Abstract
Human herpesvirus 8 (HHV-8; Kaposi's sarcoma-associated herpesvirus is linked to Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD), all of which are viewed as cytokine-driven malignancies. In particular, interleukin-6 (IL-6) has been found to promote the growth and proliferation of cells from KS and PEL. HHV-8 encodes a homologue of IL-6 (viral IL-6 [vIL-6]), which functions similarly to the cellular IL-6. Therefore, vIL-6 has been proposed to play an important role in tumor progression. Several groups have reported that vIL-6 is expressed from the HHV-8 genome at higher levels in PEL and MCD lesions than in KS lesions. However, it is not clear how vIL-6 expression is regulated. We characterized the transcription at the vIL-6 gene locus by Northern blot analysis and, in contrast to previous reports, we observed two distinct transcripts from induced PEL cell lines. This observation was confirmed by primer extension, as well as 5′ and 3′ rapid amplification of cDNA ends. Two transcription initiation sites and putative TATA boxes were mapped. A luciferase reporter system was used to show that each of the two putative TATA boxes contributed to vIL-6 promoter activity. Since virally encoded transcriptional activator Rta potently activates the viral lytic gene expression cascade, we examined the role of Rta in controlling vIL-6 gene expression and found that Rta activated the vIL-6 promoter. The Rta-responsive element was further mapped through a series of deletion constructs. Electrophoretic mobility shift assays demonstrated that Rta binds directly to the vIL-6 Rta-responsive element, and the core Rta-responsive element was mapped to a 26-bp region spanning from nucleotide 18315 to 18290 on the viral genome. We propose that the existence of two vIL-6 promoters offers opportunities for differential regulation of vIL-6 gene expression in different tissue types and may account for the variable vIL-6 levels observed in KS, PEL, and MCD.
Cytokines have long been thought to play important roles in the development of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD) (5, 14, 15). One such cytokine is interleukin-6 (IL-6), which acts through autocrine and paracrine mechanisms to stimulate proliferation, differentiation of B cells, and angiogenesis (reviewed in reference 39). Human herpesvirus 8 (HHV-8; Kaposi's sarcoma-associated herpesvirus) is associated with these three malignancies (7, 9, 35). Interestingly, HHV-8 encodes a homologue of human IL-6 (22-24; reviewed in reference 2). Viral IL-6 (vIL-6) is encoded by open reading frame (ORF) K2 and has 25% amino acid identity to human IL-6. Similar to its human counterpart, vIL-6 promotes the growth and proliferation of IL-6-dependent human and murine hybridoma B-cell lines (22, 24). vIL-6 also activates multiple signal transduction pathways, including JAK/STAT and Ras-mitogen-activated protein kinase pathways (21, 25).
IL-6 performs these functions by binding to the cell surface IL-6 receptor, which consists of two subunits, gp130 and IL-6Rα (39). Human IL-6 requires both subunits of the IL-6 receptor for its action; however, vIL-6 seems to require only gp130 (21, 40). Since gp130 is ubiquitously expressed whereas IL-6Rα is not (39), this may allow vIL-6 to stimulate a broader spectrum of cells. Recently, the crystal structure of the extracellular assembly between vIL-6 and gp130 was solved, providing a structural explanation for the ability of vIL-6 to bypass the requirement for IL-6Rα (11). These studies suggested a role for vIL-6 in tumor development, supported by the fact that the vIL-6 protein was detected in sera from AIDS-PEL patients (3). vIL-6 has been shown to be a potent inducer of vascular endothelial growth factor (1), and this induction may promote the vascular permeability and fluid accumulation observed in the body cavities of PEL patients and may also contribute to the increased angiogenesis and endothelial cell growth that is typical of MCD lesions (2).
Human B-cell lines derived from PEL predominantly carry HHV-8 in a latent state (4, 8, 32). However, treatment with a chemical such as 12-O-tetradecanoylphorbol-13-acetate (TPA) or sodium butyrate induces the virus to go to lytic replication (20). In addition, reactivation can be induced by ectopic expression of viral immediate-early gene rta, whose product mediates the switch from latency to lytic replication of HHV-8 (37). Rta is a potent transcriptional activator that autoregulates its expression and that activates downstream target genes (12, 16, 19, 37). The N terminus of the Rta protein contains a DNA binding domain, and its C terminus harbors an activation domain (19, 37). Transcriptional activation of many viral lytic genes is thought to be the mechanism by which Rta initiates viral lytic replication.
Using these PEL-derived cell lines, we and others have studied vIL-6 expression in vitro (22, 24, 33, 38). It is expressed at very low levels during viral latency, which may result from a low level of expression in all the PEL cells or spontaneous reactivation of the virus in a small percentage of the PEL cells or both. Upon reactivation of the virus, vIL-6 is highly inducible and abundantly expressed. vIL-6 expression in tumor lesions has also been examined. In situ hybridization with a riboprobe specific for the coding region of the vIL-6 gene and immunohistochemistry with a vIL-6-specific antibody showed that vIL-6 is expressed at higher levels in PEL and MCD than in KS (6, 26, 36). The average levels of vIL-6 expression in PEL and MCD in individual infected cells were greater by at least an order of magnitude than that in KS (36). However, it is not clear how vIL-6 is expressed at different levels in these three types of malignancies, and transcriptional regulation of the vIL-6 gene has not been reported.
Employing multiple techniques, we have determined that, in contrast to previous reports, two vIL-6 transcripts are expressed when latently infected PEL cells are reactivated to lytic replication, and they result from two major transcription initiation sites. Therefore, there are two promoters (distal and proximal) that regulate the vIL-6 gene. Moreover, we have shown that Rta binds directly to and strongly activates the vIL-6 promoter and have mapped a 26-bp core Rta-responsive element (RRE).
MATERIALS AND METHODS
Cell culture.
All cells were cultured at 37°C in the presence of 5% CO2. KS-1 (gift from Jonathan Said, University of California at Los Angeles) (32) and BCBL-1 (obtained through the AIDS Research and Reagent Reference Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health) (29) cell lines were derived from primary effusion lymphomas and harbor latent HHV-8 (but not Epstein-Barr virus [EBV]) genomes. DG75 is a human B-cell line that does not contain HHV-8 or EBV (gift from Sam Speck, Washington University, St. Louis, Mo.). KS-1, BCBL-1, and DG75 cells were propagated in RPMI 1640 medium (Cellgro) supplemented with fetal bovine serum (20, 15, and 10%, respectively) and antibiotics (50 U of penicillin and 50 μg of streptomycin/ml). 293T and 293 cells were grown in Dulbecco's modification of Eagle's medium (Cellgro) supplemented with 10% fetal bovine serum and antibiotics (50 U of penicillin and 50 μg of streptomycin/ml).
Plasmid construction.
Luciferase reporter plasmid pK2p(−3.2k) contains a 3.2-kb promoter region upstream of the methionine initiation codon of the vIL-6 gene. The promoter region was amplified from total cellular DNA prepared from BC-1 cells (dually infected by HHV-8 and EBV) by using primers P13a (5′-ACA GGT ACC aga tga gga tgt tcc tgt ctg c-3′) and P12b (5′-CCG AAG CTT ggc tgc taa cgc ggc ata cac-3′). For all oligonucleotide sequences, the underlined nucleotides represent restriction enzyme sites for cloning the PCR products and the lowercase letters represent viral sequences. The PCR fragment was cloned into pGL3-basic (Promega) with KpnI and HindIII cloning sites. To make 5′ promoter deletion constructs, various promoter regions were amplified by using common primer P12b and a specific primer and cloned into pGL3-basic. The specific primers were as follows: P13b (5′-ACA GGT ACC cgc caa aaa gtt att ccg tgc c-3′) for plasmid pK2p(−1.7k), P13c (5′-ACA GGT ACC gtg gtt cta agt cgc acg tta g-3′) for pK2p(−462), P13f (5′-ACA GGT ACC ttt ggc gag ttc gct cga tgc-3′) for pK2p(−414), P13d (5′-ACA GGT Acc agt tag gct att ttt aac ccg g-3′) for pK2p(−291), P13e (5′-ACA GGT ACc ata aaa gtg act cag agt tcg g-3′) for pK2p(−133), and P13g (5′-ACA GGT Acc ctt cag tga gac ttc gta ac-3′) for pK2p(−65). Deletion constructs pK2p(−1.15k), pK2p(−827), pK2p(−569), and pK2p(−366) were made by digesting pK2p(−1.7k) with KpnI plus ApaI, EcoRV, PvuII, and NheI, respectively, followed by blunt ending and self-ligations.
