Abstract
Human immunodeficiency virus (HIV) replication is linked to cellular gene transcription and requires target cell activation. The latent reservoir of HIV-1 in quiescent T cells is thought to be a major obstacle to clearance of infection by highly active antiretroviral therapy (HAART). Thus, identification of agents that can induce expression of latent virus may, in the presence of HAART, allow elimination of the infected cells by the immune response. We previously used the SCID-hu (Thy/Liv) mouse model to establish that activation-inducible HIV can be generated at high frequency during thymopoiesis. Latently infected mature thymocytes can be exported into the periphery, providing an efficient primary cell model to determine cellular activation signals that induce renewed expression of latent virus. Here we characterized the effects of prostratin, a non-tumor-promoting phorbol ester, on primary human peripheral blood lymphocytes (PBLs) and assessed its ability to reactivate latent HIV infection from thymocytes and PBLs in the SCID-hu (Thy/Liv) model. Prostratin stimulation alone did not induce proliferation of quiescent PBLs; however, it could provide a secondary signal in the context of T-cell receptor stimulation or a primary activation signal in the presence of CD28 stimulation to induce T-cell proliferation. While prostratin alone was not sufficient to allow de novo HIV infection, it efficiently reactivated HIV expression from latently infected cells generated in the SCID-hu mouse. Our data indicate that prostratin alone is able to specifically reactivate latent virus in the absence of cellular proliferation, making it an attractive candidate for further study as an adjunctive therapy for the elimination of the latent HIV reservoir.
Highly active antiretroviral therapy (HAART) often reduces human immunodeficiency virus (HIV) viral loads below detection levels and can greatly prolong the time to progression to AIDS (21). However, replication-competent HIV persists in latently infected resting CD4+ T cells despite HAART (7, 10, 26). It remains unclear to what extent the existence of viral reservoirs in latently infected resting T cells affects the prospects for long-term control or cure of HIV infection. The viral “rebound” that almost always occurs following cessation of HAART is largely due to residual replication that continues despite HAART (8, 11). However, the lifetime of the latently infected resting T-cell reservoir suggests that it could ultimately prevent the eradication of HIV from an infected individual (9). Given the lack of effective anti-HIV immune responses in infected individuals, even following effective HAART (2), long-lasting viral reservoirs represent a fundamental barrier to the eventual cure for HIV infection.
One proposed method to overcome this latent barrier is to induce the replication of HIV in latently infected resting T cells while preventing the spread of the newly produced virions to uninfected cells by providing HAART simultaneously (5, 15). For this purpose, activation of the latently infected resting T cells would accelerate the rate of decay of this viral reservoir either by the cytopathic effects of replicating virus or by exposing these cells to immune surveillance as a result of the expression of viral antigens.
Prostratin is a phorbol ester isolated from Homalanthus nutans, a plant used by healers in Western Samoa as a traditional remedy for illnesses such as yellow fever (14). Unlike many phorbol esters, prostratin is not tumor promoting (14, 23, 24, 25). It has been demonstrated that prostratin activates the replication of HIV in two latently infected cell lines (13, 14). However, a phorbol ester might not necessarily affect primary cells in the same manner as infected cell lines. Furthermore, these cell lines may not accurately reflect postintegration HIV latency in primary cells.
Recently, we reported that latently infected primary T cells can be generated in a severe combined immunodeficient mouse containing human fetal thymus and liver cells (SCID-hu [Thy/Liv] mouse) (4). During thymopoiesis, immature hematopoietic precursor cells undergo a series of replication, differentiation, and selection steps that result in the eventual export of mature CD4+ and CD8+ T lymphocytes into the peripheral blood. As thymocytes mature, the transcriptionally active immature cells become transcriptionally quiescent. We previously used the HIV-infected SCID-hu (Thy/Liv) mouse model to establish that reactivatible HIV can be generated at high frequency during thymopoiesis and that latently infected mature cells can be exported into the periphery of the animal (4). This provides a unique model to study agents that could potentially activate the latent T-cell reservoir. Therefore, to investigate the potential of prostratin to activate latent HIV infection, we evaluated the T-cell-stimulating activity of prostratin in primary cultures of human peripheral blood lymphocytes (PBLs) and in ex vivo cultures of latently infected human thymocytes and peripheral cells from the SCID-hu model.
