Abstract
Vasoactive intestinal peptide (VIP) released from some neurons and T cells affects T cell migration, cytokine generation, and other functions by binding to constitutively expressed type 1 G protein-coupled receptor (VPAC1) or activation-induced type 2 G protein-coupled receptor (VPAC2). Recently, a short-deletion (SD) splice variant of mouse VPAC2 that lacks 14 amino acids at the end of the last transmembrane domain has been identified in T cells and shown to resemble wild-type (WT) VPAC2 in affinity of VIP binding but to differ by lack of signaling of T cell adenylyl cyclase, migration, and IL-2 secretion. As Th2 cells are the principal source of immune VIP and have the greatest functional responses to VIP, the differences in signals transduced by WT and SD VPAC2 were studied in VPAC2–low D10G4.1 model Th2 cell transfectants individually expressing the respective types of VPAC2 equally. WT and SD VPAC2 Th2 cell transfectants secreted equal amounts of VIP. WT VPAC2 transfectants generated more IL-4 than did SD VPAC2 transfectants, and this increment was dependent on endogenous VIP. Exogenous VIP further increased IL-4 production by WT VPAC2 transfectants but decreased IL-4 production by SD VPAC2 transfectants. Cotransfection of the two constructs diminished VIP enhancement of IL-4 production seen with WT VPAC2 alone by preventing increases in nuclear levels of the requisite transcription factors c-Maf and Jun B. Thus the ratio of two forms of T cell VPAC2 determines the net effect of VIP on IL-4 generation by activated Th2 cells.
Vasoactive intestinal peptide (VIP)3 is generated and secreted by several sets of neurons and by T cells, of which the Th2 subset of CD4 helper-inducer T cells is the most prominent producer (1–3). VIP has broad physiological effects in many organ systems and regulates diverse functions of macrophages, dendritic cells, and B cells as well as T cells in immunity (4, 5). The principal activities of T cells that are mediated or regulated by VIP include differentiation, migration, proliferation, survival, and cytokine generation. Normal CD4 T cells of the Th2 subset not only produce much more VIP than other types of lymphocytes, but also are more responsive to effects of VIP, which results in deviation toward Th2-driven inflammatory and allergic pathways (3, 6, 7). Of the two types of native G protein-coupled receptors for VIP, type 1 (VPAC1) greatly predominates on unstimulated T cells, but its expression declines after T cell activation, and the level of type 2 (VPAC2) increases concurrently (8, 9). Results of studies of immune responses in two lines of C57BL/6 mice with T cell-selective transgenic (TG) overexpression of VPAC2 and genetic deletion or knockout (KO) of VPAC2 have shown highly abnormal immunophenotypes, which are consistent with the preferential effects of VIP on isolated Th2 cells. The VPAC2 TG mouse has increased IgE Ab responses, blood eosinophilia, heightened immediate-hypersensitivity skin testing, and reduced delayed-type hypersensitivity (DTH) (10). The VPAC2 KO mouse has markedly increased DTH and depressed allergic reactions (11). Both abnormal phenotypes are attributable to a ratio of Th2/Th1 cytokines that is different from that of wild-type (WT) mice.
Recently, it has been found that mouse T cells express an alternatively spliced form of VPAC2, as well as WT VPAC2, which has a 14-aa deletion in the seventh transmembrane domain near the cytoplasmic surface (12). This short-deletion (SD) variant of mouse VPAC2, termed VPAC2de367–380, results from a loss of exon 12. For initial studies of differences between the functions of VPAC2de367–380 and WT mouse VPAC2, each was expressed at the same level in a human VPAC2–low subline of Jurkat human T cell transfectants, as assessed by real-time PCR quantification of mRNA and Western blots of extracted VPAC2 membrane protein. Each form of VPAC2 bound VIP with a similar affinity, but only mouse WT VPAC2 transduced VIP mediation of increases in intracellular concentration of cAMP, chemotaxis, and decreases in IL-2 generation in Jurkat T cell transfectants (12).
As the highest levels of production of endogenous VIP and the greatest effects of VIP on immune responses are observed in Th2-type CD4 T cells, WT VPAC2 and SD VPAC2de367–380 were expressed equally in mouse Th2 cells of the VPAC2–low D10G4.1 line for the present investigations of their respective functions. Ab stimulation of the TCR of D10G4.1 cells now is shown to increase both the level of mRNA encoding IL-4 and the concentration of secreted IL-4 much more in WT VPAC2 transfectants than in SD VPAC2de367–380 transfectants. This is explained entirely by differences in transduction of signals from endogenous VIP through WT VPAC2, as contrasted with SD VPAC2, because the level of VIP generation after TCR stimulation is the same for both lines of transfectants. Dependence on endogenous VIP is confirmed by the capacity of an IgG VIPase to suppress the enhancement of levels of mRNA encoding IL-4 in WT VPAC2 transfectants, at concentrations that eliminated > 95% of all endogenous VIP. Further, additions of 10−9 M to 3 × 10−7 M exogenous VIP increase levels of secreted IL-4 in WT VPAC2 transfectants, whereas the opposite effect of exogenous VIP was observed in SD VPAC2 transfectants. Analyses of transcriptional mechanisms showed that SD VPAC2 fails to recruit nuclear c-Maf or Jun B, in contrast with WT VPAC2, and prevents WT VPAC2 from engaging these transcriptional factors.