To generate pK2pRRE-F and pK2pRRE-R, P25F (5′-CG GGA TCC gtg gtt cta agt cgc acg tta gaa acc ccg ccc c-3′) and P25R (5′-GA AGA TCT aaa gtg agc acc agg ggg cgg ggt ttc taa cgt g-3′) were annealed, filled in with Klenow enzyme, digested with BamHI and BglII, and cloned into vector pGL3-promoter (Promega).
pK2p(−462)M1, pK2p(−462)M2, and pK2p(−462)M12 were generated through a two-step PCR site-directed mutagenesis method. For pK2p(−462)M1, first-step PCR was performed using pK2p(−462) as a template. The first set of primers was K2P13c and K2P17b (5′-ctc tga gtc act ttC CCC Cag gtc tca aac atg ta-3′). The second set of primers was K2P16b (5′-tac atg ttt gag acc tGG GGG aaa gtg act cag ag-3′) and K2P12b. The italicized letters indicate mutated nucleotides. For pK2p(−462)M2, first-step PCR was also performed using pK2p(−462) as a template. The first set of primers was K2P13c and K2P19 (5′-gta aaa ccc ggg tta CCC CCa gcc taa ctg gcc ag-3′). The second set of primers was K2P18 (5′-ctg gcc agt tag gct GGG GGt aac ccg ggt ttt ac-3′) and K2P12b. For pK2p(−462)M12, first-step PCR was performed using pK2p(−462)M2 as a template. The first set of primers was K2P13c and K2P17b. The second set of primers was K2P16b and K2P12b. For all three constructs, both products from the first-step PCRs were gel purified and used as templates in second-step PCR, using K2P13c and K2P12b as primers. To make pK2p(T2+RRE), the promoter region was amplified by using K2P13c and K2P28 (5′-CCG AAG CTT ctc tct tgc tcg cca ggc ttg-3′) and cloned into pGL3-basic with KpnI and HindIII cloning sites.
Induction of viral lytic replication and transfections.
To induce viral lytic replication using chemicals, KS-1 or BCBL-1 cells containing latent viral genomes were resuspended in fresh media to a concentration of 106 cells/ml and treated with either 20 ng of TPA/ml or 3 mM sodium butyrate. To reactivate the lytic cycle by Rta, Rta expression plasmid pcDNA3/Rta (5 μg) (37) was transfected into KS-1 cells. Cells were washed once with 1× phosphate-buffered saline and resuspended in unsupplemented RPMI 1640 medium at a concentration of 2.5 × 107 cells/ml. Plasmid DNA was then introduced into 107 cells via electroporation (960 μF, 240 V; Bio-Rad).
Transfection in 293T cells was performed in 12-well plates by a standard calcium phosphate method (17a). A reporter plasmid (50 ng), either pcDNA3/Rta or pcDNA3 (50 ng), pRL-CMV (2 ng), and filler DNA (700 ng; plasmid DNA that lacks a mammalian promoter/enhancer) were transfected into each well. For the dose dependence experiment, each transfection sample included 50 ng of pK2p(−827), 2 ng of pRL-CMV, and 650 ng of filler plasmid DNA; also included in each sample were increasing amounts of pcDNA3/Rta (from 0 to 100 ng) and correspondingly decreasing amounts of pcDNA3 (from 100 to 0 ng). Transfection in 293 cells was performed in 12-well plates with Lipofectamine Plus (Gibco BRL) in accordance with the manufacturer's protocols. pK2pRRE-F or pK2pRRE-R (5 ng), pcDNA3/Rta, pcDNA3, or GAL4-VP16 (25 ng), pRL-CMV (2 ng), and filler DNA (700 ng) were transfected into each well.
Dual-luciferase assay.
The dual-luciferase reporter assay system from Promega was used. 293T or 293 cells were harvested at 48 h posttransfection. Cells were gently rinsed once with 1× phosphate-buffered saline. Passive lysis buffer (1×; 400 μl) was dispensed into each well, and the culture plate was placed on an orbital shaker with gentle shaking at room temperature for 15 min. Lysates (or diluted lysates, where appropriate) were assayed for dual-luciferase activities in accordance with the manufacturer's instructions by using an Optocomp I luminometer (MGM Instruments, Inc.).
RNA preparation and Northern blot analysis.
Total cellular RNAs were prepared with Tri-Reagent according to the manufacturer's suggestions (Molecular Research Center, Inc.). Total RNA (20 μg) was denatured with 1 M glyoxal-50% dimethyl sulfoxide (DMSO) and loaded onto a 1% agarose gel as described in reference 4a with modifications. A [γ-32P]dATP-end-labeled 1-kb ladder (Gibco BRL) was also treated with glyoxal-DMSO and loaded on the gel to provide size markers. Gel electrophoresis was performed in 10 mM sodium phosphate buffer (pH 6.8) with constant circulation. The separated RNAs were transferred onto a Hybond-N+ membrane (Amersham Pharmacia Biotech) with a vacuum blotter (Bio-Rad) and UV cross-linked. The blot was incubated in 20 mM Tris-HCl (pH 8.0) at 80°C for 10 min to remove glyoxal, prehybridized at 65°C, and hybridized with an [α-32P]dCTP-labeled, randomly primed probe. The vIL-6 probe was generated by using a PCR product containing the whole vIL-6 ORF as the template. The blot was washed, exposed to a phosphorimage screen, and analyzed with a STORM machine (Molecular Dynamics).
Primer extension.
Primers P14 (5′-gtt acg aag tct cac tga ag-3′) and P24 (5′-cca cgt tct tga aaa acc ctc-3′) were end labeled with [γ-32P]dATP. Total cellular RNA (15 μg) was mixed with labeled P14 or P24 in the presence of 1× cDNA synthesis buffer (Thermoscript reverse transcriptase PCR [RT-PCR] system; Gibco BRL) and denatured at 68°C for 5 min. The mixtures were slowly cooled to 50°C and incubated for 50 min. A master solution containing Thermoscript RT and deoxynucleoside triphosphates was prewarmed and incubated with the RNA-primer mixtures at 50°C for 50 min. The mixtures were then subjected to RNase digestion, extracted with phenol-chloroform, and precipitated with ethanol. Primer extension products were resolved on an 8% polyacrylamide-8 M urea gel. The gel was dried, exposed to a phosphorimage screen, and analyzed with a STORM machine.
5′ and 3′ RACE.
For 5′ rapid amplification of cDNA ends (RACE), total cellular RNA (5 μg) from induced KS-1 cells was primed with oligonucleotide P7 (5′-agc agc ggg ctc tag aat ac-3′). First-strand cDNA synthesis was carried out with ThermoScript RT (Gibco BRL) at 50°C for 50 min. The reaction was terminated by incubation at 85°C for 5 min. RNase mixture (from the 5′ RACE system, version 2.0; Gibco BRL) was then added, and incubation was performed at 37°C for 30 min. The cDNA was purified with a GlassMax DNA isolation spin cartridge (provided in the 5′ RACE system) in accordance with the manufacturer's instructions. Purified cDNA was treated with terminal deoxynucleotidyltransferase in the presence of 0.2 mM dCTP for 10 min at 37°C. PCR of dC-tailed cDNA was primed with oligonucleotides P8 (5′-gag gtc gcg gaa gca ttc atc-3′) and AAP (5′-GGC CAC GCG TCG ACT AGT ACG GGI IGG GII GGG IIG-3′, provided in the kit). Seminested PCR was performed using oligonucleotides P9 (5′-ccg cgt tcc aga tac cag cag-3′) and AUAP (5′-GGC CAC GCG TCG ACT AGT AC-3′, provided in the kit). For 3′ RACE, total cellular RNA (5 μg) from induced KS-1 cells was primed with oligo(dT)12-18 (Amersham Pharmacia Biotech). First-strand cDNA synthesis was carried out with Superscript II RT (Gibco BRL) at 42°C for 50 min. The cDNA was amplified by PCR, primed with oligonucleotides P3 (5′-gga cat aca gga aga gct caa t-3′) and P3R (5′-GCG GCC GCG GAT CCG AAT TCT TTT TTT TTT TTT TTT TT-3′). Seminested PCR was carried out using P6 (5′-gcg gtc tat tag gga ggc ttc ag-3′) and P6R (5′-GCG GCC GCG GAT CCG AAT TC-3′). Both 5′ and 3′ RACE products were cloned into the pCR-II vector (Invitrogen) and sequenced.
Electrophoretic mobility shift assay (EMSA).
