Abstract
Theiler's murine encephalomyelitis virus (TMEV) is a natural pathogen of the mouse. The different strains of TMEV are divided into two subgroups according to the pathology they provoke. The neurovirulent strains GDVII and FA induce an acute fatal encephalitis, while persistent strains, like DA and BeAn, cause a chronic demyelinating disease associated with viral persistence in the central nervous system. Different receptor usage was proposed to account for most of the phenotype difference between neurovirulent and persistent strains. Persistent but not neurovirulent strains were shown to bind sialic acid. We characterized DA and GDVII derivatives adapted to grow on CHO-K1 cells. Expression of glycosaminoglycans did not influence infection of CHO-K1 cells by parental and adapted viruses. Mutations resulting from adaptation of DA and GDVII to CHO-K1 cells notably mapped to the well-characterized VP1 CD and VP2 EF loops of the capsid. Adaptation of the DA virus to CHO-K1 cells correlated with decreased sialic acid usage for entry. In contrast, adaptation of the GDVII virus to CHO-K1 cells correlated with the appearance of a weak sialic acid usage for entry. The sialic acid binding capacity of the GDVII variant resulted from a single amino acid mutation (VP1-51, Asn→Ser) located out of the sialic acid binding region defined for virus DA. Mutations affecting tropism in vitro and sialic acid binding dramatically affected the persistence and neurovirulence of the viruses.
Viruses use a wide variety of surface molecules as receptors, including proteins, carbohydrates, and glycolipids. The receptor has been identified for several picornaviruses, which form a large family of genetically and structurally closely related positive-stranded RNA viruses. In some cases, expression of the receptor is sufficient to promote virus entry in otherwise resistant cells. This is the case for CD155 and the intercellular adhesion molecule-1 (ICAM-1), which are the receptors of poliovirus and human rhinovirus-14, respectively (7, 11, 26, 34). For other picornaviruses, such as foot-and-mouth disease virus (FMDV) and some echoviruses, there is evidence for participation of additional cell surface molecules in virus entry, heparan sulfate and β2-microglobulin, respectively (7). In the case of FMDV, it seems that the interaction between the virus and heparan sulfate is cell type dependent (14). In this case, heparan sulfate is suggested to promote the concentration of virus particles at the cell surface and to favor the interaction with the actual receptor, which has been identified as the αvβ3 vitronectin receptor (29).
Theiler's murine encephalomyelitis virus (TMEV) is a picornavirus classified in the Cardiovirus genus. This virus is a highly neurotropic natural pathogen of the mouse (36). TMEV strains are divided into two subgroups according to the disease they provoke in the central nervous system. Viruses of the first subgroup, including the GDVII and FA strains, are highly neurovirulent. These viruses replicate mostly in neurons and kill their host in a few days from fatal encephalitis.
The other subgroup of viruses includes strains DA and BeAn, which are much less virulent and have a striking ability to persist in the central nervous system of the mouse in spite of a strong and specific immune response. These viruses cause a biphasic disease (20, 30). The virus first provokes a mild and transient encephalitis. Later, the virus persists in the white matter of the spinal cord and is essentially found in macrophages, oligodendrocytes, and astrocytes (1). Viral persistence is associated with chronic inflammation and with lesions of primary demyelination, reminiscent of those observed in multiple sclerosis.
Exchanging the capsid-coding regions between neurovirulent and persistent strains resulted in almost complete exchange of the virus phenotypes (4, 9, 24). Although the viral replication signal of the VP2 region (21) and the immunogenicity of the capsid might influence the phenotype of the recombinants, the observed exchange of phenotype most likely resulted from the capsid-receptor interaction that influences the outcome of the infection by directing the tropism of the virus within the central nervous system. It has been suggested that the different strains of TMEV bind the same 34-kDa glycoprotein but in different ways (18). Neurovirulent strains would bind the protein moiety of the receptor, while persistent strains would bind both the protein and polysaccharide parts of the protein (8). On the other hand, the isolation of an L929 cell line mutant resistant to GDVII but susceptible to DA virus infection (17) pleaded in favor of distinct receptor or coreceptor usage for neurovirulent and persistent strains of TMEV.
Zhou et al. showed that persistent strains but not neurovirulent TMEV strains could bind sialic acid residues at the surface of susceptible cells (39). Crystallization of the complex between the DA virus and the sialic acid moiety-mimicking molecule sialyllactose revealed that the capsid amino acids responsible for binding are clustered in the VP2 EF loop, close to the VP1 CD loop and the VP3 carboxy terminus (40). Interestingly, in addition to their role in sialic acid binding, the VP2 EF loop (also referred to as puff B) and the VP1 CD loop have been associated with a number of properties. These loops are the most exposed regions at the surface of the virion. They differ between neurovirulent and persistent strains (27), and they correspond to major antigenic sites for neutralizing antibodies. Mutations in these loops have been associated with a dramatic attenuation of viral persistence (16, 25, 31, 32, 38, 41, 42). Mutations in these loops also occurred in a DA virus variant (KJ6) adapted to grow on L929 cells, suggesting that subtle modifications of the capsid in this region modulate tropism by altering the affinity of the capsid for the receptor present on a given cell line (16).
Here, we analyzed DA and GDVII derivatives adapted to infect CHO-K1 cells. These viruses contained mutations notably mapping to the VP1 CD loop and VP2 EF loop. Mutations in DA correlated with decreased sialic acid usage. In contrast, mutations found in the GDVII mutant promoted weak but consistent sialic acid binding. Surprisingly, this binding capacity was found not to be due to the VP2 EF loop but to result from the mutation of a single amino acid (VP1-51) located out of the sialic acid binding region defined for persistent viruses. Mutations affecting viral tropism in vitro and sialic acid binding also dramatically affected the virulence and persistence of the viruses.
MATERIALS AND METHODS
Cell culture.
L929 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 1 mM sodium pyruvate. BHK-21 cells were cultured in Glasgow's modified Eagle's medium supplemented with 10% newborn bovine serum, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 130 g of tryptose phosphate broth per liter. CHO-K1 cells and their glycosaminoglycan-deficient derivatives pgsA-745 (glycosaminoglycan deficient) (5), pgsD-677 (heparan sulfate polymerization deficient) (6), and pgsE-606 (heparan sulfate N-sulfotransferase deficient) (2) were cultured in Ham's F12 medium supplemented with 5% fetal bovine serum, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml. Sera and culture media were from Gibco-BRL (Invitrogen Life Technologies, Merelbeke, Belgium).
Cloning adapted viruses.