Materials and Methods
Genetic constructs
The full-length cDNAs encoding mouse WT VPAC2 and the SD variant of mouse VPAC2 (VPAC2de367–380) were introduced into pCMV-Tag4 vector (Stratagene), which adds a FLAG epitope tag at the C terminus. The correctness of each sequence was confirmed (ELIM Biopharmaceuticals) before large-scale production and purification (Qiagen).
Cells, nucleofection procedures, and Abs
A mouse Th2 cell line designated D10G4.1 was obtained from American Type Culture Collection. D10G4.1 cells were cultured in complete RPMI 1640 medium (Cell Culture Facility, University of California, San Francisco) containing 10% heat-treated FBS, 10% rat T-STIM factor with Con A (BD Biosciences), 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/L glucose, 1.5 g/L sodium bicarbonate, 50 μM 2-mercaptoethanol, 100 U/ml penicillin G, 100 μg/ml streptomycin, and 10 pg/ml IL-1α (Biolegend). Nucleofection was conducted with the Nucleofector kit V (Amaxa Biosystems), a total of up to 3 μg of plasmid DNA per 2.5 × 106 D10G4.1 T cells, and program T-01. Viability was ≥ 90% at 24 h after the procedure.
Anti-FLAG M2 Ab (Stratagene) is a mouse IgG1 anti-DYKDDDDK mAb, which was used for Western blots at a concentration of 2 μg/ml. Rabbit anti-mouse VPAC2 Abs, which recognize both native VPAC2 and SD VPAC2 (VPAC2de367–380), were generated with the 23-aa synthetic peptide SIHPECRFHLEIQEEETKCAELL, representing a substituent mid-sequence of the aminoterminus of VPAC2, which is the same in mouse and human VPAC2. This Ab detected only one major 48- to 55-kDa protein in Western blots of 10 μg of cellular proteins extracted from mouse WT VPAC2-human Jurkat T cell transfectants, but none in the proteins extracted from mouse VPAC1-human Jurkat T cell transfectants. Rabbit anti-VPAC2 Abs also detected one protein band of the same size in Western blots of 5 μg of selectively extracted membrane proteins from anti-CD3 plus anti-CD28 mAb-activated mouse splenic T cells (Mem-PER membrane protein extraction kit and PAGE prep Advance kit; Pierce). Other Abs were: rabbit anti-c-Maf (M-153) IgG (Santa Cruz Biotechnology), rabbit anti-Jun B (N-17) IgG (Santa Cruz Biotechnology), and mouse monoclonal anti-lamin B1 Ab (Abcam) used as a control for loading of Western blots with equal quantities of nuclear proteins. Western blots were performed and developed as described (13).
Clone c23.5 is a murine IgG2aκ with VIPase catalytic activity, which cleaves extracellular VIP with an efficiency level that removes ~95% of T cell-derived VIP, whereas at the same concentration, highly purified clone UPC10 isotype control has no effect (14, 15).
Quantification of expression of VPAC2 receptors by D10G4.1 transfectants
Total RNA was extracted from each sample with Tri-Reagent (Molecular Research Center), treated with RNase-free DNase I (Qiagen) for 30 min, and isolated on RNeasy columns (Qiagen). The mRNAs encoding mouse VPAC2 receptors and the standard mouse hypoxanthine phosphoribosyl-transferase (HPRT) were amplified in triplicate using the TaqMan reagents: 5′-AAGCAGCCAAACGGAGAATC-3′ and 5′-GGCAGGGCACTGT GACAGTT-3′ primers and 5′-FAM-CTGCAGCGGTGTCTGGGA CAACA-TAMRA-3′ -probe for mVPAC2 and 5′ CTGGTGAAAAGGAC CTCTCG-3′ and 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′ primers and 5′-TET-TGTTGGATACAGGCCAGACTTTGTTGGAT TAMRA-3′ probe for mouse HPRT (Integrated DNA Technologies) as described (16). A Prism 9700 sequence detection system (Applied Biosystems) with the recommended optimal reagents and conditions provided interanalysis coefficients of variation < 4%. The value for each unknown was determined from its optimized threshold cycle (CT) and was normalized with reference to the CT value of HPRT using the comparative CT method (User Bulletin No. 2 of ABI Prism 9700 sequence detection system). Western blots of 2–10 μg of transfectant cellular proteins, which were extracted as described (10), were developed separately with rabbit anti-mouse VPAC2 Abs and anti-FLAG M2 Ab.
Assessment of generation of VIP by D10G4.1 transfectants
Replicate suspensions of 4–5 × 106 D10G4.1 transfectants per ml of RPMI 1640 with 10% FBS, which expressed mouse WT VPAC2 or SD VPAC2 (− 367–380), were incubated for 24–72 h in 24-well plates in which each well had been coated with either 0.5 or 2 μg of anti-CD3 and of anti-CD28 mAbs (BD Pharmingen). After centrifugation of the plate at 1500 × g for 15 min at 4°C, medium samples were transferred to clean test tubes, treated with Halt protease inhibitor mixture (1/100, v/v; Pierce) for 30 min at 37°C, acidified to pH 4.5 with acetic acid, and applied to C18 reversed-phase Sep-Pak columns (Waters) for extraction of VIP, as described (15). Methanol eluates were dried in vacuo and reconstituted in 0.1 ml of PBS each for duplicate quantification of VIP by radioimmunoassay (Phoenix Pharmaceuticals).