Oligonucleotides spanning part or all of the vIL-6 RRE were annealed and end-labeled. Blunt-ended, double-stranded probes were generated by a fill-in reaction with Klenow enzyme following end labeling by T4 polynucleotide kinase to ensure similar labeling efficiencies for all oligonucleotides. Labeled (1 fmol, approximately 1 × 104 to 5 × 104 cpm) probes were incubated with different amounts (0, 20, 60, 180, or 540 ng) of FLAG- and six-His-tagged Rta DNA binding domain on ice for 20 min in binding buffer (10 mM Tris [pH 7.5], 1 mM EDTA, 150 mM KCl, 5.76 mM MgCl2, 5 μg of bovine serum albumin, 0.1 μg of poly[dI-dC], 0.38 mM dithiothreitol, 0.38 mM phenylmethylsulfonyl fluoride, 55.4 mM β-mercaptoethanol, 5% glycerol). The binding reaction mixtures were loaded onto a 4.5% native polyacrylamide gel and run at 4°C in 1× TGE buffer (5 mM Tris, 190 mM glycine, 1 mM EDTA, pH 8.3) in the presence of 50 mM β-mercaptoethanol. The Rta DNA binding domain was cloned into vector pET30b (Novagen), expressed in bacteria, and purified through a Ni+-nitrilotriacetic acid column (Qiagen) (34a). For competition assays, an excess amount of unlabeled oligonucleotides was mixed with the labeled oligonucleotide prior to the addition of the Rta DNA binding domain; 1-fold was defined as the amount of oligonucleotide equivalent to the amount of active protein present in the reaction (34a). For supershift assays, a monoclonal anti-FLAG antibody (Sigma) or a polyclonal anti-Rta antibody was included in the binding reaction. The anti-Rta antibody was generated in rabbits by using the bacterially expressed Rta DNA binding domain as an immunogen (34a).
The oligonucleotide pairs used to generate double-stranded probes for EMSA are as follows: a, P25F and P25R; b, P26F (5′-CG GGA TCC gtg gtt cta agt cgc acg tta gaa acc c-3′) and P26R (5′-GA AGA TCT agg ggg cgg ggt ttc taa cgt gcg act t-3′); c, P27F (5′-CG GGA TCC ggt gct cac ttt ttg gcg agt tcg ctc g-3′) and P27R (5′-GA AGA TCT cgc ggc atc gag cga act cgc caa aaa g-3′); d, P35F (5′-CG GGA TCC ccc cct ggt gct cac ttt ttg gcg ag-3′) and P35R (5′-GA AGA TCT agc gaa ctc gcc aaa aag tga gca cc-3′); e, P34F (5′-CG GGA TCC ccc gcc ccc tgg tgc tca ctt ttt gg-3′) and P34R (5′-GA AGA TCT aac tcg cca aaa agt gag cac cag gg-3′); f, P29F (5′-CG GGA TCC aaa ccc cgc ccc ctg gtg ctc act tt-3′) and P29R (5′-GA AGA TCT cgc caa aaa gtg agc acc agg ggg cg-3′); g, P30F (5′-CG GGA TCC tta gaa acc ccg ccc cct ggt gct ca-3′) and P30R (5′-GA AGA TCT aaa aag tga gca cca ggg ggc ggg gt-3′); h, P31F (5′-CG GGA TCC cac gtt aga aac ccc gcc ccc tgg tg-3′) and P31R (5′-GA AGA TCT agt gag cac cag ggg gcg ggg ttt ct-3′); i, P32F (5′-CG GGA TCC gtc gca cgt tag aaa ccc cgc ccc ct-3′) and P32R (5′-GA AGA TCT agc acc agg ggg cgg ggt ttc taa cg-3′); j, P33F (5′-CG GGA TCC cta agt cgc acg tta gaa acc ccg cc-3′) and P34R (5′-GA AGA TCT cca ggg ggc ggg gtt tct aac gtg cg-3′); f1, P29F and P30R; f2, P29F and P36R (5′-GA AGA TCT aaa gtg agc acc agg ggg cgg ggt tt-3′); f3, P36F (5′-CG GGA TCC aga aac ccc gcc ccc tgg tgc tca ct-3′) and P36R. Unlabeled oligonucleotides used for cross-competition experiments include the following: ORF57 RRE, ORF57F (5′-CG GGA TCC gca agt gta aca ata atg ttc cca cgg ccc-3′) and ORF57R (5′-CGA AGA TCT ggg ccg tgg gaa cat tat tgt tac act tgc-3′); polyadenylated nuclear (PAN) RNA gene RRE: GSPAN5a (5′-CG GGA TCC gct tcc aaa aat ggg tgg cta acc tgt cca aaa tat ggg aac-3′) and GSPAN5b (5′-CGA AGA TCT gtt ccc ata ttt tgg aca ggt tag cca ccc att ttt gga agc-3′).
RESULTS
Two vIL-6 transcripts are synthesized in KS-1 cells during viral lytic replication.
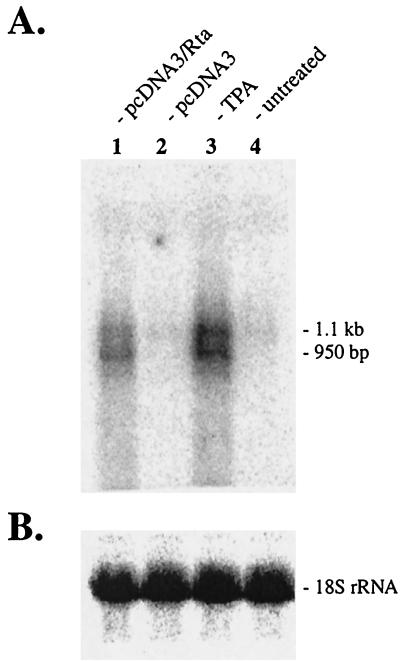
We first examined the expression of the vIL-6 gene in PEL-derived cell line KS-1. Either Rta-expressing plasmid pcDNA3/Rta or vector pcDNA3 was transfected into KS-1 cells, and total cellular RNA was harvested at 20 h posttransfection. As controls, total RNA was also prepared from untreated cells and from TPA-induced cells at 12 h postinduction. RNA was analyzed by Northern blotting for expression of the vIL-6 gene (Fig. 1). The vIL-6 gene was clearly upregulated by Rta activation (Fig. 1, lane 1) as well as by chemical induction (Fig. 1, lane 3), whereas the gene was expressed at a very low level in untreated cells (Fig. 1, lane 4) or cells transfected with pcDNA3 (Fig. 1, lane 2). A close examination of the Northern blot revealed the presence of two vIL-6 transcripts. They were expressed at similar levels during lytic replication and were approximately 950 bp and 1.1 kb. The 950-bp transcript was not expressed during latency but was strongly activated by Rta or TPA treatment. The 1.1-kb transcript was expressed at a very low level in latently infected cells and was further induced by Rta or TPA. These results indicated that two vIL-6 transcripts are synthesized in KS-1 cells during viral lytic replication.
FIG. 1.
Northern blot analysis of the vIL-6 gene transcripts. (A) pcDNA3/Rta (lane 1) or pcDNA3 (lane 2) was transfected into KS-1 cells (latently infected with HHV-8), and total cellular RNA was harvested at 20 h posttransfection. RNA was also prepared from untreated cells (lane 4) or cells induced with TPA for 12 h (lane 3). The 8-h difference was chosen to compensate for the delay of transcription and translation of Rta from the transfected pcDNA3/Rta. RNA samples were analyzed by Northern blotting using a probe containing the vIL-6 gene coding region. (B) The 18S rRNA in each sample serves as a loading control.
Mapping the polyadenylation site of the vIL-6 gene.
To gain insights into the nature of the two transcripts, we inspected the nucleotide sequence of the K2 locus that contains the HHV-8 vIL-6 gene (Fig. 2A). The coding sequence of the vIL-6 gene is located within the region spanning nucleotides (nt) 17875 to 17261 (30). The vIL-6 gene is transcribed leftward from the viral genome. Sequence analysis revealed two canonical polyadenylation signals (AATAAA motifs) downstream of the vIL-6 gene coding region, at nt 17205 and 17047. If both sites were utilized for the vIL-6 transcripts during lytic replication, the size difference between two transcripts with the same initiation site would be approximately 150 nt, consistent with the Northern blotting data. To test this possibility, we designed oligonucleotide P5 (Fig. 2B), which is complementary to the region between the two putative polyadenylation sites. The blot shown in Fig. 1 was stripped and reprobed with radioactively labeled P5. P5 would only hybridize to a transcript if the distal polyadenylation site had been utilized. However, the result suggested that the distal polyadenylation site was not used for vIL-6 transcripts (data not shown).
