Abstract
Two enzymes, soluble guanylyl cyclase and cytochrome c oxidase, have been shown to be exquisitely sensitive to nitric oxide (NO) at low physiological concentrations. Activation of the soluble guanylyl cyclase by endogenous NO and the consequent increase in the second messenger cyclic GMP are now known to control a variety of biological functions. Cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, is inhibited by NO. However, it is not clear whether NO produced by the constitutive NO synthase interacts with cytochrome c oxidase, nor is it known what the biological consequences of such an interaction might be. We now show that NO generated by vascular endothelial cells under basal and stimulated conditions modulates the respiration of these cells in response to acute changes in oxygen concentration. This action occurs at the cytochrome c oxidase and depends on influx of calcium. Thus, NO plays a physiological role in adjusting the capacity of this enzyme to use oxygen, allowing endothelial cells to adapt to acute changes in their environment.
Evidence in favor of a role of endogenous nitric oxide (NO) as a modulator of cell respiration has been derived from experiments in cells activated with cytokines and bacterial products in which NO is generated continuously in large quantities by the inducible NO synthase (NOS). In these conditions, NO-induced inhibition of cell respiration is persistent and attributable to nonselective inhibition of various mitochondrial enzymes, including complexes I-IV in the respiratory chain. Such inhibition contributes to the pathological actions of NO (1). On the other hand, experiments in animals have suggested that inhibition of endogenous generation of NO increases whole body oxygen consumption (2, 3), and bradykinin (Bk) and carbachol have been shown to reduce oxygen consumption in skeletal and cardiac muscle in a manner that could be prevented by an inhibitor of NOS (3–5).
Exogenous administration of low concentrations of NO inhibits cytochrome c oxidase (complex IV in the mitochondrial respiratory chain) in a variety of cells and isolated mitochondria. Such inhibition is competitive with oxygen and is fully reversible (6–9) even after several hours (10). These findings suggest that endogenous NO may regulate cell respiration. To test this hypothesis, we have analyzed the effect of endogenous NO, generated under basal conditions and after stimulation with Bk and ATP, on respiration in porcine aortic endothelial cells. Our results show that endogenously released NO, by acting on cytochrome c oxidase, is responsible for the physiological regulation of respiration in these cells.
MATERIALS AND METHODS
Materials.
Culture media and fetal calf serum were from GIBCO. Fura-2 acetoxymethylester was from Calbiochem. Other reagents were from Sigma.
Cell Culture and Preparation.
Endothelial cells were prepared from fresh porcine thoracic aortae obtained from the abattoir, were cultured overnight in DMEM 20% fetal calf serum, and then were grown to confluence on microcarrier beads under mild periodic stirring as described (11). After 7 days, preparations with <90% of the beads covered by a confluent layer of cells or with an initial oxygen consumption rate lower than 10 μM⋅min−1 were discarded. The beads covered with cells then were washed four times by sedimentation, were counted as described (11), and were resuspended at a density of 107 cells·ml−1 in an incubation medium consisting of (in mM): 118 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1 CaCl2, 20 glucose, 0.3 l-arginine, and 25 Hepes (pH 7.2).
Measurements of Oxygen Consumption and NO Generation.
Cell preparations (0.75 ml) were analyzed in a gas-tight vessel maintained at 37°C, equipped with both a Clark-type oxygen electrode (Rank Brothers, Cambridge, U.K.) and an NO electrode (Iso-NO, 2-mm diameter tip, World Precision Instruments, Sarasota, FL) connected to a chart recorder and calibrated as described (6). Recording was initiated immediately, and traces were analyzed when the concentration of oxygen fell below 100 μM. Release of NO and cellular oxygen consumption thus could be measured simultaneously. Preincubation with Nω-nitro-l-arginine methyl ester (l-NAME), d-NAME, or oxyhaemoglobin was for 20 min. In those experiments in which cytochrome c oxidase activity was analyzed, the complex III inhibitor myxothiazol and the cytochrome c oxidase substrates N,N,N′,N′-tetramethyl-p-phenylenediamine and ascorbic acid were added to the cells 5 min before the beginning of the recording.
Intracellular Calcium Concentration ([Ca2+]i) Measurements.