Viruses DA1 and GDVII were individually passaged on CHO-K1 cells (11 passages for DA1 and 12 passages for GDVII) until a variant virus population promoting complete cytopathic effect on these cells appeared. Adaptation to CHO-K1 cells most likely resulted from capsid mutations, as observed previously in the case of adaptation of the virus to L929 cells (16) and in the case of the adaptation of the brain-derived strain to cell culture (unpublished observations). Thus, to obtain cDNA clones of viruses with enhanced capacity to infect CHO-K1 cells, we substituted the capsid-coding regions of the adapted virus for that of the parental viruses in the parental cDNA clones.
To this end, total RNA was extracted from the supernatant of CHO-K1 cell cultures infected with these variant virus populations. A cDNA fragment containing the capsid-encoding region of the virus adapted to CHO-K1 cells was amplified by reverse transcription (RT)-PCR and cloned to replace the corresponding region in the parental full-length cDNA clones (Fig. 1). For these constructions, plasmids pVD7 and pTM380 were used as vectors instead of the parental pTMDA1 and pTMGDVII plasmids, respectively. These plasmids are pTMDA1 and pTMGDVII derivatives carrying deletions in the region to be exchanged that facilitated the identification of recombinant clones (28). Primers used for PCR amplification were TM127 (5′-AGC GCC TCT GTA GGG AAG CCA-3′) and TM107 (5′-CGC CCA TGC ACA CGA GCA TTC-3′) for the DA1 derivative and TM4 (5′-TTC CCT CCA TCG CGA CGT GGT-3′) and TM107 for the GDVII derivative. The Pfu polymerase (Stratagene) was used to minimize mutations introduced by the PCR procedure. The region amplified from the DA1 variant population was then cloned as a BsiWI-BamHI fragment (nucleotides 1265 to 3925 of pTMDA1) to replace the corresponding region in plasmid pVD7. Similarly, an MluI-BsiMI fragment (nucleotides 637 to 3930 of pTMGDVII) from the GDVII variant population was used to replace the corresponding region in plasmid pTM380.
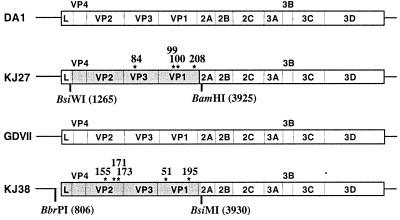
FIG. 1.
Genomes of KJ27 and KJ38. pKJ27 is a pTMDA1 derivative carrying a BsiWI-BamHI fragment cloned from the DA1 variant virus population passaged on CHO-K1 cells. pKJ38 is a pTMGDVII derivative carrying a BbrPI-BsiMI fragment from the CHO-K1-adapted GDVII variant virus population passaged on CHO-K1 cells. The fragments cloned from the CHO-K1-adapted viruses are shaded gray (nucleotide coordinates of the restriction sites are indicated in parentheses). Amino acids differing between wild-type and adapted viruses are indicated by asterisks. Their coordinates are given in amino acid numbering for each protein.
Viruses produced from four cDNA clones for the DA1 derivative and from three cDNAs for the GDVII derivative were tested on CHO-K1 cells to compare their infectivity for these cells. Two viruses, named KJ27 (DA1 derivative) and KJ28 (GDVII derivative), were retained because of their rapid cytopathic effect on CHO-K1 cells. The plasmids carrying the full-length cDNAs of these viruses were called pKJ27 and pKJ28, respectively. The regions of pKJ27 and pKJ28 that were cloned by PCR from the CHO-K1-adapted virus population were sequenced with T7 DNA polymerase (Amersham-Pharmacia Biotech). KJ28 contained one mutation in the 5′ noncoding region in addition to the mutations found in the capsid-encoding region. Analysis of recombinant viruses constructed from KJ28 indicated that this mutation had no influence on CHO-K1 cell adaptation (data not shown). Thus, the work was carried out with the recombinant virus KJ38 (and the corresponding plasmid pKJ38), lacking the mutation in the 5′ noncoding region. KJ38 carries the BbrPI-BsiMI region (nucleotides 806 to 3930) of the GDVII virus adapted to CHO-K1 cells (Fig. 1).
KJ6, KJ27, and KJ38 derivative constructions.
To evaluate the individual role of mutations present in the capsid region of the previously constructed KJ6 virus (16), recombinants were constructed by introducing different fragments from pKJ6 in pTMDA1. The exchanged fragments were the BsiWI-SphI fragment (nucleotides 1265 to 2162) for plasmid pTM745, the BsiWI-Van91I fragment (nucleotides 1265 to 2983) for plasmid pTM746, and the Van91I-BamHI fragment (nucleotides 2983 to 3925) for plasmid pTM747.
Similarly, mutations of KJ27 were separately introduced in a wild-type DA1 background to form viruses OT4, OT5, OT10, and OT11. pOT5 resulted from the insertion of the SphI-Van9I1 fragment (nucleotides 2162 to 2983) of plasmid pKJ27 in pTMDA1. pOT4 resulted from the insertion of a Van91I-BamHI fragment (nucleotides 2983 to 3925) from pKJ27 in plasmid pTMDA1. pOT10 and pOT11 were constructed by exchanging an NdeI-NdeI fragment (nucleotides 3516 to 5878) between pTMDA1 and pOT4.
Mutations from the VP1 and VP2 regions of KJ38 virus were separated by exchanging the BamHI-SgrAI fragment (5′ end to nucleotide 2947) between pTMGDVII and pKJ38 to yield plasmids pOT6 and pOT7. Two additional recombinant viruses, TM756 and TM757, were constructed to separate mutations VP1-51 and VP1-195. After several subcloning steps, these two recombinants were obtained by insertion into pTMGDVII of an SgrAI-SacII (nucleotides 2947 to 3462) fragment from pKJ38 for TM756 and of a SacII-MscI (nucleotides 3462 to 3770) fragment from pKJ38 for TM757. To facilitate the identification of recombinants, fragment replacements in pTMDA1 and pTMGDVII were done on derivatives of these plasmids carrying a deletion in the fragment to be exchanged. (Plasmid pVD6 was used to construct pOT4, pOT10, and pOT11 recombinants; pVD7 was used to construct pOT5, pTM533 and pTM564 were used to construct pTM745 and pTM746, respectively; pTM380 was used for the construction of CHO-K1-adapted GDVII derivatives [28].)
Viruses.
The DA and GDVII strains are prototypes of persistent and neurovirulent TMEV strains, respectively. The DA virus used in this study is the molecular clone DA1 (23, 28). The GDVII virus is the molecular clone produced from plasmid pTMGDVII (35). Stocks of viruses were obtained as described previously (28) by transfection of BHK-21 cells with the genomic RNA transcribed in vitro from plasmids carrying the corresponding viral cDNAs. Virus titers were determined by a standard plaque assay in BHK-21 cells.
Heparin and sialyllactose inhibition of virus attachment.