FIG. 2.
The vIL-6 gene (K2) locus on the HHV-8 genome. (A) The locus containing the vIL-6 gene. Positions of the first nucleotide of the start codon and the last nucleotide of the stop codon each of the ORFs are indicated (30). Arrowheads indicate the orientations of the ORFs. Positions of the two putative polyadenylation sites (diamonds) are also shown. DHFR, dihydrofolate reductase. (B) Locations of the probes and gene-specific primers used in this study. Diamonds, putative polyadenylation sites; arrows, transcription initiation sites mapped in this study. “Northern probe” and primer P5 were used in Northern blot analyses (Fig. 1). P14 and P24 were used in primer extension experiments (Fig. 3). P3 and P6 were used in 3′ RACE (Fig. 4A), and P7, P8, and P9 were used in 5′ RACE (Fig. 4B).
To confirm this result, we performed 3′ RACE. Total cellular RNA was harvested from induced KS-1 cells, and first-strand cDNA synthesis was carried out using oligo(dT)12-18 as a primer. PCR products were cloned into vector pCR-II and sequenced. All 3′ RACE products ended with a CA dinucleotide at nt 17182, which is 23 nt downstream of the proximal AATAAA motif (see Fig. 4A). The 30-nt sequence downstream of the polyadenylation cleavage site is T rich, conforming to the described consensus downstream elements for mammalian polyadenylation signals. These results suggest that the two vIL-6 transcripts observed by Northern blotting have the same 3′ ends and may therefore differ in the 5′ ends.
FIG. 4.
5′ and 3′ RACE. (A) Transcription termination site was determined by 3′RACE. Total cellular RNA from induced KS-1 cells was primed with oligo(dT)12-18 for first-strand cDNA synthesis. The cDNA was amplified by PCR using primers P3 and P3R. Seminested PCR was carried out with P6 and P6R. PCR products were cloned into pCR-II and sequenced. The polyadenylation signal is boxed, and dinucleotide ca at the cleavage site is double underlined. Capital letters in the second line represent the last 12 amino acids of vIL-6 (asterisk, stop codon). (B) Transcription initiation sites were confirmed by 5′ RACE. Total cellular RNA from induced KS-1 cells was primed with oligonucleotide P7 for first-strand cDNA synthesis with ThermoScript RT. cDNA was purified and tailed with dCTP and used as templates in first-round PCR, with P8 and AAP (provided in the 5′ RACE system; Gibco BRL) as primers. Seminested PCR was performed with P9 and AUAP (provided in the 5′ RACE system). PCR products were cloned into vector pCR-II and sequenced. Arrows, mapped transcription initiation sites. Proposed TATA elements are shaded. The 48-bp RRE sequence mapped by reporter studies shown in Fig. 6 is underlined. The 26-bp core RRE sequence mapped by EMSA shown in Fig. 10 is double underlined. The vIL-6 translation start codon is in boldface. In both panels, the numbers indicate the relative positions of the first nucleotide in each line, with the position of the vIL-6 translation start codon set at +1.
Mapping the transcription initiation sites of the vIL-6 gene.
To determine the 5′ ends of the vIL-6 transcripts, we performed a primer extension experiment. Total cellular RNA was prepared from uninduced or induced KS-1 cells at 24 h after induction of viral lytic replication. Primer extension experiments were performed using primer P14 (Fig. 3A). RNAs were hybridized to labeled P14 (Fig. 2B), and reverse transcription reactions were carried out at 50°C by using ThermoScript RT (Gibco BRL). Two major extension products were detected in cells induced for lytic replication (Fig. 3A, lanes 2 and 3), approximately 60 and 210 nt in length. The size difference correlates well to that observed by Northern blotting (Fig. 1). The primer extension products were specific, because analysis of RNA from DG75 cells (a virus-negative human B-cell line) yielded no products (Fig. 3A, lane 7). In addition, the pattern of vIL-6 transcripts induced by TPA (Fig. 3A, lane 2) is similar to that induced by butyrate (Fig. 3A, lane 3). The expression levels of the vIL-6 gene transcripts were higher in butyrate-induced cells, consistent with our experience that butyrate is a more potent inducer of viral lytic replication in KS-1 cells. Furthermore, this pattern of vIL-6 gene expression is not cell line specific, as similar results were obtained using another virus-positive cell line, BCBL-1 (Fig. 3A, lane 6). In our experience, BCBL-1 cells have lost responsiveness to TPA; therefore, no vIL-6 gene expression was detected when BCBL-1 cells were induced with TPA (Fig. 3A, lane 5). In addition, the vIL-6 transcript levels in butyrate-induced BCBL-1 cells were lower than those in butyrate-induced KS-1 cells (Fig. 3A, lanes 3 and 6). This could be due to differences between BCBL-1 and KS-1 cells in viral copy numbers or butyrate inducibility or both. By comparing the size of the shorter reverse transcription product (lower band) to a sequencing ladder (generated from a plasmid containing the vIL-6 gene upstream sequence, with P14 as a primer), we determined that the lower band represented a 61-nt reverse transcription product. If no splicing were involved, this would correspond to a vIL-6 transcript initiated at nt 17980.
FIG. 3.
Mapping the vIL-6 gene transcription initiation sites by primer extension. (A) RNA was prepared from untreated or TPA- or sodium butyrate-induced KS-1 cells (lanes 1 to 3, respectively) or BCBL-1 cells (lanes 4 to 6, respectively) at 24 h postinduction. RNA from virus-negative human B-cell line DG75 was also tested in parallel (lane 7) to show the specificity of the primer extension products. Primer extension experiments were performed using primer P14. Products were resolved on an 8% denaturing polyacrylamide gel, in parallel with sequencing ladders and a 10-bp DNA ladder. (B) RNA was prepared from untreated (lanes 1 and 7) or induced (lanes 4 and 10, by TPA; lanes 5 and 11, by sodium butyrate) KS-1 cells at 24 h postinduction. RNA was also harvested from pcDNA3- (lanes 2 and 8) or pcDNA3/Rta (lanes 3 and 9)-transfected KS-1 cells at 31 h posttransfection. RNA from DG75 cells was also tested in parallel (lanes 6 and 12). Primer extension experiments were performed using primers P14 (lanes 1 to 6) and P24 (lanes 7 to 12). In both panels, asterisks indicate bands corresponding to the distal transcription initiation sites and the arrowhead indicates the band corresponding to the proximal transcription initiation site. Dash, lane without an RNA sample.
To better define the distal initiation site, we designed primer P24 (Fig. 2B) based on the results from Fig. 3A. Since two vIL-6 transcripts were detected by Northern blotting when KS-1 cells were reactivated by ectopically expressed Rta, we also prepared RNA from pcDNA3/Rta- or pcDNA3-transfected KS-1 cells. Primer extension experiments were performed using primers P14 and P24 (Fig. 3B). The pattern of vIL-6 transcripts induced by Rta (Fig. 3B, lanes 3 and 9) is similar to that induced by TPA (Fig. 3B, lanes 4 and 10) or butyrate (Fig. 3B, lanes 5 and 11). By comparing the sizes of the longer reverse transcription product to the sequencing ladder, we determined that the doublet bands (Fig. 3B) represented reverse transcription products of 93 and 95 nt. These would correspond to vIL-6 transcripts initiated at nt 18126 and 18128 if no splicing events occurred.
These results from the primer extension experiments demonstrated that there are two transcription initiation sites for the vIL-6 gene during lytic replication. To determine whether any splicing event is involved in generating the 5′ end of the vIL-6 transcripts, we performed 5′ RACE using RNA from induced KS-1 cells. Sequencing the 5′ RACE products demonstrated that there is no splicing event and confirmed the transcription initiation sites mapped by primer extension (Fig. 4B). Based on these results, the lengths of the two vIL-6 transcripts, excluding the poly(A) tails, would be 799 and 946 nt. Since viral transcripts typically have poly(A) tails of 100 to 200 nt, the calculated lengths for the vIL-6 transcripts correlate with the sizes we observed in the Northern blot analysis (Fig. 1).
Rta activates the vIL-6 promoter in a reporter system.