Cell preparations were loaded with the Ca2+-sensitive dye fura-2 (4.5 μM), administered as acetoxymethylester for 40 min at 37°C. Cell preparations then were diluted (5 × 106 cells), and the concentrations of intracellular calcium ([Ca2+]i) before and after challenge with Bk and ATP were analyzed in a Perkin–Elmer LS-5B fluorimeter as described (12). Ca2+ release and Ca2+ influx were analyzed separately, resuspending cell samples in a medium supplemented with 2 mM EGTA (Ca2+-free medium; estimated concentration of extracellular calcium was <10−8 M). After stimulation with Bk or ATP, the recording was continued until the end of the first [Ca2+]i peak (intracellular Ca2+ release), at which time Ca2+ (2 mM) was reintroduced into the medium, and the second peak (Ca2+ influx) was recorded.
Statistical Analysis.
The results are expressed as mean ± SEM; n represents the number of individual experiments. Statistical analysis was performed by Student’s t test for unpaired variables (two-tailed).
RESULTS
The rate of oxygen consumption of endothelial cell suspensions respiring on glucose was assayed in a gas-tight vessel equipped with electrodes to detect oxygen and NO. Cell respiration was analyzed over a continuous gradient of oxygen concentration decreasing from 100 to 0 μM. The initial rate of oxygen consumption in control cells was 13.1 ± 1.3 μM⋅min−1 (n = 8). As the oxygen concentration fell, the rate of oxygen consumption declined (Fig. 1). On reoxygenation, the oxygen consumption was restored to 77.6 ± 5.7% (n = 3) of the original rate (Fig. 1). The oxygen concentration at which half maximum inhibition of oxygen consumption rate (p50; ref. 13) was observed was 9.8 ± 1.0 μM. Seventy-five percent inhibition of oxygen consumption occurred at 6.8 ± 0.6 μM oxygen.
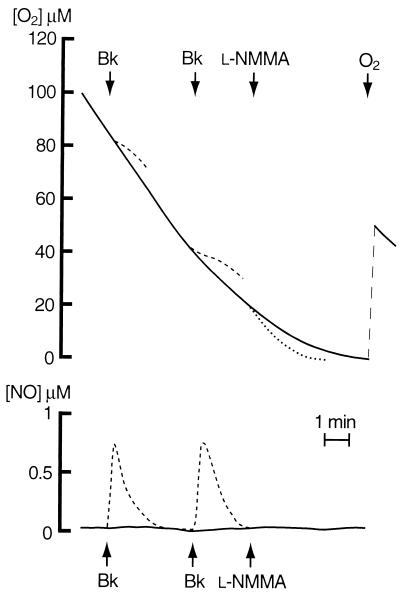
Figure 1.
Effects of exposure to a continuous gradient of oxygen and to Bk and Nω-monomethyl-l-arginine (l-NMMA) on endothelial cell respiration and NO generation. Here and in the following figures, the upper panel shows the consumption of oxygen whereas the lower panel shows the generation of NO by the cells. The continuous traces are from a representative polarographic record of cells respiring on glucose without any drug addition. The dashed traces show the effects of cell stimulation with Bk (1 μM), added as indicated by the arrows, on both oxygen consumption and NO generation. The dotted traces show the effect of addition of Nω-monomethyl-l-arginine (0.5 mM). The effect of reoxygenation on oxygen consumption also is shown.
Addition of the NOS inhibitor Nω-monomethyl-l-arginine (0.5 mM) resulted in an immediate increase in oxygen consumption (dotted line in Fig. 1). Pretreatment of the cells with l-NAME (0.5 mM), a more potent inhibitor of the endothelial NOS (Richard Knowles, personal communication), but not with d-NAME (0.5 mM), its inactive enantiomer, resulted in an initial rate of oxygen consumption that was significantly higher than that in the control cells (18.7 ± 1.93 μM⋅min−1, n = 5, P < 0.03 vs. controls). This rate was independent of the oxygen concentration down to 4 μM oxygen. Below this concentration there was an abrupt decrease in respiration rate, the nature of which is not clear at present (Fig. 2). In the presence of the NO scavenger hemoglobin (8 μM), the dependence of oxygen consumption on oxygen concentration was similar to that in the presence of NOS inhibitors. Under basal conditions, NO was not detected outside of the cells by any of the methods used, including chemiluminescence and the NO electrode (lowest level of detection, 1 and 10 nM, respectively; refs. 14 and 15).