MATERIALS AND METHODS
Isolation of quiescent peripheral blood cell population.
Peripheral blood was obtained from healthy HIV-seronegative blood donors, and peripheral blood mononuclear cells were separated over a Ficoll-Hypaque gradient. Nonadherent cells were obtained after depleting macrophages by 3 h of adherence to plastic. The enriched quiescent T-cell population was purified as previously described (17). Briefly, cells were incubated on ice with saturating amounts of antibodies to HLA-DR, CD14, CD19, CD25, and CD69 (Becton Dickinson, Mountain View, Calif.), washed extensively, resuspended in medium, and subjected to panning in goat anti-mouse immunoglobulin antibody (GAM; Sigma, St. Louis, Mo.)-coated flasks, resulting in depletion of cells expressing major histocompatibility complex class II antigen as well as depletion of B cells, macrophages, and previously activated T cells. Purified cells were 99% pure, as assessed by flow cytometry.
Cell cultures and conditions.
Cells were cultured in RPMI 1640 supplemented with 10% human AB serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM glutamine. Cells were stimulated with several concentrations of prostratin, ranging from 100 nM to 10 μM. In addition, cells were stimulated with 1 μg of immobilized anti-CD3 monoclonal antibodies per ml or 0.1 μg of soluble anti-CD28 per ml or costimulated with anti-CD3 and soluble anti-CD28 (Pharmingen, San Diego, Calif.), each at the above concentrations. In some cases, prostratin plus anti-CD3 stimulation was applied. For latency experiments, cells were cultured in the presence of 1 μM zidovudine (AZT) and 100 ng of indinavir per ml to prevent virus spread to new target cells during stimulation.
Cellular proliferation.
Cellular proliferation was assessed 3 days poststimulation by measuring DNA synthesis. A total of 105 cells were incubated for 4 h with [3H]thymidine (10 μCi/ml) and harvested onto glass fiber filters with a Classic Cell Harvester (Skatron Instruments, Lier, Norway), and thymidine incorporation into DNA was measured with a liquid scintillation counter.
Flow cytometry for surface markers.
To determine the expression levels of activation markers, 5 × 105 cells were stained with monoclonal antibodies against CD69, CD25, and CD4 (Coulter Corporation, Miami, Fla.; Becton Dickinson). For these stainings, anti-CD69 monoclonal antibodies were conjugated directly to fluorescein isothiocyanate. Anti-CD25 was directly conjugated to phycoerythrin, and anti-CD4 was directly conjugated to phycoerythrin-cyanin 5.1. Cells were acquired on a Coulter Flow Center Flow cytometer (Coulter Corporation), and data were analyzed by using the CellQuest program (Becton Dickinson). Live cells were gated by using forward-versus-side scatter dot plots.
Cells from thymocyte cultures (see below) were stained with monoclonal antibodies specific for human CD4, CD8, and CD45 and murine CD24 (muCD24, expressed by an HIV reporter virus; see below) directly conjugated to phycoerythrin, peridinin chlorophyll protein, allophycocyanin, or fluorescein isothiocyanate (Coulter), respectively. Samples were analyzed on a FACSCaliber flow cytometer with the Cell Quest program (Becton Dickinson, Mountain View, Calif.). Forward-versus-side scatter profiles and 7-amino-actinomycin D dead-cell exclusion were used to define the live population. These cells were further gated on the human CD45+ population to exclude murine cells. Quadrants were set based on isotype controls from mock-infected cells.
Cell cycle analysis.
A total of 5 × 105 cells of each condition were stained for DNA and RNA content with 7-amino-actinomycin D and pyronin Y as previously described (17). Briefly, cells were suspended in NASS buffer (0.15 M NaCl in 0.1 M phosphate citrate buffer [Sigma] containing 5 mM EDTA [Sigma] and 0.5% bovine serum albumin [fraction V; Sigma]) containing 0.03% saponin (Sigma). Fifty microliters of 400 μM 7-amino-actinomycin D (Calbiochem, La Jolla, Calif.) was added at a final concentration of 20 mM. The cells were incubated at room temperature for 30 min and cooled on ice, and 5 μl of 1.7 mM pyronin Y (Polyscience, Warrington, Pa.) was added at a final concentration of 5 μM. The cells were then incubated for an additional 10 min on ice and analyzed. Data were accumulated on a FACStar Plus flow cytometer and analyzed with the CellQuest program (Becton Dickinson).
