Abstract
Mechanisms leading to fibroblast accumulation during pulmonary fibrogenesis remain unclear. Although there is in vitro evidence of lung alveolar epithelial-to-mesenchymal transition (EMT), whether EMT occurs within the lung is currently unknown. Biopsies from fibrotic human lungs demonstrate epithelial cells with mesenchymal features, suggesting EMT. To more definitively test the capacity of alveolar epithelial cells for EMT, mice expressing β-galactosidase (β-gal) exclusively in lung epithelial cells were generated, and their fates were followed in an established model of pulmonary fibrosis, overexpression of active TGF-β1. β-gal-positive cells expressing mesenchymal markers accumulated within 3 weeks of in vivo TGF-β1 expression. The increase in vimentin-positive cells within injured lungs was nearly all β-gal-positive, indicating epithelial cells as the main source of mesenchymal expansion in this model. Ex vivo, primary alveolar epithelial cells cultured on provisional matrix components, fibronectin or fibrin, undergo robust EMT via integrin-dependent activation of endogenous latent TGF-β1. In contrast, primary cells cultured on laminin/collagen mixtures do not activate the TGF-β1 pathway and, if exposed to active TGF-β1, undergo apoptosis rather than EMT. These data reveal alveolar epithelial cells as progenitors for fibroblasts in vivo and implicate the provisional extracellular matrix as a key regulator of epithelial transdifferentiation during fibrogenesis.
Keywords: apoptosis, fibronectin, integrin αvβ6, TGF-β, usual interstitial pneumonia
Idiopathic pulmonary fibrosis (IPF) is a disease characterized by fibroblast accumulation, excessive collagen deposition, and matrix remodeling, which lead to distortion of the alveolar architecture, progressive decline in lung function, and ultimately death (1). The pathologic hallmarks of IPF include heterogeneous injury/fibrosis, scattered foci of fibroblast accumulation beneath flattened alveolar epithelial cells (AECs), and scant inflammation (1). The pathways leading to excess collagen-producing fibroblasts in either IPF or experimental models of lung fibrosis are uncertain. Although expansion of resident fibroblasts likely occurs in response to injury, several lines of evidence support the possibility that lung fibroblast development during injury responses may also be derived from bone marrow and/or epithelial cells (2, 3). A greater understanding of the cellular origin of fibroblast accumulation in response to injury is important because the regulation of cellular transdifferentiation into a fibroblast phenotype is likely very different from the regulation of fibroblast proliferation (3–5). Fibroblasts isolated from IPF lungs have heterogenous phenotypes and properties different from that of normal lung fibroblasts (6). One potential explanation for this heterogeneity is that fibroblasts are derived from multiple cell types. There have been prior suggestions that epithelial-to-mesenchymal transition (EMT) occurs in the lung during fibrogenesis, but these suggestions derive largely from studies of transformed cells or primary AECs cultured on plastic, the in vivo significance of which is unclear (7–9). Reports using primary AECs (8, 9) are more compelling, but current isolation methods do not completely remove contaminating mesenchymal cells, thus making it uncertain whether factors thought to induce EMT may not actually favor the growth of contaminating mesenchymal cells and/or promote the loss of epithelial cells leading to increased measurements of mesenchymal markers.
To address these uncertainties, we used several approaches to explore lung EMT during the pathogenesis of both experimental pulmonary fibrosis and IPF. We found that IPF lung biopsies demonstrate epithelial cells which have acquired mesenchymal features, raising the possibility of EMT during fibrogenesis. We then developed a triple transgenic mouse reporter system in which lung epithelial cells are genetically altered to permanently express β-gal, allowing us to map the fate of these cells and definitively test whether EMT occurs in vivo in an animal model of pulmonary fibrosis. These studies provide the strongest evidence to date that EMT occurs during fibrogenesis and reveals EMT to be a surprisingly robust process. Furthermore, our data show that the provisional matrix drives this process by activating the integrin αvβ6 and thereby latent TGF-β1, revealing a mechanism by which the provisional matrix initiates repair.
Results
AECs in IPF Lungs Demonstrate Features of Early EMT.
