viruses derived from intact Oka strain cosmids, as was observed in vitro. However, we found that gI expression is essential for VZV replication in both skin and T cells, which are necessary cellular targets during the pathogenesis of primary and recurrent VZV infection in the human host.
Recovery of infectious VZV from skin implants.
The gI deletion mutant virus, rOkaΔgI, has a slow-growth phenotype in cell culture compared to the rOka recombinant and the restored virus, rOka-gI@Avr, that expressed gI from a nonnative site (Fig. 1) (7). To determine whether gI was important for growth in differentiated human tissue in vivo, we investigated the relative capacity of these viruses to grow in human skin implants (Fig. 2). All procedures involving SCID mice were performed in accordance with state and federal regulations and were approved by the Committee for the Humane Use of Animals at SUNY Upstate Medical University (CHUA protocol no. 667). The SCIDhu model of VZV pathogenesis is described in detail elsewhere (8). Before inoculation into skin implants, all VZV strains were passaged three times in MRC-5 or HEL cells (primary human lung fibroblasts). VZV is highly cell associated and loses infectivity when stored frozen, thus, the inocula were freshly prepared and titers were determined for each experiment. Although this results in varied quantities of inocula, especially for the slow-growing gI deletion mutant, concentrations of ≥6 × 104 infected cells/ml are sufficient to initiate infection by wild-type and mutant VZV strains in skin and thymus-liver implants (8-10, 13).
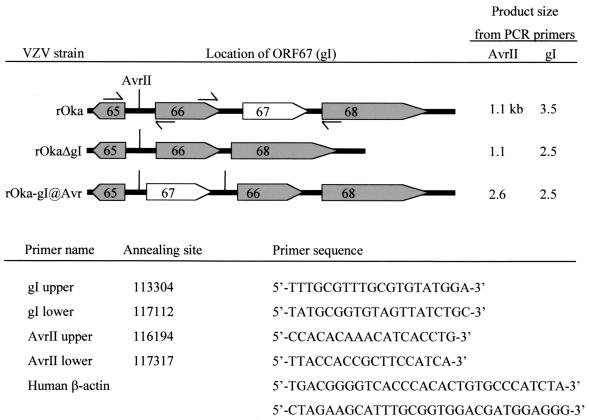
FIG. 1.
Viruses and PCR primers. Arrow boxes indicate the direction of transcription of ORFs 65, 66, 67, and 68. The short vertical lines show the positions of AvrII sites. Facing arrows show the approximate annealing sites of PCR primer pairs that span the AvrII site and ORF67. The sequences and annealing sites of all primers are listed.
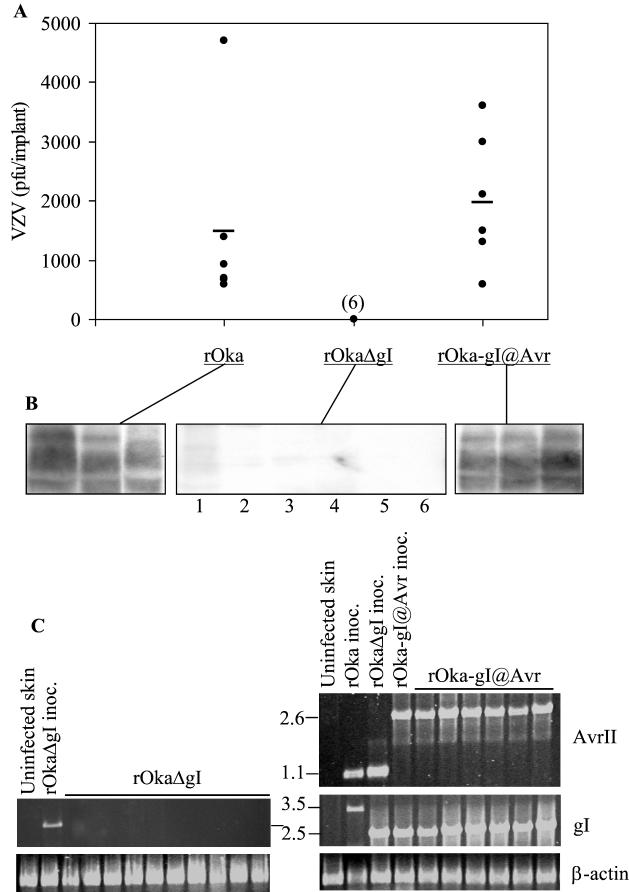
FIG. 2.