Quantification of cellular and fluid-phase concentration of IL-4 and of mRNA encoding IL-4
Replicate suspensions of 0.5–0.8 × 106 D10G4.1 transfectants per 0.6 ml of RPMI 1640 with 10% FBS, which expressed mouse WT VPAC2 or SD VPAC2 (VPAC2de367–380), were preincubated either with or without 1 μM IgG VIPase or isotype control IgG for 30 min at 37°C and incubated for 24–48 h in 24-well plates in which each well had been coated with 1 μg of anti-CD3 and 0.5 μg of anti-CD28 mAbs (15). VIP at 10−9 M to 10−6 M was added every 12 h to some wells. IL-4 in media supernatants was assayed with an ELISA (Endogen; Pierce Biotechnology). In some studies, cells were washed twice with cold PBS, incubated for 30 min at 37°C with Halt protease inhibitor mixture (1/100 in PBS, v/v; Pierce), resuspended in 0.1 ml of PBS with 1/100 Halt and 1% Nonidet P40 (v/v), and subjected to two cycles of freezing and thawing before centrifugation and removal of supernatants for ELISAs of cellular IL-4. Real-time PCR quantification of mRNA encoding IL-4 was conducted exactly as for VPAC2 mRNA with the primers 5′-CGCCATGCACGGAGATG-3′ (forward) and 5′-ACGAGCT-CACTCTCTGTGGTGTT (reverse), and probe 5′-Fam-TGCCAACGTCCTCACAGCAACG-TAm-3′ (Integrated DNA Technologies). After calculating the quantities of IL-4 mRNA in all samples with the CT method and normalizing them to that for HPRT mRNA, relative values were assigned to each based on setting that of IL-4 mRNA in WT VPAC2 transfectants to 1 and effects of anti-TCR Ab stimulation were represented by increments in these relative values.
Transcription factor assays
Replicate suspensions of D10G4.1 transfectants were pretreated and incubated as in studies of IL-4 generation for 4 h at 37°C. Then nuclear proteins were extracted with NE-PER nuclear and cytoplasmic reagents (Pierce). Western blots were performed as described using 5–20 μg of total nuclear protein for electrophoresis and developing blots with rabbit anti-mouse c-Maf and anti-mouse Jun B Abs (Santa Cruz Biotechnology) (16). Levels of active Jun B in replicate 5 μg samples of nuclear proteins were quantified with the TransAM AP-1 kit (Active Motif) in 96-well plates coated with consensus sequence oligonucleotide and using specific Ab to detect active Jun B bound to the oligonucleotide, as described (16).
Results
All studies of differences between mouse WT and SD VPAC2 in transducing signals from VIP to effects on generation of IL-4 by the VPAC2-low D10G4.1 mouse Th2 model cell line were conducted within 48 h of nucleofection with equal amounts of the corresponding expression plasmids. Real time-PCR quantification of mRNA, extracted from the respective transfectants, which encodes each type of VPAC2 GPCR showed similar high levels at 24–48 h (Fig. 1). As for the course of expression of mRNA, membrane proteins extracted from the same sets of transfectants showed similarly high levels of expression of WT and SD VPAC2 for up to 48 h, which encompasses the times of study of IL-4 generation (Fig. 2). The illustration is for samples obtained at 48 h and detected with mouse anti-FLAG M2 mAb to the epitope tag on VPAC2, but results were identical with rabbit anti-VPAC2 Abs, and the levels of WT and SD VPAC2 also were identically high at 24 h with both Abs.
FIGURE 1.
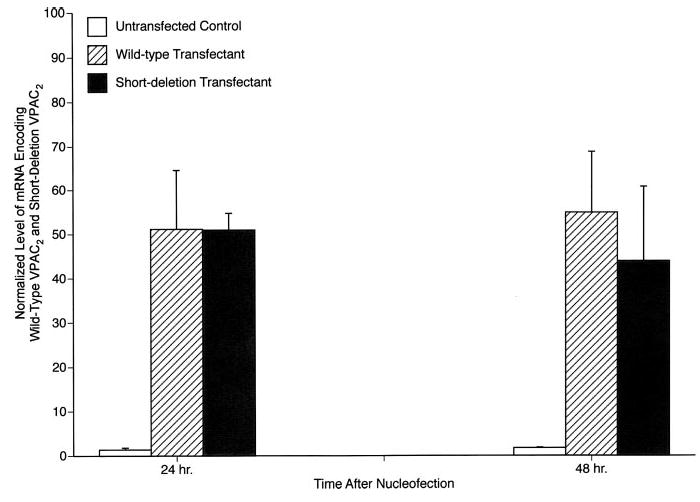
Quantification of the levels of mRNA encoding WT and SD VPAC2 in D10G4.1 cell transfectants. Each column and bar depicts the mean ± SD of the results of three individual determinations. The corresponding values for mock-transfected D10G4.1 Th2 cells were marginally detectable and were indistinguishable from those of untransfected D10G4.1 Th2 cells. Peaks of mRNA encoding each type of VPAC2 receptor are evident at 24 h and persist without significant change at 48 h. None of the differences between levels of mRNA encoding WT and SD VPAC2 were significant.
FIGURE 2.
