Abstract
Borna disease virus (BDV) is a nonsegmented negative-strand RNA virus that replicates and transcribes its genome in the nucleus of infected cells. BDV proteins involved in replication and transcription must pass through the nuclear envelope to associate with the genomic viral RNA. The RNA-dependent RNA polymerase (L) of BDV is postulated to be the catalytic enzyme of replication and transcription. We demonstrated previously that BDV L localizes to the nucleus of BDV-infected cells and L-transfected cells. Nuclear localization of the protein presupposes the presence of a nuclear localization signal (NLS) within its primary amino acid sequence or cotransport to the nucleus with another karyophilic protein. Because L localized to the nucleus in the absence of other viral proteins, we investigated the possibility that L contains an NLS. The minimal sequence required for nuclear localization of L was identified by analyzing the subcellular distribution of deletion mutants of L fused to a flag epitope tag or β-galactosidase. Although the majority of the L fusion proteins localized to the cytoplasm of transfected BSR-T7 cells, a strong NLS (844RVVKLRIAP852) with basic and proline residues was identified. Mutation of this sequence resulted in cytoplasmic distribution of L, confirming that this sequence was necessary and sufficient to drive the nuclear localization of L.
Borna disease virus (BDV) transcribes and replicates its nonsegmented negative-strand (NNS) RNA genome in the nucleus of infected cells. This nuclear phase of the virus life cycle is unique among members of the NNS RNA animal viruses (3, 5). Following translation in the cytoplasm, BDV proteins must travel through the nuclear membrane and into the nucleus in order to participate in virus transcription. Three mechanisms are described to account for cytoplasmic-nuclear trafficking: passive diffusion, active transport, and cotransport (16).
Active transport requires soluble cytoplasmic receptors called karyopherins (in yeast cells) or importins (in mammalian cells), energy in the form of GTP (in many but not all cases), and a specific nuclear localization signal(s) (NLS[s]) within the primary amino acid sequence of the karyophilic protein (6). At least four active transport pathways have been described previously (1). Each requires that importins recognize and interact directly with the NLS of the protein to be imported. The importin-NLS protein complex then docks at fibrils extending from the nuclear pore complex and is imported into the nucleus through a series of interactions that utilize energy in the form of GTP. Cotransport occurs when a protein that lacks an NLS and is too large for passive diffusion interacts with a protein that contains a functional NLS (1). The proteins are subsequently cotransported into the nucleus via active transport due to the presence of the NLS.
Functional NLSs have been mapped within the BDV nucleoprotein (N) (8, 10), phosphoprotein (P) (12, 13), and X protein (X) (17). The N-NLS is similar in sequence and amino-terminal position to the NLSs of the VP1 proteins of simian virus 40 (SV40) and polyomavirus (10). In contrast, P contains two NLSs (12, 13). The first is located near the amino-terminal portion of P and is bipartite, similar to the nucleoplasmin NLS (12). The second is located at the carboxyl-terminal portion of P (12, 13); both P-NLSs are unique in that proline residues play a central role in their activity (13). X import is mediated by interaction of a nonconventional karyophilic signal at its amino terminus with importin-α (17).
We demonstrated previously (15) that BDV P and the RNA-dependent RNA polymerase (L) interact. This interaction alone could lead to nuclear localization of L due to the P-NLS; however, immunohistochemical studies with anti-L1 antisera and BSR-T7 cells (Huh-7 cells stably transfected to express T7 RNA polymerase) transiently transfected with an L-expression plasmid revealed the presence of L protein in the nucleus (15). This finding suggested the presence of an NLS(s) in L. To characterize the putative L-NLS, immunofluorescence analyses of BSR-T7 cells transfected with wild-type or mutant forms of L fused to a flag epitope tag or β-galactosidase were performed. Analysis of amino- and carboxyl-truncation mutants fused to the flag epitope tag indicated that the central residues of L (amino acids 824 to 1062) were sufficient for nuclear localization. These results were confirmed and expanded by analysis of L-β-galactosidase fusion constructs. A strong NLS at residues 844 to 852 was identified. Mutation of 844R (arginine) and 847K (lysine) to A (alanine) led to cytoplasmic accumulation of L, confirming that these residues within the sequence 844RVVKLRIAP852 are necessary and sufficient for nuclear localization of L.