The effect of heparin on the infectivity of KJ27, KJ38, and the parental viruses was tested on CHO-K1 cells. Viruses were incubated for 30 min with 100 μg of heparin per ml before infection of CHO-K1 cell monolayers at a multiplicity of infection (MOI) of 1 PFU/cell.
The effect of sialic acid on TMEV infection was tested on different cell lines. Viruses were incubated, before infection, with sialyllactose at a final concentration of 9 mM and were then applied to the cell monolayers. One hour after infection, the culture medium was removed, and the cells were washed two or three times before being supplied with complete medium. After one viral cycle (12 to 14 h), the supernatant was collected, and the number of infectious viral particles released by the cells was quantified by plaque assay on BHK-21 cells.
Sialidase treatment.
BHK-21, L929, and CHO-K1 cell monolayers grown in 24-well plates at 400,000 cells per well were washed with their respective serum-free medium and then incubated for 1 h at 37°C with Vibrio cholerae sialidase (Roche Biochemicals catalog no. 1080725) at a final concentration of 10 mU per well. After two or three washing steps, cells were infected for 1 h at 37°C at an MOI of 2.5 PFU per cell. At this time, cells were washed again two to three times and supplied with complete medium. Inhibition of cytopathic effect was then monitored for 72 h by light microscopy. Similar experiments were performed on 24- and 96-well plates with successive (fourfold) virus dilutions. Sialidase inhibition was quantified by comparing virus dilutions that yielded an equivalent cytopathic effect on treated and untreated cells.
Immunocytochemistry, immunohistochemistry, and flow cytometry.
Detection of infected cells was performed by immunocytochemistry and by flow cytometry (fluorescence-activated cell sorter [FACS]). After 20 min of fixation with 4% paraformaldehyde and 5 min of permeabilization of the cells with 0.1% Triton X-100, intracellular viral antigen was detected with the F12B3 monoclonal antibody specific to the VP1 capsid protein (kindly provided by Michel Brahic). Detection was performed with horseradish peroxidase and diaminobenzidine staining (LSAB-2 kit; Dako) for immunocytochemistry. For FACS analysis, the secondary antibody was a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse F(ab′)2 fragment (Dako F0479). Histological sections from paraffin-embedded tissues were stained for viral antigen with an anti-TMEV capsid rabbit polyclonal antibody (gift from Michel Brahic) with the Envision kit (Dako).
Animal experiments.
Three-week-old female SJL mice were purchased from IFFA-Credo Belgium and maintained in isolators with water and chow ad libitum. Animals were inoculated intracerebrally with 40 μl of virus suspension. For OT11 virus persistence analysis, four mice were inoculated with 105 PFU of viruses DA1 and OT11, and four control mice were inoculated with virus-free culture medium. Total RNA was extracted from the brain and the spinal cord of mice sacrificed 45 days after inoculation. Dot blot hybridization was performed as previously described (28) to compare the viral RNA loads.
For KJ38 neurovirulence analysis, groups of six mice were inoculated intracerebrally with 104 PFU of KJ38 and GDVII viruses. Three mice were inoculated with 103 PFU of KJ38 in an independent experiment. Two additional mice were inoculated with 104 PFU of KJ38 and taken 6 days after infection for histological examination. RNA was isolated from the brain and spinal cord of two mice infected for 6 weeks with KJ38. The presence of viral genomes in these mice was assessed by RT-PCR.
RESULTS
Adaptation of DA1 and GDVII viruses to CHO-K1 cells.
CHO-K1 cells are hypodiploid cells that are easily amenable to mutagenesis. In addition, well-characterized CHO-K1 cell mutants are available, representing interesting tools for studying virus-cell interaction (5). To take advantage of these properties of CHO-K1 cells, and as infection of these cells by the DA1 and GDVII strains of Theiler's virus was inefficient, we selected virus variants with capsids adapted to promote infection of these cells. For this purpose, the DA1 and GDVII viruses were adapted to grow on CHO-K1 cells by serial passages. Then, the capsid-coding region of these viruses was substituted for the parental one in the pTMDA1 and the pTMGDVII full-length cDNA clones (Fig. 1). As expected, viruses KJ27 (DA1 derivative) and KJ38 (GDVII derivative) produced from recombinant clones pKJ27 and pKJ38, respectively, had enhanced ability to infect CHO-K1 cells compared to the parental viruses (Fig. 2 and 3). At 24 h after infection of CHO-K1 cell monolayers at an MOI of 2 PFU per cell, the percentages of infected cells estimated by FACS analysis were 4.6% ± 1.8% and 53.5% ± 8.8% for DA1 and KJ27, respectively, and 0.3% ± 0.1% and 14.9% ± 5.4% for GDVII and KJ38, respectively.
FIG. 2.
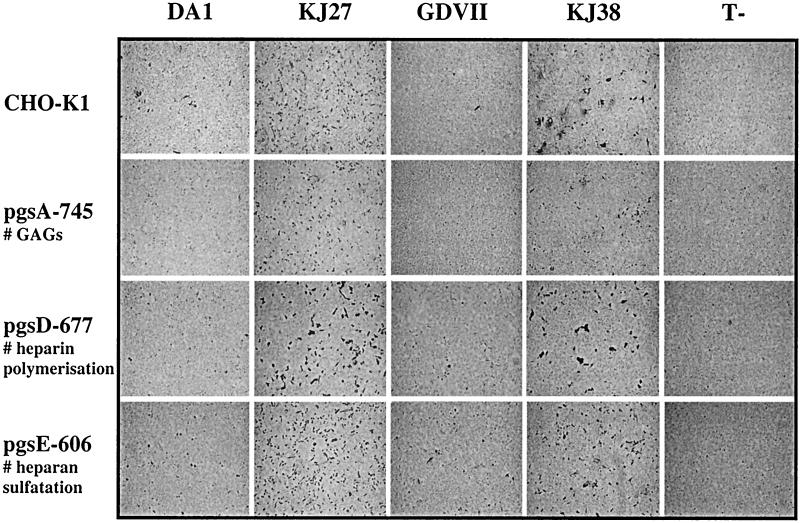
Effect of glycosaminoglycans on TMEV infection. CHO-K1 cells and their glycosaminoglycan-deficient derivatives were infected with DA1, GDVII, KJ38, and KJ27 viruses at an MOI of 5 PFU/cell or were left uninfected (T-). Infected cells (stained dark) were detected 20 h after infection by immunocytochemistry as described previously (28).
FIG. 3.