Replication of VZV rOka, rOkaΔgI, and rOka-gI@Avr in human skin. (A) Six implants infected with rOka or rOka-gI@Avr were harvested at 10 days postinfection, and six implants infected with rOkaΔgI were harvested at 21 days postinfection. The number of PFU/implant was determined in an infectious focus assay. Each point represents one human skin implant, and the bar indicates the average PFU/implant. Where points overlap, the number of implants analyzed is given in parentheses. (B) Immunoblot analysis of three skin implants infected with rOka or rOka-gI@Avr and six implants infected with rOkaΔgI. VZV proteins in the 70- to 110-kDa range were detected with high-titer human polyclonal antiserum. (C) PCR analysis of the gI region of viruses recovered from skin implants. Primers spanning the AvrII restriction site, gI, or β-actin were used, and the sizes of the fragments produced are given in kilobases (center). These results represent similar data obtained from three separate experiments.
Skin implants were inoculated with rOka (1.2 × 106 infected cells/ml), rOkaΔgI (1.1 × 105 infected cells/ml), or rOka-gI@Avr (7.3 × 105 infected cells/ml). Due to the different levels of virus in the inocula and variable implant sizes, detecting VZV replication in the implants was the measure of virulence in this experiment rather than quantitative virus yield. Implants infected with intact rOka and rOka-gI@Avr were harvested after 10 days. To allow for the potentially slower growth of the gI deletion virus, six implants inoculated with rOkaΔgI were harvested at day 21 and four implants were harvested at day 28. Comparison of the infectious virus yield per implant and the mean PFU/implant showed no detectable replication of rOkaΔgI compared with that of rOka or rOka-gI@Avr (Fig. 2A) (sensitivity of the infectious focus assay was 10 PFU per specimen). Infectious VZV was not detected even at the late day 28 time point in any of the skin implants infected with rOkaΔgI (data not shown). VZV was recovered from implants infected with rOka, with a range of infectious virus titers, which is the typical growth pattern observed when SCIDhu skin implants are inoculated with vaccine Oka (9). Importantly, the yield of infectious virus from implants infected with the restored strain, rOka-gI@Avr, was comparable to that with rOka. The fact that rOka-gI@Avr replicated like rOka indicates that the location of ORF67 within the VZV genome is not critical for skin virulence, as long as gI is produced during replication.
Immunoblot analysis of VZV-infected skin implants.
Immunoblotting was done to ensure that the inability to recover infectious VZV from implants infected with rOkaΔgI corresponded to an absence of viral protein synthesis (Fig. 2B). Protein extracts from skin implants were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes (Millipore, Inc., Bedford Mass.), and stained with amido black (1% naphthol blue black, 45% methanol, 10% acetic acid) to reveal total protein before immunoblot analysis was performed. The high-titer polyclonal human antisera used in these experiments bound to VZV proteins in the 70- to 110-kDa range, representing viral glycoproteins predominantly and were detected using ImmuneStar chemiluminescence reagent (Bio-Rad, Inc., Hercules, Calif.). Viral proteins were detected in all of the skin implants infected with rOka and rOka-gI@Avr that had also yielded virus in the infectious focus assay. Results from three representative samples are shown in Fig. 2B. As expected, VZV antigens were undetectable in five of six implants infected with rOkaΔgI. A faint trace of viral protein was visualized in lane 1 but this was probably due to overflow of the sample from the adjacent well.