Subcellular distribution of L-flag epitope tag fusions.
The subcellular distribution of L-flag epitope tag fusion proteins was determined immunohistochemically with anti-flag M2 murine antibody (Sigma) and goat anti-mouse immunoglobulin G (IgG) fluorescein isothiocyanate (Caltag) in BSR-T7 cells transiently transfected with expression plasmids encoding the flag epitope tag fused to amino- or carboxyl-deletion mutants of BDV L. Both full-length L fused to the flag epitope tag (pTM-TL) and the 394-amino-acid (aa) amino-terminal deletion mutant (pTM-TΔN394) fused to the flag epitope tag localized to the nucleus of transfected BSR-T7 cells (Fig. 1A, B, and F). In contrast, the 953-aa carboxyl-terminal deletion mutant fused to the flag epitope tag (pTM-TΔC759) (Fig. 1A and C) and the 1,062-aa amino-terminal deletion mutant fused to the flag epitope tag (pTM-TΔN1062) (Fig. 1A and E) localized to the cytoplasm. Deletion of 1,532 aa from the carboxyl region of L (pTM-ZΔC180) resulted in predominantly cytoplasmic fusion protein (Fig. 1A and D). No fluorescence was observed in cells transfected with vector (pTM1) alone (Fig. 1G).
FIG. 1.
Subcellular localization of L-flag epitope tag fusion proteins. (A) Description of expression plasmids and summary of results. Amino acid sequence of L, with a diagram of full-length and deletion mutant L-flag epitope tag fusion expression plasmids. Plasmid names and subcellular localization of fusion proteins are listed. (B through G) Subcellular localization of full-length or deletion mutants of L-flag epitope tag by indirect immunofluorescence in BSR-T7 cells transfected with expression plasmids. (B) pTM-TL; (C) pTM-TΔC759; (D) pTM-TΔC180; (E) pTM-TΔN1062; (F) pTM-TΔN394; (G) pTM1. Cells were stained with anti-flag M2 murine antibody (Sigma) and goat anti-mouse IgG fluorescein isothiocyanate (Caltag).
Subcellular localization of β-galactosidase-L fusion proteins.
The subcellular distribution of β-galactosidase-L fusion proteins was assessed by indirect immunofluorescence, using murine anti-β-galactosidase (primary antibody) (Sigma) and anti-mouse IgG fluorescein isothiocyanate (secondary antibody) (Caltag) after transient transfection of BSR-T7 cells with plasmids that encode β-galactosidase fused to amino- or carboxyl-deletion mutants of BDV L (Fig. 2A ). Whereas β-galactosidase alone was cytoplasmic (Fig. 2B, panel i), β-galactosidase fused to wild-type L (pTM-ZL) (Fig. 2B, panel a) was nuclear. Deletion of 888 (pTM-ZΔC824) (Fig. 2B, panel b), 953 (pTM-ZΔC759) (Fig. 2B, panel c), or 1,532 (pTM-ZΔC180) (Fig. 22B, panel d) aa from the carboxyl region of L led to cytoplasmic staining. In contrast, deletion of 561 (pTM-ZΔN561) (Fig. 2B, panel h) or 824 (pTM-ZΔN824) (Fig. 2B, panel g) aa from the amino region of L retained the nuclear staining pattern. Further deletion from the amino region of L again led to cytoplasmic localization of the fusion proteins, as observed upon deletion of 1,062 (pTM-ZΔN1062) (Fig. 2B, panel f) or 1,439 (pTM-ZΔN1439) (Fig. 2B, panel e) amino residues. Fusion of L aa 824 to 1062 (pTM-ZΔN824C1062) (Fig. 3C, panel a), 824 to 941 (pTM-ZΔN824C941) (Fig. 3C, panel e) or 824 to 853 (pTM-ZΔN824C853) (Fig. 3C, panel f) led to nuclear localization of β-galactosidase. In contrast, all β-galactosidase-L fusions that contained L sequence carboxyl to aa 854 localized to the cytoplasm: aa 921 to 1062 (pTM-ZΔN921C1062) (Fig. 3C, panel b), 921 to 1015 (pTM-ZΔN921C1015) (Fig. 3C, panel c), or 854 to 1,015 (pTM-ZΔN854C1015) (Fig. 3C, panel d). No fluorescence was observed in cells transfected with empty vector (pTM1) (Fig. 2B, panel j and 3C, panel g).