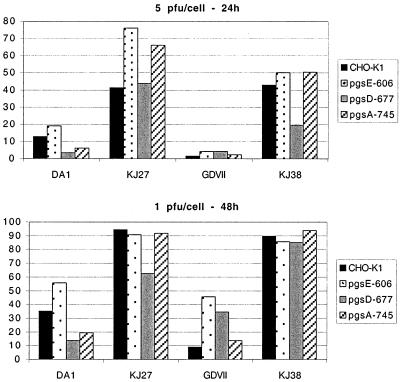
Flow cytometry measurements of wild-type and glycosaminoglycan-deficient CHO-K1 cell infections. CHO-K1 cells and their glycosaminoglycan-deficient derivatives were infected with DA1, GDVII, KJ38, and KJ27 viruses at an MOI of 5 PFU/cell for 24 h (upper panel) and at an MOI of 1 PFU/cell for 48 h (lower panel). Histograms show the percentage of infected cells determined by flow cytometry after intracellular viral antigen labeling.
Role of glycosaminoglycans as attachment molecules for TMEV.
Some viruses, such as herpes simplex virus, are known to use glycosaminoglycans, in particular heparan sulfate, for attachment to cells (33). For other viruses, such as FMDV and Sindbis virus, increased dependence on heparan sulfate for attachment has been reported to correlate with cell culture adaptation (14, 19). To test if parental and CHO-K1-adapted TMEV strains used glycosaminoglycans for cell infection, we compared the infection by DA1, KJ27, GDVII, and KJ38 of CHO-K1 cells and their glycosaminoglycan-deficient derivatives pgsA-745 cells (glycosaminoglycan deficient), pgsD-677 cells (heparan sulfate polymerization deficient), and pgsE-606 cells (heparan sulfate N-sulfotransferase deficient). Cell infection was monitored by following the cytopathic effect in the cultures (not shown), as well as by immunocytochemistry (Fig. 2) and FACS analysis (Fig. 3).
As expected, the parental DA1 and GDVII strains poorly infected the wild-type CHO-K1 cells, while the KJ27 and KJ38 viruses clearly infected these cells with a higher efficiency. None of the viruses, including the parental strains, displayed clearly reduced infectivity for the glycosaminoglycan-deficient cells. Only for the DA1 virus did we observe a slight (fivefold) reduction in the infection of heparan sulfate-deficient pgsD-677 cells compared to CHO-K1 cells. However, this effect was not seen for the pgsA-745 cell line, which is glycosaminoglycan deficient and thus also heparan sulfate deficient, nor was the effect observed when cell infection was monitored by following the cytopathic effect. Other differences were less than threefold. This does not compare to the dramatic effect of glycosaminoglycan deficiency reported for herpes simplex virus and Sindbis virus infection of these cells (19, 33).
Inhibition experiments performed on CHO-K1, BHK-21, and L929 cells with heparin as the competitor confirmed the absence of heparan sulfate binding by DA1, KJ27, GDVII, and KJ38 (data not shown).
Thus, our data suggest that adaptation of the KJ27 and KJ38 variants to CHO-K1 cells did not occur through increased glycosaminoglycan recognition. In addition, they show that infection of CHO-K1 cells by the wild-type viruses does not depend on the presence of glycosaminoglycan at the cell surface.
Identification of mutations responsible for adaptation to CHO-K1.
The cDNA fragments cloned in the parental plasmids to obtain pKJ27 and pKJ38 were sequenced to identify the capsid mutations that contributed to CHO-K1 cell adaptation (Table 1). Interestingly, mutations identified in the KJ27 and KJ38 viruses mostly affected amino acids mapping to the CD loop of VP1 and the EF loop of VP2. These two interacting loops are highly exposed on the capsid surface and correspond to antigenic determinants. In persistent strains, they form a sialic acid binding domain in which sialic acid residues contact amino acids of the VP2 EF loop. Mutations in these loops also occurred when the DA1 virus was adapted to grow on L929 cells (KJ6 virus) (16).
TABLE 1.
Mutations present in viruses adapted to CHO-K1 and L929 cellsa
Mutations that accounted for most of the capsid adaptation to L929 or CHO-K1 cells are boxed.
As some mutations of the KJ27 and KJ38 viruses also affected other parts of the VP1, VP2, and VP3 molecules, we constructed a series of recombinant viruses (Fig. 4) in order to measure the relative influence of these mutations on the adaptation of the virus to CHO-K1 cells (KJ27 and KJ38 viruses). These recombinant viruses were compared for the ability to infect CHO-K1 cells. Cell infection was monitored by following the cytopathic effect in the cultures, by immunocytochemistry, and by FACS analysis. Table 2 shows the data quantified by FACS analysis. Figure 4 presents the constructs and summarizes the data.
FIG. 4.
Role of the KJ6, KJ27, and KJ38 mutations in adaptation to L929 and CHO-K1 cells. Recombinant viruses were constructed to identify the mutations responsible for the adaptation of DA1 and GDVII viruses to CHO-K1 cells (KJ27 and KJ38) and for the adaptation of DA1 to L929 cells (KJ6). The capsid-encoding region of the recombinants is shown. Restriction sites used for cloning are indicated. Black frames identify regions cloned from the virus adapted to CHO-K1 and L929 cells. White frames identify regions from the parental DA1 and GDVII viruses. The right lanes summarize the data from at least three independent infection experiments done to compare the infectivity of recombinant viruses with that of their parental strains. Infectivity was monitored both by following the cytopathic effect (CPE) and by measuring the number of infected cells after immunostaining and flow cytometry (FACS). Relative CPEs were graded + (low) to +++ (high). ++(+) indicates a slightly weaker effect than +++. Data from FACS analysis (derived from Table 2) are percentages of infected cells normalized to the corresponding adapted virus (KJ6, KJ27, and KJ38), which was taken as 100%. ND, not done. Note that for both cytopathic effect and FACS analyses, comparisons apply independently for the three groups of recombinants (derived from KJ27, from KJ38, and from KJ6).
TABLE 2.
Percentage of infected cells measured by FACS
| Infecting virus | % of cells infected
|
|
|---|---|---|
| L929 cells (0.5 PFU/cell, 8 h postinfection) | CHO-K1 cells (2 PFU/cell, 24 h postinfection) | |
| DA1 | 3.7 ± 1.6 | |
| KJ6 | 65.6 ± 23.2 | |
| TM745 | 11.9 ± 3.3 | |
| TM746 | 11.3 ± 5.5 | |
| TM747 | 41.3 ± 15.4 | |
| DA1 | 4.6 ± 1.8 | |
| KJ27 | 53.5 ± 8.8 | |
| OT5 | 9.6 ± 1.5 | |
| OT10 | 6.1 ± 0.9 | |
| OT11 | 51.5 ± 2.1 | |
| GDVII | 0.3 ± 0.1 | |
| KJ38 | 14.9 ± 5.4 | |
| TM756 | 14.1 ± 2.3 | |
KJ27.