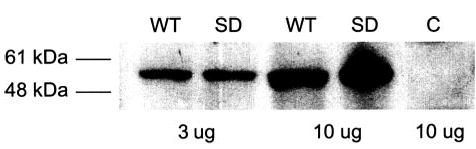
Western blot of membrane proteins extracted from mouse D10G4.1 Th2 cells transfected 48 h before with plasmids encoding mouse WT and SD VPAC2. The primary reagent for development was mouseanti-FLAG M2 mAb. The first pair of WT-SD lanes received 3 μg of extracted proteins, and the second pair 10 μg of extracted proteins; 10 μg of extract of control (C) untransfected D10G4.1 T cells were loaded into the last lane.
The Th2 subset of CD4 T cells is the principal source of T cell-derived VIP, which affects diverse functions of Th2 cells as an autocrine mediator and other immune cells through paracrine mechanisms. Therefore, it was important to document that D10G4.1 mouse Th2 cells generate VIP, and that both WT and SD VPAC2 transfectants produce identical quantities of VIP. It also was of interest to relate these quantities of VIP to the amounts produced by mouse native Th2 cells. The levels of generation of VIP by WT and SD VPAC2-transfectants of D10G4.1 mouse Th2 cells were indistinguishable after 24 h (Fig. 3) and 48 h (not shown) of incubation without and with TCR-directed Ab activation. Vector-only D10G4.1 mouse Th2 cell transfectants produced 1.3 ± 0.4 ng per 106 cells of VIP without stimulation and 2.1 ± 0.6 ng per 106 cells with 2 μg of anti-CD3 plus anti-CD-28 Abs for 24 h. The levels of VIP production by both types of D10G4.1 mouse Th2 cell transfectants were higher than that by mouse native splenic mixed CD4 T cells without stimulation but were not increased significantly by TCR-directed Ab activation, unlike the prominent increases observed with native mixed CD4 T cells (15). The identification of radioimmunoassay-detected VIP as authentic was confirmed by reversed-phase HPLC isolation and repeat RIA quantification.
FIGURE 3.
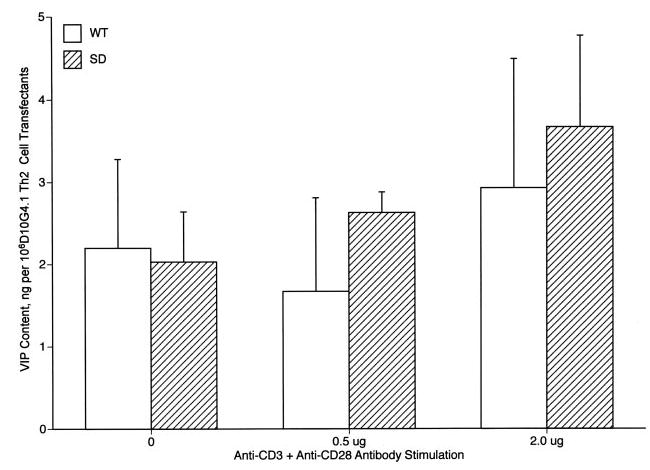
Generation of VIP by mouse D10G4.1 Th2 cell transfectants. Each column and bar represents the mean ± SD of results from two studies analyzed in duplicate. None of the differences between levels of VIP generated by WT and SD transfectants was significant.
The level of IL-4 secreted by D10G4.1 mouse Th2 cells transfected with WT VPAC2 was consistently higher than that secreted by D10G4.1 mouse Th2 cells transfected with SD VPAC2 after stimulation with adherent anti-CD3 plus anti-CD28 Abs. The mean level ± S.D. of IL-4 secreted by 106 D10G4.1 mouse Th2 cells 24 h after TCR-directed Ab activation was 8.9 ± 2.1 ng for WT VPAC2 transfectants and 5.0 ± 1.5 ng for SD VPAC2 transfectants ( p = 0.0046; paired t test). A lack of autocrine IL-4-enhancing signal from SD VPAC2 occupied by endogenous VIP, as contrasted with an effective signal from VIP-occupied WT VPAC2, was considered the most likely explanation for this significant difference. To examine this possibility, suspensions of both types of transfectants were preincubated with an optimally active concentration of 1 μM IgG VIPase for 60 min before stimulation with adherent anti-CD3 plus anti-CD28 Abs in the continued presence of VIPase for 24 h. The level of IL-4 secreted by WT VPAC2 transfectants decreased significantly by 79% from a mean of 11.3 ng/106 D10G4.1 Th2 cells with isotype control IgG to 3.2 ng/106 D10G4.1 in the presence of IgG VIPase ( p < 0.01), whereas the lower mean level secreted by SD VPAC2 transfectants of 8.1 ng/106 D10G4.1 Th2 cells with isotype control IgG was not significantly changed by IgG VIPase (28%) at 6.4 ng/106 D10G4.1 Th2 cells. Quantification of IL-4 mRNA by real-time PCR in the same VIPase protocol similarly showed a significant decrease in WT VPAC2 transfectants as a result of VIPase, whereas the significantly lower level of IL-4 mRNA in SD VPAC2 transfectants was unchanged by VIPase (Fig. 4).
FIGURE 4.
