Since the invention of the microscope, imaging has guided the mainstream of science and medicine by revealing structures not visible to the human eye. Views of single cells, organelles, molecules, and even atoms obtained by modern microscopy are now central to biological investigations. In the case of the whole body, biomedical imaging also reveals structures beneath the surface. This “occlusion problem” has been solved by techniques such as x-ray, computerized tomography (CT), positron emission tomography (PET), and MRI, which provide high-resolution views of organs and tissues within the living body. Thus, structure at the highest levels of biologically relevant detail either in the living body or in vitro is currently available for investigation of biological mechanisms and for the diagnosis and treatment of disease.
Although a picture may be worth a thousand words, current standards of clinical investigation aim beyond the view in order to understand mechanisms of action. Thus, beyond this unprecedented level of structural detail, overlays of physiological mechanisms add a necessary dimension to biomedical imaging. For example, the technical and structural challenges of imaging a moving object such as a heart must be linked to functional patterns of blood flow and turbulence in order to address more complicated problems related to treatment of heart failure. In the case of the brain, advances in imaging extend well beyond the domains of structures, regardless of size, motion, or occlusion, to the domain of function by identification of active neural tissue. This new form of functional imaging adds a novel dimension to conventional imaging by isolating the neurobiology of specific mental events. Thus, current frontiers in biomedical investigation encompass not only structures and functions of subcellular, cellular, and organ systems but also structures and neural functions that are linked to perceptions, cognition, and volitional action.
In keeping with the broad scope and fast pace of new developments in structural and functional imaging, this Perspective series aims to highlight representative new areas where developments in imaging profoundly advance and redirect our understanding and treatment of human disease. Although many potential topics would serve this objective, those included here have been selected to encompass representative advances. These topics would not conventionally fall under a single organizational scheme. The unifying goal is to illuminate frontiers in biomedical imaging that impact our understanding and treatment of human disease. Thus, complex sociomedical issues, such as the scanning of nonsymptomatic individuals to screen for indications of disease, are linked to subcellular processes that lead to gene therapy as examples of new horizons that encompass both structural and functional advances in biomedical imaging. Six emerging areas of clinical investigation and the imaging technologies that drive them are organized around this unifying theme.
The “worried healthy”: ethical and economic considerations in screening the nonsymptomatic population
Beyond the reaches of imaging technology, and as a natural consequence of success in providing widespread availability of high-quality whole-body images, the new question of efficacy and screening of the healthy population emerges. Imaging of every aspect of the human body is now considered standard in routine diagnostic and treatment practices. However, this availability of high-resolution, minimally invasive views of body parts and systems has brought new questions of use, ethics, medical practice, and policy. These issues arise, in part, from public responses to advertising that targets the “healthy but worried” population (Figure 1) and are further complicated by recent controversy concerning the benefits of many common screening procedures (1). In this Perspective series, M.G.M. Hunink and G.S. Gazelle go beyond the direct relevance of CT images for patient care to discuss the larger issues of cost, putative benefits, value of early detection, and false positive and false negative findings in general screening programs for coronary artery disease, lung cancer, colorectal cancer, and abdominal aortic aneurysms (2). These matters are raised in relation to the therapeutic advantages of medical imaging as a tool for screening. This Perspective illustrates a novel dilemma that is created when information regarding structure is offered to address questions of function: i.e., is there evidence of disease? The structure-to-function question is placed on a larger platform when it is related to healthcare-associated costs and anxiety about benign findings. It is most often assumed that a true answer to the question of whether or not there is disease will come from informed discriminations between physiological and pathophysiological conditions than from structural information alone. Thus, the Perspective by Hunink and Gazelle brings into focus the question of whether an intervention is advisable based on structural information in the absence of physiological context.
Figure 1.
Excerpts from websites that offers scanning services for elective screening for disease.
The addicted brain: advances in understanding the neurochemistry and neurobehavior of addiction using PET
Another emerging frontier of biomedical imaging integrates neurochemistry and functional neuroimaging in order to understand and treat drug addiction. N.D. Volkow and colleagues discuss the neurochemical and behavioral hallmarks of human drug addiction that can now be exposed through brain imaging using PET during various stages of addiction, craving, treatment, and recovery (3). The enabling physiology of functional neuroimaging is based on the coupling of blood volume and neural activity. Although the mechanism for this coupling is not well understood and remains an active area of research, variations in local blood volume that indicate approximate locations of active neural ensembles are detected by both PET and MRI. In the case of PET, a radioactive tracer (H215O) is injected into the arterial blood stream. Emission of the unstable positrons that are annihilated by free electrons results in detectable levels of γ rays. Elevations in γ ray counts that are detected outside the brain during cognitive-related tasks are taken as evidence for local neural activity (4). Thus, hypotheses of mechanism and potential treatment options come into view for the challenging medical problems associated with drug addiction. For example, the role of dopamine as a re-enforcer for drug abuse and subsequent decreased inhibitory control is often associated with functions of the orbitofrontal and cingulate gyri and can be observed through the virtual lens of PET imaging. Here, images of structure are integrated with knowledge of physiology and behavior to bring new hope for treatment of this devastating disease.