Four mutations occurred in KJ27. Three occurred in the VP1 coding region (VP1-99, -100, and -208), and one occurred in the VP3 coding region (VP3-84). Among the mutations of the VP1 protein, two affected amino acids (VP1-99 and -100) located in the CD loop. Two recombinant viruses, OT5 and OT4 (not shown), were first constructed to determine the relative influence of mutations in the VP3 and VP1 proteins, and the results indicated that adaptation to CHO-K1 cells was mostly due to the mutations in the VP1 region. Additional recombinant viruses, OT10 and OT11, allowed us to separate the VP1-208 mutation from the two mutations (VP1-99 and -100) present in the CD loop. Virus OT11 (mutations VP1-99 and -100) infected CHO-K1 cells almost as well as KJ27, suggesting that the mutations of the VP1 CD loop were almost entirely responsible for adaptation of DA1 virus to CHO-K1 cells. We cannot rule out an influence of the other mutations (VP1-208 and VP3-84) because, in a few experiments, KJ27 appeared slightly more infectious than OT11. However, tested individually, the VP1-208 and VP3-84 mutations hardly influenced virus infectivity.
KJ38.
KJ38 bears five mutations compared to GDVII: three in the EF loop of the VP2 protein (VP2-155, -171, and -173) and two in the VP1 protein (VP1-51 and VP1-195). We investigated the role of these two mutation clusters by constructing viruses OT6 and OT7, which contain the VP1 and the VP2 mutations, respectively. Additional recombinant viruses derived from OT6, called TM756 and TM757, were constructed to test the relative roles of the VP1-51 and VP1-195 mutations. Analysis of these recombinants revealed that the VP1-51 Asn→Ser replacement found in TM756 was sufficient to increase infectivity for CHO-K1 cells to a level similar to that of KJ38. Again, we could not totally exclude a minimal effect of the VP1-195 and VP2 mutations.
In conclusion, capsid mutations that allow DA1 adaptation to CHO-K1 cells are located in the CD loop of protein VP1. Adaptation of GDVII to these cells was mostly due to a point mutation in VP1-51.
Identification of mutations responsible for DA1 adaptation to L929 cells.
Interestingly, some of the mutations detected in KJ27 and KJ38 affected residues of the VP1 CD and VP2 EF loops that were previously found to change in the course of DA1 adaptation to L929 cells to yield the KJ6 virus (Table 1). For example, the VP1-100 Gly-to-Asp mutation is present in both KJ27 and KJ6, and the VP2-171 and VP2-173 residues were mutated in both KJ6 and KJ38 (Table 1). We were thus interested to determine which of the mutations identified in the previously characterized KJ6 virus were necessary for adaptation to L929 cells. Thus, recombinant viruses were also constructed to analyze the role of the single and clustered mutations found in KJ6 (Fig. 4).
KJ6.
Among the six mutations that occurred in the KJ6 virus, four were found in the VP2 coding region (VP2-162, -171, -173, and -256) and two were found in the VP1 coding region (VP1-100 and -102). Three of the VP2 mutations affected amino acids located in the EF loop. The fourth mutation affected codon VP2-256 and did not change the nature of the amino acid. As shown in Fig. 4 and Table 2, TM747, the virus carrying the mutations in the CD loop of VP1, infected L929 cells with high efficiency. Mutations of the VP2 EF loop also contributed significantly to L929 cell adaptation, as virus TM745 infected these cells three times better than DA1. The mutation at VP2-256 had no effect on infectivity, since the recombinant virus TM746 did not show enhanced infectivity compared to TM745.
It is noteworthy that all the DA1 variants harboring the VP1-CD loop mutations (KJ6, TM747, KJ27, and OT11) turned out to be unstable. Indeed, all these viruses were found to lose about 2 logs of infectivity upon conservation at −70°C for more than 1 year, while the titer of the other viruses was stable over the same period.
Effect of sialic acid on virus adaptation.
Since the KJ6, KJ27, and KJ38 viruses contained mutations occurring in and very close to the site of sialic acid recognition (i.e., in the VP2 EF loop and the VP1 CD loop), we were interested to test the influence of sialic acid binding on the adaptation of the virus. We therefore examined the inhibition of virus infection, either by treatment of the cells with V. cholerae sialidase or by competition of sialic acid binding with sialyllactose, an analogous soluble form of sialic acid.
Data from sialyllactose inhibition are presented in Fig. 5. As reported by Zhou et al. (39), 9 mM sialyllactose strongly inhibited infection of BHK-21 cells by DA1 (about 103-fold), while it had no effect on the infection of these cells by GDVII. Interestingly, the inhibitory effect of sialyllactose was decreased or almost completely abolished for the DA1 derivatives adapted to grow on L929 cells (KJ6) and CHO-K1 cells (KJ27). This is in agreement with the fact that these viruses had mutations in or close to the sialic acid binding site. The GDVII derivative adapted to grow on CHO-K1 cells (KJ38) was not inhibited by sialyllactose competition for BHK-21 cell infection.
FIG. 5.
Inhibition of infection by sialyllactose. Viruses were incubated with or without 9 mM sialyllactose (sial.) before infection of BHK-21 cells. Culture supernatants of infected cells were collected after one cycle of infection, and virus production was quantified by plaque assay. Infections by sialyllactose-treated (hatched columns) and untreated (black columns) viruses are compared. Virus production by cells infected with untreated viruses was taken as 100%. The histogram shows one experiment representative of three independent experiments.
Treatment of cells with V. cholerae sialidase did not influence BHK-21 cell infection by the KJ6 and KJ27 viruses, although it strongly reduced infection by the parental DA1 virus (data not shown). This confirmed the data from sialyllactose competition experiments.
As expected from the work of Zhou et al. (39), sialidase treatment did not affect infection of BHK-21 cells by GDVII. Surprisingly, although competition experiments with sialyllactose failed to indicate binding of KJ38 to sialic acid, sialidase treatment weakly but reproducibly reduced infection of BHK-21 cells by this virus (Fig. 6, Table 3). This discrepancy could result from the fact that sialyllactose does not mimic all the sialic acid forms. It could also result from a low affinity of the viral capsid for the sialic acid moiety of the molecule, although the sialyllactose concentration used (9 mM) was high.
FIG. 6.
Inhibition of KJ38 and TM756 infection by sialidase treatment. V. cholerae sialidase-treated (right panels) and untreated (left panels) BHK-21 cell cultures were infected with 2.5 PFU of viruses GDVII, KJ38, and TM756 per cell. The picture illustrates the cytopathic effect on the culture observed 48 h postinfection.
TABLE 3.