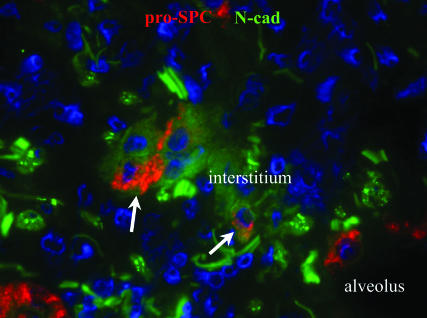
Immunofluorescent staining of lung biopsies from patients with IPF revealed cells within remodeling matrices with features of both epithelial and mesenchymal cells, suggesting active EMT. In each of four diagnostic biopsies proven to be IPF, a subset of cells stained weakly for pro-surfactant protein-C (pro-SPC), a type II AEC marker, and N-cadherin, a mesenchymal cadherin (Fig. 1). This appeared to frequently develop near sites of alveolar collapse and AEC clustering. Epithelial cells lining the lumen of injured alveoli stained much brighter for pro-SPC and had no discernible N-cadherin staining (Fig. 1). The presence of pro-SPC-positive cells within the expanded interstitium suggests AEC detachment from the basement membrane and migration within the extracellular matrix. Indeed, these epithelial cells have lost apical–basal polarity as demonstrated by diffuse staining for the integrin α3β1 (Fig. 7, which is published as supporting information on the PNAS web site), a laminin receptor normally expressed on the basal surface of AECs. Cells that might have completed EMT would be expected to have lost epithelial features, such as expression of pro-SPC. Because such fibroblasts in the human tissues could not be distinguished from resident fibroblasts, we turned to a mouse system in which AECs are marked by genetic techniques to track their fate, allowing us to more definitively address the possibility of EMT during fibrogenesis.
Fig. 1.
Evidence of early EMT in IPF lung. IPF lung biopsy demonstrating cells removed from the alveolar basement membrane within the interstitium coexpressing pro-SPC and the mesenchymal protein, N-cadherin (arrows).
Generation and Verification of Triple Transgenic Reporter Mice.
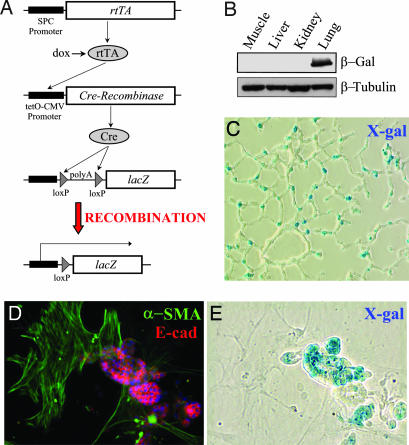
Triple transgenic reporter mice containing at least one copy of the human SPC-rtTA, tetO-CMV-Cre (10, 11) and floxed ROSA26 (12) transgenes (Fig. 2A) were generated by crossing, resulting in genetically tagged lung epithelial cells that permanently express β-gal. Mice were given doxycycline throughout gestation, and lung epithelial cell-specific expression of β-gal was confirmed by several techniques. Frozen lung sections stained with X-gal revealed robust β-gal expression in AECs (Fig. 2C), whereas mesenchymal structures within the lung (smooth muscle layers within vessels and airways) were completely X-gal-negative. Sections from kidney, liver, and muscle also revealed absence of X-gal staining. Protein lysates from these organs were analyzed by immunoblot demonstrating expression of β-gal limited to the lung (Fig. 2B). Primary AECs isolated from triple transgenic mice were immediately fixed and stained with X-gal followed by immunostaining for pro-SPC, revealing that ≈80–100% of the pro-SPC-positive cells are X-gal-positive, consistent with prior reports of in vivo Cre-mediated recombination efficiency (13). AECs from triple transgenic mice were cultured on Matrigel (Mg), which is primarily composed of laminins and collagen IV in media containing 10 ng/ml KGF (14) for 1 week, then stained with X-gal followed by immunostaining. AECs cultured on Mg form tight clusters and maintain type II AEC markers, such as pro-SPC, as has been reported. X-gal-positive AECs all stain for pro-SPC and other epithelial markers, such as E-cadherin (Fig. 2 and Fig. 8, which is published as supporting information on the PNAS web site). Contaminating fibroblasts of the type II isolation are α-SMA-positive, E-cadherin-negative, and are all X-gal-negative. Thus, the triple transgenic reporter mouse system results in lung epithelial-specific expression of β-gal. Littermate control mice lacking any of the three transgenes revealed no expression of β-gal by these techniques.
Fig. 2.
Characterization of reporter mice expressing AEC β-gal. (A) Triple transgenic mice express rtTA in lung epithelial cells using the SPC promoter. In the presence of doxycycline (dox), rtTA is an active transcriptional factor leading to expression of Cre recombinase and removal of the floxed poly(A) sequence in the ROSA allele. Thus, in cells expressing SPC, expression of lacZ is constitutively and irreversibly activated. (B) Immunoblot of lysate from different tissues demonstrating lung-specific expression of β-gal. (C) Expression of β-gal in a lung section by X-gal staining. (D and E) Type II AECs isolated from triple transgenic mice, maintained on Mg for 1 week, then stained with X-gal (E) followed by immunostaining for E-cadherin and α-SMA (D). X-gal-positive epithelial cells form clusters and stain for E-cadherin. Contaminating fibroblasts are X-gal-negative and α-SMA-positive.
AEC β-gal Reporter Mice Reveal EMT in Vivo.