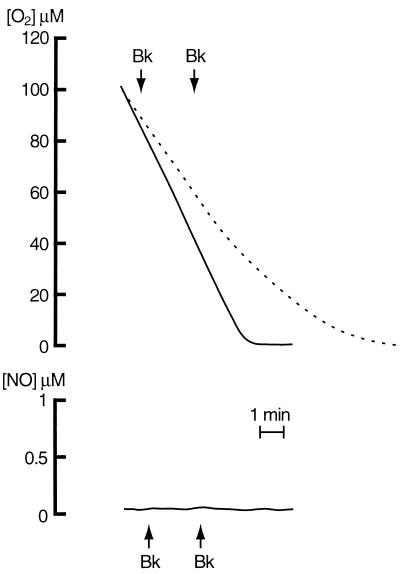
Figure 2.
Effects of l-NAME pretreatment on respiration and NO generation by endothelial cells. The continuous traces are from a representative recording by cells respiring on glucose and pretreated for 20 min with l-NAME (0.5 mM). Bk (1 μM) was added as indicated by the arrows. Oxygen consumption by cells not exposed to the NOS inhibitor is superimposed for comparison as a dashed line; these cells were not treated with Bk. In some experiments, the effect on respiration of l-NAME at 20 min was only partial.
Vasoactive agents such as Bk and ATP are known to increase the generation of NO released by endothelial NOS (16–18). Administration of Bk (1 μM) to control cells when the concentration of oxygen was 80 and 40 μM gave rise to a transient generation of NO that reached a peak concentration of 0.76 ± 0.09 μM (n = 5) and was similar at both concentrations of oxygen (Fig. 1, dashed lines). Cell respiration was inhibited in parallel with the generation of NO. This inhibition was always transient, and its degree, unlike the generation of NO, depended on the concentration of oxygen (33.9 ± 2.3 and 58.1 ± 2.7%, n = 5, P < 0.001, at 80 and 40 μM oxygen, respectively). In cells preincubated with l-NAME, Bk did not elicit any release of NO, nor did it modify the rate of oxygen consumption (Fig. 2). Similar results were obtained with ATP (100 μM; data not shown).
To investigate whether the inhibition of respiration was occurring at the level of cytochrome c oxidase, experiments such as those described in Figs. 1 and 2 were performed in the presence of myxothiazol (0.5 μM), which inhibits mitochondrial complex III. Cell respiration was maintained via the cytochrome c oxidase alone by using N,N,N′,N′-tetramethyl-p-phenylenediamine (120 μM) as the electron donor plus excess ascorbic acid (4 mM) as the primary reducing agent. In these conditions, the oxygen consumption rate was 13.64 ± 1.32 μM⋅min−1 (n = 5) and declined as the oxygen concentration fell, with a p50 of 10.2 ± 1.1 μM. Administration of Bk (1 μM) at 80 μM oxygen resulted in release of NO (0.81 ± 0.09 μM, n = 5) and in a transient inhibition of oxygen consumption (36.05 ± 2.93%, n = 5), similar to that observed in cells respiring on glucose. Pretreatment with l-NAME in these conditions increased the oxygen consumption rate to 18.77 ± 1.11 μM⋅min−1 (n = 5, P < 0.02) vs. control, which did not vary with oxygen concentrations from 100 μM down to at least 4 μM. Administration of Bk to l-NAME-treated cells did not result in the release of NO or in inhibition of respiration.