Since Rta activated the expression of the vIL-6 gene from the endogenous viral genome (Fig. 1), we further investigated the mechanism by which Rta controls vIL-6 gene expression in a reporter system. We first sought to identify the vIL-6 promoter in the region upstream of the vIL-6 ORF in the viral genome. Various upstream sequences were cloned into pGL3-basic to derive reporter plasmids pK2p(−3.2k), pK2p(−1.7k), pK2p(−1.15k), and pK2p(−827) (the numbers in parentheses indicate the lengths of the upstream regions from the vIL-6 translation initiation site). pGL3-basic contains the firefly luciferase ORF but lacks any eukaryotic promoter/enhancer element. Reporter plasmids were transfected into 293T cells with either pcDNA3/Rta or vector pcDNA3, in addition to plasmid pRL-CMV. pRL-CMV contains the coding sequence for Renilla luciferase under the control of a constitutively active human cytomegalovirus immediate-early enhancer/promoter and serves as an internal control for transfection efficiency and other experimental variations. Cell extracts were harvested and assayed for both firefly and Renilla luciferase activities at 48 h posttransfection. As shown in Fig. 5A, all four plasmids were activated 80- to 115-fold by the Rta protein. Specifically, pK2p(−827), containing the shortest fragment of the vIL-6 promoter, was activated 95-fold, a level comparable to those achieved by pK2p(−3.2k), pK2p(−1.7k), and pK2p(−1.15k).
FIG. 5.
Analysis of the vIL-6 promoter region. (A) Various regions upstream of the vIL-6 gene ORF were cloned into a luciferase reporter plasmid (pGL3-basic). Reporter plasmids were cotransfected into 293T cells with either an Rta expression plasmid (pcDNA3/Rta) or pcDNA3 and control vector pRL-CMV. Vector pRL-CMV constitutively expresses Renilla luciferase driven by a cytomegalovirus immediate-early enhancer/promoter. Cells were harvested at 48 h posttransfection, and dual-luciferase assays were performed. Firefly luciferase activities from the vIL6 promoters were normalized to the corresponding Renilla luciferase activities. Fold activation by Rta was calculated by comparing the normalized firefly luciferase activity stimulated by Rta to that stimulated by pcDNA3. Results are the averages from four transfection experiments ± standard deviations. (B) Dose-dependent activation of pK2p(−827) by Rta. A fixed amount of reporter plasmid pK2p(−827) was cotransfected into 293T cells with increasing amounts (from 0 to 100 ng) of pcDNA3/Rta, along with pRL-CMV. A decreasing amount of pcDNA3 (from 100 to 0 ng) was included in each sample so that the total amount of pcDNA3 vector backbone stayed the same. Fold activation by Rta was calculated as described for panel A. Results are representative of two triplicate experiments, with the standard deviations shown.
To confirm that activation of pK2p(−827) was mediated by the Rta protein, we tested the dose dependence of Rta activation. A fixed amount of reporter plasmid pK2p(−827) was cotransfected with increasing amounts of pcDNA3/Rta into 293T cells. As the amount of pcDNA3/Rta increased (from 0 to 100 ng), so did the normalized luciferase activity (Fig. 5B). These results indicate that the 827-nt sequence upstream of the vIL-6 ORF is sufficient to mediate dose-dependent activation by Rta and that pK2p(−827) can be used to further examine the regulation of the vIL-6 gene by Rta.
Identification of an RRE in the vIL-6 promoter.
To map the RRE in the vIL-6 promoter, we made a series of promoter deletion constructs. These constructs contain 5′ progressive deletions from the 827-nt sequence upstream of the vIL-6 ORF (Fig. 6A). These reporter plasmids were cotransfected with pcDNA3/Rta or pcDNA3 into 293T cells, as described above. As shown in Fig. 6B, when the region between −827 and −569 was deleted, the reporter response to Rta decreased 2.0-fold. However, when the region between −462 and −414 was deleted, the reporter response to Rta was reduced 8.3-fold. Deletion of other regions had minimal effect on Rta activation of the reporters. The results suggest that the 48-bp region between −462 and −414 contains an RRE (see also Fig. 4B).
FIG. 6.
Mapping the RRE of the vIL-6 promoter. (A) Diagram of the 5′ promoter deletion constructs. The vIL-6 gene ORF and the promoter region on the HHV-8 genome are shown at the top, and the promoter deletion constructs are aligned below. The deletion constructs were named by the relative positions of the 5′ end of the promoter region, with the vIL-6 translation start codon set at +1. Arrows, mapped transcription initiation sites; open boxes, proposed TATA elements (T1, proximal; T2, distal); hatched box, mapped RRE; Luc., luciferase ORF in pGL3-basic. (B) The deletion constructs were assayed for their responsiveness to Rta in reporter assays with 293T cells, as described for Fig. 5. Results are the averages of at least three experiments ± standard deviations.
The vIL-6 promoter contains two functional TATA boxes.
The identification of two transcription initiation sites suggests that there are two promoters controlling the expression of the vIL-6 gene. We analyzed the sequence upstream of the two initiation sites and identified two putative TATA boxes. The proximal TATA box is located 29 nt upstream of the initiation site for the shorter transcript. The distal TATA box is located 28 nt upstream of the initiation site for the longer transcript.
To examine the role of each putative TATA box in directing Rta-mediated expression of the vIL-6 gene, we introduced mutations into each element and tested transcriptional activation by transient-transfection experiments. Studies have shown that TATA box-binding protein (TBP) recognizes the core promoter sequence via its minor groove. Since A↔T transversions leave the minor-groove face unchanged, TBP-TATA complexes can form on many A/T-rich core promoter sequences (27). Therefore, we constructed plasmid pK2p(−462)M2, in which the distal TATA box in plasmid pK2p(−462) was mutated from tattttt to tGGGGGt through site-directed mutagenesis. Changes from A/T to G should render the distal TATA box nonfunctional, while still retaining the proximal TATA box and the RRE. To disrupt the proximal TATA box, we made two plasmids. In pK2p(−462)M1, the proximal TATA box was mutated from tacataa to tGGGGGa. Alternatively, the proximal TATA box was deleted to generate plasmid pK2p(T2+RRE). Finally, mutations in both TATA boxes were introduced into pK2p(−462) to generate pK2p(−462)M12 (Fig. 7A). We tested the Rta responsiveness of these four plasmids, in comparison to that of the parental plasmid, pK2p(−462) (Fig. 7B). pK2p(−462)M2 retained 77.7% of its Rta responsiveness, whereas the Rta responsiveness levels of pK2p(−462)M1 and pK2p(T2+RRE) were diminished to 63.4 and 56.7%, respectively. When both TATA boxes were mutated, Rta-mediated activation was further reduced to 43.6%. These results suggested that both TATA boxes contribute to activation of the vIL-6 promoter by Rta.
FIG. 7.
The vIL-6 promoter contains two functional TATA boxes. (A) Diagram of the plasmids. Boxes are as described for Fig. 6 (X, site-directed mutations in the TATA boxes). (B) The plasmids were assayed for their responsiveness to Rta in reporter assays with 293T cells, as described for Fig. 5. Fold activation of pK2p(−462) was set at 100%.
The vIL-6 RRE confers Rta responsiveness to a heterologous promoter.
To investigate whether the RRE contained between −462 and −414 of the vIL-6 promoter can mediate Rta activation when linked to a heterologous promoter, double-stranded oligonucleotide corresponding to the RRE sequence (Fig. 4B) was cloned in both orientations (forward and reverse) into vector pGL3-promoter. pGL3-promoter contains a 200-bp promoter sequence from simian virus 40 (SV40) but lacks an enhancer sequence. The resulting plasmids, pK2pRRE-F and pK2pRRE-R, were sequenced to verify both the sequence and orientation of the RRE. Because of the T antigen in 293T cells, pK2pRRE-F and -R had high basal levels in this cell line. We therefore chose to test for Rta-mediated activation in 293 cells (Fig. 8). The two plasmids were activated 8.4- and 8.5-fold by Rta, respectively. To examine the specificity of Rta activation, we also tested the ability of another potent transcriptional activator, GAL4-VP16 (31), to activate the vIL-6 RRE. We have previously demonstrated that GAL4-VP16 strongly activated pGAL4-M2-Luc, a reporter construct containing five GAL4 binding sites upstream of the luciferase ORF, whereas Rta did not (34). As shown in Fig. 8, GAL4-VP16 failed to activate pK2pRRE-F and pK2pRRE-R, indicating the specificity of Rta activation of the vIL-6 RRE. Therefore, the mapped vIL-6 RRE mediated specific activation by Rta in a promoter- and orientation-independent manner.
FIG. 8.
The vIL-6 RRE confers Rta responsiveness to a heterologous promoter. Double-stranded oligonucleotide corresponding to the RRE sequence was cloned in both orientations (forward and reverse) into vector pGL3-promoter (containing the SV40 promoter). The resulting plasmids, pK2pRRE-F and pK2pRRE-R, were then tested for Rta activation by reporter assays with 293 cells, as described for Fig. 5. To demonstrate the specificity of Rta activation, pK2pRRE-F and pK2pRRE-R were also tested for activation by transcriptional activator GAL4-VP16.