FIG. 2.
LacZ-L fusion plasmid description and subcellular distribution of full-length or deletion mutants of β-galactosidase-L by indirect immunofluorescence in BSR-T7 cells transfected with expression plasmids. (A) Amino acid sequence of L, with full-length and deletion mutant LacZ-L fusion constructs. Plasmid names and subcellular localization of fusion proteins are listed. (B) Cells were stained with murine anti-β-galactosidase antibody (Sigma) and anti-mouse IgG fluorescein isothiocyanate (secondary antibody) (Caltag). (a) pTM-ZL; (b) pTM-ZΔC824; (c) pTM-ZΔC759; (d) pTM-ZΔC180; (e) pTM-ZΔN1439; (f) pTM-ZΔN1062; (g) pTM-ZΔN824; (h) pTM-ZΔN561; (i) pTM-LacZ; (j) pTM1 (empty vector, negative control).
FIG. 3.
B-galactosidase-L fusion constructs and subcellular distribution of deletion mutants of β-galactosidase-L by indirect immunofluorescence in BSR-T7 cells transiently transfected with expression plasmids. (A) Map of the L open reading frame (ORF) (nucleotides 2472 to 3269), indicating restriction endonuclease sites, PCR primers, and subcellular localization. (B) Primer names, sequences, and nucleotide positions within the L ORF. (C) Subcellular distribution of deletion mutants of β-galactosidase-L by indirect immunofluorescence in BSR-T7 cells transiently transfected with expression plasmids. Cells were stained with anti-β-galactosidase murine antibody (Sigma) and anti-mouse IgG fluorescein isothiocyanate (secondary antibody) (Caltag). (a) pTM-ZΔN824C1062; (b) pTM-ZΔN921C1062; (c) pTM-ZΔN921C1015; (d) pTM-ZΔN854C1015; (e) pTM-ZΔN824C941; (f) pTM-ZΔN824C853 (g) pTM1 (empty vector, negative control).
L-NLS mutant localizes to the cytoplasm.
Analysis of L truncation mutants fused with the flag epitope tag and β-galactosidase suggested that an L-NLS was located between aa 824 to 853. To test this hypothesis, BSR-T7 cells were transfected with a plasmid encoding L-NLS mutants Arg844Ala and Lys847Ala (R844A/K847A). Subcellular distribution of the mutant protein within transfected cells was assessed by indirect immunofluorescence using murine anti-L1 primary antisera and anti-mouse IgG fluorescein isothiocyanate (secondary antibody) (Caltag). Whereas wild-type L localized to the nucleus (Fig. 4b), the mutant protein localized to the cytoplasm (Fig. 4a). No fluorescence was observed in cells transfected with vector (pTM1) alone (Fig. 4c).
FIG. 4.
Subcellular localization of wild-type L and L-NLS mutant by indirect immunofluorescence in BSR-T7 cells transfected with expression plasmids. Cells were stained with murine anti-L1 antibody and anti-mouse IgG fluorescein isothiocyanate (secondary antibody) (Caltag). (a) pTM-L-AVVA; (b) pTM-L1; (c) pTM1 (empty vector, negative control).
Expression of L-fusion proteins and mutant proteins of the predicted size.