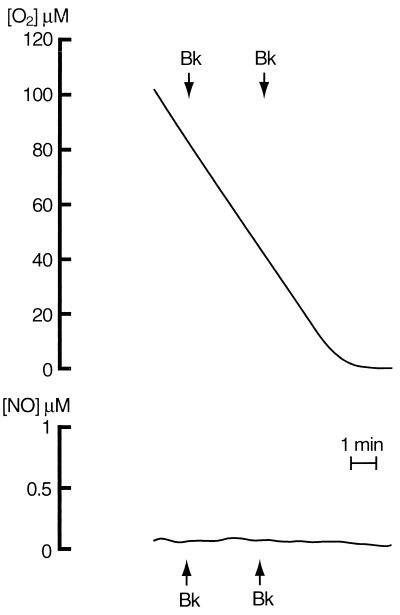
Bk and ATP are known to act by increasing [Ca2+]i, eliciting both Ca2+ release from intracellular stores and Ca2+ influx from the extracellular environment. The generation of NO, [Ca2+]i, and oxygen consumption were compared in the presence and absence of the Ca2+-chelating agent EGTA, which prevents the influx but not the release of intracellular Ca2+. Basal [Ca2+]i was 81.32 ± 3.5 nM (n = 3) and was unchanged by EGTA. However, EGTA treatment impaired the ability of the cells to modify their respiratory rate in response to different oxygen concentrations (compare Fig. 3 with Fig. 1). Readdition of Ca2+ restored the ability of the cells to control their respiration (data not shown). Administration of Bk (1 μM) or ATP (100 μM) in the presence of EGTA resulted in [Ca2+]i increases of 264 ± 2.1 and of 178 ± 0.8% (n = 3), respectively, caused by the release of intracellular Ca2+. Under these conditions, however, there was neither release of NO nor inhibition of oxygen consumption (Fig. 3 and data not shown).
Figure 3.
Effects of EGTA on respiration and NO generation by endothelial cells. EGTA (2 mM) was added immediately before the beginning of the recording. Bk (1 μM) was added as indicated by the arrows.
DISCUSSION
These results show that, in endothelial cells, the rate of oxygen consumption varies with its concentration, suggesting the existence of an intracellular mechanism that controls cell respiration. Indeed, at oxygen concentrations likely to be present in tissues (<30 μM; ref. 13) the inhibition of respiration is more evident. Endogenous generation of NO by Ca2+-dependent NOS appears to play a key role in such a mechanism because the control of oxygen consumption by cells was reduced dramatically in the presence of NOS inhibitors. Furthermore, when additional NO was generated with Bk and ATP, there was a concomitant, transient inhibition of respiration that depended on the concentration of oxygen. The experiments in which cells were respiring on N,N,N′,N′-tetramethyl-p-phenylenediamine and ascorbic acid indicate that the action of NO is exerted at the level of the cytochrome c oxidase.
Our results also show that Ca2+ influx from the extracellular environment is necessary for the control of respiration by NO both under basal conditions and after treatment with Bk and ATP. This finding is consistent with previous observations demonstrating that Ca2+ influx is crucial for NO generation by NOS in endothelial cells both under basal conditions (19) and after stimulation with vasoactive agents that increase [Ca2+]i (16–18).
Thus, cytochrome c oxidase seems to be constantly regulated by NO generated by NOS, which itself is regulated by Ca2+ influx. Although a basal generation of NO may be sufficient to exert this control during resting conditions, it is likely that increases in [Ca2+]i during cell perturbation, whether physiological or pathophysiological, may result in an increase in NO production and further inhibition of cytochrome c oxidase as an adaptive mechanism of the cell. Because it is known that increases in [Ca2+]i also can activate mitochondrial dehydrogenases (20), the way in which an equilibrium is established during cell perturbation requires further investigation. In this context, it is interesting that acute hypoxia could lead both to increases in Ca2+ influx and NO synthesis (21, 22), suggesting that, as the concentration of oxygen in the environment decreases, the cell adapts itself by reducing its oxygen requirement.
Inhibition of oxygen consumption by 50% has been reported to occur at ratios of NO:oxygen concentrations ranging from 1:500 to 1:150 (6, 23). Because half-maximal inhibition of respiration in basal conditions occurs at 9.8 μM oxygen in our experiments, a cytosolic concentration of NO in the range of 20–65 nM may be predicted. However, we were unable to detect NO in nonstimulated cells. This may indicate that NO is generated under basal conditions in the discrete cell regions in which control of respiration actually is exerted.