Rta directly binds to the vIL-6 RRE.
Rta has previously been shown to bind directly to the mapped RREs in the promoters of HHV-8 genes such as PAN RNA, Kaposin (K12), ORF57, and K-bZip (8a, 18, 34, 34a). To further define the mechanism by which Rta activates the vIL-6 promoter, we tested whether Rta can also directly bind to the vIL-6 RRE. Three double-stranded oligonucleotides, a, b, and c, were designed (see Fig. 10A). Oligonucleotide a corresponds to the 48-bp vIL-6 RRE sequence mapped by the reporter assays. Oligonucleotide b contains the 5′ 36 bp of a. Oligonucleotide c contains the 3′ 12 bp of a plus the 24-bp sequence immediately downstream of the vIL-6 promoter and was used to examine whether deletion of the vIL-6 promoter from −462 to −414 may have removed part of the sequence required for activation by Rta and therefore resulted in loss of Rta responsiveness of pK2p(−414) in reporter assays. Double-stranded oligonucleotides were labeled and tested for Rta binding by EMSA. Labeled oligonucleotide a was incubated with binding buffer alone (Fig. 9A, lane 1) or increasing amounts of purified, FLAG-tagged Rta DNA binding domain (Fig. 9A, lanes 2 to 5). Oligonucleotide a showed dose-dependent binding to Rta (Fig. 9A). To demonstrate the specificity of Rta binding to the vIL-6 RRE, we performed competition and supershift experiments. First, unlabeled a competed for this binding in a dose-dependent manner (Fig. 9A, lanes 6 and 7). Second, double-stranded oligonucleotide corresponding to the ORF57 RRE cross-competed for Rta binding to a in a dose-dependent manner (Fig. 9A, lanes 8 and 9). Third, the three Rta-bound complexes formed on oligonucleotide a were all supershifted by a monoclonal anti-FLAG antibody (Fig. 9A, lane 10). Finally, inclusion in the reaction of a polyclonal antibody against the Rta DNA binding domain abolished the binding of Rta to a (Fig. 9A, lane 11). Furthermore, the Rta DNA binding domain did not bind to an oligonucleotide containing unrelated sequences. Unrelated monoclonal or polyclonal antibodies did not have any effect on Rta binding to RRE (data not shown). These data indicated that Rta specifically binds to the vIL-6 RRE.
FIG. 10.
Mapping of a core vIL-6 RRE. (A) Schematic of the oligonucleotides tested for Rta binding by EMSAs. Their relative binding affinities for Rta are summarized on the right. Nucleotide residues of the 26-bp core site are in boldface. (B) Oligonucleotides a to j were labeled and tested. They showed dose-dependent binding to the Rta DNA binding domain, although with different affinities (lanes 7, 11, 15, 19, 23, and 27, 0 ng of protein; lanes 1, 4, 8, 12, 16, 20, 24, and 28, 60 ng of protein; lanes 2, 5, 9, 13, 17, 21, 25, and 29, 180 ng of protein; lanes 3, 6, 10, 14, 18, 22, 26, and 30, 540 ng of protein). Dashes, absence of Rta DNA binding domain in the reactions. (C) Specificities of the binding complexes formed by g and Rta. Labeled oligonucleotide g was incubated with the DNA binding buffer alone (lane 1) or with 540 ng of the Rta DNA binding domain (lanes 2 to 9). Unlabeled g (lanes 3 and 4, 4- and 20-fold, respectively) or a (lanes 5 and 6, 4- and 20-fold, respectively) oligonucleotides competed for this binding. Unlabeled oligonucleotides corresponding to the PAN RRE (lanes 7 and 8, 1- and 10-fold, respectively) competed more efficiently. The Rta-bound complexes were supershifted by a monoclonal anti-FLAG antibody (lane 9). Dash, absence of Rta DNA binding domain in the reaction; arrows, shifted complexes; arrowheads, supershifted complexes. (D) Oligonucleotides f, f1, f2, f3, and g were labeled and incubated in the absence (lanes 1, 3, 5, 7, and 9) or presence (lanes 2, 4, 6, 8, and 10) of the Rta DNA binding domain (540 ng).
FIG. 9.
Rta directly binds to the vIL-6 RRE. (A) Oligonucleotide a was labeled and tested. a showed dose-dependent binding to the purified, FLAG-tagged Rta DNA binding domain (lanes 1 to 5: 0, 20, 60, 180, and 540 ng of protein, respectively). Unlabeled oligonucleotides corresponding to a (lanes 6 and 7: 4- and 20-fold, respectively) and the ORF57 RRE (lanes 8 and 9: 4- and 20-fold, respectively) competed for this binding. The Rta-bound complexes were supershifted by a monoclonal anti-FLAG antibody (lane 10). Inclusion of a polyclonal antibody against the Rta DNA binding domain in the reaction abolished binding of Rta to the RRE (lane 11). Dash, absence of Rta DNA binding domain in the reaction; arrows, shifted complexes; arrowheads, supershifted complexes. (B) Oligonucleotide b was labeled and tested. The Rta DNA binding domain (540 ng) bound to b (lane 1). Unlabeled oligonucleotide corresponding to the PAN RRE (lanes 2 and 3: 1- and 10-fold, respectively) competed efficiently for this binding. Unlabeled oligonucleotides a or b (lanes 4 and 5: 10-fold) also competed for this binding. Dash, absence of competitor in the reaction; arrows, shifted complexes.
We next tested the binding of Rta to b and c by EMSA. Rta also bound to b (Fig. 9B, lane 1), although less efficiently than to a (Fig. 9A, lane 5), suggesting that the 3′ 12-bp sequence of a contributes to binding by Rta. The binding of Rta to b was cross-competed efficiently and in a dose-dependent manner by unlabeled oligonucleotide corresponding to the PAN RRE (Fig. 9B, lanes 2 and 3). Unlabeled a or b also competed for binding of Rta to b, although a competed more effectively than b (Fig. 9B, lanes 4 and 5). In contrast, only minimal interaction between c and Rta was detected by EMSA. Consistently, c cross-competed inefficiently with a for binding of Rta to labeled a (data not shown). Taken together, the results from reporter assays and EMSA indicated that Rta specifically binds to the vIL-6 RRE contained within 48-bp oligonucleotide a.
Mapping of a core vIL-6 RRE.
We next sought to identify the core sequence responsible for the binding of Rta to the vIL-6 promoter. A comparison of the 48-bp vIL-6 RRE to other published RREs of HHV-8 genes did not reveal any apparent homology in primary nucleotide sequence (8a, 13, 18, 34, 34a). We therefore took a systematic approach and, based on the results shown in Fig. 9, designed a series of oligonucleotides, d to j (Fig. 10A). These 32-bp oligonucleotides, staggered at 4 bp apart, span the promoter region from −456 to −401. They were labeled and tested by EMSA, in comparison to a (Fig. 10B). Although oligonucleotides d to j all showed dose-dependent binding by Rta, the binding affinities varied. In particular, the binding of Rta to oligonucleotide g was as efficient as binding of Rta to a and more efficient than the binding of Rta to f. In contrast, the binding affinities of Rta for oligonucleotides d, e, h, i, and j were significantly lower. These results, reproducible in independent experiments (data not shown), are summarized in Fig. 10A. They suggested that 32-bp oligonucleotide g, corresponding to nt −444 to −413 of the vIL-6 promoter contained a core binding site for Rta.
To examine the specificity of Rta binding to oligonucleotide g, we performed competition and supershift experiments (Fig. 10C). Incubation of labeled g with the Rta DNA binding domain resulted in shifted complexes (Fig. 10C, lane 2). Both unlabeled g (Fig. 10C, lanes 3 and 4) and a (Fig. 10C, lanes 5 and 6) competed for this binding in a dose-dependent manner and with similar efficiencies. Again, unlabeled oligonucleotide corresponding to the PAN RRE competed very efficiently for Rta binding (Fig. 10C, lanes 7 and 8). Finally, the three Rta-bound complexes formed on oligonucleotide g were all supershifted by the anti-FLAG antibody (Fig. 10C, lane 9). These data indicated that binding of Rta to oligonucleotide g is specific.