The size of proteins expressed in BSR-T7 cells transfected with plasmids encoding β-galactosidase-L fusions, L-flag epitope tag fusions, or the L-NLS mutant was determined by Western immunoblotting using murine anti-flag M2 primary antibody (Sigma) (Fig. 5), murine anti-β-galactosidase primary antibody (Sigma) (Fig. 6), or murine anti-L1 antisera (Fig. 7), followed by goat anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Sigma). L-flag epitope-tagged fusion proteins of the predicted sizes were detected as follows: pTM-TΔC180, 20 kDa (Fig. 5A, lane 1); pTM-TΔN1062, 85 kDa (Fig. 5B, lane 1); pTM-TΔC759, 95 kDa (Fig. 5B, lane 2); pTM-TΔN394, 150 kDa (Fig. 5B, lane 3);and pTM-TL, 190 kDa (Fig. 5C, lane 2). Fusions of β-galactosidase-L expressed from plasmids were also consistent as far as their predicted sizes: pTM-ZL, 310 kDa (Fig. 6A, lane 2); pTM-ZΔC824, 200 kDa (Fig. 6A, lane 3); pTM-ZΔC759, 200 kDa (Fig. 6A, lane 4); pTM-TΔC180, 140 kDa (Fig. 6A, lane 5); pTM-ZΔN1439, 150 kDa (Fig. 6A, lane 6); pTM-ZΔN1062, 190 kDa (Fig. 6A, lane 7); pTM-ZΔN824, 220 kDa (Fig. 6A, lane 8); pTM-ZΔN561, 240 kDa (Fig. 6A, lane 9); pTM-ZΔN824C1062, 146 kDa (Fig. 6B, lane 2); pTM-ZΔN921C1062, 135 kDa (Fig. 6B, lane 3); pTM-ZΔN921C1015, 130 kDa (Fig. 6B, lane 4); pTM-ZΔN854C1015, 140 kDa (Fig. 6B, lane 5); pTM-ZΔN824C941, 133 kDa (Fig. 6B, lane 6); and pTM-ZΔN824C853, 123 kDa (Fig. 6B, lane 7). L-NLS mutant protein of the predicted size (190 kDa) was expressed from pTM-L-AVVA (Fig. 7, lane 2). Proteins consistent with the size of those expressed from expression plasmids were not detected upon transfection with empty vector (pTM-1) (Fig. 5A, lane 2; Fig. 5B, lane 4; Fig. 5C, lane 1; Fig. 6, lanes 1; and Fig. 7, lane 1).
FIG. 5.
Western immunoblot analysis of L-flag epitope tag fusions. Lysates were obtained from BSR-T7 cells transfected with pTM-TΔC180 (lane 1) (A) or pTM-1 (empty vector) (lane 2); pTM-TΔN1062 (lane 1) (B), pTM-TΔC759 (lane 2), pTM-TΔN394 (lane 3), or pTM-1 (empty vector) (lane 4); or pTM-1 (empty vector) (lane 1) (C) or pTM-TL (lane 2). Blots were probed with anti-flag M2 murine primary antibody (Sigma) and goat anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Sigma). Arrows indicate protein from: pTM-TΔC180 (a), pTM-TΔN394 (b), pTM-TΔC759 (c), pTM-TΔN1062 (d), or pTM-TL (e).
FIG. 6.
Western immunoblot analysis of β-galactosidase-L fusion proteins. Lysates were obtained from BSR-T7 cells transfected with: (A) pTM-1 (empty vector) (lane 1), pTM-ZL (lane 2), pTM-ZΔC824 (lane 3), pTM-ZΔC759 (lane 4), pTM-TΔC180 (lane 5), pTM-ZΔN1439 (lane 6), pTM-ZΔN1062 (lane 7), pTM-ZDN824 (lane 8), pTM-ZΔN561 (lane 9); (B) pTM-1 (empty vector) (lane 1), pTM-ZΔN824C1062 (lane 2), pTM-ZΔN921C1062 (lane 3), pTM-ZΔN921C1015 (lane 4), pTM-ZΔN854C1015 (lane 5), pTM-ZΔN824C941 (lane 6), pTM-ZΔN824C853 (lane 7). Blots were probed with anti-β-galactosidase murine primary antibody (Sigma) and goat anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Sigma).