Inhibition of BHK-21 cell infection by sialidase treatment
| Virus | Inhibition factora
|
|||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | |
| GDVII | 1 | 1 | 1 | 1 |
| KJ38 | 16 | 16 | 4 | 4 |
| OT6 | 16 | 4 | 4 | 4 |
| TM756 | 4 | 4 | 16 | 4 |
Inhibition factors were calculated as the ratio between the virus MOIs (fourfold virus dilutions) that yielded equivalent cytopathic effects on treated and untreated cells. Data are from four independent experiments.
The KJ38 virus has mutations in the VP2 EF loop at positions 171 and 173 that precisely replaced GDVII residues with residues normally present at these positions in DA1. These mutations were thus likely to be responsible for the interaction with sialic acid. Paradoxically, the mutation that turned out to be responsible for both adaptation of the virus to CHO-K1 cells (Fig. 4) and sialic acid binding was the VP1-51 mutation. Indeed, the infectivity of both the OT6 and TM756 viruses was reduced from 4- to 16-fold upon treatment of cells with sialidase (Fig. 6; Table 3).
Influence of capsid mutations on viral persistence and neurovirulence.
We previously showed that the mutations present in virus KJ6 dramatically affected persistence of this virus in the spinal cords of infected SJL mice (16). The OT11 virus (Fig. 4) bearing the VP1-99 and -100 mutations of KJ27 that were sufficient to enhance infection of CHO-K1 cells and that decreased sialic acid binding also turned out to be affected in persistence. Dot blot experiments performed on RNA extracted from the brains and spinal cords of mice 45 days after infection showed a dramatic decrease in the viral RNA content for OT11 compared to DA1 (Fig. 7). However, OT11 was not completely cleared, as viral RNA could be detected in the brain and spinal cord samples by RT-PCR. These results confirmed previous data from other groups showing the importance of the VP1 CD loop in viral persistence (25, 31, 38, 41, 42).
FIG. 7.
Influence of VP1 CD loop mutations on viral persistence. Three-week-old female SJL mice were inoculated intracerebrally with 105 PFU of DA1 or OT11 virus. Histograms show the relative abundance of viral RNA (mean and standard error of the mean) in the spinal cords of mice 45 days postinfection (dpi) as measured by dot blot hybridization and PhosphorImager analysis. T−, data from mock-infected mice.
Six mice were infected with 104 PFU of KJ38 and with the same dose of GDVII to compare the neurovirulence of these viruses. By day 6, all the mice infected with GDVII had died, while mice infected with KJ38 survived without developing clinical symptoms. In an independent experiment, three SJL mice inoculated with 103 PFU of the KJ38 virus survived the infection without symptoms (Fig. 8). The parental GDVII virus consistently kills 100% of mice within a week of inoculation, even at lower doses.
FIG. 8.
Survival curves of SJL mice infected with the KJ38 and GDVII viruses. Three-week-old female SJL mice were inoculated intracerebrally with 103 PFU or 104 PFU of KJ38 and with 104 PFU of GDVII virus. Mice were checked daily for mortality. The graph shows the percentage of surviving mice as a function of time.
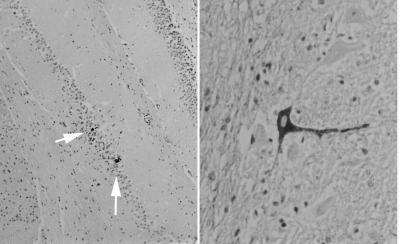
Six weeks after inoculation, RNA was prepared from the brains and spinal cords of two mice inoculated with 103 PFU of KJ38. Only traces of the virus could be detected after 40 cycles of RT-PCR amplification (data not shown), suggesting almost complete clearance of the infection. Histological examination of two mice infected for 6 days with KJ38 showed a dramatic decrease in the number of infected cells in the brain and spinal cord compared to that of GDVII-infected mice. However, detection of infected neurons in the spinal cord 6 days after infection, at a distance from the inoculation site, shows that the virus conserved some capacity to infect and multiply in neurons (Fig. 9).
FIG. 9.
Infection of central nervous system cells by KJ38. Brain and spinal cord paraffin sections were obtained 6 days after infection from SJL mice inoculated with 104 PFU of KJ38. Sections were immunostained for viral antigen (dark) and counterstained with hematoxylin. Shown is a low-magnification (×5 objective) of the hippocampal region (left panel) and a higher magnification (×40 objective) of the spinal cord, showing an infected neuron (right panel). White arrows point to a few infected cells.
DISCUSSION
The receptor of TMEV has not yet been identified. Early binding experiments suggested that the receptor for both persistent and neurovirulent TMEV strains was a 34-kDa glycoprotein (18). A recently characterized BHK-21 mutant cell line carrying a deletion in the UDP-galactose transporter resisted infection by both groups of viruses (13). Accordingly, we observed that several BHK-21 and CHO-K1 cell mutants resistant to both the DA1 and GDVII strains had glycosylation defects (unpublished observations), supporting the view that the receptors for both virus strains are glycosylated. On the other hand, studies by Zhou et al. showed that persistent but not neurovirulent strains of Theiler's virus used sialic acid for entry (39). One possibility is that the two groups of strains use the same receptor in two different ways, as proposed by Fotiadis et al. (8) on the basis of binding competition experiments. In this case, persistent but not neurovirulent strains would bind the sialic acid moiety of the receptor. Alternatively, persistent and neurovirulent strains could use different receptors or have different coreceptor requirements. The isolation of an L929 mutant cell line resistant to GDVII but susceptible to DA1 supports the latter possibility (17).
Adaptation of persistent strain through decreased sialic acid binding.
The crystal structure of the persistent DA virus complexed with sialyllactose confirmed the specificity of sialic acid binding and revealed that sialic acid interacted with a positively charged pocket on the capsid surface (40). This pocket, named the gap, is formed by the interaction between the CD loop of protein VP1 and the EF loop of protein VP2 (Fig. 10). This gap is exposed at the surface of the capsid, close to the pit, a depression of the capsid surface that was suggested to interact with the receptor (10, 12), by analogy to the canyon of poliovirus and rhinovirus (3).
FIG. 10.
Capsid mutations responsible for virus adaptation to CHO-K1 and L929 cells. Shown is a pentamer of the DA1 capsid (seen from outside) in a space-filling view generated with the Rasmol software of Roger Sayle (Biomolecular Structures Group, Glaxo Wellcome) from the structural data of the DA virus (10). The pentamer center is visible as a white hole. Shadows outline the depressions of the capsid surface. Amino acids VP1-99, -100, and -102 (CD loop of VP1), VP2-162, -171, and -173 (EF loop of VP2), and VP1-51 are shown in white. DA capsid amino acids interacting with sialic acid are VP2-161, -163, and -174 and VP3-232 (40), located in the region indicated as the VP2-EF loop. Note that the VP1-51 mutation is shown on the DA pentamer although it occurred in GDVII (at a similar location). These amino acids are all visible from outside, indicating that they are exposed at the capsid surface.