Real-time PCR.
Cells to be subjected to real-time PCR were harvested and DNAs were extracted following exposure to urea lysis buffer and subsequent phenol-chloroform extraction as previously described (27). Real-time PCR was performed to quantitate initiated and completed reverse transcripts. Amplification and detection were performed on an Applied Biosystems Prism 7700 sequence detection system with the Taqman reagent kit, following a protocol described in detail elsewhere (12).
Infection of SCID-hu mice.
SCID-hu mice were prepared by implantation of human fetal liver and thymus under the left renal capsule as previously described (1). Thy/Liv implants were mock infected with medium or directly injected with virus stocks so that a total of 20 ng of the CXCR4-tropic reporter strain HIVNL-r-HSAs (16) or 50 ng of the CCR5-tropic strain HIV-1JR-CSF (18) was introduced. In general, 1 ng of p24 contains approximately 100 infectious units. Virus stocks were prepared by electroporation of cloned proviral DNA into CEM cells. p24gag expression was assessed by enzyme-linked immunosorbent assay (ELISA; Coulter, Hialeah, Fla.) and used to quantitate virus titers and reactivation from latency.
Isolation of thymocytes and human peripheral blood mononuclear cells from SCID-hu (Thy/Liv) mice.
Thy/Liv implants from HIVJR-CSF-infected, HIVNL-r-HSAs-infected, or uninfected SCID-hu mice were harvested 5 weeks postinfection, and single-cell suspensions of thymocytes were pooled in the presence of 100 ng of indinavir (Merck, West Point, Pa.) per ml. Thymocytes were then stained with mouse antibodies to human CD8 and subjected to negative selection by panning on flasks coated with goat anti-mouse immunoglobulin antibody (Sigma). Thymocytes from HIVNL-r-HSAs-infected implants were further stained with a rat antibody against murine CD24 and placed on rabbit anti-rat immunoglobulin antibody (Sigma)-coated flasks to remove productively infected cells. Thymocytes from uninfected implants were isolated and cultured in parallel as negative controls in all experiments. Purity was determined by flow cytometric analysis with different antibody clones than were used for cell isolation and resulted in greater than 99% CD8- and muCD24-depleted subsets.
Blood from SCID-hu mice was pooled, and peripheral blood mononuclear cells were isolated by flotation over a Ficoll-Paque gradient. Splenocytes from the same mice were pooled and isolated similarly.
RESULTS
Proliferative response of PBLs to prostratin.
We used our highly purified population of quiescent T cells to study the effect of various concentrations of prostratin on the activation state of T lymphocytes. The range of these concentrations reflects plasma concentrations achieved in mice, in which no overt toxicity was seen (Michael R. Boyd, personal communication). We initially characterized the purified quiescent T-cell population for its ability to proliferate. For that purpose, we cultured highly purified quiescent T lymphocytes in the presence of various concentrations of prostratin and compared their proliferative responses to those of cells stimulated with anti-CD3 alone or costimulated with both anti-CD3 and anti-CD28 as well as to cells cultured in the absence of any stimuli.
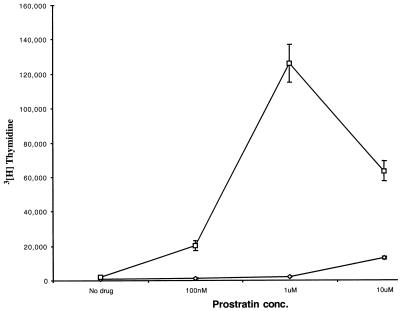
Figure 1 shows that, as was documented previously (17), the highly purified T cells did not proliferate in response to immobilized anti-CD3 alone. Similarly, these cells did not proliferate in response to treatment with various concentrations of prostratin alone. In contrast, following costimulation with anti-CD3 and anti-CD28, cells exhibited a greater than 30-fold increase in proliferation (not shown). However, the addition of increasing concentrations of prostratin to cells stimulated with anti-CD3 alone augmented the proliferation of these cells, with optimal levels of proliferation (greater than 20-fold) produced by 1 μM prostratin. The addition of prostratin to cells costimulated with anti-CD3 and anti-CD28 had a minimal effect on the proliferative ability of these cells (data not shown).