These reporter mice were next examined in a well described, cytokine-mediated model of pulmonary fibrosis (15). Triple transgenic mice were treated with intranasal instillation of AdTGF-β1 (2.5 × 108 pfu per mouse) in 30 μl of PBS or PBS alone. After 10 days, single cell suspensions of AECs were prepared from injured and control lungs. Cells were cultured on Mg-coated slides for 1 week, then stained with X-gal. AECs form tight clusters of cells that are X-gal-positive. Mesenchymal cells from control lungs are uniformly X-gal-negative (Fig. 2). In contrast, a subset of X-gal-positive cells from injured lungs demonstrated a distinct elongated mesenchymal phenotype, forming stress fibers, filopodia and lamellipodia (Fig. 9, which is published as supporting information on the PNAS web site).
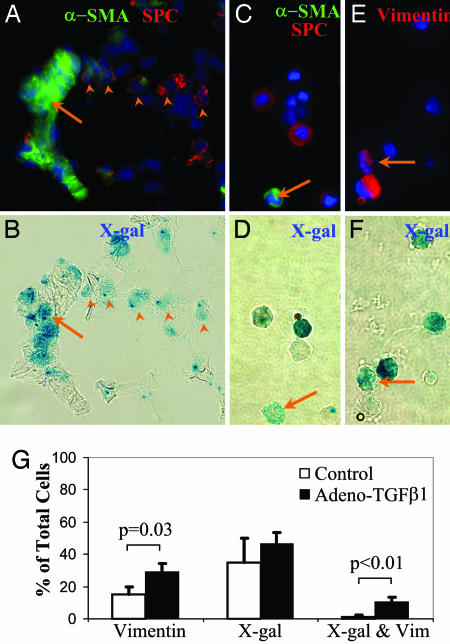
To confirm and quantify the presence of X-gal-positive epithelial-derived mesenchymal cells, adult triple transgenic mice were given either AdTGF-β1 or vehicle alone by intranasal instillation. After 21 days, lungs were analyzed for evidence of fibrosis and EMT. Lung sections revealed moderate fibrosis and whole lung lysate demonstrated increased expression of mesenchymal proteins (Fig. 9) consistent with reports using this virus (15). Clusters of X-gal-positive cells are apparent within areas of collagen deposition in injured mice. To determine whether any of these X-gal-positive cells were fibroblasts, lung sections were stained with X-gal followed by immunostaining (Fig. 3). Clusters of X-gal-positive cells are pro-SPC-negative and express the myofibroblast marker α-SMA (Fig. 3 A and B), demonstrating EMT in situ. Cellular costaining of X-gal and α-SMA was confirmed by confocal microscopy (not shown). To eliminate further the possibility that areas of costaining are due to overlapping cells rather than cells expressing both β-gal and α-SMA, single cell suspensions were prepared from control and injured lungs, then immediately fixed and stained with X-gal, followed by immunostaining for pro-SPC and α-SMA. Cells that are clearly X-gal-positive, α-SMA-positive, and pro-SPC-negative (Fig. 3 C and D) are identified from mice treated with AdTGF-β1 representing in vivo EMT. Lung sections and single cell suspensions from control mice do not show any X-gal-positive, α-SMA-positive cells. Because α-SMA expression is limited to a subset of fibroblasts (2), not all EMT-derived fibroblasts may stain for α-SMA. Indeed, <5% of X-gal-positive cells from injured mice were α-SMA-positive.
Fig. 3.
EMT develops in vivo after TGF-β1-mediated lung injury. (A and B) The same field (×60) of a lung 3 weeks after intranasal AdTGF-β1 stained by X-gal (B) then immunostained (A) for α-SMA and pro-SPC. Several X-gal-positive, pro-SPC-positive cells are demonstrated (arrowheads) as well as a cluster of X-gal-positive, α-SMA-positive, and pro-SPC-negative cells (arrow). (C and D) The same field of a whole lung single-cell suspension from an injured lung stained by X-gal (D) then immunostained (C) for α-SMA and pro-SPC. An X-gal-positive, α-SMA-positive, pro-SPC-negative cell is demonstrated (arrow). (E and F) The same field of a whole lung single cell suspension from a triple transgenic mouse three weeks after AdTGF-β1, stained by X-gal (F), and immunostained (E) for vimentin. An X-gal- and vimentin-positive cell is indicated by arrow. (G) Quantification X-gal- and vimentin-positive single cells from of AdTGF-β1-injured and control lungs (n = 3 per group).
To quantify the degree of EMT, single-cell suspensions from injured and uninjured lungs were also stained with vimentin, a more general fibroblast marker (16). Staining of murine fibroblasts and primary murine alveolar epithelial with this antibody revealed excellent specificity for fibroblasts (Fig. 10, which is published as supporting information on the PNAS web site). Many vimentin-positive cells are identified in injured lungs, approximately a third of which are also X-gal-positive (Fig. 3 E–G). There is an overall 2-fold increase in vimentin-positive cells 21 days after AdTGF-β1 instillation. Surprisingly, X-gal-positive epithelial-derived cells account for nearly all of this in vivo increase in vimentin-positive cells. Lung sections and single cell suspensions from littermate control mice which were tetO-Cre-positive and ROSA26-positive but lacked the SPC-rtTA transgene (n = 4) revealed no X-gal-positive cells 3 weeks after AdTGF-β1, excluding the possibility that AdTGF-β1 could induce native β-gal activity or lead to nonspecific recombination of the ROSA26 transgene.
