Abstract
All phases of lipopolysaccharide (LPS)-induced fever are mediated by prostaglandin (PG) E2. It is known that the second febrile phase (which starts at ~1.5 h post-LPS) and subsequent phases are mediated by PGE2 that originated in endotheliocytes and perivascular cells of the brain. However, the location and phenotypes of the cells that produce PGE2 triggering the first febrile phase (which starts at ~0.5 h) remain unknown. By studying PGE2 synthesis at the enzymatic level, we found that it was activated in the lung and liver, but not in the brain, at the onset of the first phase of LPS fever in rats. This activation involved phosphorylation of cytosolic phospholipase A2 (cPLA2) and transcriptional up-regulation of cyclooxygenase (COX)-2. The number of cells displaying COX-2 immunoreactivity surged in the lung and liver (but not in the brain) at the onset of fever, and the majority of these cells were identified as macrophages. When PGE2 synthesis in the periphery was activated, the concentration of PGE2 increased both in the venous blood (which collects PGE2 from tissues) and arterial blood (which delivers PGE2 to the brain). Most importantly, neutralization of circulating PGE2 with an anti-PGE2 antibody both delayed and attenuated LPS fever. It is concluded that fever is initiated by circulating PGE2 synthesized by macrophages of the LPS-processing organs (lung and liver) via phosphorylation of cPLA2 and transcriptional up-regulation of COX-2. Whether PGE2 produced at the level of the blood–brain barrier also contributes to the development of the first phase remains to be clarified.
The authors show that peripherally-produced COX2 plays an important role in the earliest stages of fever.
Introduction
Fever is an ancient host-defense response and a common symptom of infection and systemic inflammation. Since Milton and Wendlandt [1] discovered the pyrogenic activity of prostaglandins (PGs) of the E series, and Vane [2] found that nonsteroidal anti-inflammatory drugs block fever by inhibiting PG synthesis, it has been accepted that fever is mediated by PGs, specifically PGE2 [3–6]. PGE2 synthesis occurs in three steps: (1) membrane phospholipids are converted to arachidonic acid by phospholipase A2 (PLA2); (2) arachidonic acid is converted to PGH2 by cyclooxygenase (COX); and (3) PGH2 is isomerized to PGE2 by a terminal PGE synthase (PGES) [6,7]. It has been shown in rats [8–14] and mice [15,16] that COX-2 and microsomal PGES-1 (mPGES-1) are transcriptionally up-regulated in endothelial and perivascular cells of brain microvessels between 1.5 and 12 h after administration of pyrogenic doses of bacterial lipopolysaccharide (LPS). Furthermore, Scammell et al. [17] have shown that microinjection of the COX inhibitor ketorolac into the preoptic region attenuates the febrile response over 1.5–6 h after intravenous (i.v.) injection of LPS in rats. These results indicate that febrigenic PGE2 is produced centrally.
It should be considered, however, that the initiation of fever precedes by approximately 1 h the earliest time point at which PGE2-synthesizing enzymes have been shown to be up-regulated in the brain. In a thermoneutral environment, i.v. LPS typically causes in rats and mice a polyphasic fever, and the first phase of this response starts at approximately 0.5 h post-LPS [18,19]. Because the first phase is sensitive to ambient temperature and can be readily masked by the stress hyperthermia associated with animal handling and LPS injection [19,20], this phase often escapes detection and remains the least studied component of the febrile response. The first phase of LPS fever was not investigated in any of the abovementioned studies of the source of febrigenic PGE2. We [21–23] and others [24–26] have hypothesized that, unlike the second and subsequent febrile phases, the first phase of fever is triggered by peripherally produced PGE2. Over the last two decades, several studies have attempted to test this hypothesis, but the results obtained have been inconclusive, contradictory, or incomplete (for details, see Results and Discussion). In particular, the location (inside or outside the brain) and phenotypes of the cells involved in the initiation of fever are unknown, as are the steps of the PGE2-synthesizing cascade that are initially activated to trigger the fever response. By closing these gaps, the present study identifies the cellular and molecular bases of the initiation of fever.
Results/Discussion
The question as to whether peripherally (i.v. or intra-arterially) administered PGE2 causes fever remains controversial. Although there are reports of peripherally injected PGE1 and PGE2 being pyrogenic in several species of laboratory animals [24,27], there are at least as many documented failures to induce fever by peripheral administration of PGE [24,28,29]. The latter, negative results can be explained, at least partially, as due to self-aggregation of PGE in aqueous solutions and the subsequent loss of biological activity. Indeed, PGE2 was found to be highly pyrogenic in rabbits when infused in an albumin-bound (monomeric), but not in a free (aggregated) form [21]. Albumin is the principal carrier of PGE2 in the circulation, and up to 99% of circulating PGE2 is albumin-bound [30].