PCR analysis of VZV-infected skin implants.
To confirm the genotype of the inocula and the viruses recovered from infected implants, DNA was extracted from skin implants infected with rOka, rOkaΔgI, and rOka-gI@Avr and analyzed by PCR. Approximately 100 ng of DNA was used for PCR detection of VZV and the housekeeping gene β-actin (Stratagene, San Diego, Calif.). Primer sequences and annealing locations (Fig. 1) were derived from VZV Dumas (3). Reaction conditions were 94°C for 1 min, then 35 cycles of 94°C for 30 s, 60°C for 30 s, and 68°C for 1 min, followed by a final 10-min extension at 68°C. The sensitivity of this assay is sufficient to detect 10 pg of VZV DNA. The expected sizes of these PCR products are listed in Fig. 1.
PCR analysis of the rOka-, rOkaΔgI-, and rOka-gI@Avr-infected cell preparations used to inoculate the skin implants confirmed that the input viruses were correct (Fig. 2C). In six skin samples infected with rOka and harvested at day 10, the PCR products were identical to the inoculum (data not shown). Similarly, DNA recovered from six skin implants infected with rOka-gI@Avr had the appropriate PCR pattern. VZV DNA was not detected by PCR analysis with 10 skin implants infected with rOkaΔgI.
Recovery of infectious VZV from thymus-liver implants.
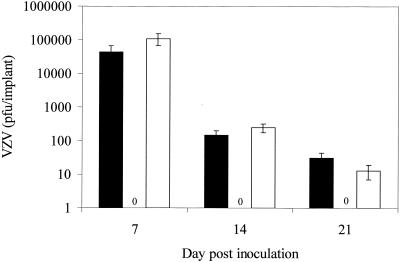
The capacity of VZV laboratory strains and cosmid-generated VZV mutants to replicate in human T cells does not always parallel their growth in skin. For example, the ORF66 kinase was required for full infectivity in T cells but not skin, whereas vaccine Oka grows more slowly in skin but is not different from parent Oka in its growth in T-cell implants (9, 10). Therefore, we tested rOkaΔgI in SCIDhu mice with thymus/liver implants to determine whether this protein was also necessary for replication in T cells. Thymus/liver implants were inoculated with rOka (8.0 × 104 infected cells/ml), rOkaΔgI (2.5 × 106 infected cells/ml), or rOka-gI@Avr (3.3 × 105 infected cells/ml) and harvested after 7, 14, or 21 days. Three or more implants were analyzed for each data point. VZV replication in the thymus/liver implants was assessed by infectious focus assay. The yields of VZV from implants infected with rOka and rOka-gI@Avr were equivalent, indicating that gI expression from either a native or nonnative locus was sufficient for VZV replication in T cells (Fig. 3). The rOka and rOka-gI@Avr viruses recovered from thymus/liver implants were analyzed by PCR to determine whether ORF67 underwent any rearrangements following in vivo replication. As expected, ORF67 was in the native locus in rOka and at the AvrII site in rOka-gI@Avr (data not shown). No infectious VZV was recovered from 12 implants infected with rOkaΔgI (Fig. 3), despite the use of an inoculum that contained 30-fold more virus than the rOka inoculum. Thus, gI was necessary for VZV replication in T cells as well as in skin.
FIG. 3.
Replication of VZV rOka, rOkaΔgI, and rOka-gI@Avr in human T cells. Thymus/liver implants infected with rOka (black), rOkaΔgI (zeros), and rOka-gI@Avr (white) were harvested at 7, 14, and 21 days after infection. The number of PFU/implant was determined using an infectious focus assay. Each bar represents the mean and standard deviation from three to five implants from two separate experiments.