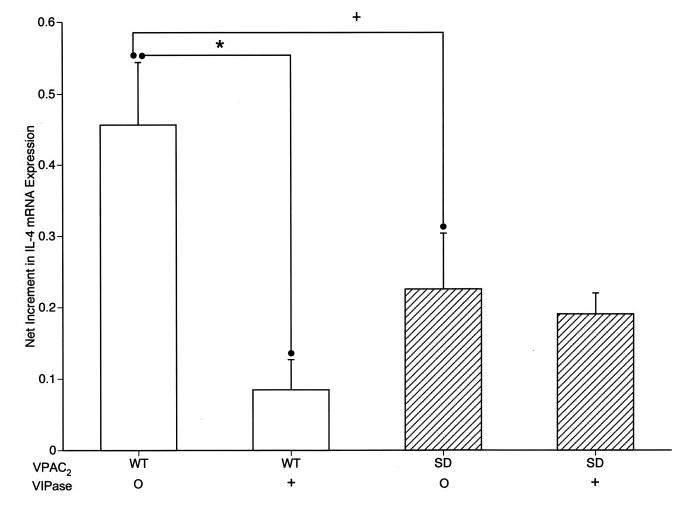
Dependence of the level of expression of mRNA encoding IL-4 by mouse D10G4.1 Th2 cell transfectants on endogenous VIP signaling through WT and SD VPAC2. The difference in corrected levels of mRNA encoding IL-4 in stimulated WT and SD VPAC2 transfectants without VIPase is significant with p < 0.05 (+) and that between WT transfectants without and with VIPase is significant with *, p < 0.01; n = 3, paired Student’s t test.
As the concentrations of endogenous VIP attained in T cell cultures are in the low nanomolar range (Fig. 3), but those in compartmental immune reactions that reflect neural and immune sources in vivo may be up to two orders of magnitude higher, effects of a broad range of concentrations of exogenous VIP on IL-4 production were investigated in D10G4.1 mouse Th2 cell transfectants. IL-4 production by WT VPAC2 transfectants stimulated with adherent anti-CD3 plus anti-CD28 Abs for 24 h was enhanced significantly by 10 nM VIP up to a maximum level at 100 nM VIP that was nearly 3-fold higher than that of controls in the absence of VIP (Fig. 5). In contrast, IL-4 production by SD VPAC2 transfectants stimulated with adherent anti-CD3 plus anti-CD28 Abs for 24 h was suppressed significantly by 30 nM VIP up to a maximum at 300 nM VIP that was a mean of ~50% of control (Fig. 5). That VIP signaling through SD VPAC2 alone decreased IL-4 generation by D10G4.1 mouse Th2 cells suggested that co-expression of SD VPAC2 with WT VPAC2 might suppress the IL-4-enhancing effect of the latter receptor. At a ratio of SD VPAC2 to WT VPAC2 of 0.5 and through a ratio of 4, the IL-4-enhancing activity of WT VPAC2 was suppressed significantly by two concentrations of exogenous VIP (Fig. 6). Thus, both the individual levels and the ratio of levels of expression of these two native forms of VPAC2 determine the net regulatory action of VIP on T cell generation of IL-4.
FIGURE 5.
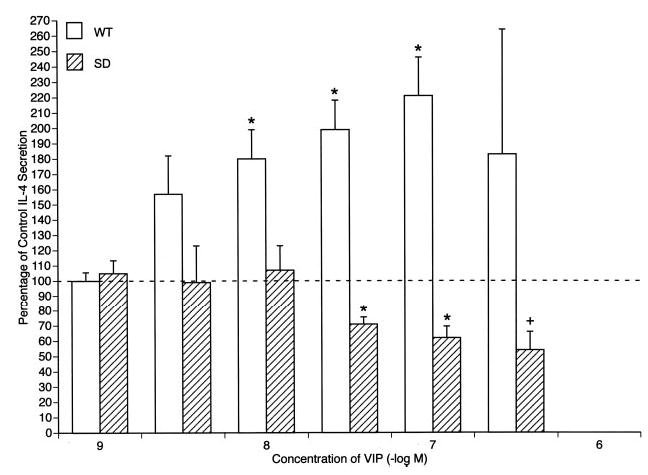
Opposite signals from WT and SD VPAC2 to IL-4 secretion by D10G4.1 Th2 cell transfectants stimulated by adherent anti-CD3 plus anti-CD28 mAbs. Control secretion of immunoreactive IL-4 by transfectants in 24 h without VIP was set at 100%. Each column and bar depicts the mean ± SD of the results of three or more studies. The concentrations of VIP between those marked were 3 × 10−9, 3 × 10−8, and 3 × 10−7 M. The significance of differences between IL-4 secretion in the presence and absence of exogenous VIP for each set of transfectants was calculated by a standard paired t test;+, p < 0.05; and *, p < 0.01.
FIGURE 6.
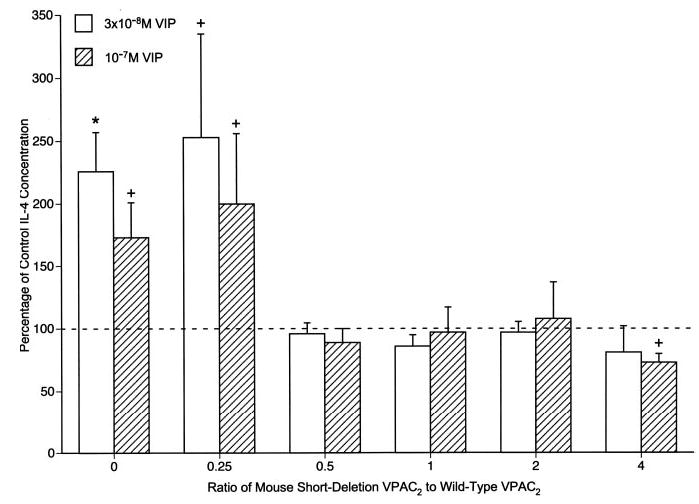
Antagonism of VIP-WT VPAC2 stimulation of IL-4 secretion by VIP-SD VPAC2 in double transfectants stimulated by adherent anti-CD3 plus anti-CD28 mAbs. Control secretion of immunoreactive IL-4 by transfectants in the absence of VIP was set at 100%. Each column and bar depicts the mean ± SD of the results of three studies. The significance of differences between IL-4 secretion in the presence and absence of exogenous VIP was calculated and shown as in Fig. 5.