FIG. 1.
Proliferative response of T-cells to prostratin. Highly purified quiescent T cells were cultured in the presence of the indicated concentrations of prostratin alone (⋄) or with the additional stimulation signals provided by anti-CD3 antibodies (□). At days 2, 3, and 4, cells were harvested in triplicate and assayed for thymidine incorporation as described in Materials and Methods. Data from day 3 poststimulation are presented. Results are the averages of triplicate wells. These results are representative of three experiments.
Expression of cell surface activation markers.
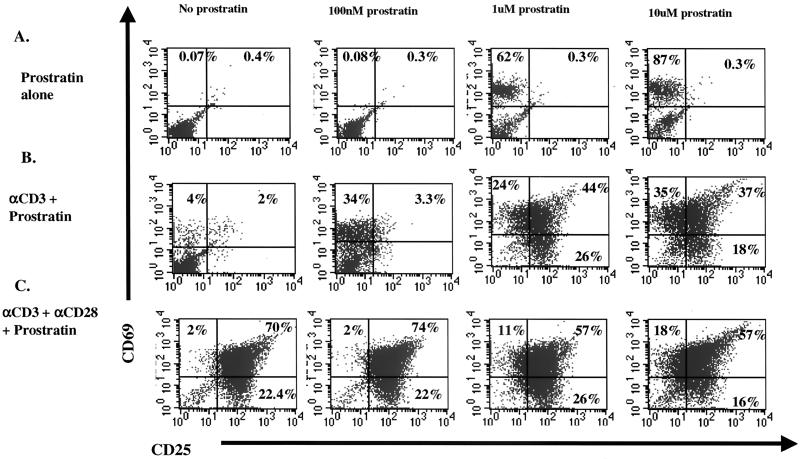
We then studied the effect of prostratin on the expression of the cell surface activation markers CD69 and CD25 in correlation with the activation state of the T cells. As shown in Fig. 2A, cells treated with concentrations of prostratin 1 μM or greater upregulated only the expression of the early activation marker CD69, which has previously been correlated to progression of quiescent T cells from G0 to the G1a phase of the cell cycle (17). In contrast, cells stimulated with anti-CD3 alone exhibited an increase in CD69 expression, with a small subset of cells also demonstrating an increase in CD25 expression. The addition of increasing concentrations of prostratin to anti-CD3-stimulated cells potentiated the anti-CD3-induced expression of CD69 and substantially upregulated the expression of CD25 (Fig. 2B). However, as shown in Fig. 2C, this increase in the expression of the CD25 activation marker fell short of the dramatic increase seen following costimulation with anti-CD3 and anti-CD28. These data suggest that prostratin exhibits an activation effect distinct from that of CD3 receptor stimulation.
FIG. 2.
Expression levels of activation markers following stimulation with various concentrations of prostratin. Highly purified quiescent T cells were cultured in the presence of the indicated concentrations of prostratin alone (A), prostratin plus anti-CD3 (B), and prostratin plus costimulation with anti-CD3 and anti-CD28 (C). Following 2 days in culture, cells were stained and analyzed as described in Materials and Methods. The cells used for this experiment were the same as those used for the experiment illustrated in Fig. 1, and results are representative of three experiments.