Brain mapping: advances in neurosurgical planning, protection of basic functions, and treatment of psychiatric disease using functional MRI
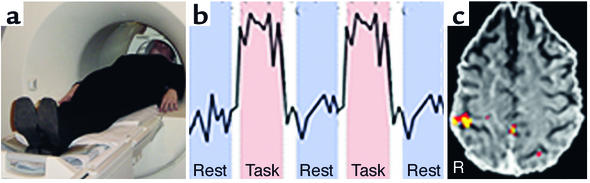
In the case of functional MRI (fMRI), detectable variations in magnetic resonance signals (reflecting variations in the magnetic susceptibility of brain tissue) are taken as evidence for blood volume changes assumed to be coupled with neural activity (5, 6). When these signals covary with the on- and offset of behavioral events, they presumably reflect the neural activity that drives cortically mediated events. Thus, current and future directions in biomedical investigation and medicine that are enabled by advances in imaging now extend to structures and functions that underlie human cognition and related disorders (Figure 2).
Figure 2.
Illustration of an fMRI procedure in which a conventional MRI scanner running an echo-planar sequence acquires brain images during performance of a task (a). The signal originating from one voxel (volume element) of 1.5 × 1.5 × 4.5 mm of brain illustrates a typical procedure in which a task is performed between alternating periods of rest (b). Typically, in a block design, each epoch is about 30–40 seconds. Post-processing of images reveals where the signal during the task is located relative to the signal during rest (c). These voxels are represented on anatomical images by a color that indicates the level of statistical significance. In this example, the task was tactile stimulation of the left hand during the task-epochs. The yellow and red colors on the brain slice indicate activity in the post-central gyrus (arrow), which was expected based on known functional specificity for this region. R, brain right.
The hypothesis of functional specificity, originally proposed by pioneering neurologists such as Wernicke (7) and Broca (8), associates specific structures of the brain with specific brain functions. For example, based on clinical observations of functional deficits, speech production was assumed to be the function of the left inferior gyrus, and comprehension of speech was assumed to be a function of the superior temporal gyrus. Penfield’s direct cortical mapping experiments in the 1950s confirmed these hypotheses and further extended notions of structure/function specificity in the brain (9). Although current models of these language functions generally assume that they are mediated by global long-range networks of multiple brain regions (10), the precise location of each component in the system is an essential aspect of planning for neurosurgical procedures (11). For example, the choice between a complete and total tumor resection and a more conservative debulking procedure often depends on the risk of potential morbidity if the adjacent function-specific cortex were to be disabled. In this Perspective series, J. Gore describes a rapidly expanding new area of biomedical imaging research that explores this essential link between structural and functional information and seeks to optimize surgical techniques and their outcome (12).
Psychiatric disorders: insights and treatment options guided by fMRI
Future directions in this new field of functional neural imaging offer significant advances in therapy based on a wide range of investigations of brain function and behavior. For example, the neural circuitry of psychiatric disorders such as anxiety, depression, post-traumatic stress disorders, attention deficit-hyperactivity disorder, and schizophrenia, as well as the specific targets of CNS drugs and other therapeutic approaches, can be assessed using fMRI. Efficient and individualized medical strategies for psychiatric illnesses based on investigations of before and after treatment effects using fMRI are on the near horizon (13, 14). Although the structural and functional determinants of schizophrenia are areas of active investigation using a wide variety of conventional techniques and approaches, functional-neuroimaging investigation now also suggests new models and possible treatment directions. T. Sharma describes insights into the treatment of schizophrenia that are emerging from neuroimaging investigations (15).
In addition to therapeutic strategies that serve patients with psychiatric illnesses, new directions for treatment of neurological disorders are equally far reaching. For example, neurological processes that underlie the rehabilitation of function that was lost secondary to stroke, brain injury, or neural degeneration can now be imaged and subjected to hypothesis-based investigations (16). Chronic pain and other disorders of sensation and perception may be better understood and treated using functional neuroimaging. Individual patient scans reveal neurophysiology that is both specific to the patient and generalizable to the diagnosis and treatment of specific disorders (17). Thus functional neuroimaging offers the hope of improving distinctions between chronic pain syndromes, and of improving treatment outcomes by more closely matching pharmacologic treatments with their targeted disorders. Genetic determinants of behavior may be similarly explored using new functional-imaging methods intended to discriminate between functional neurocircuitry that is normally conserved and that which is specific to a known genetic variant (18). Future directions of functional neuroimaging include the determination of residual cognitive function in patients who are in a vegetative state or are minimally conscious due to traumatic brain injury, oxygen deprivation, or stroke (19–21). The processes that contribute to a return to a normal level of consciousness in these patients may be elucidated by investigations that use new neuroimaging techniques based on passive stimulation of visual, verbal, and tactile systems, as previously developed for the mapping of cortical brain functions in human infants (22).