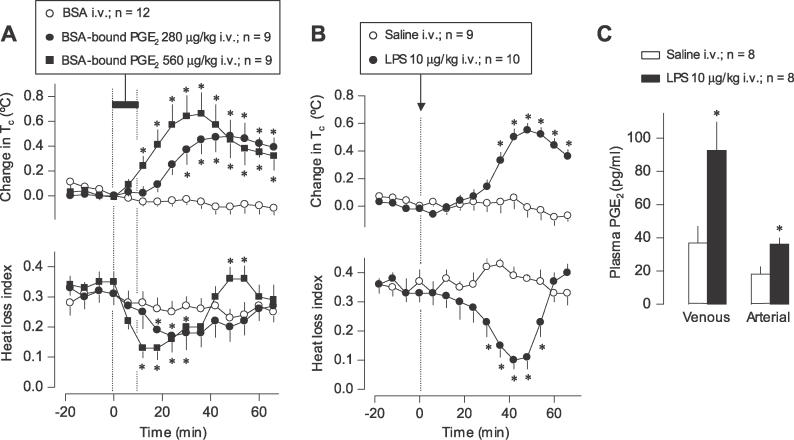
In the present study, a 2:1 (molar ratio) PGE2–albumin complex was prepared by adding PGE2 (all reagents are from Sigma-Aldrich, St. Louis, Missouri, United States, unless specified otherwise) and bovine serum albumin (BSA) to pyrogen-free saline, and then sonicating this mixture for 3 min and incubating it at 37 °C for 1 h. In a thermoneutral environment, the rats were infused i.v. with BSA-bound PGE2 (280 or 560 μg/kg, 100 μl/kg/min, 10 min). Based on the assumptions that PGE2 is evenly distributed in the extracellular compartment (20% of the body mass) and that its half-life is 1 min [31], it can be estimated that the protocol used elevates the plasma concentration of PGE2 by 350 pg/ml (low dose) or 700 pg/ml (high dose) at 12 min after the beginning of infusion. These concentrations are within the physiological range [24,32]. Whereas BSA had no thermoregulatory effect, the PGE2–BSA complex caused a dose-dependent rise in deep body (colonic) temperature (Tc; Figure 1A). This fever response was brought about, at least in part, by tail skin vasoconstriction, as evident from a decrease in the heat loss index (the quotient of two temperature gradients: skin-ambient/colonic-ambient [33]). Hence, when administered in its most relevant form (albumin complex) and at physiologically relevant doses, peripheral PGE2 is pyrogenic in rats.
Figure 1. Circulating PGE2 Initiates LPS Fever in Rats: Circumstantial Evidence.
(A) The effects of i.v. infusion (horizontal bar) of BSA-bound PGE2 or BSA on Tc and heat loss index of rats kept at a neutral ambient temperature (30 °C).
(B) The effects of i.v. bolus injection (arrow) of LPS or saline on the same parameters.
Change in Tc was calculated by subtracting the Tc value at a given point from that at the start of infusion or injection (time zero). In (A), the absolute Tcs at time zero were 38.3 ± 0.1 °C, 38.5 ± 0.1 °C, and 38.4 ± 0.2 °C for the groups treated with BSA and with the lower and higher doses of BSA-bound PGE2, respectively. In (B), initial Tcs were 38.2 ± 0.1 °C and 38.3 ± 0.1 °C for the groups treated with saline and LPS, respectively. The heat loss index was calculated as a quotient of two temperature gradients: skin-ambient and colonic-ambient; this index varies between 0 (maximal vasoconstriction) and 1 (maximal vasodilation) [33].
(C) The levels of PGE2 in the venous and arterial blood of rats 40 min after i.v. injection of LPS or saline at thermoneutrality. This time point corresponds to the maximal thermoeffector activity (minimal heat loss index) to produce the first phase of LPS fever as shown in (B). All doses are indicated. Means ± SE are presented. The number of rats in each group (n) is indicated. An asterisk (*) indicates a significant difference from the BSA- or saline-treated group (p < 0.05; two-way analysis of variance for repeated measures followed by the Tukey test in [A] and [B]; Student t-test in [C]).
How circulating, albumin-bound PGE2 causes fever remains speculative. Activation of vagal afferents by PGE2 has been proposed [34], but the fact that vagotomy does not affect the first febrile phase (for discussion, see [35,36]) makes this mechanism unlikely. An alternative scenario seems more plausible. Binding to albumin prevents the rapid enzymatic inactivation of PGE2 [37,38], thus allowing it to reach a distant site. A good candidate for such a site is the preoptic hypothalamus, which is highly sensitive to the pyrogenic effect of PGE2 [39]. Once dissociated from albumin at the target site, PGE2 may be carried into the brain tissue by transporters expressed at the blood–brain barrier (BBB) [6,26,40]. It should be noted, however, that this scenario is speculative and needs to be tested experimentally.
Having shown that peripheral PGE2 is pyrogenic in rats, we asked whether blood levels of PGE2 are elevated at the onset of the first phase of LPS fever. Fever was induced by administering 0111:B4 Escherichia coli LPS (10 μg/kg) nonstressfully via the extension of a preimplanted venous (jugular) catheter to rats kept in a thermoneutral environment (see Materials and Methods for details). The first febrile phase started at approximately 30-min post-LPS and was brought about, at least partially, by tail skin vasoconstriction (Figure 1B). At 40 min (the time corresponding both to the maximal rate of rise in body temperature and to the maximal thermoeffector activity that underlies this rise), samples of venous and arterial blood were collected from LPS-treated (febrile) and saline-treated (afebrile) rats, and the concentration of PGE2 in the venous and arterial blood was measured by enzyme immunoassay. The venous blood gathers PGE2 synthesized in the tissues, and the arterial blood delivers it to the brain, the presumptive site of the febrigenic action of circulating PGE2 [39]. Consistent with the marked catabolism of PGE2 in the lungs [41], the level of PGE2 was lower in the arterial than in the venous blood plasma in both afebrile and febrile rats (Figure 1C). However, both the venous and arterial concentrations of PGE2 were substantially (~2.5 times) higher in the febrile rats as compared to the afebrile controls. These data show that the level of circulating PGE2, most importantly in the arterial blood, is increased at the onset of the first febrile phase.