To fine map the core RRE in the vIL-6 promoter, we designed three additional oligonucleotides, f1 to f3. The 28-bp f1 corresponds to the promoter region shared by f and g. The 26-bp f2 corresponds to the region shared by a, f, and g. The 28-bp f3 differs from f2 by extra 2 bp on the 5′ end. These oligonucleotides were examined in EMSA, in comparison to f and g (Fig. 10D). f1 to f3 all demonstrated binding affinities for Rta similar to that of f, although they bound less efficiently than g. Taken together, these results (summarized in Fig. 10A) demonstrated that 26-bp oligonucleotide f2, corresponding to nt −440 to −413 of the vIL-6 promoter, or nt 18315 to 18290 on the viral genome, contains a core RRE.
DISCUSSION
vIL-6 encoded by HHV-8 has been proposed to play an important role in viral pathogenesis. Several groups have demonstrated differential expression of vIL-6 in KS, PEL, and MCD tumor lesions. However, mechanisms regulating vIL-6 gene expression have not been reported in the literature. In this study, we first characterized the transcription unit of the vIL-6 gene. Two vIL-6 transcripts were identified in PEL-derived cell lines during viral lytic replication by Northern blot analysis. Employing primer extension, 5′ RACE, and 3′ RACE, we mapped the transcription initiation and termination sites of the vIL-6 gene and demonstrated that the two transcripts resulted from two initiation sites. We also investigated the role of Rta in governing the expression of the vIL-6 gene. Rta strongly activated the vIL-6 promoter in a reporter system, and an RRE was mapped to a 48-bp region. The vIL-6 RRE conferred responsiveness to a heterologous promoter in an orientation-independent manner. Furthermore, Rta directly bound to the vIL-6 RRE, as shown by EMSA, suggesting that DNA binding is one mechanism through which Rta activates the expression of the vIL-6 gene during the viral lytic cycle.
In contrast to previous reports which have detected only one vIL-6 transcript (22, 24, 38), we identified two transcripts during viral lytic replication (Fig. 1). This discrepancy is most likely due to the small difference in sizes of the two transcripts and the high level of vIL-6 gene expression during lytic replication, such that the presence of two transcripts is only revealed on lighter exposures of the Northern blot. We have confirmed this initial observation by employing multiple techniques (Fig. 3 and 4).
Northern blot analysis demonstrated the expression of the longer transcript in uninduced or pcDNA3-transfected cells (Fig. 1, lanes 2 and 4). However, we did not detect the longer transcript in uninduced or pcDNA3-transfected cells by primer extension (Fig. 3A, lanes 1 and 4, and B, lanes 7 and 8). This may be explained by the different sensitivities of the two methods. In primer extension, each copy of the primer was end labeled with a single radioactive nucleotide. However, each copy of the 615-bp probe used in Northern blotting was labeled by a random-priming method, leading to a sensitivity much higher than that of primer extension. Furthermore, longer products are less favored in reverse transcription reactions. Other groups have also documented detection of the vIL-6 transcript during viral latency in tumor lesions (6, 36), although it was not clear whether the transcript corresponded to the longer one we observed. Identification of an RRE upstream of both TATA boxes provided a mechanism through which the two transcripts are strongly activated by Rta during lytic replication (Fig. 1 and 3). The mechanism regulating the low level of vIL-6 gene expression during latency in vivo requires further investigation.
Differential promoter usage is frequently used as a mechanism to regulate the expression of cellular and viral genes. One classic example is the EBNA-1 gene of EBV (17, 22, 24). In latency type 1 or 2, the EBNA-1 transcript is initiated at the Qp promoter. In latency type 3, EBNA-1 expression is controlled by the Cp or Wp promoter. When the viruses in latently infected cells are reactivated to lytic replication, the Fp promoter is activated and EBNA-1 expression continues. Recently, a viral interferon regulatory factor encoded by HHV-8 ORF K9 has also been shown to utilize different promoter elements to regulate its expression during latency or lytic replication (10). By applying in situ hybridization and immunohistochemistry, several groups have demonstrated different levels of vIL-6 gene expression in different HHV-8-associated diseases (6, 26, 36). In KS lesions examined, most, if not all, of the cells were latently infected, and the vIL-6 gene was expressed at very low levels in a few cells. In PEL specimens, the majority of the cells were also latently infected, but a small percentage of cells were productively infected and expressed PAN RNA. vIL-6 transcripts with a gradient of expression levels were detected in majority of the tumor cells. The frequency of cells with high levels of vIL-6 gene expression was similar to the frequency of PAN RNA-positive cells. Although not a direct demonstration, these data suggested that cells expressing low levels of vIL-6 gene but not PAN RNA harbored HHV-8 in a latent state. In contrast, in MCD samples, only a small percentage of cells located in the mantle zone surrounding the germinal centers were infected with HHV-8; however, they all seemed to express the lytic transcripts, and vIL-6 gene expression levels were very high. The riboprobe utilized in both in situ hybridization studies corresponds to the full-length vIL-6 coding region and would not differentiate between the two vIL-6 transcripts that differ at the 5′ untranslated region. Based on the studies we report here, we propose that the existence of two vIL-6 promoters allows for differential regulation of vIL-6 gene expression in cell- and tissue-specific environments, which may lead to different manifestations in HHV-8-associated malignancies. One approach to test this hypothesis is to transfect the vIL-6 promoter luciferase construct into different cell types and examine whether the relative levels of the two vIL-6 transcripts are cell type dependent or differentially regulated in distinct stages of viral replication.
The vIL-6 RRE, when linked to a heterologous promoter, conferred approximately 9-fold activation by Rta (Fig. 8), much lower than the 53-fold activation of pK2p(−462) by Rta (Fig. 6). This result may be due to the high basal activity of the SV40 promoter in plasmids pK2pRRE-F and pK2pRRE-R during the absence of Rta. The SV40 promoter in pGL3-promoter contains approximately 200 bp of sequence and is not a minimal promoter. We have previously seen a similar effect with the RRE from the PAN RNA promoter. The PAN RRE was activated 2,000- to 7,000-fold in the context of its native promoter; however, the PAN RRE yielded only approximately 25- or 12-fold activation by Rta (forward or reverse orientation, respectively) when linked to the same SV40 promoter (34). Multimerization of the PAN gene RRE enhanced the responsiveness of reporter plasmids to Rta (34). Another possibility is that sequence elements other than the RRE may be involved in enhancing the Rta responsiveness of the vIL-6 promoter.
Rta was shown to bind the vIL-6 RRE by EMSA (Fig. 9), suggesting that direct DNA binding is a mechanism through which Rta activates the vIL-6 gene. The cross-competition experiment showed that the binding affinity of Rta for the vIL-6 RRE is comparable to that for the ORF57 RRE (Fig. 9A). However, the binding affinity of Rta for the vIL-6 RRE is lower than that for the PAN RRE but higher than that for the K-bZip RRE (Fig. 9B) (M. J. Song et al., submitted for publication). In addition, we have noticed a twofold decrease in activation by Rta when the promoter fragment between −827 and −569 was deleted (Fig. 6). It is possible that Rta also acts through an indirect mechanism or that other transcription factors may play a role in Rta activation of the vIL-6 gene. It has been documented that Rta of gammaherpesviruses can activate target genes through a nonbinding mechanism, and other transcription factors (e.g., Sp1) have been shown to play a role in this scenario (28, 41).
The binding of Rta to various sequences containing the vIL-6 RRE produced three complexes with different mobilities as detected by EMSA (Fig. 9 and 10). They may result from different stoichiometries of the Rta protein in the complexes or distinct conformations of the complexes (e.g., isomers). However, all three complexes were supershifted by the anti-FLAG antibody (Fig. 9B, lane 10, and Fig. 10C, lane 9), clearly demonstrating the presence of Rta in these complexes.
We compared the 26-bp vIL-6 RRE core site mapped in this study (Fig. 10A) to other published RREs of HHV-8 genes. These include the RREs from the PAN RNA, Kaposin, ORF57, and K-bZip promoters (8a, 13, 18, 34, 34a). No significant homology in nucleotide sequence between the vIL-6 RRE and the other RREs was identified. For example, the highest homology was that between the 26-bp vIL-6 RRE, 5′-AAACCCCGCCCCCTGGTGCTCACTTT, and the PAN RRE. However, among the nine identical nucleotides (underlined), only dinucleotide TG was found to be critical for Rta binding in vitro and Rta transactivation in vivo (34a). This dinucleotide is also present in ORF57 and K-bZip RREs (18) but is not conserved in the Kaposin RRE (8a; Song et al., submitted). Furthermore, no palindromic sequence motif was found in the vIL-6 RRE, as has been noted for ORF57 and K-bZip RREs. Since these diverse sequences can serve as Rta binding sites, identification of RREs, including the vIL-6 RRE reported in this study, contributes to understanding how Rta binds to its target DNA sequences. For instance, part of Rta may bind target DNA via minor-groove as well as major-groove contacts, allowing A↔T or G↔C conversions in the target sequence to be well tolerated. This could partially explain the diversity of the RREs. Detailed analysis of the individual nucleotide residues and motifs critical for Rta binding (34a) and solution of the crystallographic structure of the Rta protein will offer insights into the mechanism of Rta binding and its role in transcriptional activation.