Sialic acid interacts directly with amino acids of the VP2 EF loop (VP2-161, -163, and -174) of virus DA (40). Although they have no direct interaction with sialic acid residues, amino acids of the CD loop of VP1 likely influence sialic acid binding. The mutations responsible for adaptation of virus DA1 to grow on L929 (KJ6) and CHO-K1 (KJ27) cells map precisely to the sialic acid binding pocket (amino acids VP1-100 and -102 and VP2-162, -171, and -173 for KJ6 and amino acids VP1-99 and -100 for KJ27) (Fig. 10). These mutations either decreased or completely abolished sialic acid binding on BHK-21 cells, as shown by the sialyllactose competition experiments. Adaptation of the KJ6 and KJ27 viruses also resulted in decreased sialic acid usage on L929 and CHO-K1 cells, respectively (data not shown).
It is known that the extent and the nature of glycosylations might vary according to the cell type. It is tempting to speculate that the protein part of the receptor would interact with the depression, while the carbohydrate moiety, in particular sialic acid for persistent viruses, would interact with the loops located at the rim of this depression. Thus, the virus would easily adapt to a new cell line by varying the flexible loops located at the surface of the capsid without affecting the structural integrity of the capsid. Adaptation of DA1 to L929 and CHO-K1 cells probably increased the affinity of the capsid for a molecule other than sialic acid. It did not promote binding to glycosaminoglycans, contrary to the situation observed in the course of Sindbis virus and FMDV adaptation to CHO-K1 cells (14, 19).
The VP1 CD and VP2 EF loops are highly exposed at the viral capsid surface and constitute an important site of recognition by neutralizing antibodies. Previous studies of recombinant viruses and of monoclonal antibody escape mutant viruses have given suggestive support for involvement of the region formed by the VP1 CD loop and VP2 EF loop in viral persistence (15, 16, 25, 31, 32, 38, 41, 42). Recently, Tsunoda et al. constructed a DA virus containing the VP1 CD loop of GDVII and an additional mutation in the VP2 EF loop at VP2-171 (pDABL2M) (37). DABL2M virus showed prolonged gray matter disease without demyelination but failed to persist in the spinal cord white matter. In agreement with the influence of this region on tropism and persistence, the KJ6 and OT11 viruses turned out to have decreased ability to persist in the central nervous system of the mouse. In conclusion, the VP1 CD loop and the VP2 EF loop could contact the carbohydrate moiety of the receptor and would contribute in this way to determining TMEV tropism and pathogenicity.
Adaptation of GDVII derivatives and increased sialic acid binding.
The adaptation of GDVII to CHO-K1 cells resulted in weak but consistent recognition of sialic acid, since treatment of cells with sialidase inhibited virus entry. Surprisingly, several mutations affected the VP2 EF loop of KJ38, but they turned out not to be responsible for sialic acid binding. The mutation that allows sialic acid binding is located at amino acid VP1-51. This mutation is also responsible for KJ38 adaptation to CHO-K1 cells. Amino acid VP1-51 is exposed at the top of the VP1 BC loop, which is close to residue VP1-101 (22). Interestingly, this loop differs between the DA1 and GDVII viruses by four residues (at positions VP1-52, -54, -57, and -63). Residue VP1-51 is located out of the defined sialic acid binding region and out of the putative receptor-binding site.
The mutations present in KJ38 dramatically attenuated neurovirulence. Infected mice showed no clinical signs. The virus was almost totally cleared from the central nervous system 42 days after inoculation. In this case, recognition of sialic acid by the virus possibly decreased binding of the virus to its actual receptor. Immunohistological data showed that KJ38 conserved some ability to infect neurons of the brain and the spinal cord, though in much reduced numbers.
In conclusion, it seems that interaction between Theiler's virus and its receptor is a complex mechanism. We and others previously showed that persistent and neurovirulent strains used different ways to enter a single cell line (8, 17). This work illustrates the polymorphism of the receptors present on different cell lines and outlines the complex influence of sialic acid in viral entry and pathogenesis.
Acknowledgments
We thank Pierre Rensonnet for expert technical assistance and Olivier Thiry for help in constructing and testing the OT recombinant viruses. We thank Michel Brahic (Pasteur Institute, Paris) for the F12B3 monoclonal antibody and for long-term collaboration. Guy Cornelis and Aoife Boyd (MIPA Unit, University of Louvain) kindly provided the CHO-K1 glycosaminoglycan-deficient cells. We thank Marie-Christine Many and Monique de Rudder (ISTO Unit, University of Louvain) for help in histology and Jean-Yves Sgro (University of Wisconsin, Madison) for help in capsid representations with the Rasmol software.
K.J. was a fellow of the Belgian FRIA (Fonds pour la Recherche dans l'Industrie et l'Agriculture) and has since been supported by the Christian de Duve Institute of Cellular Pathology. This work was supported by convention 3.4573.94F of the FRSM, by Crédit aux Chercheurs 1.5.095.00 of the FNRS, by the Charcot Foundation, and by the Fonds de Développement Scientifique (FSR) of the University of Louvain.