As c-Maf and Jun B transcription factors have been implicated in VIP-WT VPAC2 signaling of enhanced IL-4 generation, nuclear levels of these proteins were quantified to elucidate mechanisms underlying the differences between the effects of SD VPAC2 and WT VPAC2 on IL-4 generation.
Western blots were completed with nuclear proteins extracted from D10G4.1 mouse Th2 cells transfected with WT VPAC2 and SD VPAC2 alone and in combination and stimulated with anti-CD3 Ab in the absence and presence of an optimally effective concentration of exogenous VIP. These analyses revealed substantial increases in the levels of both c-Maf and Jun B when WT VPAC2 transfectants were exposed to VIP, but not for SD VPAC2 transfectants with VIP (Fig. 7, A and B). Furthermore, the coexpression of SD VPAC2 with WT VPAC2 prevented these VIP-induced increases in transcription factors. Additional evidence for the central involvement of active Jun B in WT VPAC2 signaling of enhanced IL-4 generation and the lack of recruitment of active Jun B by SD VPAC2 came from results of consensus oligonucleotide based-antibody detection assays of its nuclear levels (Fig. 8). In WT VPAC2 transfectants, the nuclear level of active Jun B was increased by a mean of 59% with 10−7 M VIP ( p < 0.01), but was not altered significantly by 10−7 M VIP in SD VPAC2 transfectants or mock-transfected control D10G4.1 mouse Th2 cells. In WT plus SD VPAC2 cotransfectants (1:2), 10−7 M VIP enhanced the increase in active Jun B less than in WT-only VPAC2 transfectants, and this 23% enhancement was not significant (Fig. 8).
FIGURE 7.
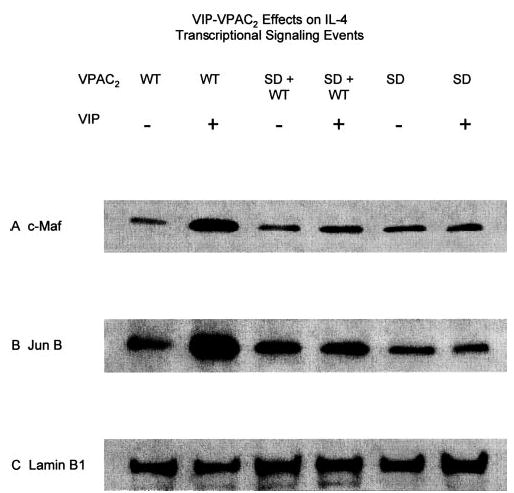
Western blot analyses of effects of WT VPAC2 and SD VPAC2 signals on nuclear levels of transcription factors required for VIP enhancement of generation of IL-4 and the Th2 phenotype. The six lanes shown in each frame of this figure are analyses of nuclear proteins from transfectants expressing: WT VPAC2 without and with 10− 7 M VIP, respectively, SD VPAC2 plus WT VPAC2 (2:1) without and with 10−7 M VIP, and SD VPAC2 alone without and with 10−7 M VIP. A, c-Maf detected by rabbit anti-c-Maf IgG Ab. B, Jun B detected by rabbit anti-Jun B IgG Ab. C, Nuclear protein lamin B1 loading control marker detected by mouse anti-lamin B1 mAb.
FIGURE 8.
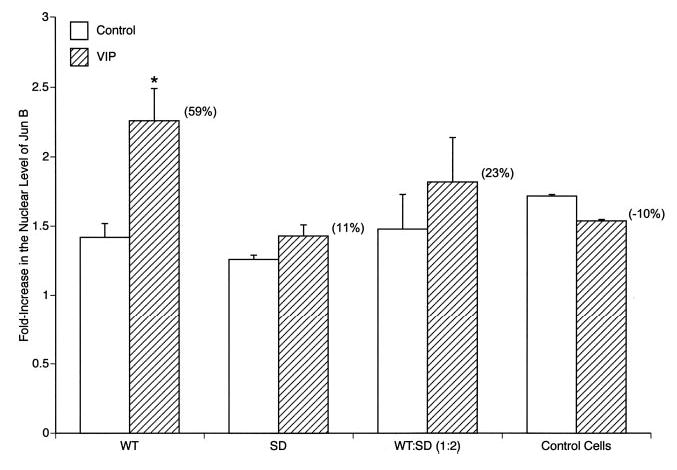
Effect of VIP on anti-CD3 stimulated increases in the nuclear level of active Jun B protein in D10G4.1 Th2 cell transfectants. Each column and bar represents the mean ± range of two studies in duplicate. Suspensions of 106 transfectants in 0.6 ml of RPMI 1640 with 10% FBS were preincubated 60 min without or with 10−7 M VIP and then incubated with 1 μg of anti-CD3 Ab for 4 h. Each number in parentheses is the mean increase (+) or decrease (−) in active nuclear Jun B attributable to VIP. The significance of differences in levels of active nuclear Jun B between samples without and with VIP was calculated by a paired t test; *, p < 0.01.