The failure of the ΔgI mutant to replicate in skin and T cells confirms that VZV genes that are dispensable in cell culture must be characterized further within intact tissues. However, examination of the effects of gI deletion on VZV replication in cell culture helps to explain why removing ORF67 is lethal in vivo. The full and partial gI deletion mutants rOkaΔgI, rOkaΔgI-C, and rOkaΔgI-N showed significant decreases in syncytium formation and infectious virus yields in melanoma cells, suggesting an inhibition of cell-cell spread (7). Wang et al. found that the TGN cisternae in human fibroblasts infected with these gI deletion mutants became adherent, viral envelopment was impaired, and virions did not reach post-Golgi structures (15). These studies demonstrated a dramatic effect of gI deletion on virion assembly and egress.
Our interpretation of the requirement for gI expression in differentiated skin and T cells is that the interactions between gI and gE are necessary for VZV replication in vivo (1, 11, 12, 14, 16). These glycoproteins form heterodimers, and gE endocytosis is enhanced substantially in the presence of gI, suggesting that gI regulates the intracellular trafficking of the gE-gI complex (11). In the absence of gI, infected cells showed an unusual punctate distribution of gE on plasma membranes, reduced syncytium formation, and diminished synthesis of the mature 94-kDa form of gE (7). VZV gE is the most abundant viral glycoprotein produced in infected cells (5), and our recent experiments document that gE is essential for VZV replication in vitro (7a). However, these experiments show that gE expression alone is not sufficient for VZV replication in human skin or T cells and that expression of both gE and gI is necessary for VZV virulence.
Acknowledgments
J.M. was supported by NRSA AI09195. The work was supported by Public Health Service grants AI20459, AI36884, and P01-CA49605 to A.M.A.
REFERENCES
- 1.Alconada, A., U. Bauer, L. Baudoux, J. Piette, and B. Hoflack. 1998. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J. Biol. Chem. 273:13430-13436. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, J. I., and H. Nguyen. 1997. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J. Virol. 71:6913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 4.Grose, C. 1990. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu. Rev. Microbiol. 44:59-80. [DOI] [PubMed] [Google Scholar]
- 5.Kinchington, P. R., and J. I. Cohen. 2000. Viral proteins, p. 74-104. In A. M. Arvin and A. A. Gershon (ed.), Varicella-zoster virus: virology and clinical management. Cambridge University Press, Cambridge, United Kingdom.
- 6.Koropchak, C. M., P. S. Diaz, and A. M. Arvin. 1989. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J. Virol. 63:2392-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallory, S., M. Sommer, and A. M. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Mo, C., J. Lee, M. Sommer, C. Grose, and A. M. Arvin. The requirement of varicella-zoster virus glycoprotein E for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology, in press. [DOI] [PubMed]
- 8.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moffat, J. F., L. Zerboni, P. R. Kinchington, C. Grose, H. Kaneshima, and A. M. Arvin. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffat, J. F., L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. USA 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson, J. K., and C. Grose. 1998. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J. Virol. 72:1542-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos, R. A., C. C. Hatfield, N. L. Cole, J. A. Padilla, J. F. Moffat, A. M. Arvin, W. T. Ruyechan, J. Hay, and C. Grose. 2000. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology 275:306-317. [DOI] [PubMed] [Google Scholar]
- 14.Wang, Z. H., M. D. Gershon, O. Lungu, Z. Zhu, and A. A. Gershon. 2000. Trafficking of varicella-zoster virus glycoprotein gI: T(338)-dependent retention in the trans-Golgi network, secretion, and mannose 6-phosphate-inhibitable uptake of the ectodomain. J. Virol. 74:6600-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, Z. H., M. D. Gershon, O. Lungu, Z. Zhu, S. Mallory, A. M. Arvin, and A. A. Gershon. 2001. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J. Virol. 75:323-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao, Z., W. Jackson, B. Forghani, and C. Grose. 1993. Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia virus-T7 RNA polymerase transfection system. J. Virol. 67:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]