FIG. 7.
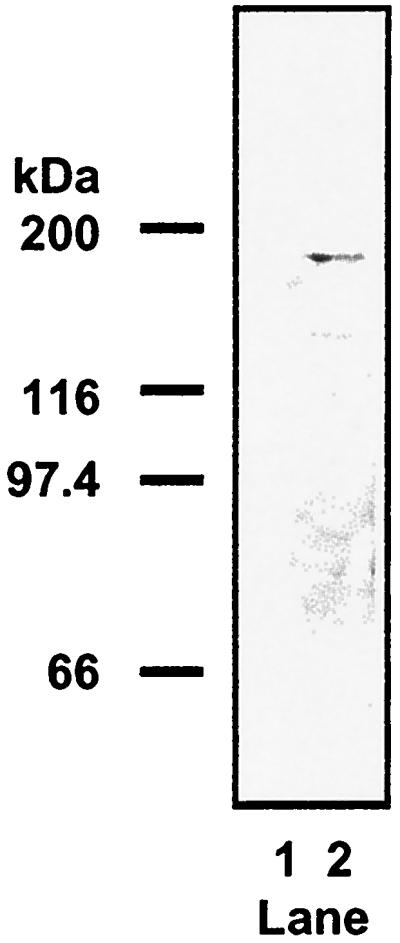
Western immunoblot analysis of L-NLS mutant. Lysates were obtained from BSR-T7 cells transfected with pTM-1 (empty vector) (lane 1) or pTM-L-AVVA (lane 2) and probed using murine anti-L1 antisera with goat anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Sigma).
BDV replicates in the nucleus of infected cells (3, 5). Thus, the BDV polymerase must localize to the nucleus of infected cells (15). Nuclear localization of BDV L could result from an NLS or from cotransport with other viral proteins (16). BDV L interacts with BDV-P (15), which has a strong NLS (12, 13); thus, it is conceivable that BDV L is cotransported into the nucleus with BDV P. However, in the absence of P, recombinant L localizes to the nucleus of transfected BSR-T7 cells (15), indicating the presence of an NLS, which we characterized.
To identify the NLS of BDV L, a flag epitope tag was fused with the wild type and with amino and carboxyl deletions of L (Fig. 1A). Indirect immunofluorescence with murine anti-flag M2 antibody (Sigma) on BSR-T7 cells transfected with expression plasmids showed clear nuclear staining when residues 759 to 1062 were present (Fig. 1B to F). Amino- and carboxyl-deletion mutants of L fused to β-galactosidase confirmed the result observed with L-flag epitope tag fusions and were used to further narrow the sequence to residues 824 to 1062 (Fig. 2B, panels a to i).
Fine mapping of the L-NLS was performed by fusion of L to β-galactosidase in order to ensure that fusion proteins were more than 60 kDa, the threshold for passive diffusion into the nucleus (14). Ultimately, residues 824 to 853 were identified as sufficient to drive nuclear localization of the β-galactosidase fusion protein (Fig. 3C, panels a to f).
Analysis of this sequence indicated a strong NLS at residues 844 to 854. The activity of the NLS was confirmed by mutating the sequence within L (R844A/K847A). This L-NLS mutant localized to the cytoplasm of BSR-T7 cells (Fig. 4a). This region contained a stretch of basic residues similar to the SV40 class of NLSs (844RVVKLR849) (9). It is likely that these residues represent the core L-NLS. Core NLSs are usually defined by a hexapeptide, with K and R residues and either V, P, or A residues interspersed (9). P, G, or acidic residues flank the SV40 type of core NLS (2). Proline residues are present flanking the L-NLS; 852P but not 854P seems to play a role in the nuclear localization of L (pTM-ZΔN824C853; Fig. 3C, panel f).
The results presented here with respect to L and elsewhere with respect to N, P, and X, confirm the intimate association between BDV and the host cell nucleus predicted by Sasaki and Ludwig (11) and Gosztonyi and Ludwig (7).