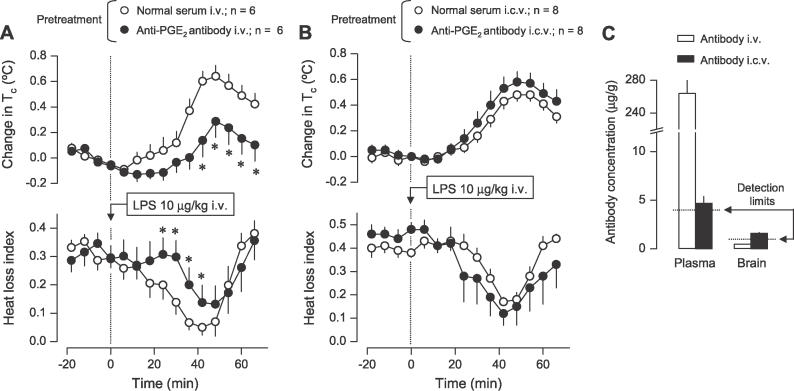
Several studies aimed at determining the source of febrigenic PGE2 have compared the antipyretic effects of nonsteroidal anti-inflammatory drugs administered peripherally (i.v. or intraperitoneally) and centrally (intracerebroventricularly [i.c.v.]). The drugs used included indomethacin [25], nimesulide [32], and keterolac (present study; unpublished data). All these studies faced multiple methodological problems, including acute thermoregulatory effects of the drug administered i.c.v. (present study), the ability of drugs to cross the BBB, and consequently, their tendency to be distributed evenly between the peripheral compartment and the brain [32]. We proposed [6] that selective neutralization of circulating PGE2 using an antibody is a better approach to test the hypothesis that peripherally produced PGE2 initiates fever. Being large proteins (160 kDa), antibodies cannot cross the BBB; this eliminates uncertainty common in experiments involving nonsteroidal anti-inflammatory drugs. The antibody used in the present study was raised against a PGE2–BSA complex in rabbits. It displayed a high affinity to PGE2 (association constant of 6.3 × 1010 M−1, as determined by Scatchard plot) and a low cross-reactivity with other prostanoids (<15% for PGF1α and PGB2, and <9% for PGA2, PGF2α, and PGB1). The rats were pretreated i.v. with the anti-PGE2 antibody (neat antiserum; 100 μl/kg/min, 120 min) or with normal rabbit serum, and LPS was injected 18 h later, i.e., at the time when the injected antibody is expected to achieve a steady-state level in the circulation [42]. The results of this experiment are shown in Figure 2A–2C. The antibody (but not normal serum) suppressed the first phase of LPS fever: both the rise in Tc and the associated decrease in the heat loss index were delayed and significantly attenuated (Figure 2A). Immediately after the temperature response was recorded, a sample of venous blood and the whole brain (cleared of blood) were collected for immunoenzymatic determination of the anti-PGE2 antibody. The antibody was found at a high concentration in the blood plasma, but was below the detection limit in the brain tissue (Figure 2C). To rule out the possibility that a minute, undetectable amount of antibody in the brain might have accounted for the suppression of fever, we administered a low dose (2.7 μl/min, 15 min) of the anti-PGE2 antibody or normal serum i.c.v., and injected the rats with LPS 18 h later. The rats injected with the anti-PGE2 antibody i.c.v. had a detectable level of the antibody in the brain (Figure 2C), but their febrile response to LPS was unaffected (Figure 2B). A large fraction of the antibody given i.c.v. leaked into the blood, presumably reflecting the asymmetric nature of the BBB (its major role is to limit transport in the blood-to-brain direction, but not in the opposite direction) or possibly because the BBB was breached in this experimental group by the implanted i.c.v. cannula. Importantly, however, the plasma antibody concentration in the rats treated with the i.c.v. antibody was approximately 60 times lower than that in the rats treated with the i.v. antibody (Figure 2C). It is concluded that minute amounts of the anti-PGE2 antibody in the brain (even when detectable) are not sufficient to suppress the initiation of fever, and that the cause of the delayed and attenuated first febrile phase observed in the rats pretreated with i.v. antibody was neutralization of PGE2 outside the BBB.
Figure 2. Circulating PGE2 Initiates LPS Fever in Rats: Direct Evidence.
(A) The effects of i.v. infusion (100 μl/kg/min, 120 min) of the anti-PGE2 antibody or normal serum 18 h before the experiment (pretreatment) on the Tc and heat loss index responses of rats injected (arrow) with LPS at thermoneutrality (30 °C).
(B) The effects of the i.c.v. infusion (2.7 μl/min, 15 min) of the same anti-PGE2 antibody or normal serum 18 h before the experiment (pretreatment) on the same responses. Note that the i.c.v. infusion was aimed at testing whether minute amounts of the antibody in the brain are sufficient to suppress LPS fever (and not at testing whether fever is altered by neutralization of PGE2 in the brain).
Change in Tc was calculated by subtracting the Tc value at a given point from that at the time of injection (time zero). In (A), the absolute Tcs at time zero were 38.2 ± 0.1 °C and 38.1 ± 0.2 °C for the groups treated with i.v. normal serum and antibody, respectively. In (B), the initial Tcs were 38.4 ± 0.1 °C and 38.2 ± 0.2 °C for the groups treated with i.c.v. normal serum and antibody, respectively.
(C) The levels of anti-PGE2 antibody in the blood plasma and whole brain of rats pretreated with i.v. or i.c.v. antibody. Blood samples and brains were collected immediately after the temperature responses were recorded, i.e., approximately 20 h after pretreatment with the antibody. Antibody levels (means ± SE) are expressed as microgram of neat antibody per gram of either plasma or brain tissue. The detection limit for each assay and the number of rats in each group (n) are indicated. An asterisk (*) indicates a significant difference from the group pretreated with normal serum (p < 0.05; two-way analysis of variance for repeated measures followed by the Tukey test).