It is not clear whether the Ca2+-dependent NOS involved in the control of respiration is the endothelial type III NOS or the recently described mitochondrial isoform, a Ca2+-dependent enzyme that is able to inhibit oxygen consumption in isolated mitochondria (24–26). Our results are consistent with both possibilities. In endothelial cells, Ca2+ influx could activate the endothelial type III NOS clustered in specific sites beneath the plasma membrane (16–18, 27), or it could activate the mitochondrial enzyme (28). If, indeed, the mitochondrial enzyme is responsible and this enzyme is present in the mitochondria of all cells, then this may be a general biological mechanism of regulation of cell respiration. This will explain, at least in part, why basal oxygen consumption is increased in a whole animal when treated with a NOS inhibitor (2, 3). If, in endothelial cells, both endothelial type III NOS and the mitochondrial enzyme are present, a cooperation between the two enzymes cannot be excluded.
The mechanism underlying sensing of acute variations in oxygen concentration has not been fully elucidated. Cumulative evidence indicates that a haem protein is involved (29), and cytochrome c oxidase has been proposed as a candidate (30, 31). However, the Km (or p50) for oxygen observed in the isolated enzyme and mitochondrial preparations is 5–10× lower than that measured in whole cells and tissues (32–36). Several hypotheses have been put forward to explain this discrepancy, such as the oxygen gradient across the plasma membrane, clustering of mitochondria, or changes in the concentrations of pyridine nucleotides (13, 37). Our data go at least some way toward explaining these differences because it is known that nanomolar concentrations of NO interact with cytochrome c oxidase to increase its apparent Km for oxygen in mitochondria (6), and we now show that endogenous NO contributes to the lower affinity of cytochrome c oxidase in endothelial cells.
In conclusion, our results suggest that cytochrome c oxidase, modulated by the NO:oxygen ratio, acts as an oxygen sensor. Stable, low concentrations of NO, continuously generated by NOS, are sufficient to explain the decrease in respiration that occurs as the concentration of oxygen decreases. In these conditions, oxygen consumption becomes dependent on oxygen concentration; the classical paradigm about the independence of oxygen consumption along a wide range of oxygen concentrations should be corrected in terms of NO metabolism. This system is enhanced on demand by an increase in Ca2+ influx, which further activates NOS. It remains to be investigated under which conditions the endothelial cells further down-regulate their oxygen requirement.
Acknowledgments
We thank Antonia Orsi for her help with the endothelial cell culture and Annie Higgs for her help in the preparation of the manuscript. This work was supported in part by grants from the European Economic Community and Consiglio Nazionale delle Ricerche (Italy) (to E.C.), the Biotechnology and Biological Sciences Research Council, Wellcome Trust and Royal Society (to G.C.B.), and Glaxo Wellcome (to S.M.).
ABBREVIATIONS
- NO
nitric oxide
- NOS
nitric oxide synthase
- Bk
bradykinin
- [Ca2+]i
intracellular Ca2+ concentration
- l-NAME
Nω-nitro-l-arginine methyl ester
References
- 1.Bolaños J P, Almeida A, Stewart V, Peuchen S, Land J M, Clark J B, Heales S J. J Neurochem. 1997;68:2227–2240. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- 2.Shen W, Xu X, Ochoa M, Zhao G, Wolin M S, Hintze T H. Circ Res. 1994;75:1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- 3.Shen W, Hintze T H, Wolin M S. Circulation. 1995;92:3505–3512. doi: 10.1161/01.cir.92.12.3505. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y W, Shen W, Zhao G, Xu X, Wolin M S, Hintze T H. Circ Res. 1996;79:381–387. doi: 10.1161/01.res.79.3.381. [DOI] [PubMed] [Google Scholar]
- 5.Poderoso J J, Peralta J G, Lisdero C L, Carreras M C, Radisic M, Schöpfer F, Cadenas E, Boveris A. Am J Physiol. 1998;274:C112–C119. doi: 10.1152/ajpcell.1998.274.1.C112. [DOI] [PubMed] [Google Scholar]