Cardiac imaging: current and future directions
Cardiac disease is a leading cause of death worldwide, and advances in treatment continue to be firmly based on recent developments that depend on biomedical imaging (23). The structural-imaging challenges include high spatial and temporal resolution of multiple tissue types and of moving targets, i.e., the cardiovascular system, heart, and blood, from inside the living body. The functional-imaging challenges include integration of these images with physiological models of cardiac disease that extend prevention strategies and treatment options. This imaging technology, emerging from within the boundaries of investigations of heart disease, promises continued and novel therapeutic benefits for cardiac patients. NMR spectroscopy and MRI together provide multiple scales of detailed information, which extend from the molecular, cellular, and organ level to whole-body investigations. In this Perspective series, J.R. Forder and G.M. Pohost discuss these and other integrated imaging techniques that aim toward a better understanding of metabolism, heart function, and the cardiovascular system (24).
Gene therapy: insights from molecular-genetic PET, MRI, and optical imaging
Also founded on multiple biomedical imaging techniques, including MRI, CT, PET, ultrasound, and optical imaging, are efforts to develop gene therapy based on an understanding of molecular and genetic processes observed by the imaging of molecular and cellular structures. R.G. Blasberg and J. Gelovani Tjuvajev discuss direct, indirect (using reporter genes), and surrogate or “downstream” imaging strategies in relation to mechanisms of regulation of endogenous gene expression. These aim toward the development of new gene therapy approaches (25).
The articles in this Perspective series link clinical investigation with conventional and novel imaging capabilities that relate molecular, cellular, and organ system physiology to human behavior and disease. Thus, gene expression, cardiovascular disease, drug addiction, schizophrenia, neurosurgical morbidity, and the medical and socioeconomic issues of mass-population screening are linked as frontiers in translational, structural, and functional imaging. The far-reaching impact of these advances, firmly grounded in interdisciplinary biomedical imaging research, will emerge from the continual development of a broad range of techniques and physiological models aimed toward treating human disease.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: computerized tomography (CT); positron emission tomography (PET); functional MRI (fMRI).
References
- 1.Pollack MN, Foulkes WD. Challenges to cancer control by screening. Nat. Rev. Cancer. 2003;3:297–303. doi: 10.1038/nrc1042. [DOI] [PubMed] [Google Scholar]
- 2.Hunink, M.G.M., and Gazelle, G.S. 2003. CT screening: a trade-off of risks, benefits, and costs. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 3.Volkow ND, Fowler JS, Wang G-J. The addicted human brain: insights from imaging studies. J. Clin. Invest. 2003;111:1444–1451. doi:10.1172/JCI200318533. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. U. S. A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa S, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U. S. A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 7.Wernicke, K. 1874. Der aphasische Symptomenkomplex. Eine psychologische Studie auf anatomischer Basis. Cohn und Weigert. Breslau, Germany.
- 8.Broca P. Remarques sur le siége de la faculté du langage articulé: suivies d’une observation d’aphémie. Bulletin de la Société Anatomique. 1861;6:330–357. [Google Scholar]
- 9.Penfield, W. 1975. The mystery of the mind. Princeton University Press. Princeton, New York, USA. 123 pp.
- 10.Hirsch J, Moreno DR, Kim KHS. Interconnected large-scale systems for three fundamental cognitive tasks revealed by functional MRI. J. Cogn. Neurosci. 2001;13:389–405. doi: 10.1162/08989290151137421. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch J, et al. An integrated fMRI procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurgery. 2000;47:711–722. doi: 10.1097/00006123-200009000-00037. [DOI] [PubMed] [Google Scholar]
- 12.Gore, J.C. 2003. Principles and practice of functional MRI of the human brain. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 13.Vaidya CJ, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein EA, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am. J. Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 15.Sharma, T. 2003. Insights and treatment options for psychiatric disorders guided by functional MRI. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 16.Mattay VS, et al. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann. Neurol. 2002;51:156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 17.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 18.Egan MF, et al. Effect of COMT Val108/158 MET genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiff ND, et al. Residual cerebral activity and behavioural fragments can remain in the persistently vegetative brain. Brain. 2002;125:1210–1234. doi: 10.1093/brain/awf131. [DOI] [PubMed] [Google Scholar]
- 20.Fins JJ, et al. Constructing an ethical stereotaxy for severe injury: balancing risks, benefits and access. Nat. Rev. 2003;4:323–326. doi: 10.1038/nrn1079. [DOI] [PubMed] [Google Scholar]
- 21.Menon DK, et al. Cortical processing in persistent vegetative state. Wolfson Brain Imaging Centre Team. Lancet. 1998;352:200. doi: 10.1016/s0140-6736(05)77805-3. [DOI] [PubMed] [Google Scholar]
- 22.Souweidane MM, et al. Brain mapping in sedated infants and young children with passive-functional magnetic resonance imaging. Ped. Neurosurgery. 1999;30:86–91. doi: 10.1159/000028768. [DOI] [PubMed] [Google Scholar]
- 23.Castillo E, Bluemke DA. Cardiac MR imaging. Radiol. Clin. North Am. 2003;41:17–28. doi: 10.1016/s0033-8389(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 24.Forder, J.R., and Pohost, G.M. 2003. Cardiovascular NMR: basic and clinical applications. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 25.Blasberg, R.G., and Gelovani Tjuvajev, J. 2003. Molecular-genetic imaging: current and future perspectives. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]