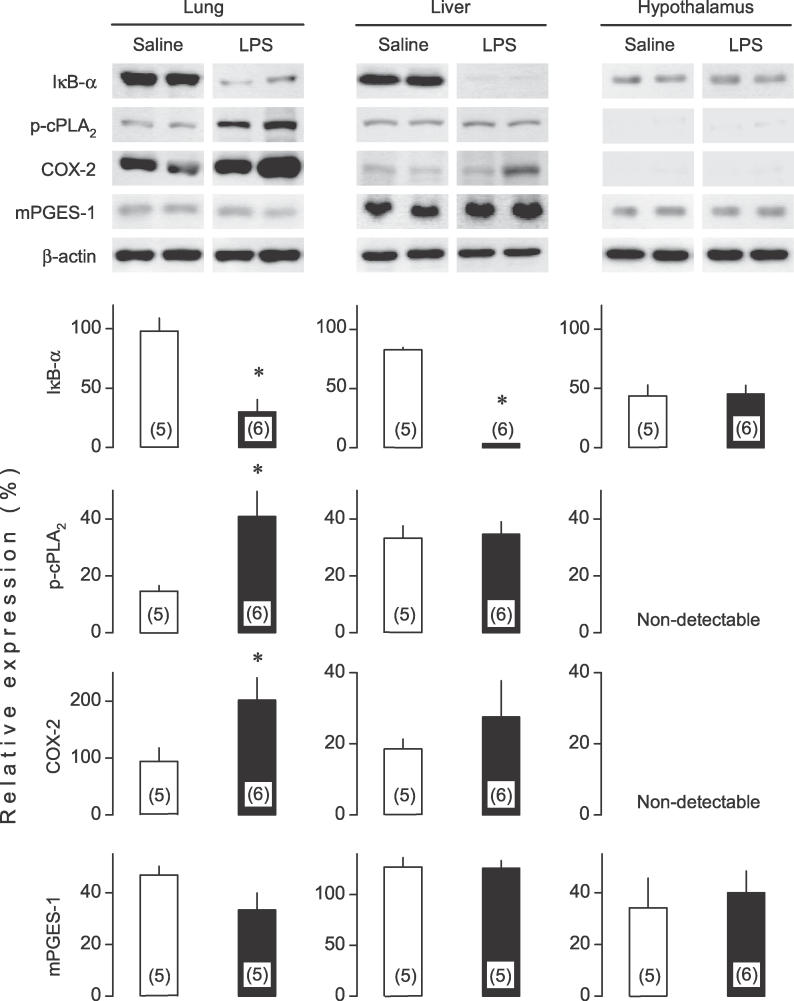
Having demonstrated that circulating PGE2 is indeed responsible, at least partially, for triggering LPS-induced fever, we investigated which step of the PGE2 biosynthetic pathway is activated at the onset of the febrile response. Previously, we reported that the onset of the first phase of LPS fever is associated with large increases of COX-2 and mPGES-1 mRNAs in the lung and liver and with a moderate increase of COX-2 (but not mPGES-1) mRNA in the hypothalamus [22]. However, it remained to be determined whether the observed transcriptional changes translate into changes in the corresponding protein contents at such an early time point (40 min) after LPS administration. We had also shown [22] that neither cytosolic PLA2-α (cPLA2-α) nor either of the two secretory PLA2 studied (II and V) is transcriptionally up-regulated at the onset of fever. This finding, however, does not exclude the possibility that cPLA2 is activated posttranscriptionally by phosphorylation, the principal mechanism of activation for this enzyme [43]. In the present study, we determined the contents of phosphorylated cPLA2 (p-cPLA2), COX-2, and mPGES-1 proteins by Western blot in the lung, liver, and hypothalamus at 40 min after injection of LPS or saline, a time that corresponds to the onset of the first febrile phase in LPS-treated rats (Figure 1B). COX-2–positive cells were also studied in all three tissues by immunohistochemistry using two different protocols of sample preparation (see Material and Methods). None of the enzymes studied was increased at the protein level in the hypothalamus of the LPS-treated rats as compared to the saline-treated controls (Figure 3). Neither did the immunohistochemical analysis reveal any increase in the number of COX-2–positive hypothalamic cells at the onset of fever, although the same antibody readily detected a surge in the number of COX-2–positive endotheliocytes in the hypothalamic microvasculature at later stages of LPS fever, in both the present study (positive controls; unpublished data) and previous studies [11,12]. In the lung, LPS increased the contents of p-cPLA2 and COX-2 (Figure 3), and augmented the number of cells containing COX-2 (Figure 4), but did not alter the protein level of constitutively expressed mPGES-1 (Figure 3). In the liver, the immunohistochemical analysis (which is more sensitive) revealed a surge in the number of COX-2–positive cells at the onset of fever (Figure 4), whereas the Western blot analysis (less sensitive) found a tendency for an increase in the overall content of COX-2 and no changes in the content of either p-cPLA2 or mPGES-1 (Figure 3). We also found that inflammatory signaling (assessed by a decrease in the content of the nuclear factor-κB inhibitor, IκB-α [44]) was activated in the lung and liver, but not in the hypothalamus, at the onset of LPS fever (Figure 3).
Figure 3. Mechanism of Activation of PGE2 Synthesis at the Onset of LPS Fever in Rats.
Tissue contents of the following proteins are shown: IκB-α (an inhibitor of nuclear factor-κB), three PGE2-synthesizing enzymes (p-cPLA2, COX-2, and mPGES-1), and β-actin (a “housekeeping” protein). These proteins were determined by Western blot in the lung, liver, and hypothalamus. The tissue samples were collected 40 min after i.v. injection of LPS (10 μg/kg) or saline at thermoneutrality. This time point corresponds to the maximal thermoeffector activity to produce the first phase of LPS fever (see Figure 1B). Electrophoretograms of two representative animals from each group are shown on top. The expression of each protein of interest (relative to the expression of β-actin) is shown on bottom (means ± SE); the number of rats (n) is shown in parenthesis. An asterisk (*) indicates a significant difference from the saline-treated group (p < 0.05; Student t-test).