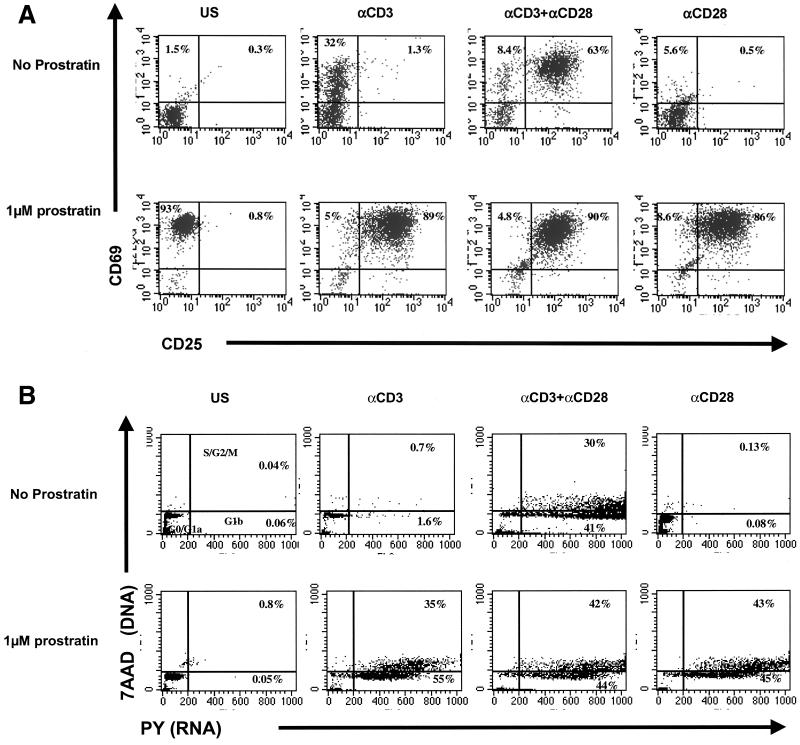
To further understand the mechanism by which prostratin stimulates T cells, we investigated the stimulatory effect of prostratin in conjunction with the effect of anti-CD28 stimulation. As shown in Fig. 3A, T cells cultured in the presence of anti-CD28 alone did not appreciably upregulate the early activation markers CD69 and CD25. However, when cultured in the presence of both prostratin and anti-CD28 antibodies, cells become fully stimulated, expressing high levels of both CD69 and CD25 on their surface (Fig. 3A).
FIG. 3.
(A) Induction of activation markers following stimulation with prostratin plus antibodies. Highly purified T cells were cultured under the indicated conditions in the absence (upper panels) or presence (lower panels) of 1 μM prostratin. Following 3 days in culture, cells were stained and analyzed as described in Materials and Methods. US, unstimulated; αCD3, stimulated with anti-CD3 antibodies; αCD28, stimulated with anti-CD28 antibodies; αCD3+αCD28, costimulated with anti-CD3 and anti-CD28. (B) Cell cycle progression following stimulation with prostratin. Highly purified T cells were cultured under the indicated conditions in the absence (upper panels) or presence (lower panels) of 1 μM prostratin. Following 3 days in culture, cells were stained and analyzed for DNA/RNA content as described in Materials and Methods. US, unstimulated; αCD3, stimulated with anti-CD3 antibodies; αCD28, stimulated with anti-CD28 antibodies; αCD3+αCD28, costimulated with anti-CD3 and anti-CD28. 7AAD, 7-amino-actinomycin D; PY, pyronin Y.
Effects of prostratin on cell cycle progression.
The interesting costimulatory effects of prostratin in conjunction with anti-CD28 antibodies were also demonstrated (Fig. 3B). Cell cycle analysis of the different conditions showed that in the presence of prostratin, anti-CD3, or anti-CD28 alone, cells remained largely in the G0/G1a phase of the cell cycle. Three days following costimulation with prostratin plus anti-CD3, cells were fully cycling and progressed through the G1, S, G2, and M phases of the cell cycle (Fig. 3B, lower panel). Thus, prostratin has stimulatory properties on quiescent T cells that are distinct from T-cell receptor-mediated signals. This phorbol ester appears to provide a secondary activation signal for T-cell receptor stimulation and can also provide a primary activation signal in conjunction with anti-CD28 but does not induce cell cycle progression on its own.
Effect of prostratin on HIV latency.
Based on the T-cell-stimulatory properties of prostratin, we determined its ability to reactivate latent HIV infection in the SCID-hu (Thy/Liv) model. This model is constructed by implanting pieces of human fetal thymus and liver under the kidney capsule of a SCID mouse, resulting in a conjoint organ (Thy/Liv) which functionally resembles a normal human thymus (20). Infection of the Thy/Liv implants with HIV-1 results in the loss of CD4-positive subsets in a manner similar to that observed in humans (1, 3, 22).