- 6.Brown G C, Cooper C E. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 7.Cleeter M W, Cooper J M, Darley-Usmar V M, Moncada S, Schapira A H. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 8.Schweizer M, Richter C. Biochem Biophys Res Commun. 1994;204:169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- 9.Brown G C. FEBS Lett. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 10.Clementi E, Brown G C, Feelisch M, Moncada S. Proc Natl Acad Sci USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gryglewski R J, Moncada S, Palmer R M J. Br J Pharmacol. 1986;87:685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clementi E, Scheer H, Zacchetti D, Fasolato C, Pozzan T, Meldolesi J. J Biol Chem. 1992;267:2164–2172. [PubMed] [Google Scholar]
- 13.Jones D P. Am J Physiol. 1986;250:C663–C675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- 14.Palmer R M J, Ferrige A G, Moncada S. Nature (London) 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 15.Malinski T, Czuchajowski L. In: Methods in Nitric Oxide Research. Feelisch M, Stamler J S, editors. New York: Wiley; 1996. pp. 319–339. [Google Scholar]
- 16.Buckley B J, Mirza Z, Whorton A R. Am J Physiol. 1995;269:C757–C765. doi: 10.1152/ajpcell.1995.269.3.C757. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Shin W S, Kawaguchi H, Inukai M, Kato M, Sakamoto A, Uehara Y, Miyamoto M, Shimamoto N, Korenaga R, et al. J Biol Chem. 1996;271:5647–5655. doi: 10.1074/jbc.271.10.5647. [DOI] [PubMed] [Google Scholar]
- 18.Lantoine F, Iouzalen L, Devynck M A, Millanvoye-Van Brussel E, David-Dufilho M. Biochem J. 1998;330:695–699. doi: 10.1042/bj3300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Jaramillo P, Gonzalez M C, Palmer R M J, Moncada S. Br J Pharmacol. 1990;101:489–493. doi: 10.1111/j.1476-5381.1990.tb12735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunter T E, Gunter K K, Sheu S-S, Gavin C E. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 21.Xu X P, Pollock J S, Tanner M A, Myers P R. Cardiovasc Res. 1995;30:841–847. [PubMed] [Google Scholar]
- 22.Cornfield D N, Stevens T, McMurtry I F, Abman S H, Rodman D M. Am J Physiol. 1994;266:L469–L475. doi: 10.1152/ajplung.1994.266.4.L469. [DOI] [PubMed] [Google Scholar]
- 23.Boveris, A., Costa, L., Cadenas, E. & Poderoso, J. J. (1999) Methods. Enzymol., in press. [DOI] [PubMed]
- 24.Ghafourifar P, Richter C. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 25.Giulivi C, Poderoso J J, Boveris A. J Biol Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 26.Giulivi C. Biochem J. 1998;332:673–679. doi: 10.1042/bj3320673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Cardena G, Oh P, Liu J, Schnitzer J E, Sessa W C. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrie A M, Rizzuto R, Pozzan T, Simpson A W. J Biol Chem. 1996;271:10753–10759. doi: 10.1074/jbc.271.18.10753. [DOI] [PubMed] [Google Scholar]
- 29.Bunn H F, Poyton R O. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 30.Duchen M R, Biscoe T J. J Physiol (London) 1992;450:13–31. doi: 10.1113/jphysiol.1992.sp019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson D F, Mokashi A, Chugh D, Vinogradov S, Osanai S, Lahiri S. FEBS Lett. 1994;351:370–374. doi: 10.1016/0014-5793(94)00887-6. [DOI] [PubMed] [Google Scholar]
- 32.Mills E, Jobsis F F. J Neurophysiol. 1972;35:405–428. doi: 10.1152/jn.1972.35.4.405. [DOI] [PubMed] [Google Scholar]
- 33.Kariman K, Hempel F G, Jobsis F F. J Appl Physiol. 1983;55:1057–1063. doi: 10.1152/jappl.1983.55.4.1057. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy F G, Jones D P. Am J Physiol. 1986;250:C374–C383. doi: 10.1152/ajpcell.1986.250.3.C374. [DOI] [PubMed] [Google Scholar]
- 35.Gnaiger E, Steinlechner-Maran R, Méndez G, Eberl T, Margreiter R. J Bioenerg Biomembr. 1995;27:583–596. doi: 10.1007/BF02111656. [DOI] [PubMed] [Google Scholar]
- 36.Budinger G R, Chandel N, Sha Z H, Li C Q, Melmed A, Becker L B, Schumacker P T. Am J Physiol. 1996;270:L44–L53. doi: 10.1152/ajplung.1996.270.1.L44. [DOI] [PubMed] [Google Scholar]
- 37.Tamura M, Hazeki O. Annu Rev Physiol. 1989;51:813–834. doi: 10.1146/annurev.ph.51.030189.004121. [DOI] [PubMed] [Google Scholar]