Discussion
The first stage of elucidation of pathways by which the VIP–VPAC2 neuroimmune axis regulates the nature of tissue compartmental immune responses consisted of in vitro studies of effects of VIP on numerous functions of cultured immune cells (5). Recognition of the capacity of the VIP–VPAC2 axis to alter the normal ratio of Th1/Th2 activities, and thereby subsequent effector events, led to the development of T cell-selective VPAC2 TG mice and VPAC2-null (KO) mice (10, 11). The findings that VPAC2 TG mice had an increased Th2:Th1 ratio, leading to high serum levels of IgE, heightened IgE Ab responses, eosinophilia, and elevated immediate-type hypersensitivity reactions, whereas VPAC2 KO mice had a decreased Th2:Th1 ratio, resulting in strikingly augmented DTH and diminished immediate-type hypersensitivity strongly supported a central role for the VIP–VPAC2 axis as a major determinant of the nature of immune effector responses. What remained to be established was whether and how regulation of secretion of VIP by Th2 cells and of VPAC2 signal transduction in Th2 cells contributed substantially to the immune effector outcome. The present studies were designed to begin to answer these questions and to delineate possible contributions to net VPAC2 signal transduction in T cells of a newly recognized and functionally different T cell SD splice-variant of mouse VPAC2.
These initial investigations were conducted in a model Th2-type D10G4.1 cell with low background expression of WT VPAC2, because native Th2 cells are the main source of immune VIP and show the greatest functional responses to VPAC2-transduced signals (3). Transfectants were created that consistently expressed similar levels of mRNA and membrane protein for WT and SD mouse VPAC2 (Figs. 1 and 2). The first point demonstrated in this system was that both types of transfectants generated and secreted similar levels of VIP that established nanomolar concentrations of extracellular VIP (Fig. 3). The remaining studies thus focused on transduction of VIP signals by the two forms of mouse VPAC2. That the VIP secreted by D10G4.1 Th2 cells expressing WT VPAC2 acts as an autocrine and possibly juxtacrine stimulus capable of signaling through WT VPAC2 to enhance IL-4 generation was shown by the very significant suppression of IL-4 secretion when VIPase eliminated endogenous VIP (Fig. 4). In contrast, IL-4 generation by D10G4.1 Th2 cells expressing SD VPAC2 was significantly lower than that by the WT VPAC2 transfectants and did not decrease as a result of VIPase treatment, indicating a lack of influence of the VIP–SD VPAC2 axis that apparently is attributable to ineffective transduction of stimulatory VIP signals by the SD VPAC2. To examine this difference further at VIP concentrations exceeding those attainable from the Th2 cells but documented in some immunological inflammatory states, the effects of signals from WT and SD VPAC2 were examined in two different protocols. In the first, 10−8 M to 3 × 10−7 M exogenous VIP enhanced significantly secretion of IL-4 by WT VPAC2 transfectants, whereas 3 × 10−8 M to 3 × 10−7 M exogenous VIP inhibited secretion of IL-4 by SD VPAC2 transfectants (Fig. 5). In the second protocol, IL-4 generation by anti-CD3 plus anti-CD28-stimulated transfectants expressing WT and SD VPAC2 together at different ratios was enhanced by WT VPAC2-predominant mixtures, not enhanced (unchanged) at WT to SD VPAC2 ratios of 0.5 to 2, and suppressed slightly by the higher concentration of VIP when the ratio was 4 (Fig. 6). Thus the SD splice-variant of VPAC2 suppresses IL-4-enhancing VIP signals from WT VPAC2 but does not suppress IL-4 generation as much below control levels in a mixture as it did when expressed alone.
To begin delineation of the signaling pathways used by SD VPAC2, as well as WT VPAC2, to regulate IL-4 generation, the transcription factors c-Maf and Jun B that are known to be involved in VIP enhancement of IL-4 generation (16) were examined in a series of VPAC2 transfectants. The VIP–WT VPAC2 axis, but not the VIP–SD VPAC2 axis, increased nuclear levels of c-Maf and Jun B proteins and of active Jun B as quantified respectively by Western blots and a plate-bound consensus oligonucleotide-active Jun B-anti-Jun B Ab assay (Figs. 7 and 8). In WT plus SD VPAC2 cotransfectants, VIP had no apparent effect on nuclear levels of c-Maf and Jun B proteins, and much less effect on nuclear levels of active Jun B than in WT VPAC2-only transfectants, suggesting competitive interactions between the two forms of VPAC2 in the same signaling pathway.