Provisional Matrices Initiate EMT.
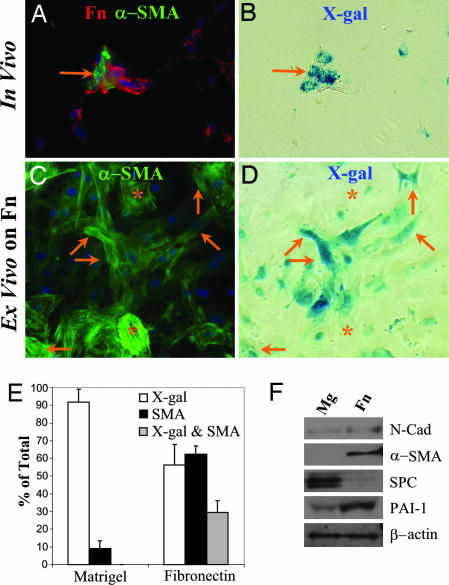
Inspection of injured lungs 21 days after exposure to active TGF-β1 indicated that clusters of X-gal-positive, α-SMA-positive cells are frequently located within areas of dense fibronectin (Fn) deposition (Fig. 4A and B). This physical association may result from Fn deposition by EMT-derived cells or from Fn-driven EMT. To test the hypothesis that matrix may regulate EMT, AECs were isolated from uninjured triple transgenic mice and cultured on Mg or Fn. After 1 week, cells were stained with X-gal, then immunostained. On Mg, no cells costaining for X-gal and α-SMA were found (Fig. 2 D and E). On Fn, the AECs undergo dramatic phenotypic changes with formation of stress fibers, lamellipodia and filopodia; numerous α-SMA-positive cells are also X-gal-positive (Fig. 4 C and D) representing epithelial-derived myofibroblasts. Quantification of cells on these surfaces staining for X-gal and α-SMA (Fig. 4E) demonstrates that this process is robust; ≈40% of all α-SMA-positive cells were X-gal-positive. X-gal-positive cells maintained on Fn for 1 week are virtually all vimentin-positive (Fig. 10) and demonstrate very weak or absent staining for epithelial markers, pro-SPC and E-cadherin (Fig. 8). Immunoblot analysis demonstrates that changes in protein expression begin within 2 days of culture; AECs maintained on Fn have increased expression of mesenchymal proteins, N-cadherin, α-SMA and PAI-1, and a marked decline in pro-SPC expression compared to the same pool of isolated AECs cultured on Mg (Fig. 4F). To determine whether other components of the provisional matrix could drive EMT, primary AECs from triple transgenic mice were cultured on surfaces coated with fibrin gel and mixtures of fibrin and Fn. Primary AECs were found to undergo a similar EMT on both fibrin-coated and Fn/fibrin-coated matrices (not shown). Thus, culturing on either of the two major components of the provisional matrix is sufficient to drive AEC EMT.
Fig. 4.
Fn drives AEC EMT ex vivo. (A and B) The same field (×60) of a lung 3 weeks after intranasal AdTGF-β1 stained by X-gal (B), then immunostained (A) for α-SMA (green) and Fn (red). A cluster of X-gal- and α-SMA-positive cells within an area of Fn deposition is demonstrated (arrow). (C and D) The same view of isolated AECs from an uninjured triple transgenic mouse cultured on Fn-coated slides for 1 week, then stained with X-gal (D) then immunostained (C) for α-SMA. Several X-gal- and α-SMA-positive cells are shown (arrows) as well as several X-gal-negative, α-SMA-positive cells (∗). (E) Quantification of primary AECs from triple transgenic mice maintained on Fn- or Mg-coated slides for 1 week and stained for X-gal and α-SMA. (F) Primary murine AECs were cultured on Fn- or Mg-coated plates for 2 days and then lysed and analyzed by immunoblot.