Acknowledgments
We are grateful to Ingo Jordan, Nicole Fisher, and B. L. Semler for helpful discussions and to Karl-Klaus Conzelmann for the gift of the BSR-T7 cell line.
This work was supported by grant NS29425 from the National Institutes of Health.
REFERENCES
- 1.Allen, T. D., J. M. Cronshaw, S. Bagley, E. Kiseleva, and M. W. Goldberg. 2000. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J. Cell Sci. 113:1651-1659. [DOI] [PubMed] [Google Scholar]
- 2.Boulikas, T. 1994. Putative nuclear localization signals (NLS) in protein transcription factors. J. Cell. Biochem. 55:32-58. [DOI] [PubMed] [Google Scholar]
- 3.Briese, T., J. C. de la Torre, A. Lewis, H. Ludwig, and W. I. Lipkin. 1992. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc. Natl. Acad. Sci. USA 89:11486-11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briese, T., A. Schneemann, A. J. Lewis, Y. S. Park, S. Kim, H. Ludwig, and W. I. Lipkin. 1994. Genomic organization of Borna disease virus. Proc. Natl. Acad. Sci. USA 91:4362-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cubitt, B., and J. C. de la Torre. 1994. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J. Virol. 68:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Görlich, D. 1997. Nuclear protein import. Curr. Opin. Cell Biol. 9:412-419. [DOI] [PubMed] [Google Scholar]
- 7.Gosztonyi, G., and H. Ludwig. 1984. Borna disease of horses. An immunohistological and virological study of naturally infected animals. Acta Neuropathol. 64:213-221. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi, T., Y. Shoya, T. Koda, I. Takashima, P. K. Lai, K. Ikuta, M. Kakinuma, and M. Kishi. 1998. Nuclear targeting activity associated with the amino terminal region of the Borna disease virus nucleoprotein. Virology 243:188-197. [DOI] [PubMed] [Google Scholar]
- 9.LaCasse, E. C., and Y. A. Lefebvre. 1995. Nuclear localization signals overlap DNA- or RNA-binding domains in nucleic acid-binding proteins. Nucleic Acids Res. 23:1647-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pyper, J. M., and A. E. Gartner. 1997. Molecular basis for the differential subcellular localization of the 38- and 39-kilodalton structural proteins of Borna disease virus. J. Virol. 71:5133-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki, S., and H. Ludwig. 1993. In Borna disease virus infected rabbit neurons 100 nm particle structures accumulate at areas of Joest-Degen inclusion bodies. Zentralbl. Veterinarmed. 40:291-297. [DOI] [PubMed] [Google Scholar]
- 12.Schwemmle, M., C. Jehle, T. Shoemaker, and W. I. Lipkin. 1999. Characterization of the major nuclear localization signal of the Borna disease virus phosphoprotein. J. Gen. Virol. 80:97-100. [DOI] [PubMed] [Google Scholar]
- 13.Shoya, Y., T. Kobayashi, T. Koda, K. Ikuta, M. Kakinuma, and M. Kishi. 1998. Two proline-rich nuclear localization signals in the amino- and carboxyl-terminal regions of the Borna disease virus phosphoprotein. J. Virol. 72:9755-9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turpin, P., B. Ossareh-Nazari, and C. Dargemont. 1999. Nuclear transport and transcriptional regulation. FEBS Lett. 452:82-86. [DOI] [PubMed] [Google Scholar]
- 15.Walker, M. P., I. Jordan, T. Briese, N. Fischer, and W. I. Lipkin. 2000. Expression and characterization of the Borna disease virus polymerase. J. Virol. 74:4425-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittaker, G. R., and A. Helenius. 1998. Nuclear import and export of viruses and virus genomes. Virology 246:1-23. [DOI] [PubMed] [Google Scholar]
- 17.Wolff, T., G. Unterstab, G. Heins, J. A. Richt, and M. Kann. 2002. Characterization of an unusual importin alpha binding motif in the Borna disease virus p10 protein that directs nuclear import. J. Biol. Chem. 277:12151-12157. [DOI] [PubMed] [Google Scholar]