The next logical step to complete these investigations will be to quantify separately the expression of WT and SD VPAC2 in native CD4 T cells under a range of conditions representing states in which the VIP–VPAC2 axis significantly influences T cell-directed immune effector reactions. These studies also could include CD4 T cells from VPAC2 TG and VPAC2-null (KO) mice to assess, respectively, any influence of elevated levels of WT VPAC2 on those of SD VPAC2 and dependence of expression of SD VPAC2 on that of WT VPAC2. As the ratio of these two forms of VPAC2 appears to determine the net effect of VIP, mechanisms controlling expression of the SD VPAC2 are important targets of research and possibly therapeutic intervention. Initial quantification of the two forms of VPAC2 relied on real-time PCR and Western blots of cellular proteins, but it would be desirable also to analyze the orientation of SD VPAC2 in plasma membranes. That only a 14-aa deletion in a transmembrane domain distinguished one form from the other suggested a daunting problem for immunochemical quantification. However, recent results of structural studies indicate that the C-terminal tail of the SD VPAC2 is extracellular rather than cytoplasmic as for the WT VPAC2. Thus, application of Abs that recognize the native C-terminal tail or a C-terminal epitope tag will permit studies of expression of each form separately in intact cells by labeling without and with permeabilization of the plasma membrane. In addition, the possibility must be considered that signal-transducing proteins interact with a different domain of SD VPAC2 than WT VPAC2. All such data will be integrated into a tentative model depicting differences between T cell SD VPAC2 and WT VPAC2.
Footnotes
This research was supported by National Institutes of Health Grant R01 AI29912.
Abbreviations used in this paper: VIP, vasoactive intestinal peptide; VPAC1, VIP type 1 receptor; VPAC2, VIP type 2 receptor; TG, transgenic; KO, knockout; DTH, delayed-type hypersensitivity; HPRT, hypoxanthine phosphoribosyltransferase; WT, wild type; SD, short deletion.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Martinez C, Delgado M, Abad C, Gomariz RP, Ganea D, Leceta J. Regulation of VIP production and secretion by murine lymphocytes. J Neuroimmunol. 1999;93:126–138. doi: 10.1016/s0165-5728(98)00216-1. [DOI] [PubMed] [Google Scholar]
- 2.Dorsam G, Voice J, Kong Y, Goetzl EJ. Vasoactive intestinal peptide mediation of development and functions of T lymphocytes. Ann NY Acad Sci. 2000;921:79–91. doi: 10.1111/j.1749-6632.2000.tb06953.x. [DOI] [PubMed] [Google Scholar]
- 3.Delgado M, Ganea D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol. 2001;166:2907–2912. doi: 10.4049/jimmunol.166.5.2907. [DOI] [PubMed] [Google Scholar]
- 4.Voice JK, Dorsam G, Chan RC, Grinninger C, Kong Y, Goetzl EJ. Immunoeffector and immunoregulatory activities of vasoactive intestinal peptide. Regul Pept. 2002;109:199–208. doi: 10.1016/s0167-0115(02)00182-9. [DOI] [PubMed] [Google Scholar]
- 5.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 6.Delgado M, Gonzalez-Rey E, Ganea D. VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. FASEB J. 2004;18:1453–1455. doi: 10.1096/fj.04-1548fje. [DOI] [PubMed] [Google Scholar]
- 7.Mathew RC, Cook GA, Blum AM, Metwali A, Felman R, Weinstock JV. Vasoactive intestinal peptide stimulates T lymphocytes to release IL-5 in murine schistosomiasis mansoni infection. J Immunol. 1992;148:3572–3577. [PubMed] [Google Scholar]
- 8.Delgado M, Martinez C, Johnson MC, Gomariz RP, Ganea D. Differential expression of vasoactive intestinal peptide receptors 1 and 2 (VIP-R1 and VIP-R2) mRNA in murine lymphocytes. J Neuroimmunol. 1996;68:27–38. doi: 10.1016/0165-5728(96)00063-x. [DOI] [PubMed] [Google Scholar]
- 9.Lara-Marquez M, O’Dorisio M, O’Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol. 2001;166:2522–2530. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- 10.Voice JK, Dorsam G, Lee H, Kong Y, Goetzl EJ. Allergic diathesis in transgenic mice with constitutive T cell expression of inducible vasoactive intestinal peptide receptor. FASEB J. 2001;15:2489–2496. doi: 10.1096/fj.01-0671com. [DOI] [PubMed] [Google Scholar]
- 11.Goetzl EJ, Voice JK, Shen S, Dorsam G, Kong Y, West KM, Morrison CF, Harmar AJ. Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC(2) receptor for vasoactive intestinal peptide. Proc Natl Acad Sci USA. 2001;98:13854–13859. doi: 10.1073/pnas.241503798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinninger C, Wang W, Oskoui KB, Voice JK, Goetzl EJ. A natural variant type II G protein-coupled receptor for vasoactive intestinal peptide with altered function. J Biol Chem. 2004;279:40259–40262. doi: 10.1074/jbc.C400332200. [DOI] [PubMed] [Google Scholar]
- 13.Goetzl EJ, Patel DR, Kishiyama JL, Smoll AC, Turck CW, Law NM, Rosenzweig SA, Sreedharan SP. Specific recognition of the human neuroendocrine receptor for vasoactive intestinal peptide by anti-peptide antibodies. Mol Cell Neurosci. 1994;5:145–152. doi: 10.1006/mcne.1994.1016. [DOI] [PubMed] [Google Scholar]
- 14.Paul S, Sun M, Mody R, Tewary HK, Stemmer P, Massey RJ, Gianferrara T, Mehrotra S, Dreyer T, Meldal M, et al. Peptidolytic monoclonal antibody elicited by a neuropeptide. J Biol Chem. 1992;267:13142–13145. [PubMed] [Google Scholar]
- 15.Voice JK, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl EJ. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J Immunol. 2003;170:308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- 16.Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl EJ. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol. 2004;172:7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]