In summary, we have defined the transcription unit of the HHV-8 vIL-6 gene and characterized the regulation of vIL-6 gene expression by Rta in vitro. Identification of two vIL-6 transcripts has laid a foundation for examining their expression in tumor lesions from KS, PEL, and MCD and will aid our understanding of the role that vIL-6 plays in viral pathogenesis.
Acknowledgments
We thank Tonia Symensma and Helen Brown for critical reading of the manuscript. We also thank Michael Carey and members of the Sun laboratory for helpful discussions.
This work was supported by NIH grant T32 AI07388 to H.D., a UCLA Chancellor's Dissertation Year Fellowship (Graduate Division) to M.J.S., NIH grants CA91791, CA83525, and DE14153, and support from the Stop Cancer Foundation, Concern Foundation, and Jonsson Cancer Center Foundation to R.S. H.D. is a Lymphoma Research Foundation Fellow.
REFERENCES
- 1.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. [PubMed] [Google Scholar]
- 2.Aoki, Y., K. D. Jones, and G. Tosato. 2000. Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. J. Hematother. Stem Cell Res. 9:137-145. [DOI] [PubMed] [Google Scholar]
- 3.Aoki, Y., R. Yarchoan, J. Braun, A. Iwamoto, and G. Tosato. 2000. Viral and cellular cytokines in AIDS-related malignant lymphomatous effusions. Blood 96:1599-1601. [PubMed] [Google Scholar]
- 4.Arvanitakis, L., E. A. Mesri, R. G. Nador, J. W. Said, A. S. Asch, D. M. Knowles, and E. Cesarman. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648-2654. [PubMed] [Google Scholar]
- 4a.Brown T. 1998. Analysis of RNA by Northern and slot blot hybridization, p. 4.9.1.-4.9.16. In F. M. Ausubel (ed.), Current protocols in molecular biology, vol. 1. John Wiley and Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 5.Burger, R., J. Wendler, K. Antoni, G. Helm, J. R. Kalden, and M. Gramatzki. 1994. Interleukin-6 production in B-cell neoplasias and Castleman's disease: evidence for an additional paracrine loop. Ann. Hematol. 69:25-31. [DOI] [PubMed] [Google Scholar]
- 6.Cannon, J. S., J. Nicholas, J. M. Orenstein, R. B. Mann, P. G. Murray, P. J. Browning, J. A. DiGiuseppe, E. Cesarman, G. S. Hayward, and R. F. Ambinder. 1999. Heterogeneity of viral IL-6 expression in HHV-8-associated diseases. J. Infect. Dis. 180:824-828. [DOI] [PubMed] [Google Scholar]
- 7.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman, E., P. S. Moore, P. H. Rao, G. Inghirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 8a.Chang, P. J., D. Shedd, L. Gradoville, M. S. Cho, L. W. Chen, J. Chang, and G. Miller. 2002. Open reading frame 50 protein of Kaposi's sarcoma-associated herpesvirus directly activates the viral PAN and K12 genes by binding to related response elements. J. Virol. 76:3168-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J., K. Ueda, S. Sakakibara, T. Okuno, and K. Yamanishi. 2000. Transcriptional regulation of the Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor gene. J. Virol. 74:8623-8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow, D., X. He, A. L. Snow, S. Rose-John, and K. C. Garcia. 2001. Structure of an extracellular gp130 cytokine receptor signaling complex. Science 291:2150-2155. [DOI] [PubMed] [Google Scholar]
- 12.Deng, H., A. Young, and R. Sun. 2000. Auto-activation of the rta gene of human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 81:3043-3048. [DOI] [PubMed] [Google Scholar]
- 13.Duan, W., S. Wang, S. Liu, and C. Wood. 2001. Characterization of Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 ORF57 promoter. Arch. Virol. 146:403-413. [DOI] [PubMed] [Google Scholar]
- 14.Ensoli, B., G. Barillari, and R. C. Gallo. 1992. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi's sarcoma. Immunol. Rev. 127:147-155. [DOI] [PubMed] [Google Scholar]
- 15.Fassone, L., G. Gaidano, C. Ariatti, D. Vivenza, D. Capello, A. Gloghini, A. M. Cilia, D. Buonaiuto, D. Rossi, C. Pastore, A. Carbone, and G. Saglio. 2000. The role of cytokines in the pathogenesis and management of AIDS-related lymphomas. Leuk. Lymphoma 38:481-488. [DOI] [PubMed] [Google Scholar]
- 16.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2627. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 17a.Kingston R. E. 1998. Transfection of DNA into eukaryotic cells, p. 9.1.1.-9.1.11. In F. M. Ausubel (ed.), Current protocols in molecular biology, vol. 1. John Wiley and Sons, Inc., New York, N.Y.
- 18.Lukac, D. M., L. Garibyan, J. R. Kirshner, D. Palmeri, and D. Ganem. 2001. DNA binding by Kaposi's sarcoma-associated herpesvirus lytic switch protein is necessary for transcriptional activation of two viral delayed early promoters. J. Virol. 75:6786-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, V. M. Kushnaryov, S. Grossberg, and Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molden, J., Y. Chang, Y. You, P. S. Moore, and M. A. Goldsmith. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272:19625-19631. [DOI] [PubMed] [Google Scholar]
- 22.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 23.Neipel, F., J. C. Albrecht, A. Ensser, Y. Q. Huang, J. J. Li, A. E. Friedman-Kien, and B. Fleckenstein. 1997. Human herpesvirus 8 encodes a homolog of interleukin-6. J. Virol. 71:839-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholas, J., V. R. Ruvolo, W. H. Burns, G. Sandford, X. Wan, D. Ciufo, S. B. Hendrickson, H. G. Guo, G. S. Hayward, and M. S. Reitz. 1997. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat. Med. 3:287-292. [DOI] [PubMed] [Google Scholar]
- 25.Osborne, J., P. S. Moore, and Y. Chang. 1999. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum. Immunol. 60:921-927. [DOI] [PubMed] [Google Scholar]
- 26.Parravicini, C., B. Chandran, M. Corbellino, E. Berti, M. Paulli, P. S. Moore, and Y. Chang. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patikoglou, G. A., J. L. Kim, L. Sun, S. H. Yang, T. Kodadek, and S. K. Burley. 1999. TATA element recognition by the TATA box-binding protein has been conserved throughout evolution. Genes Dev. 13:3217-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragoczy, T., and G. Miller. 2001. Autostimulation of the Epstein-Barr virus BRLF1 promoter is mediated through consensus Sp1 and Sp3 binding sites. J. Virol. 75:5240-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 30.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadowski, I., J. Ma, S. Triezenberg, and M. Ptashne. 1988. GAL4-VP16 is an unusually potent transcriptional activator. Nature 335:563-564. [DOI] [PubMed] [Google Scholar]
- 32.Said, J. W., K. Chien, S. Takeuchi, T. Tasaka, H. Asou, S. K. Cho, S. de Vos, E. Cesarman, D. M. Knowles, and H. P. Koeffler. 1996. Kaposi's sarcoma-associated herpesvirus (KSHV or HHV8) in primary effusion lymphoma: ultrastructural demonstration of herpesvirus in lymphoma cells. Blood 87:4937-4943. [PubMed] [Google Scholar]
- 33.Sarid, R., O. Flore, R. A. Bohenzky, Y. Chang, and P. S. Moore. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song, M. J., H. J. Brown, T. T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by Rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Song, M. J., X. Li, H. J. Brown, and R. Sun. 2002. Characterization of interactions between, R. T. A., and the promoter of polyadenylated nuclear RNA in Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 76:5000-5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 36.Staskus, K. A., R. Sun, G. Miller, P. Racz, A. Jaslowski, C. Metroka, H. Brett-Smith, and A. T. Haase. 1999. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J. Virol. 73:4181-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taga, T., and T. Kishimoto. 1997. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 15:797-819. [DOI] [PubMed] [Google Scholar]
- 40.Wan, X., H. Wang, and J. Nicholas. 1999. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J. Virol. 73:8268-8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalani, S., E. A. Holley-Guthrie, D. E. Gutsch, and S. C. Kenney. 1992. The Epstein-Barr virus immediate-early promoter BRLF1 can be activated by the cellular Sp1 transcription factor. J. Virol. 66:7282-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]