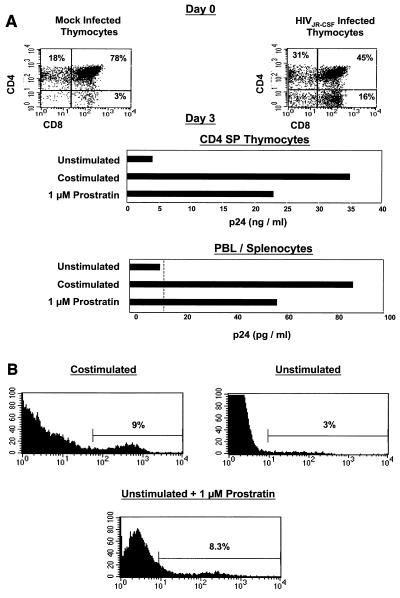
Human thymocytes and lymphocytes from SCID-hu mouse peripheral blood and spleen were harvested from animals harboring Thy/Liv implants infected with the CCR5-tropic strain HIVJR-CSF. The infected thymus demonstrated loss of CD4+ CD8+ thymocytes, indicative of active infection (Fig. 4A, upper panel). We obtained purified CD4 single-positive (SP) thymocytes, pooled peripheral blood and splenocytes from SCID-hu mice, and cultured them in the presence of AZT and a protease inhibitor to prevent viral replication. We then stimulated these cells either with antibodies to CD3 and CD28 or with 1 μM prostratin alone. Costimulation of both CD4 SP thymocytes and pooled lymphocytes resulted in increased viral p24 production from infected cells. Interestingly, the addition of prostratin alone was also able to induce substantial viral p24 expression (Fig. 4A, lower panel).
FIG. 4.
(A) Effect of prostratin on HIV latency. Upper panels, mock- and HIVJR-CSF-infected thymocytes were harvested 5 weeks postinfection (day 0), stained with antibodies to CD4 and CD8, and analyzed by flow cytometry. Lower panels, thymocytes were then cultured without stimulation, costimulated with antibodies to CD3 and CD28, or cultured in the presence of 1 μM prostratin. p24 in the supernatant was assessed as described in Materials and Methods. The dotted line indicates the level of detection of the ELISA. (B) Effect of prostratin on HIV gene expression. NL-r-HSAs-infected CD4 SP thymocytes were isolated as described for Fig. 3 and cultured under the described conditions. Three days following stimulation, cells were stained with anti-mouse CD24 to detect HIV-encoded receptor gene expression. Gates were set according to mock-infected thymocytes cultured in parallel so that the background was <1%. Percentages indicate reporter expression and induction from latency.
To define at the single-cell level the effects of prostratin on reactivation from latency, Thy/Liv implants were infected with the CXCR4-tropic reporter virus NL-r-HSAs, which contains cDNA sequences for murine CD24 inserted into the VPR region of HIVNL4-3. The viral long terminal repeat directs expression of murine CD24; thus, productively infected cells can be visualized by flow cytometry (16). CD4 SP, muCD24-negative thymocytes were isolated and cultured without stimulation, costimulated, or cultured in the presence of prostratin for 3 days. The addition of prostratin to the infected thymocytes induced viral expression from a percentage of cells similar to that observed following costimulation (Fig. 4B). These data exemplify the ability of prostratin to reactivate latent HIV expression to an extent similar to that observed following costimulation.
Effect of prostratin on de novo HIV infection.
To determine if prostratin treatment allowed de novo HIV infection, cells were treated with 1 μM prostratin for 3 days and then infected with the CXCR4-tropic strain HIVNL4-3. We then performed real-time PCR to quantitate initiated versus completed viral reverse transcripts 18 h postinfection. The data in Table 1 indicate that, in contrast to what was seen in costimulated cells, reverse transcription was not completed in prostratin-stimulated or unstimulated resting cells. Additional studies (not shown) established that viral p24 protein was not produced into the supernatant of cells stimulated with prostratin. Thus, while prostratin activation stimulates expression of integrated latent HIV, it is not sufficient to allow de novo infection.
TABLE 1.
Effect of prostratin on de novo HIV infectiona
| Stimulation | % Complete reverse transcripts
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| None | 2.5 | 5.4 |
| Prostratin | 1.8 | 3.8 |
| Anti-CD3 + anti-CD28 | 37 | 40 |
Quiescent peripheral blood cells were cultured for 3 days. Cells were either left unstimulated, costimulated with anti-CD3 and anti-CD28, or prestimulated with 1 μM prostratin. Cells were then infected with the HIV molecular clone NL4-3. DNA was extracted 18 h postinfection and subjected to real-time PCR to quantitate initiated versus completed viral reverse transcripts. Results are expressed as the percentage of initiated reverse transcripts that completed the reverse transcription process and were determined by the following formula: % reverse transcription = (completed DNA copies/initiated DNA copies) × 100; these values are indicated for each of the conditions. Results from two separate experiments are shown.