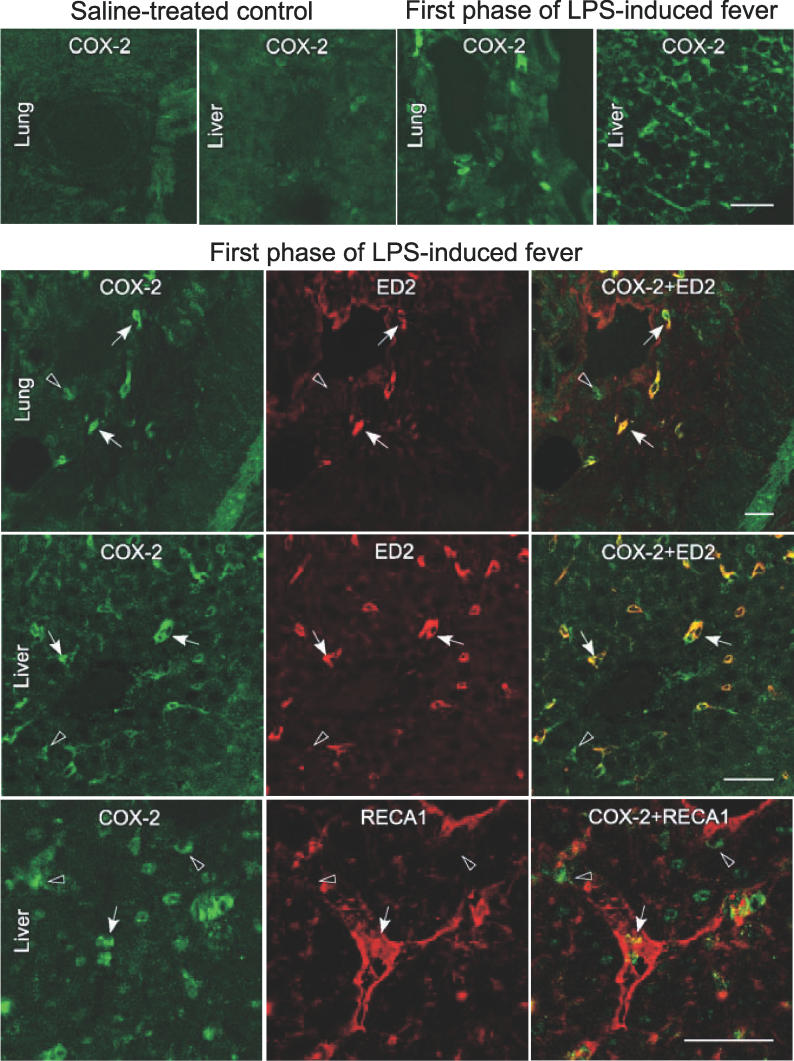
Figure 4. Identification of the Pulmonary and Hepatic Cells Producing PGE2 at the Onset of LPS Fever in Rats.
Immunolocalization of COX-2 in the lung and liver and identification of the cell types expressing this enzyme. Top row: tissue localization of COX-2 (green immunofluorescence) in the lung and liver of rats at 40 min after i.v. injection of saline or at the onset of the first febrile phase (i.e., 40 min after i.v. injection of LPS, 10 μg/kg) at thermoneutrality. Next rows: dual localization of LPS-induced COX-2–immunoreactivity (green; left column) with either the macrophage marker ED2 or the endothelial cell marker RECA1 (red; middle column) in the lung and liver at the onset of the first febrile phase. Doubly labeled cells appear yellow in the merged confocal images (right column). White arrows and black arrowheads mark examples of doubly and singly (COX-2 only) labeled cells, respectively. Scale bars represent 40 μm.
These results show that the onset of the first febrile phase is associated with activation of inflammatory signaling and increased PGE2 synthesis in the periphery. The early activation of PGE2 synthesis involves phosphorylation of cPLA2 (lung) and transcriptional up-regulation of COX-2 (lung and liver). Transcriptional up-regulation is the main (although not the only [45,46]) mechanism of activation for this enzyme [6,7]. Hence, the increased circulating level of PGE2 at the onset of the first febrile phase may be explained by the following enzymatic events in the lung and liver: production of arachidonic acid by activated (phosphorylated) cPLA2 → conversion of arachidonic acid to PGH2 by up-regulated COX-2 → isomerization of PGH2 into PGE2 by constitutively expressed mPGES-1. Whereas the physiological importance of cPLA2 and mPGES-1 in the first febrile phase remains to be confirmed in studies with pharmacological or genetic blockade of these enzymes, the indispensable role of COX-2 (and the uninvolvement of COX-1) in the first phase of LPS fever have been demonstrated in our recent study in knockout mice [47].
Preferential location of the synthesis of febrigenic PGE2 in the liver and lungs (but not in the brain) deserves special discussion. The fact that the i.v. antibody attenuated the first febrile phase but did not abolish it completely (Figure 2A) may be due to incomplete neutralization of circulating PGE2. However, it may also reflect a contribution of centrally produced PGE2 (e.g., by a small number of hypothalamic cells that express COX-2 constitutively) to the development of the first phase of LPS fever. Although we cannot rule out such a contribution, it is noteworthy that multiple methods used in our present and previous [22] studies (Table 1) found a profound activation of PGE2 synthesis in the periphery, but hardly any signs (none at the protein level) of activation of hypothalamic PGE2 synthesis.
Table 1.