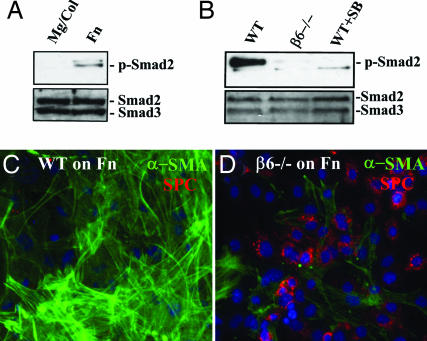
What is the mechanism by which provisional matrix proteins promote EMT ex vivo? Because activation of TGF-β1 is critical to the development of tissue fibrosis in several settings, we considered the possibility that active TGF-β1 is preferentially produced by cells contacting provisional matrix proteins. However, AECs plated on Mg and Fn secreted equal levels of total immunoreactive TGF-β1 and no detectable active TGF-β1 (not shown). Fn-driven EMT also was not blocked by adding high concentrations of a soluble TGF-β1 receptor (TGF-βRII-Fc) or antibodies against TGF-β1 (1D11), indicating that release of active TGF-β1 could not explain the process of Fn-driven EMT (not shown). However, primary AECs cultured on Fn for 2 days reveal robust levels of p-Smad2 by immunoblot (Fig. 5A and B) and nuclear localization of Smad2/3 by immunostaining (Fig. 11, which is published as supporting information on the PNAS web site), consistent with activation of endogenous TGF-β1 signaling. In contrast, AECs cultured on Mg for 2 days are quiescent, revealing no detectable p-Smad2 and only cytoplasmic Smad2/3. Addition of a low molecular weight inhibitor of TGF-β1 receptor kinase (SB431542) largely blocked the morphological responses of cells to Fn, inhibited expression of α-SMA, and allowed the maintenance of pro-SPC expression, indicating the persistence of an epithelial phenotype on Fn (not shown). These results strongly suggested that latent TGF-β1 was simultaneously being activated and sequestered, a mechanism previously ascribed to latent TGF-β1 activation through the integrin αvβ6 (17). Integrin αvβ6 expression is limited to epithelial cells and known to be required in animal models of lung fibrosis (18). To test this hypothesis, AECs were isolated from β6-null mice or wild-type controls and examined for their responses to culture on Fn. Whereas wild-type cells underwent the expected mesenchymal transition on Fn, β6-null AECs did not translocate Smad2/3 to the nucleus, developed little α-SMA, and maintained a pro-SPC-positive epithelial phenotype (Fig. 5). Thus, Fn itself leads to αvβ6-dependent activation of latent TGF-β1 with a resultant robust EMT. This finding correlates with the apparent distribution of epithelial-derived cells devoid of pro-SPC in foci of Fn accumulation in vivo during matrix remodeling and indicates that at least one component of the provisional lung matrix alone can initiate EMT.
Fig. 5.
AEC EMT requires αvβ6-dependent latent TGF-β1 activation. (A) Primary murine AECs were cultured for 2 days on Mg/Col or Fn, then lysed and analyzed by immunoblot for p-Smad2 and total Smad2/3. (B) Primary AECs from wild-type and α6-null mice were cultured on Fn ± SB431542 (10 μM) for 2 days, then lysed and immunoblotted for p-Smad2 and total Smad2/3. (C and D) AECs from wild-type (C) and β6-null (D) mice were cultured on Fn for 4 days, then stained for α-SMA and pro-SPC.
Matrigel Promotes AEC Apoptosis in Response to TGF-β1.
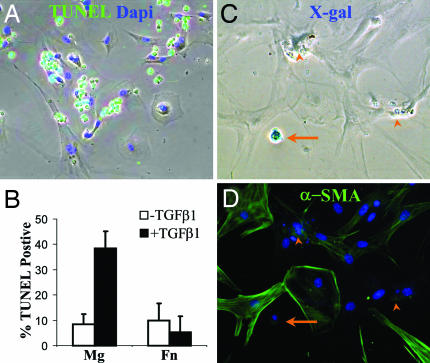
To investigate the response of AECs on Mg to exogenous TGF-β1, primary AECs from triple transgenic reporter mice were isolated and cultured on Mg or Fn. After 1 day, TGF-β1 (4 ng/ml) was added to some wells. After an additional 2 days, cells were analyzed for apoptosis by TUNEL assay. Cells on Mg treated with TGF-β1 revealed a marked increase in TUNEL-positive cells compared to cells on Mg without TGF-β1 or cells of Fn with or without TGF-β1 (Fig. 6B). The contaminating fibroblasts from the AEC isolation were resistant to TGF-β1-induced apoptosis (Fig. 6A). One week after TGF-β1 treatment, cells were stained with X-gal followed by immunostaining for α-SMA. On Fn, exogenous TGF-β1 had no effect on the number of X-gal-positive or α-SMA-positive cells. In contrast, there was a dramatic loss of X-gal-positive AECs on Mg in the presence of exogenous TGF-β1. No X-gal-positive, α-SMA-positive cells were identified within Mg-coated wells treated with TGF-β1 (Fig. 6 C and D).
Fig. 6.
TGF-β1-induced AEC apoptosis on Mg but not Fn. (A) Overlay of phase with TUNEL (green) and Dapi (blue) staining of AECs plated on Mg and treated with TGF-β1 (4 ng/ml) for 2 days. (B) Quantification of TUNEL-positive cells. (C and D) AECs from triple transgenic mice cultured on Mg then stimulated with TGF-β1 for 1 week, stained with X-gal (C) followed by immunostaining for α-SMA (D). A remaining X-gal-positive cell is shown (arrow) as well as several apoptotic remnants (arrow heads).