DISCUSSION
In the present work, we studied the ability of the naturally occurring compound prostratin to induce the expression of latent HIV from naïve CD4+ T lymphocytes. We initially determined prostratin's effect on the state of activation of quiescent peripheral T cells and evaluated optimal concentrations for use in the latency experiments. Our data show that while prostratin by itself does not induce proliferation in resting T cells, it does provide a novel signal to the cells. This effect results in no increased DNA synthesis and in little cellular RNA upregulation, and the cells progress only to the G1a phase of the cell cycle (Fig. 1 and 3B). Here, only upregulation of expression of the early activation marker CD69 is seen (Fig. 2 and 3A).
We used our recently described SCID-hu mouse system to study the effect of prostratin on HIV latency. Our data clearly show that prostratin alone is able to induce viral expression from latently infected CD4+ thymocytes and naïve lymphocytes (Fig. 4) but is not sufficient to allow de novo HIV infection (Table 1). These results are consistent with the minimal cellular activation effects of prostratin. These effects may offer prostratin an advantage as a potential treatment for individuals in addition to HAART to help reduce the latently infected cell HIV reservoir. Activation to induce productive infection in the context of HAART may allow clearance of the infected cell either by immune-mediated mechanisms or by direct viral cytopathic effects while at the same time preventing de novo infection of additional T cells. Because prostratin does not induce full activation of these cells and does not utilize the CD3 stimulation pathway, it is unlikely that anergy will be induced following stimulation with this agent. Additional studies must be performed to assess this. It will also be of interest to determine if short pulses of prostratin treatment would be sufficient to induce expression of latent virus. Nonetheless, prostratin appears to induce robust virus expression with minimal effects on T-cell activation.
Brooks et al. (4) and Chun et al. (5, 6) have previously studied the ability of several proinflammatory cytokines to induce HIV-1 replication in latently infected CD4+ T cells. In addition, Chun et al. (6) showed a decrease in the pool of latently infected resting CD4+ T cells in HIV-1-infected individuals receiving intermittent administration of interleukin-2 together with HAART. However, prostratin appears to be able to stimulate latent virus more efficiently in our system than did interleukin-2, which in our system previously showed minimal effects on virus expression (4).
Our data identify a compound that might potentially be used together with HAART in HIV-infected individuals to help eradicate virus that is hidden within latently infected, resting CD4+ T cells. The effective concentrations of prostratin used here were not overtly toxic when achieved by oral administration to mice (Michael R. Boyd, personal communication). In recent studies, Kulkosky et al. (19) confirmed the ability of prostratin to induce the expression of latent HIV-1 both from latently infected cell lines and from patient peripheral blood cells. Taken together with our studies, these results suggest that prostratin has the potential to activate the expression of latent HIV in vivo with minimal activation effects on the immune system.
Acknowledgments
This work was supported by NIH grant AI36059 (J.A.Z.), UCLA CFAR grant AI88647, the American Foundation for AIDS Research (AMFAR), the James B. Pendleton Charitable Trust, and the University of California Universitywide AIDS Research Program. Y.K. was supported by T32 DF02796.