Inflammatory Signaling and PGE2 Synthesis Are Selectively Activated at the Onset of the First Phase of LPS Fever in the Periphery (Lung and Liver) but Not in the Brain (Hypothalamus)

To identify the pulmonary and hepatic producers of PGE2, we first determined how the cells that become COX-2 positive at the onset of LPS fever relate to the histological elements revealed by eosin staining; this analysis was performed in freshly frozen samples. In the lung, COX-2–positive cells were found to cluster around alveoli, often forming what looked like cell chains (unpublished data). In the liver, the parenchyma did not stain for COX-2, and the vast majority of COX-2–positive cells were located in the stromal compartment, often in close proximity to sinusoids. Some COX-2–positive cells were also found around the central vein (a small vein that gathers the blood from sinusoids) and in the visceral peritoneum covering the liver (unpublished data). We then double-stained lung and liver for COX-2 and either the macrophage marker ED2 [48] or the endothelial marker RECA1 [49]; this analysis was performed in paraformaldehyde-fixed samples (Figure 4). In the lung, 89 ± 6% (mean ± standard error [SE] of five samples) of COX-2–positive cells were macrophages (ED2 positive), and 11% were unidentified (ED2 and RECA1 negative). In the liver, 83 ± 2% of the COX-2–positive cells were macrophages (ED2 positive), 9 ± 1% were endotheliocytes (RECA1 positive), and 8% remained unidentified. The key role of macrophages in the initiation of fever agrees with our recent finding that the first febrile phase depends entirely on the recognition of LPS (via the Toll-like receptor-4) by bone marrow-derived cells [50].
In summary, the present study shows that the first phase of LPS fever is initiated (at least partially) by PGE2 that originated in peripheral tissues. Activation of PGE2 synthesis at the onset of the first phase of LPS fever involves phosphorylation of cPLA2, transcriptional up-regulation of COX-2, and possibly other mechanisms. The vast majority of the PGE2-producing cells are macrophages. These findings challenge the predominant view that fever is initiated exclusively by inflammatory mediators produced at the level of the BBB. These findings, however, do not contradict the principal role of the centrally produced PGE2 in the second and subsequent febrile phases.
Materials and Methods
Animals.
The study was conducted in male Long-Evans rats weighing 300–400 g (Charles River, Wilmington, Massachusetts, United States). The rats were habituated (seven daily training sessions, 4 h each) to spending time in artificial “rat holes,” cylindrical confiners made of stainless steel wire [20,22,23]. The same confiners were used later in the experiments. Each rat was used in only one experiment. The protocols were approved by the St. Joseph's Hospital Animal Care and Use Committee.
Surgery and instrumentation.
Under ketamine-xylazine-acepromazine anesthesia (55.6, 5.5, and 1.1 mg/kg, respectively, intraperitoneally) and antibiotic (enrofloxacin, 1.1 mg/kg, subcutaneously) protection, each rat was subjected to chronic catheterization of the jugular vein as described elsewhere [22]. The catheters were flushed with heparinized (10 U/ml) saline on Days 1 and 3 postsurgery. The experiments were performed on Day 5. On the day of the experiment, each rat was placed in a wire confiner and equipped with two copper-constantan thermocouples: one for recording Tc and the other for recording tail skin temperature. The colonic thermocouple was inserted 10 cm beyond the anal sphincter and fixed to the base of the tail with adhesive tape. The skin thermocouple was positioned at the boundary of the proximal and middle thirds of the tail, on its lateral surface, and was insulated from the environment with tape. The thermocouples were plugged in to a data logger (Cole-Parmer, Vernon Hills, Illinois, United States), which was connected to a personal computer. The rats in their confiners were placed in a climatic chamber (Forma Scientific, Marietta, Ohio, United States) set to a neutral ambient temperature of 30.0 °C. Their jugular catheters were extended with lengths of PE-50 tubing filled with saline, thus permitting i.v. drug administration to be performed in a stress-free fashion, from outside the chamber.
Tissue harvesting.
Immediately before collection of blood and tissue specimens, the rats were anesthetized with ketamine-xylazine-acepromazine (5.56, 0.55, and 0.11 mg/kg, respectively, i.v.). Blood samples were collected either from the inferior vena cava (venous blood) or left ventricle (arterial blood). Each sample was transferred to an eppendorf tube containing EDTA and indomethacin (final concentrations: 1 mg/ml and 10 μM, respectively). The collected blood was immediately centrifuged (3,000 g, 10 min, 4 °C), and the resulting plasma was stored at −80 °C.
For collection of tissue samples for the immunoassay and Western blot protocols, each rat was perfused through the left ventricle (right atrium cut) with 100 ml of 10 mM phosphate-buffered saline (PBS; pH 7.4). The right medial lobe of the liver, the medial lobe of the right lung, and either the hypothalamus (for Western blot) or the entire brain were collected rapidly and snap frozen in liquid nitrogen. The samples were stored at −80 °C.
Tissue specimens for the immunohistochemistry protocols were collected using two methods. For immunohistochemical analysis of freshly frozen (non-fixed) tissues, a rat was perfused with PBS (50 ml). The liver, right lung, and brain were excised, frozen in dry ice powder, and stored at −80 °C. The specimens were cryosectioned (section thickness, 14 μm) immediately before the analysis. For immunohistochemical analysis of fixed tissues, a rat was perfused with 50 ml of heparinized (10 U/ml) PBS followed by 200 ml of a 4% paraformaldehyde solution in PBS. The liver, right lung, and brain were excised, postfixed in the paraformaldehyde solution for 2 h, transferred to PBS containing 20% sucrose, and kept in this solution at 4 °C for 24 h. The specimens were then frozen, cryosectioned (section thickness, 30 μm), and immediately subjected to the immunohistochemical analysis.
Immunoassays.