Discussion
Type II AECs have long been considered the precursor of alveolar type I cells, and are known to possess proliferative potential (19). In this report, we are able to address several major points of uncertainty in identifying AECs as precursors to fibroblast-like cells and a mechanism regulating this transition. By using genetically modified mice in which AEC fate can be tracked, we have been able to identify epithelial-derived mesenchymal cells, in vivo and ex vivo, that have lost epithelial features and would have otherwise been indistinguishable from other mesenchymal cells. We verified histological costaining with staining from single-cell suspensions to definitively eliminate the possibility of overlapping cells leading to apparent costaining. Our observations that these cells undergo EMT both ex vivo and in vivo provides direct evidence that AECs, and type II cells in particular, are progenitors for mesenchymal cells, which can contribute significantly to the pool of expanded fibroblasts after lung injury. AECs appear to be positioned in the alveolus to respond to injurious events by both proliferating to replace alveolar type I cell loss and by transdifferentiating to promote repair.
The model system described here not only reveals the intrinsic potential of AECs for mesenchymal transdifferentiation, as had been suspected (2, 8), but also allows quantification of how robust this process may be and provides insight in the mechanisms involved. In the context of AdTGF-β1-mediated lung injury, most of the expansion of vimentin- and α-SMA-positive cells appears to derive from epithelial cells at 21 days after the initiation of injury. Although more subjective, inspection of the histopathology of these injured lungs indicates that one common site of epithelial-derived fibroblasts in vivo is at sites of Fn accumulation (Fig. 4). This observation could result from Fn-deposition by EMT-derived cells, or more interestingly, suggest that the provisional matrix could activate AEC transdifferentiation. The ability of provisional matrix proteins to drive EMT was demonstrated in our ex vivo system. AECs maintained on Fn or fibrin, major components of the provisional matrix, surprisingly undergo spontaneous EMT without the need for exogenous TGF-β1, unlike prior reports of other in vitro models of EMT (8, 9). Addition of TGF-β1 to cells on Fn had virtually no effect because of robust αvβ6 integrin-dependent activation of endogenous TGF-β1 signaling. This observation alone invites interpretation of a large body of prior work on cultured AEC. Although AECs cultured on Fn have been known to spread and change their phenotype for many years (20), our data reveal that this is actually EMT and is driven by a specific signaling pathway initiated by engagement of fibrin or Fn. In contrast, AECs maintained on Mg, composed primarily of alveolar basement membrane components, do not activate TGF-β1 and maintain an AEC phenotype.
TGF-β1 signaling has been implicated in both AEC apoptosis (21) and EMT (8, 9), making the in vivo response of AECs to TGF-β1 during fibrogenesis unclear. Indeed, epithelial cell death is a prominent feature of fibrotic lung diseases, and our current data support a prominent role for AEC EMT, suggesting that both responses occur during fibrogenesis and that the determinants of the AEC response to TGF-β1 may be critical to the progression of fibrogenesis. Prior obscurity on the AEC response to TGF-β1 stems from the limitation of previous techniques. Immortalized AEC-like cells may have a blunted or absent apoptotic response, potentially blocking an important physiologic response to TGF-β1. Culture of primary AECs with even a small amount of fibroblast contamination may ultimately result in a pure fibroblast culture through either EMT or preferential epithelial cell death. By using a system in which primary AECs are permanently tagged, it is clear that, at least ex vivo, the content of the matrix contacting AECs determines the response to TGF-β1. Treatment of AECs on Mg with exogenous TGF-β1 leads to AEC apoptosis, whereas AECs on Fn undergo EMT through activation of endogenous TGF-β1. Collectively, these findings suggest a model of fibrosis in which an initiating stimulus leads to AEC apoptosis and enhanced alveolar permeability, then later, AECs within a Fn/fibrin-rich provisional matrix, undergo EMT and directly contribute to fibrosis. This finding is consistent with previous reports that demonstrate that alveolar accumulation of Fn precedes fibrosis, implicating Fn in the accumulation of fibroblasts in IPF and in animal models of pulmonary fibrogenesis (22). The combined in vivo and ex vivo findings in this report provide a clear link between formation of a provisional matrix in response to injury, focal activation of the repair process, and accumulation of EMT-derived fibroblasts.
To what extent does EMT contribute to the progression of fibrotic lung disease in humans? Elevated levels of active TGF-β1 and deposition of a provisional matrix rich in Fn and fibrin are prominent components of fibrotic lung disease, supporting the potential for EMT by the mechanism described in this report. Immunostaining of IPF lung biopsies also supports the possibility of EMT (ref. 8 and Figs. 1 and 7) and isolated primary human type II cells undergo an apparently identical conversion on Fn (not shown). However, detection of putative transitional cells in vivo by immunostaining requires cells to express detectable levels of epithelial markers. Our data (Fig. 4) show that AECs rapidly lose these markers on Fn. If EMT-derived fibroblasts are found to have a unique expression profile, they might be distinguished from other fibroblasts, allowing further validation and quantification of EMT in humans.