REFERENCES
- 1.Aubert, C., M. Chamorro, and M. Brahic. 1987. Identification of Theiler's virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb. Pathog. 3:319-326. [DOI] [PubMed] [Google Scholar]
- 2.Bame, K. J., and J. D. Esko. 1989. Undersulfated heparan sulfate in a Chinese hamster ovary cell mutant defective in heparan sulfate n-sulfotransferase. J. Biol. Chem. 264:8059-8065. [PubMed] [Google Scholar]
- 3.Belnap, D. M., B. M. McDermott, D. J. Filman, N. Cheng, B. L. Trus, H. J. Zuccola, V. R. Racaniello, J. M. Hogle, and A. C. Steven. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. USA 97:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calenoff, M. A., K. S. Faaberg, and H. L. Lipton. 1990. Genomic regions of neurovirulence and attenuation in Theiler murine encephalomyelitis virus. Proc. Natl. Acad. Sci. USA 87:978-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esko, J. D., K. S. Rostand, J. L. Weinke. 1988. Tumor formation dependent on proteoglycan biosynthesis. Science 241:1092-1096. [DOI] [PubMed] [Google Scholar]
- 7.Evans, D. J., and J. W. Almond. 1998. Cell receptors for picornaviruses as determinants of cell tropism and pathogenesis. Trends Microbiol. 6:198-202. [DOI] [PubMed] [Google Scholar]
- 8.Fotiadis, C., D. K. Kilpatrick, and H. L. Lipton. 1991. Comparison of binding characteristics to BHK-21 cells of viruses representing the two Theiler's virus neurovirulence groups. Virology 182:365-370. [DOI] [PubMed] [Google Scholar]
- 9.Fu, J., S. Stein, L. Rosenstein, T. Bodwell, M. Routbort, B. L. Semler, and R. P. Roos. 1990. Neurovirulence determinants of genetically engineered Theiler viruses. Proc. Natl. Acad. Sci. USA 87:4125-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant, R. A., D. J. Filman, R. S. Fujinami, J. P. Icenogle, and J. M. Hogle. 1992. Three-dimensional structure of Theiler virus. Proc. Natl. Acad. Sci. USA 89:2061-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. McClelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 12.Hertzler, S., M. Luo, H. L. Lipton. 2000. Mutation of predicted virion pit residues alters binding of Theiler's murine encephalomyelitis virus to BHK-21 cells. J. Virol. 74:1994-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertzler, S., P. Kallio, H. L. Lipton. 2001. UDP-galactose transporter is required for Theiler's virus entry into mammalian cells. Virology 286:336-344. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarousse, N., R. A. Grant, J. M. Hogle, L. Zhang, A. Senkowski, R. P. Roos, T. Michiels, M. Brahic, and A. McAllister. 1994. A single amino acid change determines persistence of a chimeric Theiler's virus. J. Virol. 68:3364-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jnaoui, K., and T. Michiels. 1998. Adaptation of Theiler's virus to L929 cells: mutations in the putative receptor binding site on the capsid map to neutralization sites and modulate viral persistence. Virology 244:397-404. [DOI] [PubMed] [Google Scholar]
- 17.Jnaoui, K., and T. Michiels. 1999. Analysis of cellular mutants resistant to Theiler's virus infection: differential infection of L929 cells by persistent and neurovirulent strains. J. Virol. 73:7248-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilpatrick, D. R., and H. L. Lipton. 1991. Predominant binding of Theiler's virus to a 34-kilodalton receptor protein on susceptible cell lines. J. Virol. 65:5244-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton, H. L. 1975. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect. Immun. 11:1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobert, P.-E., N. Escriou, J. Ruelle, and T. Michiels. 1999. A coding RNA sequence acts as a replication signal in cardioviruses. Proc. Natl. Acad. Sci. USA 96:11560-11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo, M., K. S. Toth, L. Zhou, A. Pritchard, and H. L. Lipton. 1996. The structure of a highly virulent Theiler's murine encephalomyelitis virus (GDVII) and implications for determinants of viral persistence. Virology 220:246-250. [DOI] [PubMed] [Google Scholar]
- 23.McAllister, A., F. Tangy, C. Aubert, and M. Brahic. 1989. Molecular cloning of the complete genome of Theiler's virus, strain DA, and production of infectious transcripts. Microb. Pathog. 7:381-388. [DOI] [PubMed] [Google Scholar]
- 24.McAllister, A., F. Tangy, C. Aubert, and M. Brahic. 1990. Genetic mapping of the ability of Theiler's virus to persist and demyelinate. J. Virol. 64:4252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCright, I. J., I. Tsunoda, F. G. Whitby, and R. S. Fujinami. 1999. Theiler's virus with mutations in loop I of VP1 lead to altered tropism and pathogenesis. J. Virol. 73:2814-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendelsohn, C. L., E. Wimmer, and V. R. Racaniello. 1989. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 56:855-865. [DOI] [PubMed] [Google Scholar]
- 27.Michiels, T., N. Jarousse, and M. Brahic. 1995. Analysis of the leader and capsid coding regions of persistent and neurovirulent strains of Theiler's virus. Virology 214:550-558. [DOI] [PubMed] [Google Scholar]
- 28.Michiels, T., V. Dejong, R. Rodrigus, and C. Shaw-Jackson. 1997. Protein 2A is not required for Theiler's virus replication. J. Virol. 71:9549-9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neff, S., D. Sá-Carvalho, E. Rieder, P. W. Mason, S. D. Blystone, E. J. Brown, and B. Baxt. 1998. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αvβ3 as its receptor. J. Virol. 72:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez, M., E. Olezak, and J. Leibowietz. 1987. Theiler's murine encephalomyelitis: a model of demyelination and persistence of virus. Crit. Rev. Immunol. 7:325-365. [PubMed] [Google Scholar]
- 31.Roos, R. P., S. Stein, M. Routbort, A. Senkowski, T. Bodwell, and R. Wollmann. 1989. Theiler's murine encephalomyelitis virus neutralization escape mutants have a change in disease phenotype. J. Virol. 63:4469-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato, S., L. Zhang, J. Kim, J. Jakob, R. A. Grant, R. Wollmann, and R. P. Roos. 1996. A neutralization site of DA strain of Theiler's murine encephalomyelitis virus important for disease phenotype. Virology 226:327-337. [DOI] [PubMed] [Google Scholar]
- 33.Sieh, M-T., D. WuDunn, R. I. Montgomery, J. D. Esko, and P. G. Spear. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stauton, D. E., V. J. Merluzzi, R. rothlein, R. Barton, S. D. Marlin, and T. A. Springer. 1989. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 56:849-853. [DOI] [PubMed] [Google Scholar]
- 35.Tangy, F., A. McAllister, and M. Brahic. 1989. Molecular cloning of the complete genome of strain GDVII of Theiler's virus and production of infectious transcripts. J. Virol. 63:1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theiler, M., and S. Gard. 1940. Encephalomyelitis of mice: characteristics and pathogenesis of the virus. J. Exp. Med. 72:49-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsunoda, I., Y. Wada, J. E. Libbey, T. S. Cannon, F. G. Whitby, and R. S. Fujinami. 2001. Prolonged gray matter disease without demyelination caused by Theiler's murine encephalomyelitis virus with a mutation in VP2 puff B. J. Virol. 75:7494-7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada, Y., M. L. Pierce, and R. S. Fujinami. 1994. Importance of amino acid 101 within capsid protein VP1 for modulation of Theiler's virus-induced disease. J. Virol. 68:1219-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou, L., X. Lin, T. J. Green, H. L. Lipton, and M. Luo. 1997. Role of sialyloligosaccharide binding in Theiler's virus persistence. J. Virol. 71:9701-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, L., Y. Luo, Y. Wu, J. Tsao, and M. Luo. 2000. Sialylation of the host receptor may modulate entry of demyelinating persistent Theiler's virus. J. Virol. 74:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zurbriggen, A., M. Yamada, C. Thomas, and R. Fujinami. 1991. Restricted virus replication in the spinal cords of nude mice infected with a Theiler's virus variant. J. Virol. 65:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zurbriggen, A., C. Thomas, M. Yamada, R. P. Roos, and R. S. Fujimami. 1991. Direct evidence of a role for amino acid 101 of VP1 in central nervous system disease in Theiler's murine encephalomyelitis virus infection. J. Virol. 65:1929-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]