PGE2 was measured in plasma by enzyme immunoassay using a commercially available kit (Cayman, Ann Arbor, Michigan, United States); the samples were prepared according to the manufacturer's instructions. The levels of anti-PGE2 antibody in the plasma and brain were assayed by enzyme immunoassay involving a conjugate of four proteins: goat anti-mouse IgG (which coated all wells of a 96-well plate), mouse anti-rabbit IgG, rabbit anti-PGE2 IgG (sample or standard), and PGE2-horseradish peroxidase complex (Amersham, Piscataway, New Jersey, United States). The conjugate was developed by adding the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine; the reaction product had maximal absorbance at 630 nm. Samples of blood plasma were diluted (1:256) in 100 mM Tris-buffered saline (pH 7.4) before the assay. Each brain was homogenized in 4 ml of a 100 mM NaOH solution containing 0.2% sodium dodecyl sulfate. After the homogenate was cleared by centrifugation (14,000 g, 10 min, 6 °C), the supernatant was neutralized with 100 mM HCl and diluted (1:10) with Tris-buffered saline. The detection limits for blood plasma (4 μg/g) and brain tissue (1 μg/g) were determined as the triple noise-level concentrations.
Western blot.
The Western blot protocol was identical to that used to measure IκB-α and β-actin in our earlier study [51], except that here we also measured p-cPLA2, COX-2, and mPGES-1. The following primary rabbit polyclonal antibodies were used (dilutions indicated): anti-IκB-α (1:1,000; Cell Signaling, Beverly, Massachusetts, United States), anti-β-actin (1:1,000), anti–p-cPLA2 (1:500; Cell Signaling), anti–COX-2 (1:1,000; Cayman), and anti-mPGES-1 (1:1,000; Cayman). The primary antibodies revealed immunoblot bands corresponding to the following molecular masses: 36 kDa for IκB-α; 42 kDa for β-actin; 105 kDa for p-cPLA2; 72 kDa for COX-2; and 16 kDa for mPGES-1.
Immunohistochemistry.
Single immunofluorescence was used to visualize and localize COX-2–positive cells in freshly frozen [11,12] or paraformaldehyde-fixed [52] samples of lung, liver, and hypothalamus. Dual immunofluorescence protocols were used to verify co-localization of COX-2 with ED2 (a macrophage marker) or RECA1 (an endotheliocyte marker) in paraformaldehyde-fixed samples of lung and liver [52]. The fraction of COX-2–immunoreactive cells that were also immunoreactive to ED2 or RECA1 was determined in serial (10–15) sections (30 μm-thick) collected at 150-μm intervals through the right lobe of the liver or right lung. Images of merged confocal channels were collected from 104-μm2 fields (nine for each section).
Acknowledgments
Technical assistance of D. L. Oliveira is appreciated.
Abbreviations
- BBB
blood–brain barrier
- BSA
bovine serum albumin
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- i.c.v.
intracerebroventricular(ly)
- i.v.
intravenous(ly)
- LPS
lipopolysaccharide
- mPGES
microsomal prostaglandin E synthase
- PBS
phosphate-buffered saline
- p-cPLA2
phosphorylated cytosolic phospholipase A2
- PG
prostaglandin
- PLA2
phospholipase A2
- SE
standard error, Tc, colonic temperature
Footnotes
Author contributions. AAS, AII, SK, KM, PES, and AAR conceived and designed the experiments. AAS, AII, JS, HH, ANP, JRR, and JLR performed the experiments. AAS, AAI, JS, HH, ANP, SK, KM, PES, and AAR analyzed the data. AAS and AAR wrote the paper.
Funding. The study was funded by the National Institutes of Health (grants NS41233 to AAR and NS21182 to PES) and Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan (KM). Additional funding was provided by the St. Joseph's Foundation and Sigma-Aldrich Corporation.
Competing interests. The authors have declared that no competing interests exist.
References
- Milton AS, Wendlandt S. A possible role for prostaglandin E1 as a modulator for temperature regulation in the central nervous system of the cat. J Physiol. 1970;207:76P–77P. [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: The febrile response. Trends Neurosci. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, et al. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med. 2002;80:5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Kobayashi S. Signaling the brain in inflammation: The role of endothelial cells. Front Biosci. 2004;9:2819–2826. doi: 10.2741/1439. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: Synthesis and catabolism. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am J Physiol. 1997;272:R1712–R1725. doi: 10.1152/ajpregu.1997.272.6.R1712. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, et al. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381:119–129. doi: 10.1002/(sici)1096-9861(19970505)381:2<119::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, et al. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: Light and electron microscopic immunocytochemical studies. J Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, et al. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue W, Matsumura K, Yamagata K, Takemiya T, Shiraki T, et al. Brain-specific endothelial induction of prostaglandin E2 synthesis enzymes and its temporal relation to fever. Neurosci Res. 2002;44:51–61. doi: 10.1016/s0168-0102(02)00083-4. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Lu J, Elmquist JK, Saper CB. Specific roles of cyclooxygenase-1 and cyclooxygenase-2 in lipopolysaccharide-induced fever and Fos expression in rat brain. J Comp Neurol. 2003;463:3–12. doi: 10.1002/cne.10743. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: Relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- Li S, Wang Y, Matsumura K, Ballou LR, Morham SG, et al. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2−/−, but not in cyclooxygenase-1−/− mice. Brain Res. 1999;825:86–94. doi: 10.1016/s0006-8993(99)01225-1. [DOI] [PubMed] [Google Scholar]
- Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, et al. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci. 2003;6:1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Griffin JD, Elmquist JK, Saper CB. Microinjection of a cyclooxygenase inhibitor into the anteroventral preoptic region attenuates LPS fever. Am J Physiol. 1998;274:R783–R789. doi: 10.1152/ajpregu.1998.274.3.R783. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Kulchitsky VA. “Biphasic” fevers often consist of more than two phases. Am J Physiol. 1998;275:R323–R331. doi: 10.1152/ajpregu.1998.275.1.R323. [DOI] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: Dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1244–R1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N. Methodology of fever research: Why are polyphasic fevers often thought to be biphasic? Am J Physiol. 1998;275:R332–R338. doi: 10.1152/ajpregu.1998.275.1.R332. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Karman EK. Blood-borne, albumin-bound prostaglandin E2 may be involved in fever. Am J Physiol. 1999;276:R1840–R1844. doi: 10.1152/ajpregu.1999.276.6.R1840. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Pero RS, Scheck AC, Romanovsky AA. Prostaglandin E2-synthesizing enzymes in fever: Differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1104–R1117. doi: 10.1152/ajpregu.00347.2002. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Steiner AA, Patel S, Rudaya AY, Romanovsky AA. Albumin is not an irreplaceable carrier for amphipathic mediators of thermoregulatory responses to LPS: compensatory role of α1-acid glycoprotein. Am J Physiol Regul Integr Comp Physiol. 2005;288:R872–R878. doi: 10.1152/ajpregu.00514.2004. [DOI] [PubMed] [Google Scholar]
- Skarnes RC, Brown SK, Hull SS, McCracken JA. Role of prostaglandin E in the biphasic fever response to endotoxin. J Exp Med. 1981;154:1212–1224. doi: 10.1084/jem.154.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto A, Murakami N, Nakamori T, Watanabe T. Evidence for separate mechanisms of induction of biphasic fever inside and outside the blood-brain barrier in rabbits. J Physiol. 1987;383:629–637. doi: 10.1113/jphysiol.1987.sp016433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J, Abul HT, Milton AS, Rotondo D. Cytokines and cytokine inducers stimulate prostaglandin E2 entry into the brain. Pflugers Arch. 2001;442:526–533. doi: 10.1007/s004240100572. [DOI] [PubMed] [Google Scholar]
- Eguchi N, Hayashi H, Urade Y, Ito S, Hayaishi O. Central action of prostaglandin E2 and its methyl ester in the induction of hyperthermia after their systemic administration in urethane-anesthetized rats. J Pharmacol Exp Ther. 1988;247:671–679. [PubMed] [Google Scholar]
- Milton AS, Wendlandt S. Effects on body temperature of prostaglandins of the A, E and F series on injection into the third ventricle of unanaesthetized cats and rabbits. J Physiol. 1971;218:325–336. doi: 10.1113/jphysiol.1971.sp009620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT. Effects of intravenous and intraventricular prostaglandin E1 on thermoregulatory responses in rabbits. J Pharmacol Exp Ther. 1978;204:39–45. [PubMed] [Google Scholar]
- Unger WG. Binding of prostaglandin to human serum albumin. J Pharm Pharmacol. 1972;24:470–477. doi: 10.1111/j.2042-7158.1972.tb09034.x. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Neufang B. Elimination from the circulation of cats of 6-keto-prostaglandin E1 compared with prostaglandins E2 and I2 . J Pharm Pharmacol. 1983;35:724–728. doi: 10.1111/j.2042-7158.1983.tb02878.x. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Li S, Llanos QJ, Blatteis CM. Differential inhibition by nimesulide of the early and late phases of intravenous- and intracerebroventricular-LPS-induced fever in guinea pigs. Neuroimmunomodulation. 2001;9:263–275. doi: 10.1159/000054289. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: Ambient temperature for experiments in rats: A new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1β: Role of endogenous prostaglandins. J Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan MD, Patel S, Rudaya AY, Steiner AA, Szekely M, et al. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol. 2004;143:1023–1032. doi: 10.1038/sj.bjp.0705977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA. Signaling the brain in the early sickness syndrome: Are sensory nerves involved? Front Biosci. 2004;9:494–504. doi: 10.2741/1247. [DOI] [PubMed] [Google Scholar]
- Heirwegh KP. Effects of binding of amphipathic compounds to carrier proteins on their enzymic biotransformation by membrane-bound enzymes. Biochem Soc Trans. 1984;12:11–13. doi: 10.1042/bst0120011. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr . All about albumin: Biochemistry, genetics, and medical applications. San Diego (California): Academic Press; 1996. 432. p. [Google Scholar]
- Scammell TE, Elmquist JK, Griffin JD, Saper CB. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J Neurosci. 1996;16:6246–6254. doi: 10.1523/JNEUROSCI.16-19-06246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis B, Isse T, Snipes JA, Chen L, Yamashita H, et al. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol. 2006;100:1392–1399. doi: 10.1152/japplphysiol.01259.2005. [DOI] [PubMed] [Google Scholar]
- Piper PJ, Vane JR, Wyllie JH. Inactivation of prostaglandins by the lungs. Nature. 1970;225:600–604. doi: 10.1038/225600a0. [DOI] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, et al. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kB and IkB proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Balabanova L, Leffler CW. Posttranslational regulation of cyclooxygenase by tyrosine phosphorylation in cerebral endothelial cells. Am J Physiol. 1998;274:C72–C81. doi: 10.1152/ajpcell.1998.274.1.C72. [DOI] [PubMed] [Google Scholar]
- Baber SR, Champion HC, Bivalacqua TJ, Hyman AL, Kadowitz PJ. Role of cyclooxygenase-2 in the generation of vasoactive prostanoids in the rat pulmonary and systemic vascular beds. Circulation. 2003;108:896–901. doi: 10.1161/01.CIR.0000084536.87322.BB. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Rudaya AY, Robbins JR, Dragic AS, Langenbach R, et al. Expanding the febrigenic role of cyclooxygenase-2 to the previously overlooked responses. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1253–R1257. doi: 10.1152/ajpregu.00371.2005. [DOI] [PubMed] [Google Scholar]
- Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: Distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, et al. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992;66:459–466. [PubMed] [Google Scholar]
- Steiner AA, Chakravarty S, Rudaya AY, Herkenham M, Romanovsky AA. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood. 2006;107:4000–4002. doi: 10.1182/blood-2005-11-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Dogan MD, Ivanov AI, Patel S, Rudaya AY, et al. A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. FASEB J. 2004;18:1949–1951. doi: 10.1096/fj.04-2295fje. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]