The high prevalence of EMT during experimental lung injury suggests that this is a previously unrecognized component of a normal remodeling process. If so, what normally limits and reverses this process? Preliminary observations indicate that EMT-derived fibroblasts are more prone to cell death than resident lung fibroblasts in culture, suggesting a mechanism for limiting physiologic repair after injury and inviting the speculation that, in pathologic conditions, an inherent tendency of EMT-derived fibroblasts to die may be suppressed leading to dysregulated and progressive fibrosis. Elucidation of additional determinants of AEC EMT and their subsequent cell survival and function could provide new therapeutic insights for fibrotic disorders.
Materials and Methods
Genetically Modified Mice.
The conditional ROSA26 reporter mouse (12), was a generous gift from Brian Black (University of California, San Francisco, CA). Briefly, this reporter mouse contains a transgene (ROSA) consisting of a lacZ gene and an upstream floxed triple polyadenylation [poly(A)] sequence preventing transcription of the lacZ gene. The poly(A) sequence is flanked by loxP sites subjecting it to removal by Cre recombinase, resulting in transcription and constitutive expression of lacZ in cells expressing Cre. Lung epithelial specific recombination between the loxP sites was achieved by using the tetO-CMV-Cre transgene and the surfactant protein-C promoter-reverse tetracycline transactivator transgene (SPC-rtTA) (10). Compound mice containing at least one copy of all three transgenes were obtained by breeding and were genotyped by PCR (10–12). Pregnant females were maintained on doxycycline containing water (1 mg/ml) throughout gestation. Freshly frozen lung samples for staining were obtained from mice anesthetized with a mixture of ketamine and xylazine. Mice were exsanguinated and then perfused with PBS. Lungs were lavaged twice with PBS and then frozen in OCT for sectioning and staining. Lysate from lung, kidney, and liver were routinely screened by immunoblot to ensure lung-specific expression of β-gal in all mice used for experiments. Integrin β6-null mice and matched controls were generated as described (17).
X-gal Staining.
Expression of lacZ in 5- to 7-μm-thick freshly frozen tissue sections or isolated primary AECs was determined by X-gal staining (23). In some samples, X-gal staining was followed by immunofluorescent staining (23) or picosirius red collagen staining performed by the University of California Research Morphology Core Facility (San Francisco, CA). For cellular quantification, 300 cells per sample stained for β-gal and for mesenchymal proteins were visualized and quantified independently by two investigators in blinded fashion. For evaluation of group differences, the two-tailed Student’s t test was used assuming equal variance. A P value of <0.05 was accepted as significant.
Type II Cell Isolation and Culture.
Murine alveolar epithelial type II cells were isolated from adult mice (6–12 weeks old) following the method of Corti et al. (20) with minor modifications and were cultured on tissue culture plates and chamber slides coated with Mg, supplemented with 5% collagen type I and 25% SABM (Cambrex, East Rutherford, NJ) or coated with 100 μg/ml Fn in PBS. Cells were maintained in SAGM without hydrocortisone containing 5% charcoal/dextran-treated FBS and 10 ng/ml KGF in a 37°C, 5% CO2 incubator as described (14). For further details, see Supporting Text, which is published as supporting information on the PNAS web site.
Whole Lung Single-Cell Suspensions.
Single-cell suspension was obtained from murine lungs as described (24) with minor modifications. Cells were either cultured or immediately fixed and stained with X-gal in suspension. Cells stained with X-gal in suspension were then centrifuged onto superfrost-coated slides (Fisher) and immunostained. For further details, see Supporting Text.
Intranasal Adeno-TGF-β1.
A replication-deficient adenovirus encoding a mutated TGF-β1 (AdTGF-β1) as described (15) was a generous gift from Jack Gauldie (McMaster University, Hamilton, ON, Canada). This adenovirus encodes porcine TGF-β1 with two point mutations (C223S and C225S) that prevent TGF-β1 binding to latency-associated protein and thus produces a constitutively active TGF-β1. Adult (6–12 weeks old) triple transgenic mice were lightly anesthetized with isoflurane, then instilled with 2.5 × 108 pfu of AdTGF-β1 in 30 μl of PBS through intranasal delivery. This dose was empirically derived based on preliminary experiments and prior reports. Littermate control triple transgenic mice received PBS alone. Mice were killed and analyzed 10–21 days after AdTGF-β1 instillation, as indicated.
Immunohistochemistry.
Cryosections (5–7 μm) of tissue and cultured cells were stained by immunofluorescence as described (17, 25). Stained sections were visualized on a Nikon fluorescent microscope, and images were captured with a SPOT 2.3.1 camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed by using SPOT 4.0.9 software (Diagnostic Instruments).