REFERENCES
- 1.Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 2.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. [DOI] [PubMed] [Google Scholar]
- 3.Bonyhadi, M. L., L. Rabin, S. Salimi, D. A. Brown, J. Kosek, J. M. McCune, and H. Kaneshima. 1993. HIV induces thymus depletion in vivo. Nature 363:728-732. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, D. G., S. G. Kitchen, C. M. Kitchen, D. D. Scripture-Adams, and J. A. Zack. 2001. Generation of HIV latency during thymopoiesis. Nat. Med. 7:459-464. [DOI] [PubMed] [Google Scholar]
- 5.Chun, T. W., D. Engel, S. B. Mizell, L. A. Ehler, and A. S. Fauci. 1998. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J. Exp. Med. 188:83-91. (Erratum, 188:614.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun, T. W., D. Engel, S. B. Mizell, C. W. Hallahan, M. Fischette, S. Park, R. T. Davey, Jr., M. Dybul, J. A. Kovacs, J. A. Metcalf, J. M. Mican, M. M. Berrey, L. Corey, H. C. Lane, and A. S. Fauci. 1999. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat. Med. 5:651-655. [DOI] [PubMed] [Google Scholar]
- 7.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davey, R. T., Jr., N. Bhat, C. Yoder, T. W. Chun, J. A. Metcalf, R. Dewar, V. Natarajan, R. A. Lempicki, J. W. Adelsberger, K. D. Miller, J. A. Kovacs, M. A. Polis, R. E. Walker, J. Falloon, H. Masur, D. Gee, M. Baseler, D. S. Dimitrov, A. S. Fauci, and H. C. Lane. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 96:15109-15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-517. [DOI] [PubMed] [Google Scholar]
- 10.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 11.Furtado, M. R., D. S. Callaway, J. P. Phair, K. J. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perelson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 12.Gorry, P. R., G. Bristol, J. A. Zack, K. Ritola, R. Swanstrom, C. J. Birch, J. E. Bell, N. Bannert, K. Crawford, H. Wang, D. Schols, E. De Clercq, K. Kunstman, S. M. Wolinsky, and D. Gabuzda. 2001. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J. Virol. 75:10073-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulakowski, R. J., J. B. McMahon, R. W. Buckheit, Jr., K. R. Gustafson, and M. R. Boyd. 1997. Antireplicative and anticytopathic activities of prostratin, a non-tumor-promoting phorbol ester, against human immunodeficiency virus (HIV). Antiviral Res. 33:87-97. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson, K. R., J. H. Cardellina II, J. B. McMahon, R. J. Gulakowski, J. Ishitoya, Z. Szallasi, N. E. Lewin, P. M. Blumberg, O. S. Weislow, J. A. Beutler, et al. 1992. A nonpromoting phorbol from the Samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. J. Med. Chem. 35:1978-1986. [DOI] [PubMed] [Google Scholar]
- 15.Ho, D. D. 1998. Toward HIV eradication or remission: the tasks ahead. Science 280:1866-1867. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson, B. D., and J. A. Zack. 1998. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J. Virol. 72:6520-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korin, Y., and J. A. Zack. 1998. Progression to the G1 phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 72:3161-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyanagi, Y., S. Miles, R. T. Mitsuyasu, J. E. Merrill, H. V. Vinters, and I. S. Chen. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819-822. [DOI] [PubMed] [Google Scholar]
- 19.Kulkosky, J., D. M. Culnan, J. Roman, G. Dornadula, M. Schnell, M. R. Boyd, and R. J. Pomerantz. 2001. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 98:3006-3015. [DOI] [PubMed] [Google Scholar]
- 20.Namikawa, R., K. N. Weilbaecher, H. Kaneshima, E. J. Yee, and J. M. McCune. 1990. Long-term human hematopoiesis in the SCID-hu mouse. J. Exp. Med. 172:1055-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powderly, W. G., A. Landay, and M. M. Lederman. 1998. Recovery of the immune system with antiretroviral therapy: the end of opportunism? JAMA 280:72-77. [DOI] [PubMed] [Google Scholar]
- 22.Stanley, S. K., J. M. McCune, H. Kaneshima, J. S. Justement, M. Sullivan, E. Boone, M. Baseler, J. Adelsberger, M. Bonyhadi, J. Orenstein, et al. 1993. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp. Med. 178:1151-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szallasi, Z., and P. M. Blumberg. 1991. Prostratin, a nonpromoting phorbol ester, inhibits induction by phorbol 12-myristate 13-acetate of ornithine decarboxylase, edema, and hyperplasia in CD-1 mouse skin. Cancer Res. 51:5355-5360. [PubMed] [Google Scholar]
- 24.Szallasi, Z., K. W. Krausz, and P. M. Blumberg. 1992. Non-promoting 12-deoxyphorbol 13-esters as potent inhibitors of phorbol 12-myristate 13-acetate-induced acute and chronic biological responses in CD-1 mouse skin. Carcinogenesis 13:2161-2167. [DOI] [PubMed] [Google Scholar]
- 25.Szallasi, Z., L. Krsmanovic, and P. M. Blumberg. 1993. Nonpromoting 12-deoxyphorbol 13-esters inhibit phorbol 12-myristate 13-acetate induced tumor promotion in CD-1 mouse skin. Cancer Res. 53:2507-2512. [PubMed] [Google Scholar]
- 26.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 27.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]