Immunoblot.
Tissue and cells were lysed in RIPA buffer (150 mM NaCl/50 mM Tris, pH 8.0/1% Triton X-100/0.5% sodium deoxycholate/0.1% SDS, supplemented with protease and phosphatase inhibitors) and analyzed by immunoblot as described (25).
TUNEL Assay.
Assessment of AEC apoptosis was performed by TUNEL assay (Roche, Basel, Switzerland) according to package insert. Percentages of TUNEL-positive cells were quantified from five low-power fields per sample.
Supplementary Material
Acknowledgments
We thank Jack Gauldie and Jeffrey Whitsett (Cincinatti Children’s Hospital Medical Center, Cincinatti, OH) and Paul Weinreb (Biogen Idec, Cambridge, MA) for contributing valuable reagents for these studies. This work was supported in part by National Institutes of Health Grants GM-075419 (to K.K.K.), HL-04055 (to P.J.W.), HL-53949 (to D.S.), and HL-44712 (to H.A.C.).
Abbreviations:
- IPF
idiopathic pulmonary fibrosis
- AEC
alveolar epithelial cell
- EMT
epithelial-to-mesenchymal transition
- pro-SPC
pro-surfactant protein-C
- Fn
fibronectin
- Mg
matrigel.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.American Thoratic Society & European Respiratory Society. Am. J. Respir. Crit. Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R., Neilson E. G. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips R. J., Burdick M. D., Hong K., Lutz M. A., Murray L. A., Xue Y. Y., Belperio J. A., Keane M. P., Strieter R. M. J. Clin. Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore B. B., Kolodsick J. E., Thannickal V. J., Cooke K., Moore T. A., Hogaboam C., Wilke C. A., Toews G. B. Am. J. Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisberg M., Hanai J., Sugimoto H., Mammoto T., Charytan D., Strutz F., Kalluri R. Nat. Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 6.Thannickal V. J., Toews G. B., White E. S., Lynch J. P., III, Martinez F. J. Annu. Rev. Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 7.Kasai H., Allen J. T., Mason R. M., Kamimura T., Zhang Z. Respir. Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis B. C., Liebler J. M., Luby-Phelps K., Nicholson A. G., Crandall E. D., du Bois R. M., Borok Z. Am. J. Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao H. W., Xie Q. M., Chen J. Q., Deng Y. M., Tang H. F. Life Sci. 2004;76:29–37. doi: 10.1016/j.lfs.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Mucenski M. L., Wert S. E., Nation J. M., Loudy D. E., Huelsken J., Birchmeier W., Morrisey E. E., Whitsett J. A. J. Biol. Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- 11.Tichelaar J. W., Lu W., Whitsett J. A. J. Biol. Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 12.Soriano P. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 13.Lewandoski M. Nat. Rev. Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- 14.Rice W. R., Conkright J. J., Na C. L., Ikegami M., Shannon J. M., Weaver T. E. Am. J. Physiol. 2002;283:L256–L264. doi: 10.1152/ajplung.00302.2001. [DOI] [PubMed] [Google Scholar]
- 15.Bonniaud P., Kolb M., Galt T., Robertson J., Robbins C., Stampfli M., Lavery C., Margetts P. J., Roberts A. B., Gauldie J. J. Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 16.Radisky D. C., Levy D. D., Littlepage L. E., Liu H., Nelson C. M., Fata J. E., Leake D., Godden E. L., Albertson D. G., Nieto M. A., et al. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munger J. S., Huang X., Kawakatsu H., Griffiths M. J., Dalton S. L., Wu J., Pittet J. F., Kaminski N., Garat C., Matthay M. A., et al. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 18.Breuss J. M., Gallo J., DeLisser H. M., Klimanskaya I. V., Folkesson H. G., Pittet J. F., Nishimura S. L., Aldape K., Landers D. V., Carpenter W., et al. J. Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 19.Adamson I. Y., Bowden D. H. Lab. Invest. 1974;30:35–42. [PubMed] [Google Scholar]
- 20.Corti M., Brody A. R., Harrison J. H. Am. J. Respir. Cell Mol. Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 21.Solovyan V. T., Keski-Oja J. J. Cell Physiol. 2006;207:445–453. doi: 10.1002/jcp.20607. [DOI] [PubMed] [Google Scholar]
- 22.Limper A. H., Roman J. Chest. 1992;101:1663–1673. doi: 10.1378/chest.101.6.1663. [DOI] [PubMed] [Google Scholar]
- 23.Snyder E. Y., Deitcher D. L., Walsh C., Arnold-Aldea S., Hartwieg E. A., Cepko C. L. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 24.Kim C. F., Jackson E. L., Woolfenden A. E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R. T., Jacks T. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F., Tom C. C., Kugler M. C., Ching T. T., Kreidberg J. A., Wei Y., Chapman H. A. J. Cell Biol. 2003;163:177–188. doi: 10.1083/jcb.200304065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.